Abstract
ClC-Ka and ClC-Kb Cl− channels are pivotal for renal salt reabsorption and water balance. There is growing interest in identifying ligands that allow pharmacological interventions aimed to modulate their activity. Starting from available ligands, we followed a rational chemical strategy, accompanied by computational modeling and electrophysiological techniques, to identify the molecular requisites for binding to a blocking or to an activating binding site on ClC-Ka. The major molecular determinant that distinguishes activators from blockers is the level of planarity of the aromatic portions of the molecules: only molecules with perfectly coplanar aromatic groups display potentiating activity. Combining several molecular features of various CLC-K ligands, we discovered that phenyl-benzofuran carboxylic acid derivatives yield the most potent ClC-Ka inhibitors so far described (affinity <10 μM). The increase in affinity compared with 3-phenyl-2-p-chlorophenoxy-propionic acid (3-phenyl-CPP) stems primarily from the conformational constraint provided by the phenyl-benzofuran ring. Several other key structural elements for high blocking potency were identified through a detailed structure–activity relationship study. Surprisingly, some benzofuran-based drugs inhibit ClC-Kb with a similar affinity of <10 μM, thus representing the first inhibitors for this CLC-K isoform identified so far. Based on our data, we established a pharmacophore model that will be useful for the development of drugs targeting CLC-K channels.
Keywords: kidney, molecular modeling, pharmacology
The CLC Cl− channels ClC-Ka and ClC-Kb, as their correspondent murine orthologues ClC-K1 and ClC-K2, are members of the CLC family that are essential for Cl− absorption in various sections of the nephron as well as for endolymph production in the stria vascularis and the vestibular labyrinth (1, 2). The importance of these channels for the kidney is evidenced by the fact that mutations in the genes coding for them cause human genetic diseases and by the phenotypes of mouse models. Loss-of-function mutations in ClC-Kb lead to severe renal salt loss in Bartter syndrome type III (3), whereas the disruption of ClC-K1 in mice causes a defect in urinary concentration (4). Human mutations in barttin, an accessory β subunit modulating trafficking and function of CLC-K channels (5–7), lead to Bartter syndrome type IV, which combines severe renal salt loss with congenital deafness (8). Importantly, beyond the rare inherited diseases, accumulating data point to a possible role of CLC-Ks in blood-pressure regulation (9–11).
High-affinity CLC-K ligands could result in clinically useful drugs. On the one hand, activators (12, 13) could be used to boost ClC-Kb channels in Bartter syndrome; on the other hand, specific blockers of either ClC-Ka or ClC-Kb could be used for the development of agents capable of regulating diuresis (14, 15). Indeed, blockers of ClC-Kb should be effective diuretic and natriuretic drugs given the phenotype associated with loss-of-function mutations in Bartter syndrome (3, 16). By contrast, selective inhibitors of ClC-Ka might primarily interfere with water reabsorption, as suggested by the phenotype resulting from targeted inactivation of the homologous gene in mice (4).
In the last few years, working with two structurally unrelated classes of compounds (CPP derivatives and fenamates), we identified CLC-K activators and inhibitors with moderate affinity (12, 13, 17–20). The only CLC-K opener identified was niflumic acid (NFA). Molecular modeling revealed that NFA shows a planar conformation accordingly to crystallographic studies (21), whereas flufenamic acid (FFA) derivatives and 3-phenyl-CPP, the most potent ClC-Ka blockers, are forced to assume a noncoplanar arrangement of the aromatic rings. Thus, the spatial geometry profile associated with each molecule seemed to be the main determinant of the final effect (activating or blocking).
Based on these observations, we performed rationally designed chemical maneuvers on the available ligands to preferentially direct drug binding toward a blocking or an activating binding site. Using computational modeling and electrophysiological techniques, here we report the successful transformation of an activator into a pure blocker and vice versa. Besides unequivocally correlating the coplanar/noncoplanar conformation of the aromatic portions of the molecules to the activating/blocking effect, this experimental approach revealed a class of ClC-K inhibitors, which were characterized further in terms of potency, mechanism of action, and CLC-K isoform specificity. Finally, to define the chemical features of drugs for producing CLC-K channels block or activation, a pharmacophore model was developed.
Results
Effect of Rationally Designed Molecules on ClC-Ka.
Effect of an NFA analog with noncoplanar conformation.
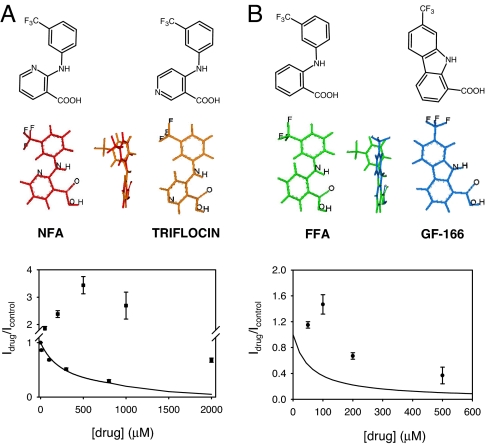
Recently, we showed that NFA (Fig. 1A) has a biphasic behavior on ClC-Ka, being able to activate the channel at low concentrations (50–500 μM) and block it at higher concentrations (1–2 mM) (12, 13). In contrast, FFA (Fig. 1B) and other fenamates block ClC-Ka currents with an apparent Kd in the high micromolar range (12). The major difference between the NFA structure and the FFA structure is the planar arrangement of the two aromatic moieties. It is thus reasonable to hypothesize that a distortion of the NFA molecule should convert it into a pure blocker. The coplanar conformation of the two aromatic rings is the result of the intramolecular hydrogen bond between the amino group and the oxygen atoms of the carboxylic moiety. To induce a distortion of this coplanar arrangement, we synthesized triflocin, a blocker of Na-HCO3 cotransporters that is used as a diuretic (22), in which the pyridine nitrogen atom is shifted from the ortho to the para position with respect to the amino group, thus allowing a noncoplanar arrangement of the two aromatic moieties due to the steric hindrance resulting from the introduction of an additional hydrogen atom in the ortho position to the aniline nitrogen atom (Fig. 1A). In agreement with our hypothesis, triflocin blocked ClC-Ka at all concentrations tested (10 μM to 1 mM) (Fig. 1A; compare with the NFA effect shown as squares). The dose–response curve was well fitted by a simple titration curve, with an apparent Kd of 283 ± 50 μM, suggesting 1:1 binding (Fig. 1A). These results strongly indicate that a more flexible noncoplanar conformation confers a larger affinity toward the inhibitory binding site on ClC-Ka.
Fig. 1.
Effect of rationally designed molecules on ClC-Ka. (A) Conversion of the coplanar NFA structure into a noncoplanar analog (triflocin). The chemical structure and lowest-energy conformation of each molecule are shown. Dose–response relationship of the block at 60 mV by triflocin (circles). The solid line is drawn according to Eq. 1 with Kd = 283 μM. Squares represents the NFA data reported in ref. 12. (B) Conversion of the noncoplanar FFA structure into the coplanar carbazole structure (GF-166). Dose–response relationship of GF-166 at 60 mV (circles). Solid line represents the fit to FFA data reported in ref. 12.
Effect of an FFA analog with coplanar conformation.
Next, we investigated the inverse hypothesis, i.e., if constraining the aromatic rings of FFA into a coplanar conformation converts it into an activator at low concentrations. To this end, we synthesized derivative GF-166 (Fig. 1B) in which the two phenyl groups are forced to assume a coplanar conformation by a cyclization to give a carbazole structure. Indeed, GF-166 showed a biphasic effect, displaying an activator behavior at low concentrations (Fig. 1B), similar to NFA (12, 13), but qualitatively different from the pure block induced by FFA (solid line in Fig. 1B).
Modification of the Flexibility of the 3-Phenyl-CPP Molecule: Insertion of the Chloro-Phenoxy Moiety into a Benzofuran System.
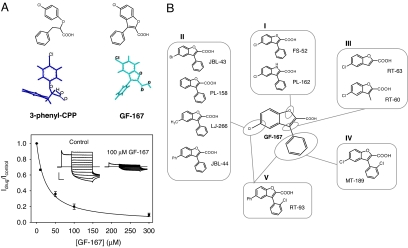
3-Phenyl-CPP (Fig. 2A, top left) is one of the most potent blockers of ClC-Ka described so far (12, 19, 20). To explore whether modification of the flexibility and the degree of planarity of the 3-phenyl-CPP structure could produce an alteration in drug blocking potency, we synthesized a molecule (GF-167; Fig. 2A, top right) in which the side chain of the stereogenic center was conformationally constrained, while maintaining the chlorine atom in the para position of the phenoxy moiety. Interestingly, GF-167 reversibly blocked ClC-Ka from the extracellular side with an apparent Kd of 24 ± 2 μM at 60 mV (Fig. 2A), a value that is ≈4-fold lower than that of 3-phenyl-CPP (12, 19).
Fig. 2.
Effect of rationally designed molecules on ClC-Ka. (A) Insertion of the chlorophenoxy moiety of 3-phenyl-CPP into a benzofuran system. The chemical structure and the lowest energy conformation of each molecule are shown. Dose–response relationship of the block at 60 mV by GF-167. The line is drawn according to the equation reported in Methods. Voltage–clamp traces of ClC-Ka currents before and after application of 100 μM of GF-167 are shown in Inset. (Horizontal scale bar, 50 ms; vertical scale bar, 5 μA.) (B) Chemical structure of GF-167 derivatives. I, compounds with isosteric substitutions of the oxygen atom of the benzofuran nucleus; II, compounds with different substituents on the benzofuran nucleus; III, compounds with elimination of the phenyl group; IV, compound with a chlorine atom on the phenyl group; V, compound with an additional phenyl group on the benzofuran nucleus and a chlorine atom on the phenyl group.
Structure–Activity Relationship by Benzofuran Derivatives.
Encouraged by this result, we synthesized and tested the effect of a series of analogues of GF-167 (Fig. 2B) to define the structural requisites for block as well as to improve drug potency.
Isosteric Substitutions of the Oxygen Atom.
To study the role of the oxygen atom of the benzofuran system, we substituted it by an isosteric sulfur (FS-52) or amino (PL-162) group (Fig. 2 BI). Both derivatives continued to maintain the increased potency of the strictly related parent compound GF-167 with respect to 3-phenyl-CPP [supporting information (SI) Fig. 6A; apparent Kd values: 23 ± 3.7 μM and 21 ± 4 μM for FS-52 and PL-162, respectively].
Substitutions on the Benzofuran Nucleus.
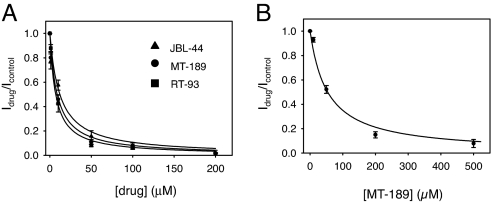
Having established no critical need for the oxygen atom of the benzofuran nucleus within the isosteric series, we began to explore the effects of various substituents on the benzofuran system in the place of the chlorine atom (Fig. 2BII). First, the kind of substituent did not affect drug-blocking potency, derivative JBL-43 in which the chlorine atom was substituted with a bromine atom, showed a Kd of 20 ± 2.8 μM (SI Fig. 6B and data not shown). Additionally, the presence of an electron-attractive group seemed to be not a stringent structural requisite: substitution of the chlorine atom with a methyl group (LJ-266; Fig. 2BII) or a hydrogen atom (PL-158; Fig. 2BII) did not produce any significant change of drug potency with respect to JBL-43 (SI Fig. 6B). Thus, we next substituted the chlorine atom with a bulky phenyl group (JBL-44; Fig. 2BII). Interestingly, this chemical maneuver increased the blocking potency 2-fold with respect to GF-167 (Kd = 12 ± 4 μM; Fig. 3A).
Fig. 3.
Structure–activity study by benzofuran derivatives on ClC-Ka and mechanism of action. Dose–response relationship of the block at 60 mV by JBL-44, MT-189, and RT-93 on ClC-Ka wild-type (A) and MT-189 on ClC-Ka N68D mutant (B). The ratio of the current in the presence and absence of drug is plotted vs. drug concentration. The lines are drawn according to the equation reported in Materials and Methods.
Elimination of the Phenyl Group.
Eliminating the phenyl group on the benzofuran nucleus (RT-63; Fig. 2BIII), drastically reduced the blocking potency (SI Fig. 6C; Kd = 267 ± 19 μM), showing that the benzofuran skeleton by itself is not sufficient to generate an efficient blocker. Also when the phenyl group was substituted with a methyl group (RT-60; Fig. 2BIII) the drug potency drastically decreased with respect to GF-167 (data not shown).
Introduction of a Chlorine Atom on the Phenyl Group.
Given the pivotal role of the phenyl group on the benzofuran structure, the possible influence of a substituent on the electric cloud rearrangement was next examined (Fig. 2BIV). To this end, an electron-withdrawing chlorine atom was introduced on the phenyl group in ortho position with respect to the benzofuran ring (MT-189). MT-189 shows a substantial higher potency than the parent compound GF-167 with an apparent Kd value of 7.0 ± 1.0 μM (Fig. 3A).
Introduction of a Phenyl Group and a Chlorine Atom.
As shown above, the potency of GF-167 was increased by the enlargement of the aromatic moiety of the benzofuran nucleus through the substitution of the chlorine atom with a phenyl group (JBL-44; Fig. 2BII) and by the introduction of a chlorine atom in the ortho position of the phenyl group (MT-189; Fig. 2BIV). These results prompted us to investigate the effect of compound RT-93 (Fig. 2BV) in which both modifications were combined. Somewhat surprisingly, however, derivative RT-93 had the same potency as MT-189 (Kd = 7.0 ± 0.9 μM, Fig. 3a).
Effect of MT-189 on CLC-Ka N68D.
We have previously demonstrated that ClC-Ka possesses different blocking binding sites, the inhibitory binding site of NFA being different from that of 3-phenyl-CPP and FFA derivatives (12–13, 19). To start to explore the binding site of the benzofuran derivatives we used the ClC-Ka mutant N68D, that is significantly less sensitive to 3-phenyl-CPP and FFA induced block (12, 19) but not to NFA-induced block (13). The mutant was ≈8-fold less sensitive to MT-189 compared with WT ClC-Ka (Fig. 3b, Kd = 54 ± 8 μM and SI Fig. 7). Also, block by RT-93 was significantly reduced (Kd = 31 ± 4 μM; data not shown). These results suggest that benzofuran derivatives bind to the previously identified 3-phenyl-CPP and FFA blocking site.
Effect of Benzofuran Derivatives on ClC-Kb.
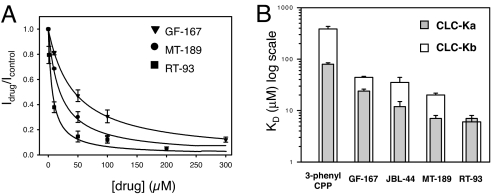
We next investigated the effect of the benzofuran derivatives on the highly homologous human isoform ClC-Kb for which, to date, no inhibitor with a Kd < 100 μM is known. Interestingly, the application of the lead benzofuran compound, GF-167, at a concentration of 50 μM produced a marked decrease of ClC-Kb sustained currents (SI Fig. 8A). Fitting the data obtained at various concentrations resulted in an apparent Kd for GF-167 of 44 ± 2.2 μM (Fig. 4A). Successively, we paid particular attention to JBL-44, MT-189, and RT-93, the compounds with the highest affinity for ClC-Ka. The presence of a phenyl group on the benzofuran nucleus (JBL-44) or the introduction of a chlorine atom on the phenyl group (MT-189) produced a slight (Fig. 4B) and a substantial (Fig. 4 A and B and SI Fig. 8B) increase in drug potency respectively (Kd = 35 ± 7 μM for JBL-44; Kd = 20 ± 2 μM for MT-189).
Fig. 4.
Effect of benzofuran derivatives on ClC-Kb. (A) Dose–response relationship of the block at 60 mV by GF-167, MT-189, and RT-93. The ratio of the current in the presence and absence of drug is plotted vs. drug concentration. The lines are drawn according to the equation reported in Materials and Methods. (B) Comparison of CLC-K isoform drug blocking affinity. Mean values of the apparent inhibition constant at 60 mV, obtained by fitting the equation reported in Methods to the inhibition data, for each indicated derivative.
Unlike what we found for ClC-Ka, the presence of both chemical modifications enhanced drug affinity toward ClC-Kb, with an apparent Kd of 6.0 ± 0.9 μM for RT-93 (Fig. 4 A and B and SI Fig. 8C). Thus, this benzofuran derivative represents the first ClC-Kb inhibitor with a relatively high affinity identified so far.
Effect of Benzofuran Derivatives on Other Renal CLC Proteins.
To test whether these compounds act specifically on CLC-K channels, we investigated the effect of several key substances on two CLC members that are expressed in the kidney: the ubiquitously expressed ClC-2 Cl− channel (23) and the renal Cl−/H+ antiporter ClC-5 (24–25). GF-167, JBL-44, and MT-189 were almost without effect at 50 μM or 200 μM, resulting in Kd values >1 mM. RT-93 showed a slight inhibitory action on both CLC proteins, however, with apparent Kd values that were >30-fold larger for ClC-5 and >40-fold larger for ClC-2 than the value obtained for CLC-Ka and CLC-Kb (SI Table 1). These results support the conclusion that the newly discovered compounds are specific for CLC-K channels.
Molecular Modeling Study and Pharmacophore Hypothesis.
Conformational search studies showed that triflocin, FFA, and NFA exhibit a restricted number of low-energy conformers characterized by the presence of an intramolecular hydrogen bond between the amino group and the oxygen atoms of carboxylic moiety. The calculated low-energy conformations include the ones found in the x-ray crystal structure (12, 21).
In triflocin and FFA, however, the presence of an additional hydrogen atom in the ortho-position to the amino group introduces a steric hindrance in the resulting biaryl-amino system that forces the noncoplanar arrangement of the aromatic rings. The lowest-energy conformer of triflocin shows a 22.4° dihedral angle between the aromatic rings, similar to 23.6° found for FFA, and therefore quite different from the NFA planar arrangement (SI Fig. 9A).
GF-166 may be considered a constrained analogue of FFA with a planar structure similar to that of NFA (SI Fig. 9B). In the same way, benzofuran derivatives may be considered as semirigid analogues of 3-phenyl-CPP, showing a limited number of low-energy conformations and noncoplanar orientation of the aromatic rings, with dihedral angles ranging from 60° to 80° (SI Fig. 9C).
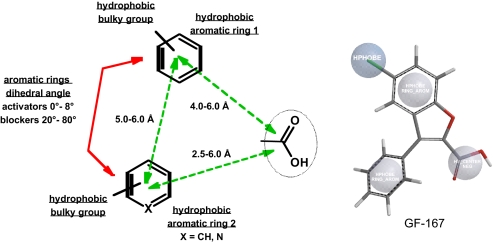
On the basis of the structural requirements and of the structure–activity relationship related to the principal classes of the studied compounds, namely flexible 3-phenyl-CPP derivatives (19), fenamates (12, 13), 3-phenyl-1-benzofuran-2-carboxylic acid derivatives, and 7-(trifluoromethyl)-9H-carbazole-1-carboxylic acid (GF-166), we hypothesize the ClC-Ka/Kb blockers/activators pharmacophore topography. The pharmacophore model (Fig. 5) shows the essential requirements for activating/blocking action, based on the presence of a carboxylic group and of two hydrophobic aromatic rings (hydrophobic rings 1 and 2), one of which can be an heterocyclic ring; a heteroatom (O, N, or S) bridges the two aromatic rings, as in fenamates, or the aromatic ring 1 and the carboxylic moiety, as in 3-phenyl-CPP and derivatives or in phenyl-benzofurane derivatives and isosters. The dihedral angle (d.a.) of the aromatic rings represents the discriminating function for activating or blocking action: activating molecules show 0–8° d.a., while blocking molecules exhibit 20–80° d.a. Interestingly, more flexible molecules, like 3-phenyl-CPP, show a higher Ka/Kb activity ratio, whereas, on the other hand, more rigid ones, like GF-167, are more potent but with a reduced Ka/Kb activity ratio. In benzofurane derivatives, the presence of a bulkier phenyl substituent on the aromatic ring 1 increases the potency, whereas the presence of a 3-(2-chloro-phenyl) substituent of benzofurane derivatives gives an almost 1:1 Ka/Kb activity ratio.
Fig. 5.
Pharmacophore model. Essential requirements for activating/blocking action (see above for description). For comparison, we reported the stick model and the chemical function descriptors of the master compound GF-167.
Discussion
Searching for potent and specific small molecules able to modulate CLC-K Cl− transporting proteins provides useful tools to explore their physiological role in cellular functions and is crucial for the development of molecules of therapeutic interest for human diseases caused by mutations of these proteins (Bartter syndrome type III and IV) or, more generally, for disorders of salt balance (1, 2, 14, 15). In the last few years, the study of 2-(p-chlorophenoxy)propionic acid (CPP) derivatives and fenamates, allowed us to open the way toward a pharmacological characterization of heterologously expressed CLC-K channels (12, 13, 17–20). Particularly, we discovered the presence of an activating and a blocking extracellular drug-binding site. In an attempt to explain the opposite drug effects mediated by molecules showing no marked structural differences, we proposed that the spatial geometry profile associated to each molecule was the main determinant of the final effect (12).
The findings in this report strongly demonstrate our hypothesis that a coplanar conformation of the aromatic moieties favors the interaction with the activating binding site, whereas a noncoplanar conformation is required for a high-affinity binding with a blocking binding site. Indeed, we showed here that triflocine, the 4-amino pyridine ring analogue of NFA, shows a noncoplanar arrangement of the aromatic rings and a blocking activity similar to FFA. Conversely, GF-166, a cyclized FFA homologue, with a planar structure, exhibited an activation behavior like NFA.
Furthermore, here we report the discovery of a family of benzofuran compounds as structurally general blockers of CLC-K channels. The lead compound GF-167, rationally designed via cyclization of 3-phenyl-CPP, was found to be an efficient ClC-Ka blocker with a gain of affinity of ≈3-fold with respect to 3-phenyl-CPP. Starting from GF-167, our structure–activity analysis led to the discovery of three benzfuran derivatives (JBL-44, MT-189, and RT-93) that showed an elevated blocking potency for ClC-Ka with Kd values down to 7 μM. JBl-44 is characterized by an additional phenyl ring that could supply a further molecular point for drug-binding interaction, whereas MT-189 bears an additional chlorine atom compared with GF-167. The increased potency of MT-189 may be caused by the electron negative effect of the chlorine atom. Alternatively, it may be due to the marked noncoplanar arrangement of the aromatic rings induced by the substituent on the phenyl group. Surprisingly, RT-93, which combines these two chemical maneuvers, was not more potent but had the same apparent Kd as MT-189.
Importantly, these benzofuran derivatives revealed themselves as efficacious blockers also of ClC-Kb, the other human CLC-K isoform for which no inhibitor has yet been described. RT-93 blocks ClC-Kb with the same affinity <10 μM as ClC-Ka.
Based on the results with the large number of structurally related molecules obtained in this and a previous study (12, 19), a pharmacophore model was successfully generated that will be helpful for the development of even more potent drugs. In fact, although the insertion of the chloro-phenoxy moiety of 3-phenyl-CPP into a benzofuran system results in more potent CLC-K inhibitors, further optimization may be necessary to improve the potency and selectivity of these derivatives. The crystal structure of the complete protein would be extremely helpful for the development of high-affinity compounds. The recent determination of the x-ray structure of the soluble cytoplasmic domain of ClC-Ka (26) is a step in that direction.
Diuretics are the mainstay of therapy for a variety of conditions associated with expanded extracellular volume, including hypertension, congestive heart failure, and renal insufficiency. For their relatively wide expression pattern along the tubule, CLC-K channels represent an attractive pharmacological target for a powerful diuretic. Among renal CLC proteins, benzofuran derivatives are highly specific inhibitors of CLC-K channels, being practically inactive on ClC-2 and ClC-5 at the tested concentrations. Only RT-93, the most effective benzofuran derivative, showed a slight blocking effect, however with an apparent Kd in the 200–400 μM range. Thus, the concentrations required for producing a significant ClC-2 or -5 block are far away from the concentration that produces an almost complete block of CLC-K channels.
The pharmacological targeting of ClC-Kb of the distal tubule will result in an intermediate diuretic response without major drug-induced imbalance in renal calcium handling. Because of its capability of blocking ClC-Kb with a micromolar range affinity, RT-93 represents a promising candidate as a lead for the design of new diuretic drugs. Such a drug could be of therapeutic interest also for the various form of volume-dependent/salt-sensitive hypertension (9–11) due to gain-of-function polymorphisms within the CLCNKA, CLCNKB, or BSND genes. Furthermore, high-affinity CLC-K inhibitors may permit the pharmacological creation of animal models that mimic, in dependence on the specific or unspecific CLC-K isoform block, Bartter syndrome type III or IV, respectively. A similar animal model would be of great value in elucidating the pathophysiology of tubule disease in Bartter syndrome as well as in evaluating the efficacy of possible therapies.
Materials and Methods
Expression in Xenopus laevis Oocytes and Voltage-Clamp Analysis.
Wild-type ClC-Ka, ClC-Kb, and their mutants, obtained as described (19), were coexpressed with the activating mutant Y98A of human barttin (5). Some of the most potent drugs were tested on two other renal CLC proteins, human ClC-5 and rat ClC-2, containing an N-terminal deletion (Δ16–61, ΔN-ClC-2) (27). Expression in oocytes and electrophysiological measurements was performed as described (27). Detailed electrophysiological methods are provided in SI Text.
Apparent dissociation constants for drugs showing blocking activity, Kd, were determined by calculating the ratio of the steady-state current in the presence and absence of the drug and fitting the ratios to the equation: I(c)/I(0) = 1/(1 + c/Kd), where c is the concentration (Eq. 1). Errors in figures and in the text are indicated as SEM.
Drugs.
With the exception of NFA and FFA purchased from Sigma–Aldrich, all tested compounds were synthesized in our laboratory. Methods for synthesis procedures are provided in SI Text.
For the electrophysiological recordings, compounds were daily dissolved in DMSO solutions, and the final concentrations were obtained by appropriate dilution with the standard extracellular solution. DMSO never exceeded 0.1%, a concentration without effect on CLC-K channels by itself.
Modeling Study.
Quantum chemical modeling of compounds is described in detail in SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
Financial support from the Italian Research Ministry (Grant MIUR COFIN 2005, to D.C.C.) and Telethon Italy (Grant GGP04018, to M.P.) is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708977105/DC1.
References
- 1.Jentsch TJ. J Am Soc Nephrol. 2005;16:1549–1561. doi: 10.1681/ASN.2005020207. [DOI] [PubMed] [Google Scholar]
- 2.Uchida S, Sasaki S. Annu Rev Physiol. 2005;67:759–778. doi: 10.1146/annurev.physiol.67.032003.153547. [DOI] [PubMed] [Google Scholar]
- 3.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alay H, Bakkaloglu A, et al. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 4.Akizuki N, Uchida S, Sasaki S, Marumo F. Am J Physiol. 2001;280:F79–F87. doi: 10.1152/ajprenal.2001.280.1.F79. [DOI] [PubMed] [Google Scholar]
- 5.Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, Jentsch TJ. Nature. 2001;414:558–561. doi: 10.1038/35107099. [DOI] [PubMed] [Google Scholar]
- 6.Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW. Pfügers Arch. 2002;444:411–418. doi: 10.1007/s00424-002-0819-8. [DOI] [PubMed] [Google Scholar]
- 7.Scholl U, Hebeisen S, Janssen AG, Müller-Newen G, Alekov A, Fahlke C. Proc Natl Acad Sci USA. 2006;103:11411–11416. doi: 10.1073/pnas.0601631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkenhäger R, Otto E, Schurmann MJ, Vollmer M, Ruf E-M, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, et al. Nat Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 9.Jeck N, Waldegger S, Lampert A, Boehmer C, Waldegger P, Lang PA, Wissinger B, Friedrich B, Risler T, Moehle R, et al. Hypertension. 2004;43:1175–1181. doi: 10.1161/01.HYP.0000129824.12959.f0. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo Y, Iwai N, Tago N, Inamoto N, Okayama A, Yamawaki H, Naraba H, Tomoike H. Circ J. 2005;69:138–142. doi: 10.1253/circj.69.138. [DOI] [PubMed] [Google Scholar]
- 11.Barlassina C, Dal Fiume C, Lanzani C, Manunta P, Guffanti G, Ruello A, Bianchi G, Del Vecchio L, Macciardi F, Cusi D. Hum Mol Genet. 2007;16:1630–1638. doi: 10.1093/hmg/ddm112. [DOI] [PubMed] [Google Scholar]
- 12.Liantonio A, Picollo A, Babini E, Carbonara G, Fracchiolla G, Loiodice F, Tortorella V, Pusch M, Conte Camerino D. Mol Pharmacol. 2006;69:165–173. doi: 10.1124/mol.105.017384. [DOI] [PubMed] [Google Scholar]
- 13.Picollo A, Liantonio A, Babini E, Conte Camerino D, Pusch M. J Membr Biol. 2007;216:73–82. doi: 10.1007/s00232-007-9034-z. [DOI] [PubMed] [Google Scholar]
- 14.Fong P. EMBO Rep. 2004;5:565–566. doi: 10.1038/sj.embor.7400168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sile S, Vanoye CG, George AL., Jr Curr Opin Nephrol Hypertens. 2006;15:511–516. doi: 10.1097/01.mnh.0000242177.36953.be. [DOI] [PubMed] [Google Scholar]
- 16.Konrad M, Vollmer M, Lemmink HH, van den Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschênes G, Antignac C, et al. J Am Soc Nephrol. 2000;11:1449–1459. doi: 10.1681/ASN.V1181449. [DOI] [PubMed] [Google Scholar]
- 17.Liantonio A, Accardi A, Carbonara G, Fracchiolla G, Loiodice F, Tortorella P, Traverso S, Guida P, Pierno S, De Luca A, et al. Mol Pharmacol. 2002;62:265–271. doi: 10.1124/mol.62.2.265. [DOI] [PubMed] [Google Scholar]
- 18.Liantonio A, Pusch M, Picollo A, Guida P, De Luca A, Pierno S, Fracchiolla G, Loiodice F, Tortorella P, Conte Camerino D. J Am Soc Nephrol. 2004;15:13–20. doi: 10.1097/01.asn.0000103226.28798.ea. [DOI] [PubMed] [Google Scholar]
- 19.Picollo A, Liantonio A, Didonna MP, Elia L, Conte Camerino D, Pusch M. EMBO Rep. 2004;5:584–589. doi: 10.1038/sj.embor.7400169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusch M, Liantonio A, De Luca A, Conte Camerino D. In: Chloride Transport Across Biological Membranes. Pusch M, editor. Amsterdam: Elsevier; 2007. pp. 83–108. [Google Scholar]
- 21.Dhanaraj V, Vijayan M. Acta Crystallogr B. 1988;44:406–412. doi: 10.1107/s0108768188001107. [DOI] [PubMed] [Google Scholar]
- 22.Belachgar F, Hulin P, Anagnostopoulos T, Planelles G. Br J Pharmacol. 1994;112:465–470. doi: 10.1111/j.1476-5381.1994.tb13096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiemann A, Gründer S, Pusch M, Jentsch TJ. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 24.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ. Nature. 2005;436:424–427. doi: 10.1038/nature03860. [DOI] [PubMed] [Google Scholar]
- 25.Picollo A, Pusch M. Nature. 2005;436:420–423. doi: 10.1038/nature03720. [DOI] [PubMed] [Google Scholar]
- 26.Markovic S, Dutzler R. Structure (London, UK) 2007;15:715–725. doi: 10.1016/j.str.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Pusch M, Liantonio A, Bertorello L, Accardi A, De Luca A, Pierno S, Tortorella V, Conte Camerino D. Mol Pharmacol. 2000;58:498–507. doi: 10.1124/mol.58.3.498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.