Abstract
We report that administration of the low-molecular-weight thiol pantethine prevented the cerebral syndrome in Plasmodium berghei ANKA-infected mice. The protection was associated with an impairment of the host response to the infection, with in particular a decrease of circulating microparticles and preservation of the blood–brain barrier integrity. Parasite development was unaffected. Pantethine modulated one of the early steps of the inflammation–coagulation cascade, i.e., the transbilayer translocation of phosphatidylserine at the cell surface that we demonstrated on red blood cells and platelets. In this, pantethine mimicked the inactivation of the ATP-binding-cassette transporter A1 (ABCA1), which also prevents the cerebral syndrome in this malaria model. However, pantethine acts through a different pathway, because ABCA1 activity was unaffected by the treatment. The mechanisms of pantethine action were investigated, using the intact molecule and its constituents. The disulfide group (oxidized form) is necessary to lower the platelet response to activation by thrombin and collagen. Thio-sensitive mechanisms are also involved in the impairment of microparticle release by TNF-activated endothelial cells. In isolated cells, the effects were obtained by cystamine that lacks the pantothenic moiety of the molecule; however, the complete molecule is necessary to protect against cerebral malaria. Pantethine is well tolerated, and it has already been administered in other contexts to man with limited side effects. Therefore, trials of pantethine treatment in adjunctive therapy for severe malaria are warranted.
Keywords: blood–brain barrier, phosphatidylserine, Plasmodium, platelet activation
Low-molecular-weight thiols, widely distributed in the living world, show broad physiological activity involving multiple targets. Among them, the dietary provitamin pantethine, a dimer of pantothenic acid linked by disulfide cystamine (1), has been the subject of much research. As a part of CoA, pantethine is a key regulator of lipid metabolism (2–4). Pantethine has been shown also to inhibit in vitro platelet aggregation in a dose-dependent manner (5–7), an action that was not clearly understood (5).
Because platelets play a central role in the pathological process associated with cerebral malaria (CM), we examined the effects of pantethine administration to mice infected with Plasmodium berghei strain ANKA (PbA). The processes identified by using this model are relevant to the human pathology (8). Susceptible strains of mice infected with PbA develop neurological manifestations rapidly followed by death. The pathology is due not to the direct action of the parasite but to a deleterious immune response of the infected host, involving cerebral vascular inflammation with microcirculatory dysfunction (9–13). The ultimate consequence is disruption of the brain microvasculature, enhancement of blood–brain barrier (BBB) permeability, and edema formation leading to major hemodynamic dysfunction (14–16).
One of the earliest steps of the inflammation–coagulation cascade is the translocation of the aminophospholipid phosphatidylserine (PS) from the inner to the outer membrane leaflet (17). The asymmetric distribution of PS is maintained by specific mechanisms (18–20) and collapses as a result of cell activation and subsequent elevation of intracellular calcium levels. At the cell surface, PS provides the physical support for activation of coagulation factors leading to thrombin generation (21); exposed PS causes also the shedding of microparticles (MPs) (22–25) that are normally engulfed by macrophages. It has been showed that the targeted deletion of the ATP-binding cassette transporter A1 (ABCA1) locus in the mouse leads to reduced PS at the red blood cell surface after stimulation and decreased MP generation (26). ABCA1-null mice are completely resistant to cerebral malaria (CM) associated with PbA infection (27). The protection of ABCA1−/− mice against cerebral malaria is associated with the impairment of cellular responses to the parasite (27), highlighting the potential therapeutic interest of a compound able to mimic transporter A1 gene deletion.
We show here that daily injections of pantethine to PbA-infected mice prevented the cerebral syndrome with avoidance of BBB leakage. The protection was associated with down-regulation of platelet responsiveness and impairment of endothelial cell activation. In vitro experiments were performed with isolated platelets, red blood cells, and endothelial cells in an attempt to highlight the presumptive mechanisms of the protection.
Results
Protection Against the Malaria-Associated Neurological Syndrome.
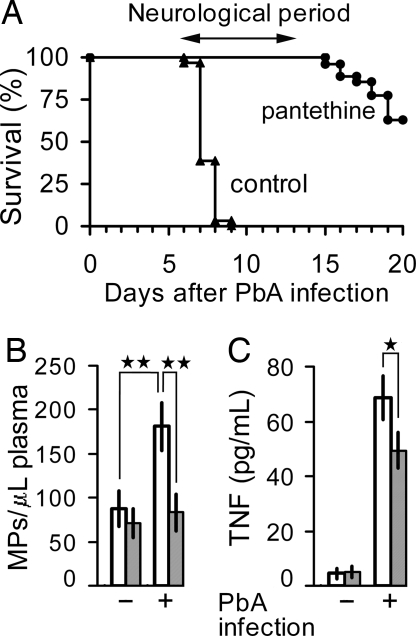
Starting 7 days after infection, PbA-infected mice presented with the neurological signs of cerebral malaria, i.e., ataxia, convulsions followed by death. The syndrome was not seen in mice receiving daily i.p. injections of 30 mg of pantethine, starting on day 1 after infection. At the stage when control infected mice were moribund, pantethine-treated mice were in every aspect similar to uninfected controls (Fig. 1A). Complete protection was obtained with a much lower dose (5 mg) provided that the treatment started earlier, i.e., 8 days before infection. Mice that escaped the cerebral syndrome eventually developed a high parasitemia of >60% of parasitized red blood cells (RBCs) and they died at week 3 after infection and later, without, however, any neurological syndrome. The log-rank test showed that the survival curves of the pantethine-treated and controls groups differed significantly (P < 0.001). Pantethine treatment did not affect parasite proliferation. On day 6 of infection, levels of parasitemia were comparable in all groups (6–8%). PbA infection was associated with a dramatic increase of circulating MP levels in infected-control mice, whereas pantethine-treated ones showed MP levels comparable to that of uninfected animals (Fig. 1B). Protection against the cerebral syndrome was also associated with a significant lower level of circulating TNF in pantethine-treated compared with untreated animals (P < 0.05) (Fig. 1C). Under our experimental conditions, total cholesterol levels in plasma were unchanged by the treatment [supporting information (SI) Table 2]. Other conditions of treatment, such as a dose lower than that mentioned above or applied over a shorter period, led to partial protection, i.e., occurrence of the cerebral syndrome in part of the animals (data not shown). The following pantethine constituents were also tested: cystamine, cysteamine, and pantothenic acid. They were administered at a molar concentration equivalent to the dose of pantethine that gave complete protection, except for cystamine, which was used at 3.75 mg/day, the highest nontoxic dose. None of these compounds showed any protective effect (data not shown).
Fig. 1.
Prevention of the cerebral syndrome by pantethine treatment in PbA-infected mice. (A) cumulative survival analysis of infected CBA/J mice, either treated or not treated with pantethine. Control mice received saline injections (n = 31). Death in this group was due to cerebral complications. The syndrome did not occur in pantethine-treated animals. The treatment consisted in daily i.p. injections of either 30 mg of pantethine, starting on day 1 after infection (n = 20), or 5 mg, starting 8 days before infection (n = 7). The two treated groups are combined in the graph. Beyond the neurological phase, surviving mice died from hyperparasitemia with consequent severe anemia, without cerebral syndrome. (B and C) plasma MP and TNF levels on day 7 after infection in control and treated mice (n = 10 in each group). Open bars, untreated; hatched bars, pantethine treatment. +, infected; −, noninfected. Results are expressed as means ± SD; *, P < 0.05; **, P < 0.01.
Brain Protection by Pantethine in PbA-Infected Mice: Avoidance of BBB Permeability.
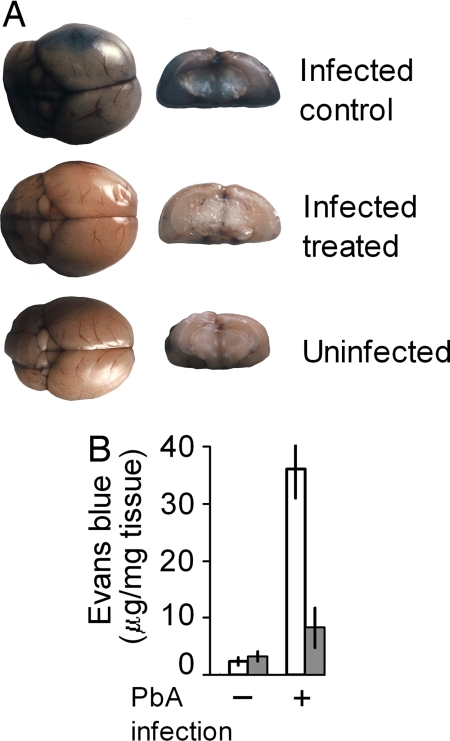
The brains of infected mice were stained intensely by Evans blue administered intravenously on day 7 after infection, indicating a widespread increase in vascular permeability and rupture of the BBB. In contrast, there was only little dye leakage in infected mice treated with pantethine and the brain tissue looked similar to that of the uninfected control (Fig. 2A). The inhibition of the diffusion of the stain in treated animals was confirmed by the measurement of Evans blue in brain extracts (Fig. 2B).
Fig. 2.
Prevention of blood–brain barrier leakage by pantethine treatment in PbA-infected mice. (A) BBB leakage as revealed by intravenously injected Evans blue in an untreated PbA-infected mouse; in contrast the brain of an infected pantethine-treated mouse was not stained and was comparable to the uninfected. (Left) Whole brain. (Right) Frontal section. (B) Evans blue concentration in brain extracts from uninfected and PbA-infected mice, treated or untreated with pantethine (n = 4 in each group). Open bars, untreated; hatched bars, pantethine treatment. +, infected; −, uninfected. Results are means ± SD.
Assessment of Cellular Pantethine Effects: Decrease of PS Exposure.
Platelets were collected from uninfected animals either treated or not treated with pantethine. PS exposure was determined by flow cytometric analysis of annexin V–FITC binding and by measurement of prothrombinase activity, which reflects PS-supported activation of procoagulant factors. After activation with thrombin plus collagen, the percentage of annexin-binding platelets and the prothrombinase activity were significantly lower in samples collected from pantethine-treated mice (SI Fig. 5A). A similar decrease of prothrombinase activity was obtained when platelets were collected from untreated mice and incubated with increasing concentrations of pantethine (ex vivo treatment) (SI Fig. 5A). Similar results were obtained with RBCs (SI Fig. 5B). Moreover, pantethine treatment led to a defective outward movement of the fluorescent tracer NBD-PS in response to Ca2+ stress, whereas NBD-PC randomization was unaffected (SI Fig. 6). Pantethine effects on PS distribution is unlikely to be mediated by ABCA1, because ABCA1 activity was unaffected by the treatment (SI Fig. 7).
Decreased Platelet Reactivity by Pantethine in Healthy and PbA-Infected Mice.
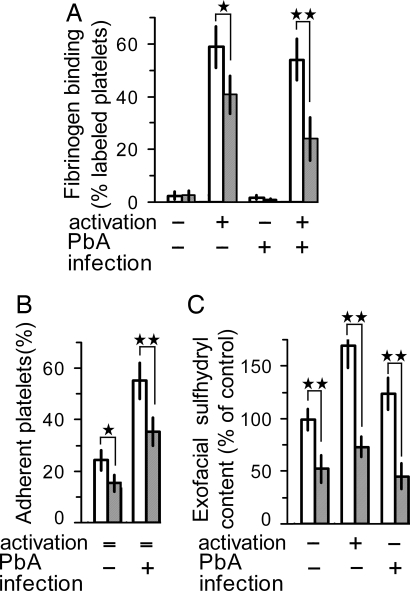
We examined the effect of in vivo pantethine treatment on platelet responsiveness to thrombin or collagen. After activation by thrombin, 58% of the platelets collected from control mice bound fibrinogen-Cy5; the value significantly decreased to 41% on platelets collected from pantethine-treated mice (P < 0.05) (Fig. 3A). Pantethine effect was even stronger on platelets from PbA-infected mice, with 24% of fibrinogen-labeled platelets compared with 55% on controls (P < 0.01). Activation with collagen instead of thrombin produced comparable effects (data not shown). Fibrinogen binding was barely detected in unstimulated platelets.
Fig. 3.
Decreased platelet responsiveness after in vivo treatment by pantethine. Platelets were isolated from healthy and in PbA-infected mice on day 6 after infection; in both conditions, mice received either pantethine or saline injections (n = 6 in each group). (A) fibrinogen-Cy5 binding to nonactivated or thrombin-activated platelets. (B) platelet adhesion to collagen-coated plates. Adherent platelets were quantified by phosphatase activity and expressed as percentage of the total platelet population. (C) platelet activation was associated with an increase in exofacial sulfhydryl content; the increase was significantly weaker in platelets from pantethine-treated mice than in controls. A significant decrease of exofacial sulfhydryls by pantethine treatment was observed also in PbA-infected mice. Sulfhydryl content is expressed as percentage of control. Open bars, untreated; hatched bars, pantethine treatment. +, activated and/or infected; −, nonactivated and/or noninfected; =, nonapplicable. Results are means ± SD. *, P < 0.05; **, P < 0.01.
Moreover, treatment with pantethine significantly reduced platelet adhesion to collagen. Under our experimental conditions, ≈22% of circulating platelets collected from healthy mice adhered to collagen; the percentage was significantly lower (15%) with platelets from pantethine-treated mice (P < 0.05) (Fig. 3B). Circulating platelets showed a higher reactivity in PbA-infected mice, with 56% of collagen-adherent ones; the percentage was reduced significantly (P < 0.01) to 35% in treated mice (Fig. 3B).
In an attempt to highlight the presumptive mechanisms of pantethine action, ex vivo experiments were performed by using the chemical constituents of the molecule. Table 1 shows that fibrinogen binding to activated platelets and platelet adhesion to collagen were significantly reduced after treatment with pantethine or cystamine. Conversely, pantethine derivatives lacking the disulfide group, such as pantothenic acid and cysteamine, had no effect.
Table 1.
Effects of pantethine constituents on platelet reactivity
| Platelets incubated with | Fibrinogen binding, % labeled platelets |
Adherent platelets, % | |
|---|---|---|---|
| Without activation | With activation | ||
| Control | 2.6 ± 1.1 | 50.4 ± 5.5 | 30.8 ± 3.2 |
| Pantethine | 2.9 ± 0.9 | 28.8 ± 7.2** | 24.6 ± 2.2* |
| Cysteamine | 3.5 ± 0.8 | 48.7 ± 6.1 | 36.5 ± 5.0 |
| Cystamine | 2.9 ± 0.9 | 38.9 ± 4.5* | 17.0 ± 2.3** |
| Pantothenic acid | 2.3 ± 1.2 | 51.6 ± 4.7 | 33.1 ± 4.1 |
Effects of reduced/oxidized pantethine constituents on platelet fibrinogen binding and platelet adhesion to collagen. Platelets were incubated with the compounds at a concentration of 1 mM for 30 min at 37°C. They were then activated with thrombin plus collagen for determination of fibrinogen binding or transferred to collagen-coated wells for adhesion assay. Values are means ± SD of quadruplet assays in each of two independent experiments. Values significantly different from the control: *, P < 0.05 and **, P < 0.01.
Platelet function involves sulfhydryl-dependent reactions on exofacial proteins. As a matter of fact, we observed that platelet activation resulted in a significant increase in surface sulfhydryl levels. Redox status was preserved in platelets from pantethine-treated mice; in this case, the amount of -SH moieties was less than half of that observed in untreated controls, either in resting or in activated platelets. Similar effects of pantethine treatment were observed also in platelets from PbA-infected mice (P < 0.01) (Fig. 3C).
Down-Regulation of MP Release from TNF-Activated Endothelial Cells.
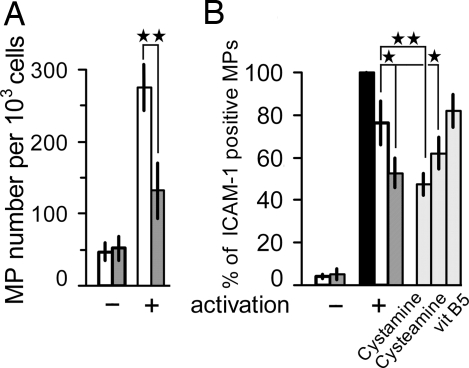
We evaluated the shedding of microvesicles as a consequence of plasma membrane budding induced by increased levels of phosphatidylserine on the outer leaflet. Mouse brain endothelial cells (MBECs) and human umbilical vein endothelial cells (HUVECs) were incubated with 1 mM pantethine for 24 h, then stimulated for 24 h with TNF and compared with untreated activated cells for the concentration of MP in the culture supernatant. The number of annexin V-positive MPs released from MBECs incubated with pantethine was reduced by 51% relative to the control (Fig. 4A). HUVEC-derived MPs were double-labeled with annexin V and anti-ICAM-1. Seventy-six percent of annexin-positive MPs expressed ICAM-1 in controls. The percentage decreased significantly to 52% after incubation with pantethine. Incubation with cystamine had a similar effect, whereas compounds lacking the -SS- bond, vitamin B5 and cysteamine were ineffective or had a slight but significant effect, respectively (Fig. 4B). The effect of cysteamine may have resulted from the partial oxidation of the compound during the 24-h exposure of the cells.
Fig. 4.
Pantethine reduced microparticle (MP) release by TNF-activated endothelial cells. MBECs (A) and HUVECs (B) were incubated with pantethine and then activated with TNF. MPs were collected by centrifugation, processed, and quantified by flow cytometry. MPs derived from MBECs were labeled with annexin V–FITC. Results are expressed as MP number per 1 × 103 cells. MPs derived from HUVEC were double-labeled with annexin V–FITC and mAb directed against ICAM-I; results are expressed as the percentage of mAb-stained MPs within the total annexin V-positive elements. Open bars, untreated control; filled bars, annexin V-positive MPs; hatched bars, incubation with pantethine; gray bars, incubation with pantethine constituents as mentioned. +, TNF-activated; −, nonactivated. Results are expressed as means ± SD; *, P < 0.05; **, P < 0.01.
Discussion
We found that the administration to mice of the low-molecular-weight thiol pantethine prevented the occurrence of cerebral malaria (CM) after infection with P. berghei ANKA. The protection was associated with the down-regulation of two key cellular responses to the infection, i.e., platelet reactivity and microparticle release by the endothelial cells. The most striking sign of the attenuated pathological process was the maintenance of the blood–brain barrier.
The protection obtained by pantethine treatment was comparable to that observed in ABCA1−/− mice (27). Both the transporter A1 and pantethine are known to regulate lipid metabolism and reduce total cholesterol levels. However, because the transfer of cellular cholesterol to apolipoproteins is mediated by ABCA1, HDL levels decrease in ABCA1−/− mice (27), whereas they increase in pantethine-treated animals (28, 29). This suggests that ABCA1 activity is not altered by pantethine, and, in effect, we showed that ApoA1 binding to RAW cells expressing ABCA1 was not affected by pantethine. Under our experimental conditions, total cholesterol levels remained unchanged in pantethine-treated mice on day 6 after infection, i.e., at the onset of the cerebral syndrome period.
To better understand the protection by pantethine, we explored its effects on platelets, red blood cells, and endothelial cells under physiological and pathological conditions. Platelets play a dual role in the development of CM (30, 31). On the one hand, they initiate the repair of the injured endothelial barrier; on the other hand, they trigger multiple pathogenic processes, leading to microvascular alteration (10, 32). Platelet reactivity involves sulfhydryl-dependent reactions on exofacial proteins. Disruption of disulfide bonds in the αIIb/β3 integrin is associated with activation of this receptor so that it binds fibrinogen, leading to platelet aggregation (33–35). We showed that actions of pantethine, a dimer of pantothenic acid linked by a disulfide cystamine, involve thio-dependent mechanisms. Platelet redox status was preserved by pantethine treatment in normal conditions and PbA-infected mice. In vitro experiments, using pantethine constituents, showed that the observed down-regulation of platelet reactivity, i.e., fibrinogen binding and platelet adhesion to collagen, was mediated by the -SS- group, because pantethine derivatives lacking the disulfide bond were ineffective.
MPs play an amplifying pathogenic role because they express the same surface determinants as the activated cells of origin and thus disseminate intravascular coagulation (36, 37). We found that pantethine treatment reduced MP levels in PbA-infected mice. In vitro, pantethine and cystamine and to a lesser extent cysteamine impaired MP release by TNF-activated endothelial cells, suggesting that the compounds exerted their effect through a thio-sensitive mechanism. In agreement, intramolecular and/or intersubunit disulfide bonds are important for the activity of enzymes that control membrane phospholipid organization and, therefore, PS exposure and microparticle generation (38).
Cystamine effects in vitro indicate that the pantothenic moiety is not involved in the pantethine activity on isolated cells. However, the presence or absence of pantothenic moiety dramatically changes the in vivo effects. When administered to mice, cystamine exerts a “biochemical shock” (39) with pathogenic consequences. Inhibition of γ-glutamylcysteine synthetase activity by cystamine but not by pantethine (40) is an illustration of the function of the pantothenic moiety. In PbA-infected mice, cystamine did not prevent the cerebral syndrome at the highest nonlethal dose administered. Therefore, the protective action of pantethine requires the entire molecule.
In conclusion, despite the similarities between ABCA1 inactivation and pantethine treatment, protection against cerebral malaria seems to go through two different routes. We show that pantethine effects on platelets are mediated primarily by the maintenance of the redox status, whereas impaired platelet activation associated with ABCA1 defects is mediated by ineffective maturation of dense granules (41). The central role played by platelets in the processes leading to endothelial cell injury may then result in convergent protective effects.
Several compounds have been shown to protect against CM in the mouse, the steroid dexamethasone (42), which, however, proved deleterious in patients (43), anti-CD41 antibody (44), recombinant human EPO (45), and recombinant human IFN-α (46). The antioxidant N-acetylcysteine has been tested in patients with some success but shows hepatic toxicity (47, 48). Carbon monoxide may be an attractive candidate (49), but its potential toxicity needs to be considered carefully (50). Pantethine may offer a mechanism of protection against CM, without noticeable side effects. Pantethine exerts only moderate activity on isolated cells that may be sufficient to halt the pathological cascade in the organism. The possibility that, in combination with anti-parasite treatment, pantethine may be an effective therapeutic agent for CM should be considered.
Materials and Methods
Pantethine Treatment of Mice.
Eight-week-old CBA/J females (Charles River Laboratories) were used and handled according to the rules of “Décret no. 87–848 du 19/10/1987, Paris”; approval no. 13.306. Pantethine was indicated by the manufacturer (Sigma–Aldrich) to be 97% pure. Purity of the preparation was further ascertained, using HPLC-mass spectrometry detection (LC-UV-MSD) analysis. Pantethine was administered by daily intraperitoneal injections; unless otherwise mentioned, the dosage regimen used was 30 mg for 5 days, according to preliminary experiments that showed that maximal cellular effects were obtained under these conditions, without side effects. The cellular effects of the treatment were still at a plateau value 24 h after pantethine withdrawal and decreased gradually, disappearing after 4 days. Under these conditions, blood samples were collected 24 h after the last pantethine injection. The blood was taken from the retro-orbital plexus of halothane-anesthetized mice to capillary tubes containing 3.8% trisodium citrate.
Infection of Mice and Treatment.
Mice received intraperitoneal injections of 1 × 106 red blood cells infected with P. berghei ANKA (PbA) (11). The cerebral syndrome (i.e., ataxia and convulsions, rapidly followed by death) occurred on days 7–8 after infection. Starting on the day after infection, mice received daily intraperitoneal injections of either pantethine (30 mg) or the pantethine metabolites cysteamine and pantothenic acid (vitamin B5), administered at the same molar concentration as pantethine. Cystamine was administered to another group of mice, but at a much lower dose (3.75 mg) because of its pathogenic side effects. Control mice received saline injections. An additional group of mice received daily injections of 5 mg pantethine starting 8 days before infection. All compounds were administered throughout the experiment. Parasitemia was determined by using blood samples collected from the tail and blood smears stained with Giemsa. Circulating MP levels (27) and plasma TNF concentration in infected mice were determined on day 7 postinfection on additional experimental groups. TNF levels were measured by using the Quantikine ELISA kit (R&D Systems) according to the recommendations of the manufacturer.
Blood–Brain Barrier Permeability.
Vascular permeability related to brain injury was assessed by Evans blue extravasation. For this test, four animals were taken randomly in each of the groups on day 7 after infection, i.e., at the onset of the neurological phase. In addition, four healthy, noninfected, mice were used as controls. Animals received 0.1 ml of 1% Evans blue by retro-orbital injection and were killed 1 h later. Evans blue extravasation was evaluated by examination of the brain and quantified on brain extracts by monitoring the OD640 nm (51).
Platelet Preparation.
Platelet-rich plasma (PRP) was obtained by centrifugation of whole blood for 15 min at 200 × g at room temperature. To prepare washed platelets, PRP was centrifuged at 1,500 × g for 10 min. Sedimented platelets were then washed in Hepes-buffered saline and resuspended in the appropriate buffer. Purity of the preparation obtained was checked by double labeling with anti-CD41-FITC and anti-CD61-PE mAbs (BD Biosciences). Samples were analyzed by using a FACSCalibur flow cytometer and CellQuest software. Anti-CD61-labeled events within the platelet gate were <1%, indicating minimal platelet activation. Platelets were activated with 0.5 units/ml thrombin plus 10 μg/ml collagen for 10 min in buffer containing 1 mM CaCl2. Under these conditions, no platelet lysis was observed.
Platelet Responsiveness.
For in vivo experiments, blood samples were collected from healthy or infected mice at day 6 after infection; platelets were isolated and washed as described above. Platelets (5 × 106) were then incubated with 150 μg/ml of cyanin V-labeled fibrinogen for 30 min, either without or with addition of 0.5 units/ml of thrombin. Fibrinogen binding was analyzed by flow cytometry. Adherence of platelets to immobilized type 1 collagen was determined according to Lahav et al. (52). Briefly, isolated platelets (5 × 106) were resuspended in adhesion buffer and transferred to collagen-coated wells for 1 h at room temperature. After washing, adherent platelets were quantified through the determination of acid phosphatase activity according to Bellavite (53). Results are expressed as percentage of control. For ex vivo experiments, platelets collected from healthy mice were incubated with 1 mM pantethine and its constituents for 30 min at 37°C and then processed for fibrinogen binding and adhesion assay.
Determination of Platelet Redox Status.
Exofacial sulfhydryls were quantified by using the membrane-impermeant reagent 5,5′-dithio-bis-(2-nitrobenzoic) acid (DTNB; Ellman's reagent). Platelets (1 × 108 per milliliter in Tyrode's buffer, pH 7.4) were collected from untreated and pantethine-treated mice then washed. In PbA-infected mice, platelets were collected on day 6 after infection. Platelets were either activated or not activated with 0.5 units/ml thrombin plus 10 μg/ml collagen for 10 min. DTNB (0.1 mM) was added to platelet suspensions for 15 min at 25°C. Platelets were pelleted by centrifugation and washed three times, and the concentration of the 2-nitro-5-thiobenzoic acid generated in the supernatant was measured by absorbance at 412 nm.
Measurement of Plasma Cholesterol Levels.
Total cholesterol levels were determined as described in SI Materials and Methods.
Assessment of Membrane Phosphatidylserine Movement in RBCs and Platelets.
PS exposure on the of platelets and RBCs membrane was measured as described in SI Materials and Methods.
Generation and Flow Cytometric Analysis of Endothelial Microparticles (MPs).
For the determination of endothelial cell vesiculation, we used immortalized mouse brain microvascular endothelial cells (MBEC) (line B3) and human umbilical vein endothelial cells (HUVECs) (Cambrex). MBECs were grown in DMEM supplemented with 450 mg/dl glucose, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% FBS. HUVECs were maintained in EGM2 medium with supplements (Cambrex). Confluent cell monolayers were incubated with 1 mM pantethine and its constituents for 24 h (because of its cytotoxicity, cystamine was used at 250 μM). Preliminary experiments, using Trypan blue dye exclusion, had shown that, under these conditions, there was no cytotoxic effect. Cells were then washed and incubated for 24 h with 100 ng/ml TNF (Cliniscience). Generated MPs were determined according to Sabatier et al. (54). MPs were separated by centrifugation and resusupended in PBS and used for cytometric analysis. The MP gate was defined by reference to 1-μm latex beads as described in ref. 36. MBEC-derived MPs were labeled with annexin V–FITC for 15 min at room temperature in the dark and then enumerated as described above. Results are expressed as the number of counted MPs for 103 MBECs. Aliquots of HUVEC-derived MP suspensions were double-labeled with ICAM mAb (Cliniscience) for 30 min and with annexin V–FITC for 15 min in the dark; MPs were then analyzed by using flow cytometry. In this case, results are expressed as the proportion of ICAM-1 labeled MPs in the total number of annexin V-positive MPs.
Statistics.
Nonparametric Mann–Whitney U tests were performed by using GraphPad Prism software; survival curves were compared by using the Log-rank test; P < 0.05 was considered significant. Displayed values are means ± 1 SD.
Supplementary Material
ACKNOWLEDGMENTS.
The authors thank Howard Rickenberg for useful comments and criticisms. Part of the work was performed in Institut National de la Santé et de la Recherche Médicale research unit U399. Institutional support was obtained from Unite Mixte de Recherche Centre National de la Recherche Scientifique 6184 and Institut National de la Santé et de la Recherche Médicale U399.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706867105/DC1.
References
- 1.Dupre S, Graziani MT, Rosei MA, Fabi A, Del Grosso E. Eur J Biochem. 1970;16:571–578. doi: 10.1111/j.1432-1033.1970.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 2.Branca D, Scutari G, Siliprandi N. Int J Vitam Nutr Res. 1984;54:211–216. [PubMed] [Google Scholar]
- 3.Cighetti G, Del Puppo M, Paroni R, Galli Kienle M. Biochim Biophys Acta. 1988;963:389–393. doi: 10.1016/0005-2760(88)90306-2. [DOI] [PubMed] [Google Scholar]
- 4.Wittwer CT, Graves CP, Peterson MA, Jorgensen E, Wilson DE, Thoene JG, Wyse BW, Windham CT, Hansen RG. Atherosclerosis. 1987;68:41–49. doi: 10.1016/0021-9150(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 5.Bon BG, Ballerini PF, Pitteri C, Cerni G, Avogaro P. Curr Ther Res. 1986;40:464–470. [Google Scholar]
- 6.Hofmann B, Lang A, Ostermann G, Arese P, Biffignandi P, Till U. Curr Ther Res. 1987;41:791–801. [Google Scholar]
- 7.Hiramatsu K, Nozaki H, Arimori S. Tokai J Exp Clin Med. 1981;6:49–57. [PubMed] [Google Scholar]
- 8.Schofield L, Grau GE. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- 9.Bagot S, Idrissa Boubou M, Campino S, Behrschmidt C, Gorgette O, Guenet JL, Penha-Goncalves C, Mazier D, Pied S, Cazenave PA. Infect Immun. 2002;70:2049–2056. doi: 10.1128/IAI.70.4.2049-2056.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunt NH, Grau GE. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 11.Lou J, Lucas R, Grau GE. Clin Microbiol Rev. 2001;14:810–820. doi: 10.1128/CMR.14.4.810-820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Francischetti IM, Seydel KB, Monteiro RQ, Whitten RO, Erexson CR, Noronha AL, Ostera GR, Kamiza SB, Molyneux ME, Ward JM, et al. J Thromb Haemost. 2007;5:155–165. doi: 10.1111/j.1538-7836.2006.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanni LA. Redox Rep. 2001;6:137–142. doi: 10.1179/135100001101536238. [DOI] [PubMed] [Google Scholar]
- 15.van der Heyde HC, Bauer P, Sun G, Chang WL, Yin L, Fuseler J, Granger DN. Infect Immun. 2001;69:3460–3465. doi: 10.1128/IAI.69.5.3460-3465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penet MF, Viola A, Confort-Gouny S, Le Fur Y, Duhamel G, Kober F, Ibarrola D, Izquierdo M, Coltel N, Gharib B, et al. J Neurosci. 2005;25:7352–7358. doi: 10.1523/JNEUROSCI.1002-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmon CT. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 18.Devaux PF. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 19.Wolfs JL, Comfurius P, Rasmussen JT, Keuren JF, Lindhout T, Zwaal RF, Bevers EM. Cell Mol Life Sci. 2005;62:1514–1525. doi: 10.1007/s00018-005-5099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwaal RF, Schroit AJ. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 21.Heemskerk JW, Bevers EM, Lindhout T. Thromb Haemost. 2002;88:186–193. [PubMed] [Google Scholar]
- 22.Sims PJ, Wiedmer T, Esmon CT, Weiss HJ, Shattil SJ. J Biol Chem. 1989;264:17049–17057. [PubMed] [Google Scholar]
- 23.Freyssinet JM. J Thromb Haemost. 2003;1:1655–1662. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- 24.Comfurius P, Senden JM, Tilly RH, Schroit AJ, Bevers EM, Zwaal RF. Biochim Biophys Acta. 1990;1026:153–160. doi: 10.1016/0005-2736(90)90058-v. [DOI] [PubMed] [Google Scholar]
- 25.Zwaal RF, Comfurius P, Bevers EM. Cell Mol Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, et al. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 27.Combes V, Coltel N, Alibert M, van Eck M, Raymond C, Juhan-Vague I, Grau GE, Chimini G. Am J Pathol. 2005;166:295–302. doi: 10.1016/S0002-9440(10)62253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomikawa M, Nakayasu T, Tawara K, Kameda KY, Abiko Y. Atherosclerosis. 1982;41:267–277. doi: 10.1016/0021-9150(82)90191-5. [DOI] [PubMed] [Google Scholar]
- 29.Carrara P, Matturri L, Galbussera M, Lovati MR, Franceschini G, Sirtori CR. Atherosclerosis. 1984;53:255–264. doi: 10.1016/0021-9150(84)90126-6. [DOI] [PubMed] [Google Scholar]
- 30.Combes V, Coltel N, Faille D, Wassmer SC, Grau GE. Int J Parasitol. 2006;36:541–546. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Sun G, Chang WL, Li J, Berney SM, Kimpel D, van der Heyde HC. Infect Immun. 2003;71:6553–6561. doi: 10.1128/IAI.71.11.6553-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassmer SC, de Souza JB, Frere C, Candal FJ, Juhan-Vague I, Grau GE. J Immunol. 2006;176:1180–1184. doi: 10.4049/jimmunol.176.2.1180. [DOI] [PubMed] [Google Scholar]
- 33.Filizola M, Hassan SA, Artoni A, Coller BS, Weinstein H. J Biol Chem. 2004;279:24624–24630. doi: 10.1074/jbc.M400243200. [DOI] [PubMed] [Google Scholar]
- 34.Essex DW, Li M. Biochemistry. 2003;42:129–136. doi: 10.1021/bi0205045. [DOI] [PubMed] [Google Scholar]
- 35.Yan B, Smith JW. J Biol Chem. 2000;275:39964–39972. doi: 10.1074/jbc.M007041200. [DOI] [PubMed] [Google Scholar]
- 36.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, Hack CE, Sturk A. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 38.Kamp D, Sieberg T, Haest CW. Biochemistry. 2001;40:9438–9446. doi: 10.1021/bi0107492. [DOI] [PubMed] [Google Scholar]
- 39.Bacq ZM, Alexander P. Nature. 1964;203:162–164. doi: 10.1038/203162a0. [DOI] [PubMed] [Google Scholar]
- 40.Martin F, Penet MF, Malergue F, Lepidi H, Dessein A, Galland F, De Reggi M, Naquet P, Gharib B. J Clin Invest. 2004;113:591–597. doi: 10.1172/JCI19557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nofer JR, Herminghaus G, Brodde M, Morgenstern E, Rust S, Engel T, Seedorf U, Assmann G, Bluethmann H, Kehrel BE. J Biol Chem. 2004;279:34032–34037. doi: 10.1074/jbc.M405174200. [DOI] [PubMed] [Google Scholar]
- 42.Neill AL, Hunt NH. Parasitology. 1995;111(Pt 4):443–454. doi: 10.1017/s003118200006594x. [DOI] [PubMed] [Google Scholar]
- 43.Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, Harinasuta T. N Engl J Med. 1982;306:313–319. doi: 10.1056/NEJM198202113060601. [DOI] [PubMed] [Google Scholar]
- 44.van der Heyde HC, Gramaglia I, Sun G, Woods C. Blood. 2005;105:1956–1963. doi: 10.1182/blood-2004-06-2206. [DOI] [PubMed] [Google Scholar]
- 45.Kaiser K, Texier A, Ferrandiz J, Buguet A, Meiller A, Latour C, Peyron F, Cespuglio R, Picot S. J Infect Dis. 2006;193:987–995. doi: 10.1086/500844. [DOI] [PubMed] [Google Scholar]
- 46.Vigario AM, Belnoue E, Gruner AC, Mauduit M, Kayibanda M, Deschemin JC, Marussig M, Snounou G, Mazier D, Gresser I, et al. J Immunol. 2007;178:6416–6425. doi: 10.4049/jimmunol.178.10.6416. [DOI] [PubMed] [Google Scholar]
- 47.Treeprasertsuk S, Krudsood S, Tosukhowong T, Maek ANW, Vannaphan S, Saengnetswang T, Looareesuwan S, Kuhn WF, Brittenham G, Carroll J. Southeast Asian J Trop Med Public Health. 2003;34:37–42. [PMC free article] [PubMed] [Google Scholar]
- 48.Watt G, Jongsakul K, Ruangvirayuth R. Qjm. 2002;95:285–290. doi: 10.1093/qjmed/95.5.285. [DOI] [PubMed] [Google Scholar]
- 49.Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, et al. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 50.Hunt NH, Stocker R. Nat Med. 2007;13:667–669. doi: 10.1038/nm0607-667. [DOI] [PubMed] [Google Scholar]
- 51.Green TP, Johnson DE, Marchessault RP, Gatto CW. J Lab Clin Med. 1988;111:173–183. [PubMed] [Google Scholar]
- 52.Lahav J, Wijnen EM, Hess O, Hamaia SW, Griffiths D, Makris M, Knight CG, Essex DW, Farndale RW. Blood. 2003;102:2085–2092. doi: 10.1182/blood-2002-06-1646. [DOI] [PubMed] [Google Scholar]
- 53.Bellavite P, Andrioli G, Guzzo P, Arigliano P, Chirumbolo S, Manzato F, Santonastaso C. Anal Biochem. 1994;216:444–450. doi: 10.1006/abio.1994.1066. [DOI] [PubMed] [Google Scholar]
- 54.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, et al. Diabetes. 2002;51:2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.