Abstract
Nutrition recommendations worldwide emphasize ingestion of plant-based diets rather than diets that rely primarily on animal products. However, this plant-based diet could limit the intake of essential nutrients such as calcium. Osteoporosis is one of the world's most prevalent nutritional disorders, and inadequate dietary calcium is a known contributor to the pathophysiology of this condition. Previously, we have modified carrots to express increased levels of a plant calcium transporter (sCAX1), and these plants contain ≈2-fold-higher calcium content in the edible portions of the carrots. However, it was unproven whether this change would increase the total amount of bioavailable calcium. In randomized trials, we labeled these modified carrots with isotopic calcium and fed them to mice and humans to assess calcium bioavailability. In mice feeding regimes (n = 120), we measured 45Ca incorporation into bones and determined that mice required twice the serving size of control carrots to obtain the calcium found in sCAX1 carrots. We used a dual-stable isotope method with 42Ca-labeled carrots and i.v. 46Ca to determine the absorption of calcium from these carrots in humans. In a cross-over study of 15 male and 15 female adults, we found that when people were fed sCAX1 and control carrots, total calcium absorption per 100 g of carrots was 41% ± 2% higher in sCAX1 carrots. Both the mice and human feeding studies demonstrate increased calcium absorption from sCAX1-expressing carrots compared with controls. These results demonstrate an alternative means of fortifying vegetables with bioavailable calcium.
Keywords: absorption, bioavailability, radioactive isotope, stable isotope
Low dietary calcium intake can negatively impact health and increase the risk of diseases such as osteoporosis (1, 2). Fruits and vegetables offer a diverse mixture of nutrients that promote good health, and it is generally thought that they will be more beneficial to human health than dietary supplements (3). One way to increase the nutrient content of some vegetables is to increase their bioavailable calcium levels (4, 5). Carrots are among the most popular vegetables in the United States and contain high levels of beta carotene (the precursor to Vitamin A) and other vitamins and minerals; however, like many vegetables, they are a poor source of dietary calcium (5, 6). By engineering carrots and other vegetables to contain increased calcium levels, we may boost calcium uptake and reduce the incidence of calcium deficiencies (7).
Previously, we have demonstrated that the calcium (Ca) levels in plants can be engineered through high-level expression of a deregulated Arabidopsis calcium transporter. An Arabidopsis vacuolar calcium antiporter, termed Cation exchanger 1 (CAX1), contains an N-terminal autoinhibitory domain (8, 9). Expression of N-terminal truncations of CAX1 (sCAX1) in plants such as potatoes, tomatoes, and carrots increases the calcium content in the edible portion of these foods (7, 10, 11). Presumably, these sCAX1-expressing plants have heightened sequestration of calcium into the large central plant vacuoles. However, we have not established that this modification leads to increased calcium absorption.
The creation of genetically modified plants with increased nutritional benefits is an expanding field (12, 13). The term “nutritional genomics” has been used to describe various studies that implement some form of plant biochemistry, genomics, or human nutrition. Transgenic plants are frequently analyzed only for changes in plant metabolism. Ideally, these genetically modified plants need to be labeled and used in controlled animal and human feeding studies to assess nutritional impacts. Often, this type of analysis to assess the nutrient value of transgenic foods is not reported.
Here, we analyze sCAX1 carrots that express increased levels of a plant calcium transporter for improved calcium absorption by using both mice and human feeding trials. The experimental design provides a rigorous platform to validate the nutritional impact of engineered foods. Furthermore, our findings offer a unique mechanism to enhance calcium absorption in numerous agriculturally important crops.
Results
Labeling Carrots with 45Calcium and Stable Isotopes.
Previously, we had demonstrated that sCAX1-expressing carrots contain 2-fold more calcium than vector control lines (7). These lines are fertile and display no adverse phenotypes in the greenhouse growth conditions tested. In hydroponics, because of the shallow nature of the growth containers, the control and sCAX1-expressing carrots were both short and wide compared with the soil-grown carrots. As a first step toward determining bioavailability, we labeled the edible portions of theses carrots with 45Ca and 42Ca. In the labeling growth conditions, we were again able to measure a 2-fold increase in the Ca2+ content of the sCAX1-lines and no increase in the content of other minerals (Cd2+, Cu2+, Fe2+ Mn2+, and Zn2+) compared with control lines (data not shown). Both the control and sCAX1 lines had similar mineral profiles to published values listed in the U.S. Department of Agriculture (USDA) food composition guide (www.ars.usda.gov/main/site_main.htm?modecode=12354500). The percentage of 45Ca activity of the administered dose that accumulated in the edible carrot tissues was 14.86% for control and 30.83% for sCAX1-expressing carrots (Table 1). Using stable isotopes, we measured the concentration of 42Ca enrichment in the edible portions of the control carrots at 2.5% and the sCAX1-expressing carrots at 2.4% (Table 1). Thus, calcium labeling by using either radioactive or stable isotopes in the carrots can be used to study calcium bioavailability in feeding studies.
Table 1.
Labeling efficiency of radioactive and stable calcium isotopes in hydroponically grown carrots
| Carrot type | 45Ca activity, %* | 42Ca enrichment, %† |
|---|---|---|
| Control | 14.86 | 2.5 |
| sCAX1-expressing carrot | 30.83 | 2.4 |
*45Ca isotope activity in edible portion of carrot.
†42Ca in edible portion of carrot.
Mice Fed sCAX1-Expressing Carrots.
Previously, we have used both extrinsic and intrinsic 45Ca labeling of diets in mouse feeding study models to measure tracer incorporation into hind limbs (14). We have validated this mouse model by using various diets, and our results compared favorably to previous feeding studies in rats (15). Here, mice were fed both extrinsically and intrinsically labeled diets containing control and sCAX1-expressing carrots. Potentially, sCAX1 expression may alter calcium partitioning within the plant. Initially, we determined whether sCAX1 expression altered oxalic acid concentration, a known inhibitor of calcium absorption (15). The oxalate concentration was 1.88% ± 0.08% mM for the control carrots compared with 1.46% ± 0.10% mM for the sCAX1-expressing carrots. This finding suggests that sCAX1 expression does not cause an increase in oxalate levels. However, these measurements do not directly address calcium bioavailability issues.
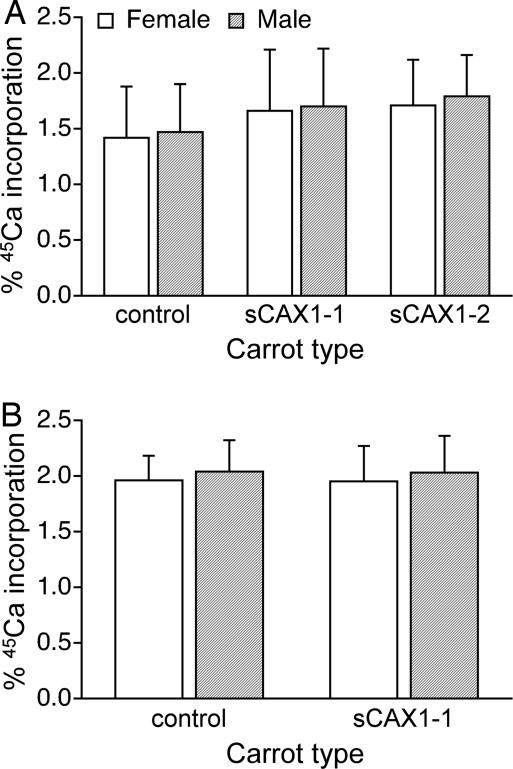
To study bioavailability, we measured calcium incorporation by extrinsically labeling the carrots. Extrinsic labeling can give meaningful bioavailability data for foods like milk (16, 17); however, it does not work for spinach (15). In general, extrinsic labeling should be expected to work for any food in which the various food calcium species are readily exchangeable. Because we are interested in addressing this exchangeable issue with the sCAX1-expressing carrots, we made side-by-side tests of the intrinsically and extrinsically labeled carrots. In the feeding studies, both control and sCAX1-expressing extrinsically labeled carrots showed no difference in 45Ca absorption. Furthermore, the sex of the mice did not alter the absorption rate of the labeled diets. The extrinsically labeled control carrots had incorporation of 1.42% ± 0.46% in female and 1.47% ± 0.43% in male mice of the 45Ca activity, compared with 1.66% ± 0.55% in females and 1.70% ± 0.52% in males fed sCAX1–1 carrots and 1.71% ± 0.41% in females and 1.79% ± 0.37% in males fed the sCAX1–2 carrots (Fig. 1A).
Fig. 1.
Percentage of incorporation of 45Ca into female and male mice bones after a single meal from extrinsically and intrinsically labeled carrot diets. (A) Thirty mice were fed extrinsically labeled control and sCAX1–1 diets, and 10 mice were fed sCAX1–2 carrots. (B) Thirty mice were fed intrinsically labeled control and sCAX1–1-expressing carrot-containing diets.
Intrinsic labeling is more useful when studying the bioavailability of labeled calcium in vegetable-based diets. Many vegetables contain antinutritients that can interfere with calcium absorption in the gut (18). These compounds can bind the calcium freely, and some plants also can bind calcium during the growth process (18–20). In the case of spinach, a poor source of bioavailable calcium, the incorporation of a 45Ca tracer in bone is reduced by 50% in the intrinsically labeled diets compared with the extrinsic diets when fed to rats or mice (14, 15). Although sCAX1-expressing carrots contained very low concentrations of oxalic acid, it is still imperative to measure calcium bioavailability from intrinsically labeled carrots. In the intrinsically labeled diets, the incorporation from the control carrots was 1.96% ± 0.22% in females and 2.04% ± 0.28% in males, compared with 1.95% ± 0.32% in females and 2.03% ± 0.33% in males for the sCAX1–1-expressing carrots (Fig. 1B). Again, these incorporation rates were not sex linked. Thus, in both the intrinsically and extrinsically labeled diets, the mice get equivalent amounts of bioavailable calcium from half the quantity of sCAX1-expressing carrots compared with controls.
Humans Fed sCAX1-Expressing Carrots.
To determine the bioavailability in humans, we intrinsically labeled a single homozygous carrot line (sCAX1–1) with stable isotopes and measured the calcium absorption in 15 males and 15 females from various ethnicities (Table 2). To ensure adequate calcium intake, the subjects were asked to report their intake on the day of the study. Human subjects consumed 796 ± 97 mg of Ca on the first study day and 797 ± 103 mg of Ca on the second study day, 2 weeks later. Based on averaged data from the two 24-h dietary recalls, subjects consumed 1,165 ± 461 mg of Ca. Therefore, subjects maintained a calcium intake within the enrollment criteria of 600–1,200 mg/day over the 2 weeks between trials.
Table 2.
Characteristics of human subjects at baseline
| Characteristics | Males | Females |
|---|---|---|
| n | 15 | 15 |
| Age, years | 24.2 ± 1.6 | 25.7 ± 2.8 |
| Weight, kg | 80.2 ± 12.0 | 62.8 ± 7.0 |
| Height, cm | 181.0 ± 9.7 | 168.3 ± 5.8 |
| BMI, kg/m2 | 24.5 ± 3.2 | 22.3 ± 3.0 |
| Ethnicity* | 11W/1H/3A/0ME | 11W/3H/0A/1ME |
| Calcium intake, mg/day | 817 ± 246 | 913 ± 206 |
All data are mean ± SD. BMI, body mass index.
*W, White; H, Hispanic; A, Asian; ME, multiethnic.
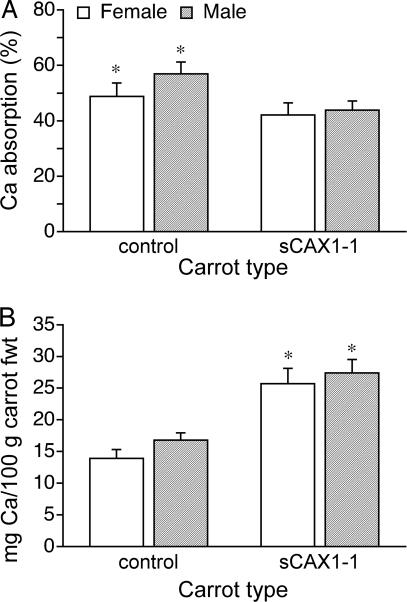
In the mouse study, we used radioactive isotopes and measured incorporation into bone; for the human study, fractional calcium absorption was measured. Using 42Ca-labeled carrots, we found a higher fractional (P < 0.001) absorption in control carrots. The fractional absorption of calcium from the control carrots was 48.8% ± 4.81% for females and 56.9% ± 4.29% for males, compared with 42.1% ± 4.32% for females and 43.8% ± 3.37% for males (P < 0.001) (Fig. 2A) for the sCAX1-expressing carrots. Although fractional calcium absorption is lower in the sCAX1-expressing carrots, the total calcium absorbed per 100 g of fresh carrots is 45.9% higher for females and 38.7% higher for males from the sCAX1-expressing carrots (P < 0.001) (Fig. 2B). Therefore, the sCAX1-expressing carrots contain more bioavailable calcium in both the mouse and human models.
Fig. 2.
Calcium absorption in female and male human subjects fed single meals containing labeled carrots. (A) Fractional absorption of 42Ca from control and sCAX1-expressing carrots. *, P < 0.001. (B) Total calcium absorbed from 100 g of fresh control and sCAX1-expressing carrots. *, P < 0.001.
Discussion
We have modified carrots to express increased levels of a plant calcium transporter (sCAX1), and these plants contain higher calcium content in the edible portions of the carrots. Mouse and human feeding studies demonstrated that sCAX1-expressing carrots had increased calcium absorption. In the human feeding studies, calcium absorption efficiency was 42.6% ± 2.8% and 52.1% ± 3.2% (P < 0.001) for the sCAX1 carrots and control carrots, respectively. However, total calcium absorption per 100 g of carrots was 41% ± 2% higher in sCAX1 carrots compared with control carrots (26.50 vs. 15.34 mg of Ca per 100 g) (P < 0.001).
Poor diets and exercise habits prevent many people from achieving optimal bone health. In fact, in the United States, dietary calcium intake has decreased, such that 90% of adolescent girls and 50% of adolescent boys consume less than the optimal amount of calcium (21). To help compensate for this deficiency, one strategy is to increase the calcium content of the foods they do eat. Here, we have shown the ability to improve the bioavailable calcium content of a staple food; when applied to a wide variety of fruits and vegetables, this strategy could lead to more calcium consumption in the diet.
In addition to the nutritional benefits, the use of genetic engineering to increase calcium levels could improve plant productivity and extend product shelf life. Calcium has long been used to combat many postharvest issues (22). For example, apples are immersed in a calcium solution to maintain firmness in shipping and prolong shelf life (23). Calcium also is added to soil to reduce the incidences of pathogen attack on potato tubers (24) and to combat heat stress (25, 26). All of these measures require the application of calcium-containing solutions to the soil or fruits. Recently, sCAX1 expression has been shown to increase calcium level in tomatoes that increased fruit firmness and prolonged shelf life (10). Aside from the nutritional impact shown here, using sCAX1 expression in a variety of fruits and vegetables also could positively impact plant productivity while decreasing labor costs.
Testing the nutritional qualities of genetically modified foods is a rigorous process. Any initial mineral nutrition study requires labeling of the foods with either radioactive isotopes for animal studies or stable isotopes for human trials. To date, there have been no reports of stable isotopes used to study the effects of nutrient availability from genetically modified foods. Most of the isotope research has been done with existing plants or hybrid varieties (27, 28). Here, we have developed a labeling protocol for carrots by using both radioactive and stable isotopes (Table 1). We hypothesize that the 2-fold-higher 45Ca activity in the sCAX1-expressing carrots is a function of using the same activity of 45Ca for each type of carrot compared with using 2-fold-higher 42Ca concentration in the solution for the control carrots compared with the modified carrots.
We used a mouse model to initially assess the bioavailability of calcium in the transgenic carrot lines (Fig. 1). In previous work using this protocol, we demonstrated that mice fed spinach had absorption values of ≈0.24% for intrinsically and ≈0.44% for extrinsically labeled diets. These values are consistent with similar studies using rats, validating our procedures (14) and further demonstrating that spinach, which contains high oxalate levels, is a poor source of bioavailable calcium. The sCAX1-expressing carrot lines did not contain more oxalate, and this finding may explain why both intrinsically and extrinsically labeled carrot diets showed reasonable calcium absorption. Because of the relative ease in preparing the extrinsic diets, we were able to analyze two sCAX1-expressing lines to demonstrate equivalent calcium bioavailability among different lines.
Although animal models provide evidence related to bioavailability, there are fundamental differences in the mechanism of calcium absorption between humans and small animals. In particular, humans use a greater proportion of calcium absorption in the upper small intestine than small animals (29). Therefore, it was necessary to demonstrate the bioavailability of the calcium-containing carrot in a human study. We chose young adults who were healthy and represent a typical population that might use vegetable sources to obtain a substantial amount of their calcium intake.
Although both the mouse and human feeding studies demonstrate that sCAX1-expressing carrots have increased calcium absorption (Figs. 1 and 2), the experiments measure different end points. With the mice, we are using a single meal and assessing the absorption of the tracer into bone. By using this methodology, the entire increase in calcium in the sCAX1-expressing carrots appears to be bioavailable. That is, the calcium found in 100 g of normal carrots can be obtained from 50 g of sCAX1-expressing carrots. Meanwhile, in the human feeding, we are comparing the fractional absorption of calcium from each of the carrots. Although there is a 10% reduction in absorbed calcium from the sCAX1-expressing carrots, the total concentration of calcium absorbed from the sCAX1-expressing carrots is 42% ± 2% higher compared with an equal quantity of control carrots (Fig. 2B). This finding demonstrates that we have reduced bioavailability as a function of total calcium content and emphasizes the fact that not all genetic engineering increases translate into enhanced bioavailability. Plant biologists often disregard this issue. Our working hypothesis to explain the differences between controls and sCAX1 lines is that not all of the calcium sequestered in the vacuole by ectopic expression of sCAX1 is bioavailable. It may be conjugated to phytates, phosphates, or other antinutrients within the edible carrot. In the future, maintaining a positive gain in total calcium despite reduced bioavailability will be critical when modifying plants by using sCAX1.
Acceptability of sCAX1-expressing crops demands that the product be safe. Here, we have presented preliminary human nutrition studies by using a single carrot line grown under controlled laboratory conditions. Future work needs to be done testing multiple lines and various growth regimes. Previously, using biochemical approaches, we have shown that sCAX1 can transport a wide range of substrates (9, 30). Potentially, CAX-expressing crops could be used for enrichment of other nutrients (e.g., CAX-mediated Zn2+ accumulation). However, these types of manipulations will require different growth conditions and modifications in CAX transport properties (31). Given that the ionic radius of Ca2+ is almost identical to that of Cd2+, sCAX1-expressing plants also can potentially sequester increased levels of cadmium. Here, we used cadmium-free hydroponic solutions to avoid any adverse metal accumulation in the carrots. Careful monitoring of the growth conditions and nutrient composition of the food will have to be taken with any crop expressing CAX transporters.
Our findings directly evaluate the nutritional consequence of transgenic foods in animal and human feeding studies. We establish unequivocally that modifying a single plant calcium transporter improves plant calcium absorption. Although this work represents initial studies toward understanding the nutritional impact of transgenic foods, the technology may be eventually applied to various crops because it involves the overexpression of a gene found in all plants. Additionally, the approach in this work can serve as a paradigm for related similar hypotheses about the role of other plant alterations to the bioavailability of nutrients contained within the plant matrix.
Materials and Methods
Mice Feeding Study.
Animal protocols were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. The C57BL/6 (Charles River Laboratories) mice were housed in cages with ad libitum access to water and food (AIN93G diet; ref. 32), and the feeding protocols have been described previously (14). The mice ate ≈3.5–4 g of diet per day at 6–7 weeks of age (n = 120). The mice were stratified and held without food for 24 h and separated into treatment groups by using a randomized block design. After 24 h, 3.5 g of the carrot diets was placed in a glass food dish and placed into the cage. The amount of diet fed to each mouse represented 14.0 kcal, which is similar to the 14.4–16 kcal/day that control mice of identical age consume and ensured that the mice ate the majority of the meal in 24 h. All mice were killed with CO2 after 24 h of feeding and placed in a cooler at 4°C for 24 h. The remaining diet, if any, was placed in a bag and saved for analysis.
Growth of Carrots and Preparation of 45Ca-Labeled Plants.
Carrots (Daucus carota L. var. Danver 60) and single-copy homozygous sCAX1-expressing carrot (sCAX1–1 and sCAX1–2) (7) lines were germinated on paper, and plants were subsequently transferred to hydroponic growth containers (33, 34). Carrot plants were grown in hydroponics for 60 days, and then the solution was supplemented with 1μCi 45Ca per liter. The carrots were then grown an additional 30 days.
The plant material was harvested and dried at 25°C. Once dried, the material was frozen in liquid nitrogen and ground to a powder with a mortar and pestle.
Preparation of 42Ca-Labeled Plants.
Carrots were germinated and grown hydroponically (14). After 90 days, the solution was supplemented with 5 mg 42Ca per liter for the control carrots and 2.5 mg42Ca per liter for the sCAX1-expressing carrots. This label was added to 21 liters of hydroponic solution, and the carrots were grown until all of the solution was absorbed by the plants. A second round of 42Ca labeling was done in 10 liters of hydroponic solutions. This two-part labeling took 14–18 days. Fresh carrots were harvested, weighed, sliced, and stored at −20°C.
One 0.25- to 0.50-inch latitudinal slice from control and sCAX1-expressing carrots was dried at 70°C for 24 h and then ashed at 700°C for 15 h (Thermolyne Furnace, Barnsted International). These carrot pieces were then dissolved in 3 M HCl, neutralized with 1 M NaOH, dried for 24 h at 70°C, and resuspended in 0.1 M HCl. Total calcium was determined by inductively coupled plasma-atomic emission spectrometry (ICP) (35), and 42Ca isotopic enrichment was determined with the use of a magnetic sector thermal ionization mass spectrometer (Finnigan MAT 261; Finnigan) (36).
Oxalic Acid Determination.
Oxalic acid concentration of the carrot samples was determined by methods outlined in Nakata and McConn (37).
Mouse Diet Preparation.
Initially, we determined the nutritional composition of carrots for the following constituents: fiber, total protein, calcium, potassium, and magnesium (35, 38, 39). Two standard diet mixes were then obtained from Research Diets and used for mice feeding. These mixes allowed for the addition of 1.0 g of dried ground control carrot and 0.5 g of dried ground sCAX1-expressing carrots to 2.5–3.0 g of prepared diet mix. This 3.5 g of composite diet was nutritionally equivalent (including 5.0 mg/g calcium) to the standard AIN93G diet eaten by mice during a given 24-h period (32). Before feeding, diets were mixed to homogeneity with 2 ml of dH2O. For the extrinsically labeled diet, the 45Ca label was a component in the water.
Mice Bioavailability Analysis.
The mice bones were analyzed as described previously (14). Briefly, the hind limbs (femur, tibia, and fibula) were removed and the bones ashed in a muffle furnace (Thermolyne Furnace, Barnsted International). The activity of 45Ca incorporated in the bones was determined as described previously (14).
First, ≈95% of the mice ate the entire labeled diet. If any diet was left uneaten, it was processed and analyzed as previously described. Then, the 45Ca activity in the uneaten diet was subtracted from the total amount of 45Ca label in the diets.
The percent dose of 45Ca in the hind limbs was calculated by using Excel (Microsoft), and statistical differences were calculated by using ANOVA in SPSS.
Subject Screening.
Healthy adults 21–29.9 years of age were recruited by public advertising and word of mouth. The Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals approved this protocol, and informed written consent was obtained from all subjects. Subjects were eligible for enrollment if they had no underlying health conditions that may affect calcium absorption, had an average Ca intake of 600–1,200 mg/day based on three separate days of 24-h dietary recalls, and had a body mass index less than the 95th percentile for age and gender (Table 2).
Isotope Preparation and Mineral Absorption Study.
We procured 42Ca (94% enrichment) and 46Ca (6% enrichment) from Trace Sciences, which were prepared for human use as the chloride salt by the Investigational Pharmacy Service of Texas Children's Hospital [(TCH) Houston, TX]. All isotopes were tested for sterility and pyrogenicity before use (36). On the morning of the study, subjects arrived at the General Clinical Research Center of TCH (National Institutes of Health Grant M01-RR00188, General Clinical Research Center) having not ingested any food or water since midnight. Subjects were randomized to receive either the control or modified carrots at visit 1 (with the alternate being consumed at visit 2). Diets were controlled so that each breakfast provided approximately one third of the daily calcium intake of the subjects' mean intake (≈900 mg of Ca on the study day). The remaining intake was divided between lunch and dinner, with one optional snack of negligible calcium content. Subjects consumed either 65 g of the modified carrot or 120 g of the control carrot to provide ≈38 mg of Ca. Subjects also consumed 170 g of calcium-fortified orange juice (Minute Maid, Coca-Cola), providing ≈225 mg of Ca. After finishing the meal, the subjects were given 15 μg of 46Ca i.v. This process was repeated at visit 2 to ensure that the subjects received the reciprocal carrot meal; however, i.v. injection was not given. Lunch and the snack were provided to the subjects as a predetermined sack lunch. Dinner was provided in the form of a fast-food gift card from one of three locations. Subjects were instructed by the study dietitian as to what they were allowed to eat at each location to maintain the 300 mg of Ca per meal requirement. Subjects notified the study dietitian the following day as to what they consumed. After the morning test meal, subjects were instructed to collect their urine in 8-h pools for 24 h. Subjects were contacted between the two visits for 24-h dietary recalls on two nonconsecutive days to ensure that their calcium intake was maintained during the study.
Calculation of Mineral Absorption.
Urine samples were prepared for mass spectrometric analysis by using an oxalate precipitation technique (16). Samples were analyzed for isotopic enrichment with the use of a magnetic sector thermal ionization mass spectrometer (Finnigan MAT 261; Finnigan). Each sample was analyzed for the 42Ca/48Ca and 46Ca/48Ca ratios with correction for fractionation to the reference 44Ca/43Ca. The accuracy and precision of this technique for natural abundance samples compared with those of standard data are 0.15% depending on the ratio being measured (16). Calcium absorption was calculated as the relative recovery in the urine of the oral isotope divided by the recovery of the i.v. isotope during the 24 h after isotope administration (from time of the first oral dose until 24 h after the last oral dose).
ACKNOWLEDGMENTS.
We thank Drs. Ian Griffin, Paul Nakata, and Dennis Bier for critical reading and helpful comments with this manuscript; Gloria Orozco, Adrianne Morse, Sevahn Allahverdian, Maria Hamzo, and Zhensheng Chen for subject assistance and sample analysis; Jenell Dancy and staff for help with subject assistance during their time at the General Clinical Research Centers; and Adam Gillum for graphic design assistance. This work was supported by National Institutes of Health Grant IR01 DK 062366 and U.S. Department of Agriculture Grant CSRESS#2005-34402-16401 Designing Foods for Health (to K.D.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Duque G, Mallet L, Roberts A, Gingrass S, Kremer R, Sainte-Marie LG, Kiel DP. J Am Med Dir Assoc. 2000;8:67–73. doi: 10.1016/j.jamda.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Kin CF, Shan WS, Shun LJ, Chung LP, Jean W. Int J Epidemiol. 2007;36:1143–1150. doi: 10.1093/ije/dym149. [DOI] [PubMed] [Google Scholar]
- 3.Copper DA. J Nutr. 2004;134:221–224. [Google Scholar]
- 4.Bachrach LK. Trends Endocrinol Metab. 2001;12:22–28. doi: 10.1016/s1043-2760(00)00336-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller GD, Jarvis JK, McBean LD. Am Col Nutr. 2001;20:168S–185S. doi: 10.1080/07315724.2001.10719029. [DOI] [PubMed] [Google Scholar]
- 6.Gueguen L, Pointillart A. J Am Col Nutr. 2000;19:119S–136S. doi: 10.1080/07315724.2000.10718083. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Kim C, Pike L, Smith R, Hirschi K. Mol Breeding. 2004;14:275–282. [Google Scholar]
- 8.Pittman JK, Hirschi KD. Plant Physiol. 2001;127:1020–1029. [PMC free article] [PubMed] [Google Scholar]
- 9.Mei H, Zhao J, Pittman J, Lachmansingh J, Park S, Hirschi KD. J Exp Bot. 2007;58:3419–3427. doi: 10.1093/jxb/erm190. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH, Hirschi KD. Plant Physiol. 2005;139:1194–1206. doi: 10.1104/pp.105.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S, Kang TS, Kim CK, Han JS, Kim S, Smith RH, Pike LM, Hirschi KD. J Agric Food Chem. 2005;53:5598–5603. doi: 10.1021/jf050531c. [DOI] [PubMed] [Google Scholar]
- 12.Freese W, Schubert D. Biotechnol Genet Eng Rev. 2004;21:299–324. doi: 10.1080/02648725.2004.10648060. [DOI] [PubMed] [Google Scholar]
- 13.DellaPenna D. Proc Natl Acad Sci USA. 2007;104:3675–3676. doi: 10.1073/pnas.0700640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris J, Nakata P, McConn M, Brock A, Hirschi KD. Plant Mol Biol. 2007;64:613–618. doi: 10.1007/s11103-007-9180-9. [DOI] [PubMed] [Google Scholar]
- 15.Weaver CM, Martin BR, Ebner JS, Krueger CA. J Nutr. 1987;117:1903–1906. doi: 10.1093/jn/117.11.1903. [DOI] [PubMed] [Google Scholar]
- 16.Abrams SA. Forum Nutr. 2003;56:312–313. [PubMed] [Google Scholar]
- 17.Avalos Mishaan AM, Zavaleta N, Griffin IJ, Hilmers DC, Hawthorne KM, Abrams SA. J Pediatr. 2004;145:26–31. doi: 10.1016/j.jpeds.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Heaney RP, Weaver CM, Recker RR. Am J Clin Nutr. 1988;47:707–709. doi: 10.1093/ajcn/47.4.707. [DOI] [PubMed] [Google Scholar]
- 19.Hanes DA, Weaver CM, Heaney RP, Wastney ME. J Nutr. 1999;129:170–173. doi: 10.1093/jn/129.1.170. [DOI] [PubMed] [Google Scholar]
- 20.Hanes DA, Weaver CM, Wastney ME. J Nutr. 1999;129:165–169. doi: 10.1093/jn/129.1.165. [DOI] [PubMed] [Google Scholar]
- 21.Matkovic V, Heaney RP. Am J Clin Nutr. 1992;55:992–996. doi: 10.1093/ajcn/55.5.992. [DOI] [PubMed] [Google Scholar]
- 22.Raz V, Fluhr R. Plant Cell. 1992;4:1123–1130. doi: 10.1105/tpc.4.9.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dris R, Niskanen R. Plant Foods Hum Nutr. 1999;54:159–171. doi: 10.1023/a:1008171909458. [DOI] [PubMed] [Google Scholar]
- 24.Tani S, Yatzkan E, Judelson HS. Mol Plant Microbe Interact. 2004;17:330–337. doi: 10.1094/MPMI.2004.17.3.330. [DOI] [PubMed] [Google Scholar]
- 25.Kleinhenz MD, Palta JP. Physiol Plant. 2002;115:111–118. doi: 10.1034/j.1399-3054.2002.1150113.x. [DOI] [PubMed] [Google Scholar]
- 26.Sterrett SB, Haynes KG, Yencho GC, Henninger MR, Vinyard BT. Crop Sc. 2006;46:1471–1478. [Google Scholar]
- 27.Patterson KY, Veillon C. Exp Biol Med. 2001;226:271–282. doi: 10.1177/153537020122600403. [DOI] [PubMed] [Google Scholar]
- 28.Weil JH. IUBMB Life. 2005;57:311–314. doi: 10.1080/15216540500092252. [DOI] [PubMed] [Google Scholar]
- 29.Abrams SA, Hawthorne KM, Aliu O, Hicks PD, Chen Z, Griffin IJ. J Nutr. 2007;137:2208–2212. doi: 10.1093/jn/137.10.2208. [DOI] [PubMed] [Google Scholar]
- 30.Shigaki T, Pittman JK, Hirschi KD. J Biol Chem. 2003;278:6610–6617. doi: 10.1074/jbc.M209952200. [DOI] [PubMed] [Google Scholar]
- 31.Shigaki T, Barkla BJ, Miranda-Vergara MC, Zhao J, Pantoja O, Hirschi KD. J Biol Chem. 2005;280:30136–30142. doi: 10.1074/jbc.M503610200. [DOI] [PubMed] [Google Scholar]
- 32.Reeves PG, Nielsen FH, Fahey GC., Jr J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 33.Grusak MA, Pezeshgi S, O'Brien KO, Abrams SA. J Sci Food Agric. 1996;70:11–15. [Google Scholar]
- 34.Grusak MA. J Nutr Biochem. 1997;8:164–171. [Google Scholar]
- 35.Franson MAH. Standard Methods for the Examination of Water and Wastewater. Washington, DC: American Public Health Association; 1989. sect. 3120. [Google Scholar]
- 36.Chen Z, Griffin IJ, Kriseman YL, Liang LK, Abrams SA. Clin Chem. 2003;49:2050–2055. doi: 10.1373/clinchem.2003.025692. [DOI] [PubMed] [Google Scholar]
- 37.Nakata PA, McConn MM. Plant Physiol Biochem. 2003;41:325–329. [Google Scholar]
- 38.Sheldrick BH. Can J Soil Sci. 1986;66:543–545. [Google Scholar]
- 39.Komarek AR. Publication #101. Fairport, NY: Ankom; 1993. [Google Scholar]