Abstract
Convergent evidence has revealed an association between insulin resistance and Alzheimer's disease (AD), and the peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, rosiglitazone, an insulin sensitizer and mitochondrial activator, improves cognition in patients with early or mild-to-moderate AD. Apolipoprotein (apo) E4, a major genetic risk factor for AD, exerts neuropathological effects through multiple pathways, including impairment of dendritic spine structure and mitochondrial function. Here we show that rosiglitazone significantly increased dendritic spine density in a dose-dependent manner in cultured primary cortical rat neurons. This effect was abolished by the PPAR-γ-specific antagonist, GW9662, suggesting that rosiglitazone exerts this effect by activating the PPAR-γ pathway. Furthermore, the C-terminal-truncated fragment of apoE4 significantly decreased dendritic spine density. Rosiglitazone rescued this detrimental effect. Thus, rosiglitazone might improve cognition in AD patients by increasing dendritic spine density.
Keywords: Alzheimer's disease, mitochondria, peroxisome proliferator-activated receptor-γ, apolipoprotein E fragment, synaptogenesis
Alzheimer's disease (AD), a devastating neurodegenerative disease that usually develops in the sixth decade of life, affects millions of people globally (1). Alarmingly, currently available treatments provide no more than a temporary improvement of functional deficits. Thus, AD cases will increase disproportionately as the global elderly population increases. A major risk factor for late-onset AD is apolipoprotein (apo) E4, which increases the risk and lowers the age of onset in a gene dose-dependent manner (2, 3). The three isoforms of human apoE (apoE2, apoE3, and apoE4) have different roles in lipid metabolism and neurobiology (4–8). ApoE3 promotes neurite outgrowth, dendritic arborization, and synaptogenesis, whereas apoE4 inhibits these processes both in vitro (9–11) and in vivo (12–14). Furthermore, apoE4 transgenic mice have deficits in synaptic plasticity, synaptic terminal remodeling, synaptogenesis, and learning and memory (12–16). Loss of synaptophysin-immunoreactive presynaptic terminals, indicating synaptic loss (12, 17), occurs early in AD and is considered the best pathological correlate of cognitive decline (18–20).
Previously, we showed that neurons express apoE in response to injury (21) and that neuronal apoE is cleaved into C-terminal-truncated fragments resembling those in AD brains (22). ApoE4 is more susceptible to this cleavage than apoE3 (23, 24). ApoE4 fragments are neurotoxic in vitro and cause neurodegeneration and behavioral deficits in transgenic mice (22–24). In neurons, truncated apoE4 escapes the secretory pathway, enters the cytosol, and interacts with tau, increasing its phosphorylation and causing preneurofibrillary tangles (22, 23). In the cytosol, apoE4 fragments also interact with the mitochondria, impairing their membrane potential and function (25). Mitochondrial impairment in AD is greater in apoE4 than in apoE3 carriers (26). Thus, apoE4 may contribute to AD pathogenesis by causing mitochondrial dysfunction and synaptic deficits (6).
Type 2 diabetes also increases the risk of developing AD (27–29), particularly among diabetic patients carrying apoE4 (30). Insulin resistance, the core defect in type 2 diabetes, results in hyperinsulinemia to compensate for the reduced action of insulin in peripheral tissues (31). Correspondingly, AD patients are at increased risk for elevated plasma insulin levels and insulin resistance (32, 33). In addition, AD brains from autopsied patients have markedly lower levels of insulin mRNA and tyrosine phosphorylation than control brains (34). Indeed, inhibition of the neuronal insulin receptor has been proposed as a model for sporadic AD (35). Thus, type 2 diabetes and AD might share a common pathogenic feature that can be modified by apoE genotype.
The thiazolidinediones (e.g., troglitazone, pioglitazone, and rosiglitazone) are agonists of the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ). Because they increase peripheral insulin sensitivity and stimulate mitochondrial biogenesis and function (36, 37), thiazolidinediones are widely used to treat type 2 diabetes (38, 39). In clinical trials, rosiglitazone improved cognition in a subset of AD patients (40, 41) and also reduced learning and memory deficits in a mouse model of AD (42). However, the mechanisms underlying the potential beneficial effects of rosiglitazone in AD remain unclear. In this study, we tested the hypothesis that rosiglitazone increases dendritic spine density and rescues the dendritic spine loss caused by apoE4.
Results
Rosiglitazone Increases Dendritic Spine Density.
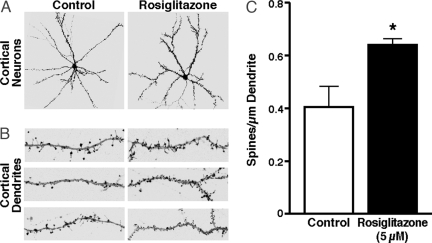
To assess the effect of the PPAR-γ agonist, rosiglitazone, on dendritic spine density, we incubated primary cortical rat neurons, previously cultured for 14–17 days in vitro, with DMSO control or 5 μM rosiglitazone for 24 h. Four to 7 days before the experiment, the cells were transfected with EGFP-tagged β-actin (EGFP–β-actin), a cytoskeletal protein that is abundant in dendritic spines (43). EGFP–β-actin expression does not impair neuronal function or synaptic morphology (44). Rosiglitazone increased dendritic spine density, visible at both low (Fig. 1A) and high (Fig. 1B) magnification of representative neurons and dendrites, respectively, as shown by microscopic analysis. Quantitative analyses showed 58.5 ± 3% greater dendritic spine density in treated neurons than in controls (Fig. 1C). However, rosiglitazone did not affect other parameters of neuronal plasticity, including the area of the dendritic field (estimated by counting the proximal extensions of the dendritic tree), dendrite length (longest distance between the soma and the proximal dendritic extension), and the number of dendritic branch points (extensions originating from primary dendrites) (Fig. 2).
Fig. 1.
Rosiglitazone increases dendritic spine density in rat primary cortical neurons. Spine density is defined as the number of spines per micrometer of dendrite length. The cells, transiently transfected with the synaptic marker EGFP–β-actin and cultured for 14–17 days, were incubated with 5 μM rosiglitazone for 24 h or DMSO only as a control. (A) Representative images showing the stimulatory effect of rosiglitazone on dendritic spine density. (B) Representative dendrites from three different neurons. (C) Dendritic spine densities of eight randomly selected cells per condition were quantified. Values are mean ± SEM. *, P < 0.01 versus control (two-tailed t test).
Fig. 2.
Rosiglitazone does not alter the area of the dendritic field, dendrite length, and the number of dendritic branch points. The cells, transiently transfected with the synaptic marker EGFP–β-actin and cultured for 14–17 days, were incubated with 5 μM rosiglitazone for 24 h or DMSO only as a control. The area of dendritic field (A), dendrite length (B), and the number of dendritic branches (C) from 15 randomly selected cells per condition were quantified. Values are mean ± SEM.
Rosiglitazone's Effects on Dendritic Spine Density Are Dose-Dependent.
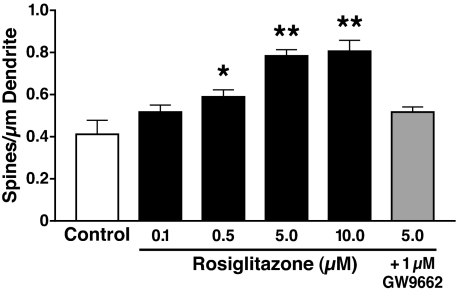
Next, we performed dose–response studies to test the efficacy of rosiglitazone in increasing dendritic spine density. After 14–17 days in culture, primary cortical neurons were incubated with 0.1, 0.5, 5, and 10 μM rosiglitazone at 37°C for 24 h. A comparison of the dentritic complexity of neurons incubated with rosiglitazone versus control suggested no toxic effects at all concentrations used. Dendritic spine density was increased by 29.6 ± 5.2% at a dose of 0.5 μM and by 47.03 ± 3.4% at 5 μM (Fig. 3). No further increase was seen at 10 μM.
Fig. 3.
Dose-dependent effect of rosiglitazone on dendritic spine density in rat primary cortical neurons. The cells, transiently transfected with the synaptic marker EGFP–β-actin and cultured for 14–17 days, were incubated for 24 h with rosiglitazone in the presence or absence of 1 μM PPAR-γ antagonist GW9662 or DMSO only as a control. Dendritic spine densities of eight randomly selected cells per condition were quantified. Values are mean ± SEM. *, P < 0.05; **, P < 0.01 versus control (two-tailed t test).
Rosiglitazone Increases Dendritic Spine Density by Activating the PPAR-γ Pathway.
Depending on the concentration, thiazolidinediones exert both PPAR-γ-dependent and PPAR-γ-independent effects (45). To assess the specificity of rosiglitazone's effect on dendritic spine density, we cultured primary cortical neurons for 14–17 days and incubated them with 5 μM rosiglitazone and 1 μM PPAR-γ-specific antagonist GW9662 at 37°C for 24 h. GW9662 abolished the rosiglitazone-induced increase in dendritic spine density (Fig. 3).
Rosiglitazone Rescues the Dendritic Spine Loss Caused by ApoE4.
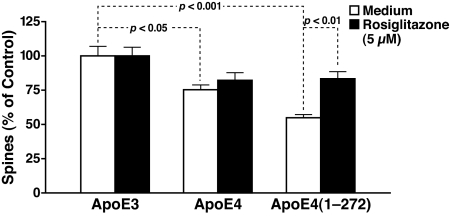
ApoE4 and C-terminal-truncated fragments of apoE4 [apoE4(1–272), lacking the C-terminal 27 aa] impair cytoskeletal structure and mitochondrial function (12, 22, 23, 25). Because cytoskeletal integrity and mitochondrial function are critical for normal synaptic morphology and function (46, 47), we tested the effects of various forms of apoE on dendritic spine density in primary cortical neurons. ApoE4 significantly reduced dendritic spine density by 25.5 ± 4%, and the apoE4(1–272) fragment reduced it by 45.6 ± 3%, compared with apoE3 (Fig. 4). Thus, apoE4 and, to a greater extent, its fragment appear to impair synaptogenesis or synaptic maintenance. Rosiglitazone rescued the dendritic spine loss caused by apoE4(1–272) (Fig. 4). Rosiglitazone also abolished the difference in dendritic spine density between apoE4 and apoE3, although the effect did not reach statistical significance, compared with apoE4 alone (Fig. 4).
Fig. 4.
Rosiglitazone rescues dendritic spine loss caused by the apoE4 fragment. Rat primary cortical neurons, transiently transfected with the synaptic marker EGFP–β-actin and cultured for 14–17 days, were incubated with 7.5 μg/ml apoE (various forms) for 24 h in the presence or absence of 5 μM rosiglitazone. Dendritic spine densities of 10 cells per condition were quantified. Values are mean ± SEM.
Discussion
This study shows that the PPAR-γ agonist, rosiglitazone, increases the density of dendritic spines on cultured primary rat neurons in a dose-dependent manner. In addition, rosiglitazone rescued the loss of dendritic spines caused by the C-terminal-truncated fragments of apoE4.
In clinical trials, rosiglitazone had beneficial effects in patients with early or mild-to-moderate AD (40, 41). Rosiglitazone also reduced learning and memory deficits in a mouse model of AD (42). Our data suggest that rosiglitazone improves cognition by increasing dendritic spine density. In one trial, rosiglitazone at once-daily doses of 2, 4, and 8 mg improved cognition in AD patients carrying apoE3, but not in those carrying apoE4 (41). However, rosiglitazone clearly prevented the dendritic spine loss caused by the apoE4 fragments in primary neuronal cultures. Because rosiglitazone does not readily cross the blood–brain barrier at least in rodents (40), this discrepancy may indicate that the lower doses of rosiglitazone used in AD patients might not achieve brain levels high enough to overcome the detrimental effects of apoE4 and its fragments despite a potentially compromised blood–brain barrier.
PPAR-γ agonists, such as rosiglitazone, have a complex pharmacology beyond their established peripheral effect in activating the PPAR-γ pathway. For instance, they appear to have diverse roles in neuroprotection, such as promoting the expression of the mitochondrial uncoupling protein 2 after ischemia-induced hippocampal injury (48) and suppressing the proinflammatory transcription factor NF-κB through PPAR-γ-dependent (49, 50) or PPAR-γ-independent pathways (51). This finding raises the question of whether the effects of rosiglitazone on dendritic spine density are related to its ability to activate PPAR-γ or other actions. We found that the PPAR-γ-specific antagonist, GW9662, abolished the rosiglitazone-induced increase in dendritic spine density, strongly suggesting that it exerts beneficial effects by activating the PPAR-γ pathway. Further studies are required to elucidate the precise mechanism.
How does rosiglitazone increase dendritic spine density and rescue dendritic spine loss induced by apoE4? One possibility is that rosiglitazone stimulates mitochondrial biogenesis and function (37, 52, 53). Normal mitochondrial dynamics and function are essential for generating and maintaining distinct axonal and dendritic microdomains in response to local metabolic demand (47). Moreover, failure of mitochondria to traffic to appropriate sites causes energy starvation and impairs synaptogenesis and memory formation (47, 54). Thus, rosiglitazone might increase mitochondrial biogenesis or function, thereby improving synaptogenesis or maintenance of dendritic spines. Furthermore, because apoE4 fragments impair mitochondrial integrity and function (25), the rescue of dendritic spine loss also might reflect the beneficial effects of rosiglitazone on mitochondrial biogenesis or function.
Materials and Methods
Reagents.
Rosiglitazone maleate was provided by GlaxoSmithKline. Recombinant apoE3, apoE4, and apoE4(1–272) were provided by Karl Weisgraber (The J. David Gladstone Institutes). The pPDGF–EGFP–β-actin construct was a gift of Yukiko Goda (University College London, London, U.K.). All plasmids were purified with the Plasmid Maxi kit from Qiagen. GW9662, a PPAR-γ antagonist, was from Sigma–Aldrich.
Primary Neuron Culture and Transfection.
Cortices from neonatal rat pups postnatal day 1 were dissected, treated for 30 min with 10 units/ml papain (Worthington Biochemical), and incubated with 10 μg/ml trypsin inhibitor for 15 min. After trituration, dissociated neurons were plated on 12-mm glass coverslips (Fisher Scientific) coated with poly-l-lysine at 8 × 105 cells per square centimeter. After 2 h, cells were transferred into neurobasal medium supplemented with B27, 1 mM l-glutamine, and 100 μg/ml penicillin/streptomycin (Invitrogen). Neurons were routinely transfected after 10 days in culture and used for experiments 4–7 days after transfection. Cells were maintained in a humidified incubator with 5% CO2 at 37°C. The neurons were transfected with 2 μg of pPDGF–EGFP–β-actin construct with 3 μl of Lipofectamine 2000 (Invitrogen).
Treatment with Rosiglitazone and ApoE.
After 14–17 days in culture, primary cortical rat neurons were incubated with the rosiglitazone concentrations indicated in Fig. 3 or DMSO as a control with or without 1 μM GW9662 or 7.5 μg/ml apoE (various forms) at 37°C for 24 h.
Confocal Microscopy.
After treatment, the neurons were fixed for 20 min in ice-cold 4% paraformaldehyde in PBS (pH 7.4) and mounted on microscope slides with VECTAshield (Vector Laboratories). Digital images of EGFP were collected on a laser-scanning confocal microscope with a Bio-Rad Radiance 2000 scanhead mounted on an Optiphot-2 microscope (Nikon) with a ×60 oil objective lens.
Image Analyses.
To determine dendritic spine densities, high-resolution digital images were analyzed with National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij). The length of dendrites was measured by tracing their extension using the segmented line selection. Dendritic spines were counted manually by using the point picker function of the particle analysis plug-in. The complexity of the dendritic tree was quantified by using the ImageJ NeuronJ plug-in to trace dendritic branches. The area of the dendritic field was estimated by connecting the outmost dendritic extensions and calculating the area of the resulting polygon.
Statistical Analysis.
A two-tailed t test assuming equal variances was used for statistical analyses. P < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS.
We thank Dr. Karl Weisgraber for providing purified apoE3, apoE4, and apoE4(1–272); Dr. Yukiko Goda for providing the EGFP–β-actin construct; Karina Fantillo and Sylvia Richmond for manuscript preparation; John Carroll for graphics; and Stephen Ordway and Gary Howard for editorial assistance. This work was supported by The J. David Gladstone Institutes and GlaxoSmithKline Research and Development.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mayeux R. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 2.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 3.Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 4.Mahley RW. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Weisgraber KH, Mucke L, Mahley RW. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 6.Mahley RW, Weisgraber KH, Huang Y. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang Y. Neurology. 2006;66(Suppl 1):S79–S85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y. Curr Opin Drug Discovery Dev. 2006;9:627–641. [PubMed] [Google Scholar]
- 9.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 10.Bellosta S, Nathan BP, Orth M, Dong L-M, Mahley RW, Pitas RE. J Biol Chem. 1995;270:27063–27071. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- 11.Teter B, Xu P-T, Gilbert JR, Roses AD, Galasko D, Cole GM. J Neurochem. 1999;73:2613–2616. doi: 10.1046/j.1471-4159.1999.0732613.x. [DOI] [PubMed] [Google Scholar]
- 12.Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mucke L, Mahley RW. J Neurosci. 1999;19:4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttini M, Yu G-Q, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. J Neurosci. 2002;22:10539–10548. doi: 10.1523/JNEUROSCI.22-24-10539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levi O, Jongen-Relo AL, Feldon J, Roses AD, Michaelson DM. Neurobiol Dis. 2003;13:273–282. doi: 10.1016/s0969-9961(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 15.Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, Mucke L. Proc Natl Acad Sci USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi O, Jongen-Relo AL, Feldon J, Michaelson DM. J Neurol Sci. 2005;229–230:241–248. doi: 10.1016/j.jns.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 17.Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeKosky ST, Scheff SW. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 19.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 20.Masliah E, Mallory M, Alford M., DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Proc Natl Acad Sci USA. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, et al. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brecht WJ, Harris FM, Chang S, Tesseur I, Yu G-Q, Xu Q, Fish JD, Wyss-Coray T, Buttini M, Mucke L, et al. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang S, Ma TR, Miranda RD, Balestra ME, Mahley RW, Huang Y. Proc Natl Acad Sci USA. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson GE, Haroutunian V, Zhang H, Park LCH, Shi Q, Lesser M, Mohs RC, Sheu RK-F, Blass JP. Ann Neurol. 2000;48:297–303. [PubMed] [Google Scholar]
- 27.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ. Am J Epidemiol. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Tang M-X, Stern Y, Shea S, Mayeux R. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 29.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 30.Peila R, Rodriguez BL, Launer LJ. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein BJ. Am J Cardiol. 2002;90:3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 32.Craft S, Dagogo-Jack SE, Wiethop BV, Murphy C, Nevins RT, Fleischman S, Rice V, Newcomer JW, Cryer PE. Behav Neurosci. 1993;107:926–940. doi: 10.1037//0735-7044.107.6.926. [DOI] [PubMed] [Google Scholar]
- 33.Watson GS, Craft S. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 35.Hoyer S, Lee SK, Löffler T, Schliebs R. Ann NY Acad Sci. 2000;920:256–258. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 36.Day C. Diabetic Med. 1999;16:179–192. doi: 10.1046/j.1464-5491.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 37.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Russo CD. Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Wilson TM, Kliewer SA. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 39.Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, Saperstein R, Smith RG, Leibowitz MD. Endocrinology. 1996;137:4189–4195. doi: 10.1210/endo.137.10.8828476. [DOI] [PubMed] [Google Scholar]
- 40.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, et al. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 41.Risner ME, Saunders AM, Altman JFB, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Exp Neurol. 2006;199:265–273. doi: 10.1016/j.expneurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Dillon C, Goda Y. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 44.Morales M, Colicos MA, Goda Y. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 45.Lea MA, Sura M, Desbordes C. Anticancer Res. 2004;24:3–9. [PubMed] [Google Scholar]
- 46.Tesseur I, Van Dorpe J, Bruynseels K, Bronfman F, Sciot R, Van Lommel A, Van Leuven F. Am J Pathol. 2000;157:1495–1510. doi: 10.1016/S0002-9440(10)64788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Okamoto K-I, Hayashi Y, Sheng M. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Chen S-D, Wu H-Y, Yang D-I, Lee S-Y, Shaw F-Z, Lin T-K, Liou C-W, Chuang Y-C. Biochem Biophys Res Commun. 2006;351:198–203. doi: 10.1016/j.bbrc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 49.Daynes RA, Jones DC. Nature. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 50.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. J Clin Endocrinol Metab. 2004;89:2728–2735. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 51.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 52.Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hondares E, Mora O, Yubero P, Rodriguez de la Concepción M, Iglesias R, Giralt M, Villarroya F. Endocrinology. 2006;147:2829–2838. doi: 10.1210/en.2006-0070. [DOI] [PubMed] [Google Scholar]
- 54.Sullivan PG, Brown MR. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:407–410. doi: 10.1016/j.pnpbp.2004.12.007. [DOI] [PubMed] [Google Scholar]