Abstract
Dysregulation of brain serotonin (5-HT) neurotransmission is thought to underlie mental conditions as diverse as depression, anxiety disorders, bipolar disorder, autism, and schizophrenia. Despite treatment of these conditions with serotonergic drugs, the molecular mechanisms by which 5-HT is involved in the regulation of aberrant emotional behaviors are poorly understood. Here, we generated knockin mice expressing a mutant form of the brain 5-HT synthesis enzyme, tryptophan hydroxylase 2 (Tph2). This mutant is equivalent to a rare human variant (R441H) identified in few individuals with unipolar major depression. Expression of mutant Tph2 in mice results in markedly reduced (≈80%) brain 5-HT production and leads to behavioral abnormalities in tests assessing 5-HT-mediated emotional states. This reduction in brain 5-HT levels is accompanied by activation of glycogen synthase kinase 3β (GSK3β), a signaling molecule modulated by many psychiatric therapeutic agents. Importantly, inactivation of GSK3β in Tph2 knockin mice, using pharmacological or genetic approaches, alleviates the aberrant behaviors produced by 5-HT deficiency. These findings establish a critical role of Tph2 in the maintenance of brain serotonin homeostasis and identify GSK3β signaling as an important pathway through which brain 5-HT deficiency induces abnormal behaviors. Targeting GSK3β and related signaling events may afford therapeutic advantages for the management of certain 5-HT-related psychiatric conditions.
Keywords: GSK-3, mood disorders, serotonin, Tph2, functional polymorphism
Serotonin (5-HT) is involved in multiple aspects of normal brain functions ranging from the regulation of mood to the control of appetite and social interactions (1–3). Several studies have suggested a contribution of abnormal 5-HT transmission in various human psychiatric conditions and drugs acting on 5-HT neurotransmission are commonly used for the management of major depression, anxiety disorder, obsessive-compulsive disorder, autism, and schizophrenia (1–3). Although drugs that influence the 5-HT system can affect histone acetylation, modulate production of brain-derived neurotrophic factor, increase neural progenitor cell proliferation, and inhibit glycogen synthase kinase 3β (GSK3β) in certain brain areas (4, 5), the mechanisms underlying the regulation of behavior by 5-HT are still obscure.
There are indications that pharmacologic manipulations of 5-HT levels by different classes of drugs can affect distinct neuronal signaling mechanisms (6–9). For instance, multiple classes of 5-HT drugs, including selective 5-HT reuptake inhibitors (SSRIs), tricyclic antidepressants, monoamine oxidase inhibitors, and atypical antipsychotics (5, 7, 9), inhibit brain GSK3β signaling. GSK3β is also inhibited in vivo by lithium (6, 10–12), which is often used in combination with antidepressants for the management of certain mood disorders (13). However, whether these changes in GSK3β are incidental or contribute to the regulation of 5-HT-related abnormal behaviors is unexplored (5). GSK3β is a constitutively active kinase that is inhibited following the phosphorylation of the Ser-9 residue located in its amino-terminal domain (14). In mouse brain, pharmacological stimulation of 5-HT1 receptors enhances phosphorylation of the Ser-9 residue, thus leading to inhibition of the kinase (7). In contrast, 5-HT2 receptor signaling reduces GSK3β phosphorylation thus leading to kinase activation (7). Hence, a general 5-HT deficiency may exert differential effects on GSK3β activity in brain regions expressing diverse sets of 5-HT receptors.
Several lines of evidence indicate that the rate-limiting enzyme for 5-HT synthesis in adult brain is tryptophan hydroxylase 2 (Tph2) (15, 16). This enzyme catalyzes the conversion of the amino acid tryptophan to 5-hydroxytryptophan (5-HTP) that is subsequently decarboxylated to 5-HT by l-aromatic amino acid decarboxylase. Recent investigations of naturally occurring genetic polymorphisms in humans and mice have identified functional mutations in Tph2 that lead to pronounced reduction in enzyme activity (15, 17–19). A rare R441H TPH2 variant has been identified in individuals from a small cohort of elderly patients with major unipolar depression. This mutation causes a reduction of ≈80% of enzyme activity when expressed in transfected PC12 cells (18). To investigate more directly the effects of this mutation on in vivo 5-HT synthesis, cellular signaling, and behavior, we generated mice where the R441H TPH2 mutation was engineered at the equivalent R439H amino acid residue of the mouse Tph2. Here we show that this mutation recapitulates in the mouse the neurochemical changes predicted from cellular studies of the human mutant Tph2 and reveals an important role of the GSK3 signaling pathway in the behavioral abnormalities associated with reduced brain 5-HT levels.
Results
Generation of R439H Tph2 Knockin Mice.
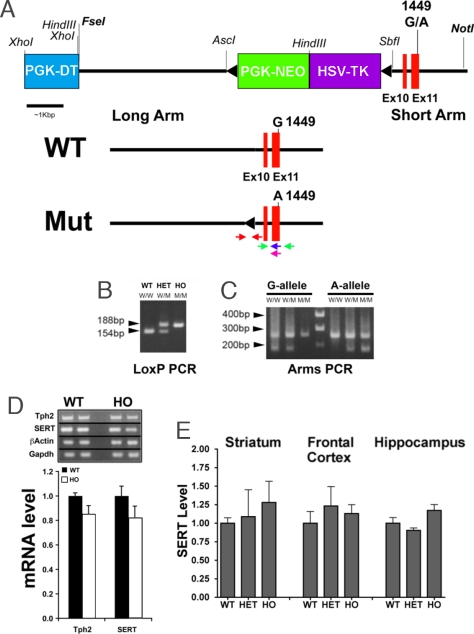
R439H Tph2 knockin mice were generated by using a homologous recombination strategy that resulted in the insertion of the mutation in exon 11 and of a residual loxP site in the ninth intron of the gene (Fig. 1A). Homozygous (HO) and heterozygous (HET) mutant mice were first identified using a PCR genotyping protocol detecting the presence of the LoxP insertion in intron 9 (Fig. 1B). Subsequently, the presence of the mutated tph2 alleles in both HO and HET knockin mice was confirmed (Fig. 1C) by ARMS-PCR (15, 20). Mice carrying the R439H mutation were viable, developed without overt phenotype, and reproduced normally. RT-PCR confirmed the integrity of the mutated R439H Tph2 transcript and semiquantiative RT-PCR analyses showed no differences in expression of Tph2 between wild type (WT) and HO R439H Tph2 mice (Fig. 1D). Importantly, expression of the 5-HT transporter (SERT), a target for most antidepressants and a potential contributing factor to the regulation of behavior by 5-HT (1, 2, 21), was also unaffected in these mice as determined by RT-PCR and Western blots (Fig. 1 D and E).
Fig. 1.
Generation of mice expressing the R439H allele of Tph2. (A) Diagram of the Tph2 locus and the targeting vector. The floxed PGK-NEO/HSV-TK cassette, flanked by the PGK-DT cassette and targeting arms, is illustrated. The G1449A coding mutation was engineered in exon 11 of the mouse Tph2 gene. After homologous recombination, ES cell clones carrying the mutated exon 11 were selected using ARMS-PCR and transfected with a CRE recombinase expression vector to remove the PGK-NEO/HSV-TK cassette resulting in a Tph2 mutant allele carrying a mutated exon 11 and a residual LoxP site in intron 9. The WT and mutant (Mut) alleles are shown; the location of PCR primers is indicated, red arrows: LoxP primers, green arrows: ARMS-PCR external primers, blue or purple arrows: ARMS-PCR allele-specific primers. (B and C) PCR genotyping of R439H Tph2 knockin mice. Two genotyping protocols were used, one detected the residual loxP element in intron 9 (B) and the other detected the G1449A mutation in exon 11 (C). W, G-allele; M, A-allele; the molecular weight product sizes for the G- and A-alleles are noted. WT, wild type; HET, heterozygote; HO, homozygous mutant. (D) Semiquantitative RT-PCR of Tph2 and SERT normalized to β-actin, and GADPH mRNA levels in the brainstem of WT and HO R439H Tph2 mice (n = 4 mice per group). (E) Densitometric Western blot analyses of SERT expression in different brain areas of WT, HET, and HO Tph2 knockin mice. Data are normalized to optical densities for WT animals, β-actin was used as a loading control (n = 4 mice per group). Data are means ± SEM.
5-HT Deficiency in R439H Tph2 Knockin Mice.
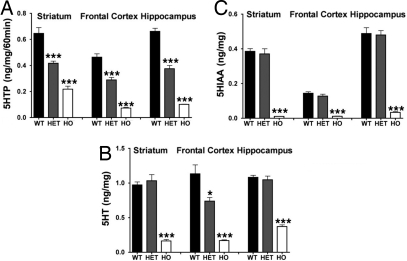
The effect of the R439H mutation on 5-HT brain tissue contents and synthesis in vivo was evaluated. Monitoring the accumulation of the 5HT precursor and Tph2 product, 5HTP, following treatment of mice with the l-aromatic amino acid decarboxylase inhibitor, m-hydroxybenzylhydrazine, provides a direct assessment of Tph2 activity in vivo (15). Synthesis rates in the striatum, frontal cortex (FC), and hippocampus were reduced by ≈40% and ≈80% in HET and HO R439H Tph2 knockin mice, respectively (Fig. 2A). Due to decreased synthesis, tissue contents of 5-HT were also substantially reduced in these same brain regions of HO R439H mice (Fig. 2B). However, in HET mice, a significant reduction in 5-HT contents was only apparent in the FC, suggesting that homeostatic regulation of 5-HT levels may differ among various brain regions. Finally, no differences between WT and HET R439H mice were evident in tissue levels of the 5-HT degradation product 5-hydroxyindoleacetic acid (5-HIAA), whereas the metabolite was reduced to almost undetectable levels in all brain regions examined in HO Tph2 knockin mice (Fig. 2C). Taken together, these neurochemical data indicate that the R439H mutation markedly reduces Tph2 activity in vivo and they provide further direct evidence for the critical role of this enzyme in the regulation of 5-HT synthesis in brain (15).
Fig. 2.
Neurochemical measurements of 5-HT synthesis rates and contents in R439H Tph2 knockin mice. (A) 5-HTP synthesis rates in striatum [ANOVA, FGenotype(2,21) = 5.412, P < 0.012], frontal cortex [ANOVA, FGenotype(2,21) = 11.791, P < 0.003], and hippocampus [ANOVA, FGenotype(2,21) = 17.426, P < 0.001] from WT, HET and HO Tph2 mice. (B) 5-HT tissue contents in striatum [ANOVA, FGenotype(2,21) = 19.236, P < 0.001], frontal cortex [ANOVA, FGenotype(2,21) = 17.675, P < 0.001], and hippocampus [ANOVA, FGenotype(2,21) = 15.319, P < 0.001]. (C) 5-HIAA tissue contents in striatum [ANOVA, FGenotype(2,21) = 9.678, P < 0.001], frontal cortex [ANOVA, FGenotype(2,21) = 6.238, P < 0.007], and hippocampus [ANOVA, FGenotype(2,21) = 8.112, P < 0.002] of WT, HET and HO Tph2 mice (n = 4–11 mice per group). Data are means ± SEM. Bonferroni corrected pair-wise comparisons: *, P ≤ 0.05; ***, P ≤ 0.005 from the WT control.
Activation of GSK3β in the Frontal Cortex of R439H Tph2 Knockin Mice.
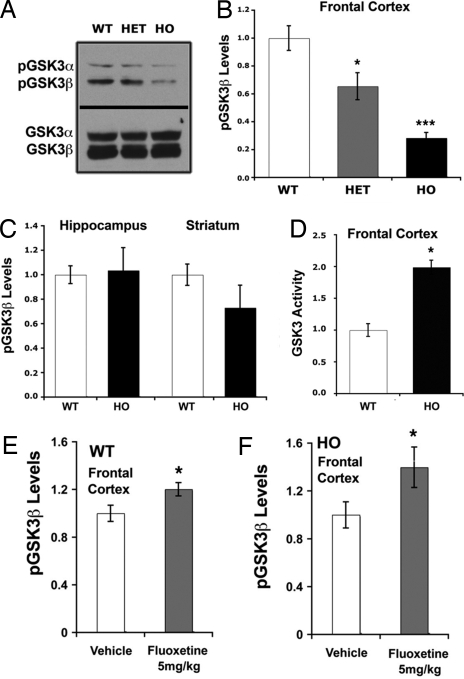
Because GSK3 activity is modulated by pharmacological manipulations of 5-HT levels (5, 7, 22), we assessed the effects of 5-HT deficiency on GSK3β. Ser-9 phosphorylation of GSK3β was measured in the FC, hippocampus, and striatum of WT and R439H Tph2 knockin mice (Fig. 3 A–C). Although overall GSK3β levels were similar in these brain regions for all genotypes, levels of phospho-Ser-9-GSK3β were specifically reduced in the FC of Tph2 knockin mice, suggesting activation of the enzyme (Fig. 3 A–C). Moreover, in FC, decreases in GSK3β phosphorylation were directly proportional to levels of 5-HT synthesis as compared across WT, HET, and HO R439H Tph2 knockin mice, with the latter having the lowest phospho-GSK3β levels and 5-HT synthesis (Figs. 2A and 3B). These data suggest that GSK3β activity within the FC is directly regulated by the availability of 5-HT. To verify this hypothesis, a kinase assay (11) was performed to directly measure GSK3 activities within the FC of WT and HO R439H Tph2 knockin mice. This assay confirmed that reduced GSK3β phosphorylation resulted in an ≈2-fold increase in kinase activity in the FC of HO R439H knockin mice (Fig. 3D). To further evaluate the contribution of 5-HT to the regulation of GSK3β, the SSRI fluoxetine was used (23) to augment extracellular 5-HT levels. Administration of fluoxetine at a dose (5 mg/kg) that normally induces at least a 2-fold increase in extracellular 5-HT levels in the FC (23) resulted in enhanced GSK3β phosphorylation in FC of WT and HO R439H Tph2 knockin mice (Fig. 3 E and F); thus, further supporting a role of 5-HT in the inhibition of GSK3β in this brain region.
Fig. 3.
Regulation of cortical GSK3β activity and levels by 5-HT and fluoxetine. (A–C) Western blot (A) and densitometric analyses (B and C) of phospho-GSK3β (Ser-9) in the frontal cortex (A and B) and hippocampus and striatum (C) of WT and HO R439H Tph2 mice (n = 5 mice per group). Densitometic data were normalized to the average optical density in WT mice, and total GSK3 levels in extracts were used as loading controls for measurement of phospho-protein levels [ANOVA frontal cortex, FGenotype (2,12) = 9.951, P < 0.003]. (D) Kinase activity assays performed after immunoprecipitation of GSK3 from protein extracts prepared from the frontal cortex of WT and HO R439H Tph2 mice [t test, t (1,8) = 4.883, P < 0.006]. Kinase assays were performed using recombinant inhibitor 2 as a substrate and kinase activity in the assay was sensitive to the GSK3 inhibitor kenpaullone (11). (E and F) Phospho-GSK3β levels in the frontal cortex of WT (E) [t test, t (1,8) = 2.768, P < 0.012] and HO R439H Tph2 knockin mice (F) [t test, t (1,8) = 2.639, P < 0.029] (n = 5 mice per group) 30 min after administration of vehicle or 5 mg/kg fluoxetine (s.c.). Data are normalized to the average signal obtained from vehicle-treated mice. Data are means ± SEM. *, P ≤ 0.05; ***, P ≤ 0.005 from the WT or vehicle control; one-way ANOVA with Bonferroni corrected pair-wise comparisons or t test.
GSK3β Contributes to Behavioral Changes in 5-HT-Deficient R439H Tph2 Knockin Mice.
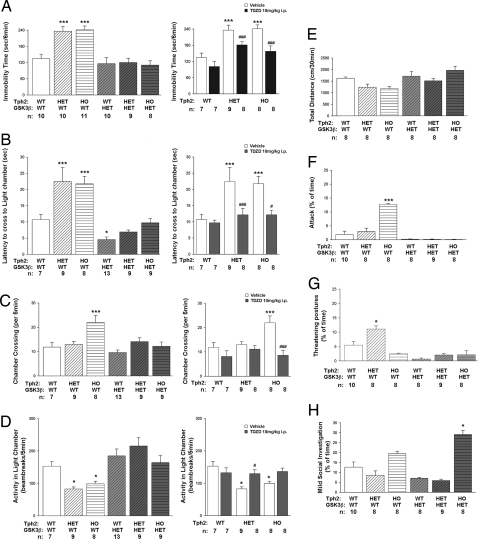
The availability of haploinsufficient GSK3β mice (24) allowed us to assess the contribution of GSK3β activation to behavioral changes induced by 5-HT deficiency. Thus, R439H Tph2 knockin mice were bred with GSK3β+/− animals (24) to produce six genotypes: WT, HET, and HO R439H Tph2 knockin mice, as well as each of the same Tph2 genotypes displaying ≈50% reduction in GSK3β expression. These mice were evaluated on a battery of behavioral tests designed to analyze 5-HT-related emotional states in rodents (Fig. 4). To control for possible developmental differences in haploinsufficient GSK3β mice, some tests were conducted also with WT, HET, and HO R439H Tph2 knockin mice treated acutely with the brain permeable GSK3β inhibitor thiadiazolidinone (TDZD-8) (11, 25).
Fig. 4.
Reduction of GSK3β activity antagonizes behavioral changes in R439H Tph2 mice. (A) Immobility times in tail suspension for vehicle-treated mice from the six genotypes (Left) and for Tph2 knockin mice treated with 10 mg/kg TDZD-8 (i.p.) the GSK3β inhibitor (Right). Data are presented as total immobility time for the 5-min test. [ANOVA, FGenotype(5,52) = 11.481, P < 0.001; TDZD ANOVA, FGenotype(2,41) = 21.775, P < 0.001; FDrug = ns; FGenotypexDrug(2,41) = 11.196, P < 0.001]. (B–D) Dark–light emergence test evaluating latency to the first cross to the lighted chamber [ANOVA, FGenotype(5,49) = 13.564, P < 0.001; TDZD ANOVA, FGenotype(2.41) = 4.595, P < 0.016; FDrug(1.41) = 10.986, P < 0.002; FGenotypexDrug(2,41) = 3.601, P < 0.036] (B), number of crosses to lighted chamber [ANOVA, FGenotype(5,49) = 6.301, P < 0.001; TDZD ANOVA, FGenotype(2.41) = 3.477, P < 0.040; FDrug(1.41) = 14.920, P < 0.001; FGenotypexDrug(2.41) = 4.980, P < 0.012] (C), and locomotor activity in the lighted chamber [ANOVA, FGenotype(5,49) = 6.493, P < 0.001; TDZD ANOVA, FGenotype(2.41) = 5.456, P < 0.008; FDrug(1.41) = 3.982, P < 0.049; FGenotypexDrug(2,41) = 4.794, P < 0.013] (D) in vehicle-treated mice from the six genotypes (Left) and for Tph2 knockin mice treated with 10 mg/kg TDZD-8 (i.p.) (Right). (E) Basal locomotor activities in vehicle-treated mice from the six genotypes. Mice were placed into the open field, and the distance traveled was monitored over 30 min. (F–H) Parameters of social interaction in the dyadic test. (F) Attacks [ANOVA, FGenotype(5,45) = 8.101 P < 0.001]. (G) Threatening postures [ANOVA, FGenotype(5,45) = 2.875 P < 0.025]. (H) Mild exploratory behaviors [ANOVA, FGenotype(5,45) = 24.722 P < 0.001]. For all behavioral tests, balanced groups of male and female mice were used, with the exception of the social interaction test, where only males where used. Data are shown as means ± SEM. *, P < 0.05; ***, P < 0.005, as compared with WT mice; #, P < 0.05; ###, P < 0.005, as compared with vehicle-treated mice from the same genotype, ANOVA with Bonferoni corrected pair-wise comparisons. Numbers of animals for each condition (n) are indicated.
Reduction of immobility times in the tail suspension test, is a paradigm commonly used to evaluate effects of antidepressants in rodents (26). It is well-known that behavioral responses in this test are affected by acute reductions in 5-HT synthesis or its synaptic release (27). Immobility times were prolonged for both HET and HO Tph2 knockin mice (Fig. 4A Left). Strikingly, whereas GSK3β haploinsufficiency and TDZD-8 had no significant effect in animals carrying WT Tph2 alleles, both genetic and pharmacological inhibition of GSK3β activity significantly reversed immobility in HET and HO Tph2 knockin mice (Fig. 4A).
The dark–light emergence test is a 5-HT receptor-responsive behavioral assay used to assess anxiolytic efficacies of drugs such as SSRIs and benzodiazepines in rodents (28, 29). In this test, mice are placed in a darkened compartment and allowed free exploration of the darkened and adjoining lighted compartments. HET and HO Tph2 knockin mice displayed longer latencies to cross to the lighted compartment (Fig. 4B Left), as well as reduced activity in this compartment (Fig. 4D Left). Furthermore, HO Tph2 knockin mice also exhibited more transitions between the two compartments (Fig. 4C). Together, these results indicate that expression of R439H Tph2 results in enhanced anxiogenesis in mice. In contrast, both genetic and pharmacological reductions in GSK3β activity antagonized “anxiety-like” responses induced by the expression of R439H Tph2, and they restored behaviors essentially to levels comparable to those of the WT controls (Fig. 4 B–D).
Because changes in general activity can affect results from these tests (26, 30), basal locomotor activity in the open field was monitored. No differences in basal locomotion were discerned among mice from the six genotypes (Fig. 4E). These findings indicate that reduced 5-HT synthesis in R439H Tph2 mice and GSK3β haploinsufficiency do not affect general motor activity, whereas 5-HT deficiency selectively modulates behavioral responses in the tail suspension and the dark–light emergence tests that are rescued by genetic or pharmacologic inhibition of GSK3β.
Besides exerting multiple effects on the behaviors described above, 5-HT-mediated mechanisms are also associated with changes in more complex behavioral paradigms involving the regulation of emotionality and sociability (31, 32). To assess the impact of reduced 5-HT synthesis and enhanced GSK3β signaling on emotional and social functioning, males from the six genotypes were examined in a test of social interaction in a neutral arena (33). Levels of physical attacks against a nonaggressive male were dramatically enhanced in HO Tph2 knockin males (Fig. 4F). Although HET Tph2 mice did not display increased attacks, these animals did show a significant increase in threatening postures (Fig. 4G), suggesting that aggressive behavior is also enhanced in these mice, albeit to a lesser degree than in the homozygous mutants. Importantly, attacks were completely suppressed in HO Tph2 knockin mice that were also haploinsufficient for GSK3β (Fig. 4F). Reduced GSK3β expression also resulted in decreased threatening postures in HET Tph2 knockin mice (Fig. 4G). Interestingly, GSK3β haploinsufficient HO R439H Tph2 mice displayed markedly enhanced social investigation (Fig. 4H), suggesting that their reduction in numbers of attacks was not due to disruption of investigative social behaviors. Collectively, these results suggest that the well known effects of 5-HT on aggression may be controlled at least in part through regulation of GSK3β activity.
Taken together, our results identify multiple abnormal behaviors in R439H Tph2 mice that are consistent with a generalized reduction in brain 5-HT function. Furthermore, the rescue of these behavioral aberrations by genetic or pharmacologic inhibition of GSK3β indicates that control of the phosphorylation/activity status of this enzyme constitutes an important, and previously unrecognized, mechanism of 5-HT action in brain.
Discussion
The results presented here show that a functional Tph2 gene mutation produces profound reductions of brain 5-HT synthesis in mice. Furthermore, reduced 5-HT levels lead to an activation of cortical GSK3β and the development of behavioral abnormalities in tests used to model endophenotypes of 5-HT associated disorders in rodents. These observations provide direct functional evidence for a contribution of GSK3β mediated signaling in 5-HT functions and further establish Tph2 as an important determinant of brain 5-HT synthesis and mood regulation.
Although further genetic studies will be needed to firmly link this functional R441H Tph2 polymorphism with human depression (34, 35), large-scale genetic analyses have associated several noncoding polymorphisms in the human TPH2 gene with depression, bipolar disorder, and suicidality (36–41). Furthermore, several naturally occurring functional polymorphisms in the Tph2 gene have also been identified in mice (15), rhesus monkeys (42), chimpanzees (43), and humans (17–19). The existence of multiple rare variants in a single gene leading to the development of human pathologies is not a rare occurrence. For example, mainly because of the availability of a readily measurable biological marker in phenylketonuria, as many as 307 missense mutations have been reported in the Tph2-related enzyme phenylalanine hydroxylase (PAH) (34, 44). This suggests that, although the human R441H mutation may contribute to the development of major depression in some individuals, multiple other functional TPH2 mutations can also exist and potentially play a role in the etiology of this and other psychiatric conditions (34).
We previously identified a naturally occurring P447R variant of Tph2 in specific inbred mouse lines. This variant results in a reduction of enzyme activity by ≈50% when expressed in PC12 cells. Furthermore, expression of this Tph2 variant in these inbred mouse lines is associated with reduced brain 5-HT synthesis (15) and differences in aggressive behavior (45), pre-mRNA editing of the 5-HT2C receptor (46), as well as responsiveness to SSRIs (47, 48). However, these studies can only be viewed as correlative because they were all conducted by comparing different strains of inbred mice and the potential contribution of other genetic variations in these mice cannot be ruled out. In contrast, the results presented here provide direct in vivo evidence that a Tph2 genetic variation is sufficient to affect brain 5-HT synthesis and induce biochemical and behavioral changes associated with reduced 5-HT neurotransmission. As such, R439H Tph2 knockin mice represent a unique animal model to study the biological functions of Tph2 and brain 5-HT.
In addition, the R439H Tph2 knockin mice have allowed a direct assessment of the contribution of GSK3β signaling in the regulation of behavior by 5-HT. GSK3β is an established target of lithium, which inhibits this kinase through direct and indirect mechanisms (10, 11). GSK3β has also recently been identified as a common target for SSRIs, tricyclic antidepressants, and antipsychotics (5, 7, 9). Furthermore, reduction of GSK3β phosphorylation, resulting in kinase activation, in the prefrontal cortex has been associated with major depressive disorders in a cohort of suicide victims (49). Although these observations suggest an involvement of GSK3β signaling in the etiology of various disorders and the action of psychoactive drugs, a direct role for GSK3β in the regulation of 5-HT related behaviors or the effect of drugs enhancing 5-HT neurotransmission has remained unclear.
Here we show that 5-HT deficiency leads to a dose-dependent activation of GSK3β in the frontal cortex of mice carrying the R439H Tph2 mutation. In addition, either pharmacological or genetic inhibition of this kinase prevents the expression of behavioral changes induced by 5-HT deficiency in these mice. These results provide direct in vivo evidence for a role of GSK3β activation in behavioral changes associated with low 5-HT synthesis and suggest that drugs that enhance 5-HT neurotransmission may exert some of their actions by inhibiting this kinase. Furthermore, the involvement GSK3β in the regulation of 5-HT-related behavior suggests that inhibition of GSK3β may also contribute to the enhancing effect of lithium and antipsychotics, which are often used as augmentation therapies to increase the efficacy of antidepressants (13, 50). Further studies using tissue specific deletion of GSK3 or other signaling components may be useful to address some of these interesting issues.
In conclusion, our results directly demonstrate the involvement of GSK3β in the regulation of behavior controlled by 5-HT and Tph2. These observations provide a basis for a cell signaling mechanism by which a reduction of 5-HT synthesis leads to the expression of behavioral abnormalities in the animals. Furthermore, these results directly implicate a functional Tph2 gene polymorphism identified in humans in the expression of these behavioral changes and suggest a potential role of Tph2 variants in the etiology of 5-HT related brain disorders. Investigations of the function and mechanisms of GSK3β mediated signaling in 5-HT synaptic transmission should provide research avenues to understand and potentially manage human disorders such as mood disorders and schizophrenia.
Methods
Generation of R439H Tph2 Knockin Mice.
Knockin mice carrying the R439H Tph2 allele (GenBank accession no. NM_173391), equivalent to the human R441H TPH2 allele (GenBank accession no. NM_173353) identified in major unipolar depression patients (18), were derived as follows. A 4.6-kb “long arm” and a 2.0-kb “short arm” were cloned by PCR with EXL DNA polymerase (Stratagene, La Jolla, CA) using genomic DNA obtained from 129S6/SvEv mice as template. The long arm corresponded to sequences from Tph2 intron 9, whereas the short arm comprised intron 9 sequences as well as exon 10, intron 10, exon 11, and ≈1 kb of the 3′UTR of the mouse Tph2 gene. To engineer the R439H mutation, guanine 1449 in exon 11 was changed to an adenosine using site-directed mutagenesis. The long and short arms were then subcloned into a targeting vector (51) resulting in the insertion of a floxed herpes virus thymidine kinase/neomicin (TK/NEO) selection cassette in the ninth intron of the gene. The targeting construct was transfected into 129S6/SvEv mouse ES cells. Clones carrying recombinant Tph2 alleles were selected using a standard diphtheria toxin (DT)/G418 double selection protocol. Positive ES cell clones were subsequently karyotyped, and the occurrence of homologous recombination was confirmed by PCR. Four recombinant ES cell lines were transiently transfected with a CRE recombinase expression construct to remove the TK/NEO selection cassette (Fig. 1A). Ganciclovir-negative selection followed by PCR amplification using primers (5′-CACCCAATTTGCCTGCCGTAGGGA-3′; 5′-GCTGCAAAACATATCACAGAACTCATTCAAGACCA-3′) flanking the TK/NEO cassette were used to select clones lacking the selection cassette. These (LoxP) primers were also used for routine genotyping of Tph2 knockin mice. Moreover, a G-allele/A-allele-specific ARMS-PCR protocol was used to confirm the presence of the G1449A mutation in the selected ES cell clones. The following primers were used for ARMS-PCR: mOuter/Forward primer (5′-TGGTCTTGAATGAGTTCTGTGATATGTTTTGCA-3′); mOuter/Reverse primer (5′-TCATGCTGACAATAACTCTGGTTCTAGGC-3′); G-allele specific primer (5′-TAGGGGTTGAAGTATACCGAGAAGGCAC-3′); A-allele specific primer (5′-TAGGGGTTGAAGTATACCGAGAAGGCAT-3′). Chimeras generated from selected ES cells were intercrossed with C57BL6/J mice; offspring (F1) that inherited the mutant Tph2 allele were identified by PCR analysis (Fig. 1B). Finally, expression and integrity of the mutant Tph2 transcripts in F1 mice carrying the engineered A-allele was confirmed by RT-PCR cloning of brainstem Tph2 full-length mRNA followed by sequencing as described in ref. 15.
Additional Experimental Animals.
GSK3β mice were described in ref. 24. HET R439H TpH2 mice were mated following a two-step breeding protocol (52) with GSK3β-HET animals to obtain littermate WT, HET, or HO R439H TpH2 mice having one or two functional copies of the GSK3β gene. C3H/HeJ mice for dyadic testing were obtained from The Jackson Laboratory (Bar Harbor, MA). Experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee according to National Institutes of Health guidelines.
Neurochemistry.
Methods used to analyze levels of 5-HT and 5-HTP using HPLC and electrochemical detection are described in ref. 15. 5-HT synthesis in vivo was measured in mice treated with 100 mg/kg (i.p.) m-hydroxybenzylhydrazine (NSD-1015) for 1 h (15).
Western Blot Analysis and Kinase Activity Assay.
Briefly, mice were killed by decapitation, after which the heads of the animals were immediately cooled by immersion in liquid nitrogen for 6 s. The right hemisphere FC, striatum, and hippocampus were rapidly dissected (within 60 s) on an ice-cold surface and frozen in liquid nitrogen before protein extraction. Western blot analysis and kinase activity assays were carried out as described in ref. 11. Results were normalized to respective control conditions and presented as means ± SEM.
Measurement of Locomotor Activity.
Locomotion was monitored for 30 min under illuminated conditions in an automated Omnitech Digiscan apparatus (AccuScan Instruments, Columbus, OH). Activity was measured in terms of the total distance traveled (horizontal activity) (11).
Tail Suspension Test.
Mice were tested in a tail suspension apparatus (Med-Associates, St. Albans, VT) as described (26). Behavior was scored as time spent in immobility (sec).
Dark–Light Emergence Test.
The test was performed over a period of 5 min with mice initially placed in the center of the darkened chamber (29). Tests were conducted using a passive-avoidance chamber with activity monitors (Med-Associates). The scored behaviors were: initial latency to enter the lighted compartment, number of compartment entries, and total locomotor activity in each compartment.
Social Interaction Test.
Dyadic tests were conducted as described in ref. 33.
Statistical Analysis.
Data from the neurochemical and biochemical studies were analyzed by univariate ANOVA or two-tailed t tests. ANOVA with Bonferroni corrected pair-wise comparisons were applied to behavioral studies using the Statistical Package for the Social Sciences (SPSS), Version 11.0 (Chicago, IL).
Detailed methods are available in supporting information (SI) Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Cheryl B. Bock from the Duke Comprehensive Cancer Center Transgenic Facility for transgenic services, Wendy Roberts for assistance in the maintenance of mouse colonies, and Yuxin Ma for his unstinting assistance with behavioral testing. GSK3β haploinsufficient mice were a kind gift from James R. Woodgett (Samuel Lunenfeld Research Institute, Toronto, Canada). This work was supported in part by National Institutes of Health Grants MH-79201, MH-73853, MH-60451, and NS19576 (to M.G.C.). Support from the Lennon Family Foundation is also greatly appreciated. M.G.C. is the NARSAD Lattner Foundation Distinguished Investigator. J-M.B. is NARSAD Southwest Florida Investigator and the recipient of a fellowship from the Canadian Institutes of Health Research (CIHR), and X.Z. has a NARSAD Young Investigator award.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711496105/DC1.
References
- 1.Bonasera SJ, Tecott LH. Mouse models of serotonin receptor function: toward a genetic dissection of serotonin systems. Pharmacol Ther. 2000;88:133–142. doi: 10.1016/s0163-7258(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 2.Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berlin) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- 3.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu JM. Not only lithium: regulation of glycogen synthase kinase-3 by antipsychotics and serotonergic drugs. Int J Neuropsychopharmacol. 2007;10:3–6. doi: 10.1017/S1461145706006857. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem. 2006;281:32072–32080. doi: 10.1074/jbc.M606062200. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 10.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 13.De Montigny C, Grunberg F, Mayer A, Deschenes JP. Lithium induces rapid relief of depression in tricyclic antidepressant drug non-responders. Br J Psychiatry. 1981;138:252–256. doi: 10.1192/bjp.138.3.252. [DOI] [PubMed] [Google Scholar]
- 14.Cross DA, et al. The inhibition of glycogen synthase kinase-3 by insulin or insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin, but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J. 1994;303(Pt 1):21–26. doi: 10.1042/bj3030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
- 16.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 17.Lin YM, et al. Association of functional polymorphisms of the human tryptophan hydroxylase 2 gene with risk for bipolar disorder in han chinese. Arch Gen Psychiatry. 2007;64:1015–1024. doi: 10.1001/archpsyc.64.9.1015. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Cichon S, et al. Brain-specific tryptophan hydroxylase 2 (TPH2): A functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm286. Advance Access published September 27, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:E88. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesch KP, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- 24.Hoeflich KP, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 25.Martinez A. TDZD: Selective GSK-3 inhibitor with great potential for Alzheimer disease. Neurobiol Aging. 2006;27:S13. [Google Scholar]
- 26.Crowley JJ, Jones MD, O'Leary OF, Lucki I. Automated tests for measuring the effects of antidepressants in mice. Pharmacol Biochem Behav. 2004;78:269–274. doi: 10.1016/j.pbb.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 27.O'Leary OF, et al. Depletion of serotonin and catecholamines block the acute behavioral response to different classes of antidepressant drugs in the mouse tail suspension test. Psychopharmacology (Berl) 2007;192:357–371. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- 28.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 29.Weisstaub NV, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 30.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl) 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 31.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Saudou F, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–1878. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Beaulieu JM, Gainetdinov RR, Caron MG. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell Mol Life Sci. 2006;63:6–11. doi: 10.1007/s00018-005-5417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blakely RD. Overview: A rare opportunity or just one less reason to be depressed. Neuron. 2005;48:701–702. doi: 10.1016/j.neuron.2005.11.029. author reply, 705–706. [DOI] [PubMed] [Google Scholar]
- 36.Van Den Bogaert A, et al. Association of brain-specific tryptophan hydroxylase, TPH2, with unipolar and biopolar disorder a Northern Swedish, isolated population. Arch Gen Psychiatry. 2006;63:1103–1110. doi: 10.1001/archpsyc.63.10.1103. [DOI] [PubMed] [Google Scholar]
- 37.Harvey M, et al. Support for the involvement of TPH2 gene in affective disorders. Mol Psychiatry. 2004;9:980–981. doi: 10.1038/sj.mp.4001557. [DOI] [PubMed] [Google Scholar]
- 38.Zill P, et al. SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry. 2004;9:1030–1036. doi: 10.1038/sj.mp.4001525. [DOI] [PubMed] [Google Scholar]
- 39.Harvey M, Gagne B, Labbe M, Barden N. Polymorphisms in the neuronal isoform of tryptophan hydroxylase 2 are associated with bipolar disorder in French Canadian pedigrees. Psychiatr Genet. 2007;17:17–22. doi: 10.1097/YPG.0b013e3280111877. [DOI] [PubMed] [Google Scholar]
- 40.Lopez de Lara C, et al. Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2006.09.008. in press. [DOI] [PubMed] [Google Scholar]
- 41.Lopez VA, Detera-Wadleigh S, Cardona I, Kassem L, McMahon FJ. Nested association between genetic variation in tryptophan hydroxylase II, bipolar affective disorder, and suicide attempts. Biol Psychiatry. 2007;61:181–186. doi: 10.1016/j.biopsych.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Chen GL, Novak MA, Hakim S, Xie Z, Miller GM. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: association with hypothalamic-pituitary-adrenal axis function and in vitro gene expression. Mol Psychiatry. 2006;11:914–928. doi: 10.1038/sj.mp.4001870. [DOI] [PubMed] [Google Scholar]
- 43.Hong KW, et al. A new gain-of-function allele in chimpanzee tryptophan hydroxylase 2 and the comparison of its enzyme activity with that in humans and rats. Neurosci Lett. 2007;412:195–200. doi: 10.1016/j.neulet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Pey AL, Desviat LR, Gamez A, Ugarte M, Perez B. Phenylketonuria: Genotype-phenotype correlations based on expression analysis of structural and functional mutations in PAH. Hum Mutat. 2003;21:370–378. doi: 10.1002/humu.10198. [DOI] [PubMed] [Google Scholar]
- 45.Kulikov AV, Osipova DV, Naumenko VS, Popova NK. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005;4:482–485. doi: 10.1111/j.1601-183X.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 46.Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25:648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cervo L, et al. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 49.Karege F, et al. Alteration in kinase activity but not in protein levels of protein kinase B, glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2006;62:72–80. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 50.Valenstein M, et al. What happened to lithium? Antidepressant augmentation in clinical settings. Am J Psychiatry. 2006;163:1219–1225. doi: 10.1176/ajp.2006.163.7.1219. [DOI] [PubMed] [Google Scholar]
- 51.Gainetdinov RR, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- 52.Beaulieu JM, Julien JP. Peripherin-mediated death of motor neurons rescued by overexpression of neurofilament NF-H proteins. J Neurochem. 2003;85:248–256. doi: 10.1046/j.1471-4159.2003.01653.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.