Abstract
Was the mortality associated with the deadliest known epidemic in human history, the Black Death of 1347–1351, selective with respect to preexisting health conditions (“frailty”)? Many researchers have assumed that the Black Death was so virulent, and the European population so immunologically naïve, that the epidemic killed indiscriminately, irrespective of age, sex, or frailty. If this were true, Black Death cemeteries would provide unbiased cross-sections of demographic and epidemiological conditions in 14th-century Europe. Using skeletal remains from medieval England and Denmark, new methods of paleodemographic age estimation, and a recent multistate model of selective mortality, we test the assumption that the mid-14th-century Black Death killed indiscriminately. Skeletons from the East Smithfield Black Death cemetery in London are compared with normal, nonepidemic cemetery samples from two medieval Danish towns (Viborg and Odense). The results suggest that the Black Death did not kill indiscriminately—that it was, in fact, selective with respect to frailty, although probably not as strongly selective as normal mortality.
Keywords: frailty, paleodemography, paleoepidemiology, selective mortality
Paleodemography and paleoepidemiology, which use skeletons from archaeological sites to study the population and health characteristics of past human communities, have the potential to answer important questions about past population dynamics (1–3). Both fields, however, suffer from fundamental methodological and interpretive problems that have only recently received serious attention (4–9). One basic problem derives from the fact that paleodemographers, paleoepidemiologists, and other skeletal biologists observe only dead individuals from the populations of interest, not living ones (4, 9). This would pose no difficulty if the dead were an unbiased sample of the living, i.e., if mortality were not selective for the sorts of health characteristics we are trying to study. But in fact people die for a reason, and those dying at any given age are demonstrably not a random sample of the living population at risk of death at that age (10, 11). Although all people die eventually, their health characteristics just before the time of death are unlikely to be representative even of their own physical condition over most of their lifetimes. The dying, on average, are less healthy than the rest of the living.
This problem is now widely acknowledged (12–15). But several researchers hope that it can be circumvented by examining so-called “catastrophic” skeletal samples. Such samples are produced at more or less one point in time by massive, population-wide die-offs, caused, for example, by natural disasters, famines, epidemics, or war (15, 16). Catastrophic mortality, in this view, is far less selective for health characteristics, age, or sex than is the noncatastrophic, “attritional” mortality that creates most skeletal samples, and catastrophic samples can provide an unbiased picture of the demographic and epidemiological characteristics of past populations. Introduced diseases of high virulence are often pointed to as potential causes of catastrophic mortality, and few diseases have received as much attention from this perspective as the first known outbreak of the Black Death in Europe during the mid-14th century (16–18).
The Black Death of 1347–1351 was one of the most devastating epidemics in human history; it killed an estimated 30–50% of the European populations affected and initiated or exacerbated important demographic, economic, and social changes (19–22). During the epidemic, mass burial grounds were established throughout Europe to accommodate the enormous number of victims, who rapidly overwhelmed local parish cemeteries. One such special-purpose burial ground, the East Smithfield cemetery in London, was established in late 1348 or early 1349 for the express purpose of burying Black Death victims, and there is no evidence that it was used after the epidemic ended in 1350 (23, 24). East Smithfield is one of very few excavated mass burial grounds in Europe that has clear documentary and archaeological evidence linking it to the mid-14th century Black Death (24). Given the extent to which Black Death mortality overwhelmed normal mortality (21), most if not all of the individuals buried in East Smithfield must have died from the disease. The skeletons excavated at the East Smithfield site thus provide an excellent opportunity to explore questions about the patterns of Black Death mortality.
We examined a total of 490 skeletons from East Smithfield to test whether mortality associated with the 1349–1350 outbreak of the Black Death in London was selective with respect to preexisting health conditions—or what demographers often call “frailty.” Frailty was originally defined as an individual's age-adjusted relative risk of death compared with the rest of his or her birth cohort (25). In this study, frailty refers specifically to an individual's age-adjusted relative risk of death before the Black Death (i.e., during normal, nonepidemic times) compared with the rest of the living population of the period. Frailty will be indicated by the presence of at least one skeletal lesion (porotic hyperostosis, cribra orbitalia, linear enamel hypoplasia, periosteal lesions of the tibia, or short femur length) known from prior research to be associated with earlier episodes of infection, under-nutrition, or other forms of physiological stress (10, 12, 14). The purpose here was to test whether the Black Death killed people indiscriminately—i.e., regardless of frailty as indicated by the presence of skeletal lesions—or whether Black Death mortality behaved like normal, nonepidemic mortality in which individuals with the highest frailty were at the highest risk of death.
To answer this question, we need to compare the East Smithfield skeletal sample to a nonepidemic, attritional control sample. The control sample should come from an urban community as similar as possible to London ca. 1345, i.e., just before the epidemic. Ideally, the only difference between the East Smithfield cemetery and the control cemetery would be that the Black Death affected the former but not the latter. The further we stray from this (unattainable) ideal, the less confident we can be about attributing any observed differences specifically to the Black Death. No post-Black Death burials will meet this ideal, for the Black Death itself initiated profound demographic and economic changes throughout Europe (19, 20, 26–30). Moreover, any selective mortality associated with the epidemic would immediately make the surviving population unrepresentative of pre-Black Death conditions, and catastrophic Black Death mortality could have had large effects on age-at-death distributions that lasted for several generations (31). Most previous studies comparing East Smithfield to normal-mortality cemeteries have used comparison samples that were either partly or entirely post-Black Death (16, 17). For the current study, appropriate comparison samples have been identified from cemeteries in two medieval Danish towns, Viborg and Odense (32). During the Middle Ages, Denmark and southern England were similar socially, economically, and demographically, at least up to the time of the Black Death (33–36). According to recent studies of Y-chromosome haplotypes (37), the two populations also share genetic similarities, presumably reflecting the pre-Conquest Danish settlement of eastern England. Denmark's medieval urban cemeteries are likely to provide as close an approximation to the ideal control group for East Smithfield as can be hoped for. In addition, a well validated method now exists for dating medieval Danish burials (38, 39), making it possible to select a predominantly (if not exclusively) pre-Black Death sample of 291 Danish skeletons, one large enough for the current analyses. The possible effects of any uncontrolled population differences on the results are discussed below.
A newly developed method of paleodemographic age-at-death estimation, the latent-trait method (40), has been applied to the East Smithfield and Danish skeletons; this method is a form of Bayesian age estimation and is consistent with the recent Rostock protocol for skeletal age estimation (7, 8). This analysis also uses a new multistate model of morbidity and mortality (10) to estimate the selective mortality associated with various skeletal lesions.
The Model
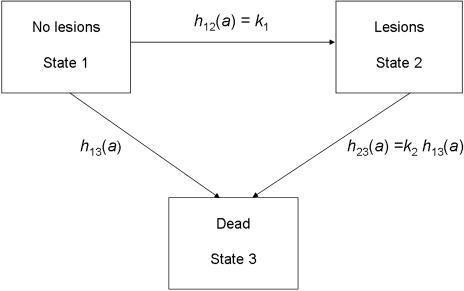
To estimate the effects of selective mortality, we use a reduced form of the multistate model of morbidity and mortality developed by Usher for paleoepidemiological analysis (10). In the three-state form of Usher's more general model, individuals are in one of three nonoverlapping “states” at any one age (Fig. 1): State 1 includes individuals with no detectable skeletal lesions, State 2 includes those with lesions, and State 3 is death. Although all individuals are observed in State 3, i.e., as skeletons, the distribution of ages and lesion status among skeletons provides the information needed to estimate the parameters governing all of the transitions (on the usual assumption that the model is correct). The Usher model is well suited for investigating selective mortality because it allows for differences in the risk of death associated with each of the two living states. The model thus enables us to estimate the force of mortality associated with each lesion type—that is, whether an individual with a certain lesion is more or less likely to die than a similarly aged individual without that lesion.
Fig. 1.
Three-state model of morbidity and mortality. Individuals are born into State 1, and transitions between States i and j occur at age-specific hazard rates hij(a), where a is age in years. The transition between States 1 and 3 follows the baseline Siler hazard function h13(a) = α1 exp(−β1a) + α2 + α3 exp(β3a), and the relative risk of death (frailty) associated with a given lesion type, k2, acts proportionately to modify this baseline rate. The hazard of developing a detectable bony lesion is set equal to the constant k1 at all ages. In general, estimates of k1 and k2 will vary across different types of lesions and different populations. (Redrawn from ref. 10.)
The baseline risk of death from State 1, h13(a), is specified as a five-parameter Siler mortality function (41–43). Because the age at onset of conditions resulting in skeletal lesions is generally unknown in paleoepidemiological studies, age at development of lesions is modeled as an exponential random variable with parameter k1. The risk of death from State 2, h23(a), is assumed to be proportional to the baseline age-specific risk of dying from State 1. Under this specification, k2 is a proportional term acting on the Siler function and is thus independent of age; k2 estimates the proportional difference in risk of death between individuals with and without lesions. When k2 is larger than one, individuals with lesions faced an elevated risk of dying compared with similarly aged individuals without lesions.
To determine whether the Black Death was selective for frailty, the Usher model was applied separately to the East Smithfield and Danish samples, permitting the relative risk of death associated with each bony lesion to be compared across samples. In earlier analyses, Usher applied the model to observations on most of the lesions examined here from a normal-mortality sample of 468 skeletons excavated at the medieval Danish village site of Tirup, and she found that many were associated with substantial increases in the risk of death (10). In light of these results, it was expected that lesions would be associated with an excess risk of death in the urban Danish samples and thus be valid proxies for frailty under conditions of normal, attritional mortality. To determine whether the Black Death was selective with respect to frailty, estimates of excess mortality associated with lesions observed in the East Smithfield sample were compared with those from the Danish cemeteries. If the Black Death killed indiscriminately, skeletal lesions known to be indicative of an increased risk of death in Denmark should not be associated with an increased risk of death in East Smithfield.
Results
Table 1 presents maximum likelihood estimates of the excess mortality k2 associated with each lesion within East Smithfield and Denmark, along with nominal standard errors and likelihood ratio tests of whether k2 differs from one (i.e., no selectivity). These standard errors and likelihood ratio tests should be interpreted with great caution. When fitting the Usher model, we used the point estimate of each skeleton's age at death derived from the latent-trait method; although these estimates are expected to be unbiased, they still have large estimation errors associated with them. Those errors were not taken into account when the Usher model was fit, which means that the reported standard errors are likely to be substantial underestimates and the likelihood ratios are likely to be biased to an unknown degree. Thus, these indicators of “significance” can be misleading if interpreted too literally: They are presented purely as a crude yardstick (at best) for informal model assessment. This same problem, of course, applies to a greater or lesser extent whenever error-prone measures are used, which is one reason epidemiology journals have started to discourage the reporting of statistical significance (e.g., ref. 64). (We also note that we perform multiple tests, a process that further distorts the notional significance level of any one test.) What is important, we would argue, is whether the parameter estimates show a consistent pattern that is interpretable in terms of our model of selective mortality.
Table 1.
Maximum likelihood estimates of k2 with nominal SE and likelihood ratio tests of H0: k2 = 1
| Lesion type | East Smithfield (n = 490) |
Denmark (n = 291) |
||
|---|---|---|---|---|
| k̂2 (SE) | −2LLR | k̂2 (SE) | −2LLR | |
| Periosteal lesions of the tibia | 1.5 (0.3) | 27.80 | 5.3 (2.0) | 61.74 |
| Porotic hyperostosis | 1.8 (0.3) | 8.84 | 2.3 (0.7) | 12.54 |
| Cribra orbitalia | 1.7 (0.4) | 2.30 | 3.6 (2.1) | 53.57 |
| Mandibular canine LEH | 2.9 (1.1) | 13.16 | 7.6 (14.4) | 11.96 |
| Maxillary canine LEH | 2.0 (0.5) | 32.29 | 2.7 (3.0) | 16.74 |
| Femur length | 1.2 (0.2) | 0.12 | 2.7 (0.7) | 0.09 |
LLR, ln(likelihood ratio), full model against reduced model in which k2 = 1 (df = 1); LEH, linear enamel hypoplasia.
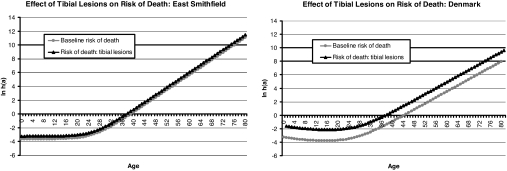
We suggest that Table 1 does show such a pattern. The presence of every lesion type considered in this study is associated with an increased risk of death (k̂2 > 1) in both cemeteries, and most of the estimates are reasonably large (>2). Without exception, the estimates of k2 are higher in Denmark than in East Smithfield. Together, these results suggest that mortality was selective for frailty during the Black Death in London, but probably not as strongly selective as normal, attritional mortality in medieval Denmark. As an example, Fig. 2 compares the elevation in risk of death associated with periosteal lesions of the tibia in East Smithfield and Denmark based on the respective estimates of k2. Insofar as tibial lesions are a proxy for frailty, the Danish curves suggest much greater selectivity than the East Smithfield curves, although selectivity does appear to be operating in the Black Death cemetery as well.
Fig. 2.
The estimated effect of periosteal lesions of the tibia on the baseline risk of death within the East Smithfield Black Death cemetery (Left) and the two Danish attritional mortality cemeteries (Right).
According to the −2LLR criterion, one skeletal feature, femur length, did not seem to improve model fit in either sample, compared with a nested model without selectivity. In other words, the evidence for selective mortality, whether epidemic or attritional, acting on femur length is weak in this analysis. Femur length was used here as an indicator of overall stature; short stature may imply high frailty if, for example, it results from repeated rounds of interrupted growth, but it may also be partly genetic and more or less unassociated with frailty. It could also be that any association between growth interruption and elevated risk of death attenuates over time if the individual survives and resumes normal growth (although the elevated risk associated with linear enamel hypoplasias would argue against this). At any rate, femur length as measured here does not seem to be a good proxy for frailty under either normal or catastrophic mortality.
Discussion
All of the lesions included in this study, with the exception of short femurs, were strongly associated with excess mortality in the normal-mortality Danish cemeteries. This finding for the Danish samples suggests that these particular skeletal lesions really are informative about frailty; i.e., individuals in Denmark with the lesions had higher frailty than their peers without them. All of the lesions except femur length were also associated with substantial excess mortality in East Smithfield, except perhaps for cribra orbitalia, which does poorly according to the −2LLR criterion. This finding suggests that the Black Death probably was selective with respect to at least some of the skeletal indicators of frailty, as individuals who had those lesions before the Black Death appear to have been more likely to die during the epidemic than individuals without them.
The level of excess mortality for each lesion was, without exception, higher in Denmark than in East Smithfield, suggesting that the Black Death was not as strongly selective as normal mortality. For example, the k2 estimate for proliferative tibial lesions was >5 for the Danish sample, but only 1.5 for the East Smithfield sample. This finding suggests that in Denmark, during times of normal mortality, individuals with periosteal lesions of the tibia were more than five times more likely to die than their peers without such lesions; however, during the Black Death, individuals who had tibial lesions before the outbreak were only ≈50% more likely to die than their unaffected peers. During the Black Death, frail individuals were more likely to die, but these results suggest that their relative risk was not as high as it would have been under normal-mortality conditions.
The large differences between East Smithfield and Denmark in the excess mortality associated with skeletal lesions presumably reflect a situation in which a greater number of healthy individuals (those without lesions) died during the Black Death than would have been the case under normal conditions. The Black Death was highly virulent and undoubtedly killed many otherwise healthy people who would have been unlikely to die under normal-mortality conditions. This situation would produce a reduction in the excess mortality of individuals with lesions in East Smithfield (compared with that in Denmark), not because individuals with lesions were less likely to die during the Black Death than under normal conditions, but because the risk of death for otherwise healthy people increased during the epidemic. In other words, the results suggest that the Black Death did not kill indiscriminately, but it did discriminate less sharply than death normally does.
It is important to note that the estimated differences between the two samples may reflect the use of an inappropriate comparison sample rather than actual differences in the strength of selectivity in normal versus Black Death mortality. The samples used in this study were drawn from two different geographic areas. Differences in estimates of excess mortality might arise if the populations differed with respect to susceptibility to death from the conditions that caused the lesions or if the lesions were caused by very different conditions in each population. Suppose, for example, that periosteal lesions (which are not specific to a particular infection) were caused by a disease with high virulence in Denmark but low virulence in East Smithfield. Under these circumstances, the lower estimate of k2 associated with these lesions in East Smithfield could reflect the lower virulence of the associated cause rather than weaker selectivity of the Black Death. That there was a pattern of excess mortality associated with several lesions in the Black Death cemetery suggests the epidemic really was selective with respect to frailty. In addition, finding a completely consistent pattern of higher k2 values in Denmark across a wide variety of lesions with different etiologies suggests that mortality was more strongly selective under normal, nonepidemic conditions in Northern Europe. Nonetheless, conclusions about differences in the strength of selectivity between the Black Death and normal mortality should be tempered by the possibility of population differences, as well as by the dubious values of the reported standard errors and LLRs.
It is known from chronicles written at the time of the Black Death that some people managed to survive the disease, despite clear evidence that they contracted it (see ref. 19 for numerous examples). These survivors must ultimately have been able to mount an effective primary immune response to the disease. Individuals unable to mount a full response presumably succumbed to the disease in disproportionate numbers. Because most of the lesions included in our analyses can reflect under-nutrition, we suggest that we may be capturing some of the effect of poor nutritional status on immune function during the Black Death (as well as during normal mortality). But whatever the immediate mechanism, it is important to emphasize that it was unlikely to have been the observed lesions per se that put people at higher risk; rather, it is because the presence of one or more lesions is correlated with some aspect of poor underlying physical condition, perhaps malnutrition, perhaps impaired immunocompetence, or perhaps something else entirely.
Finally, our results suggest that we can rarely if ever assume that mortality is not selective with respect to existing health conditions, even in the case of a catastrophe as devastating as the Black Death. These results are consistent with previous studies of the catastrophic mortality associated with natural disasters and warfare (44–51). It has been found, for example, that a 1995 earthquake in Japan disproportionately killed individuals with physical disabilities (47). Paleodemographic studies of Native American massacres have similarly shown that mortality in warfare can be selective for various conditions that may compromise the victims' ability to flee or defend themselves (49, 50). Severe famine increases mortality at all ages but disproportionately affects adult males (51). Thus, despite the obvious virulence of the Black Death, we should not be surprised that it did not affect all individuals equally. As a consequence, cemeteries associated with the Black Death cannot be expected to provide a perfect, easy-to-interpret cross-section of the living population immediately before the outbreak.
Materials and Methods
Skeletal Samples.
The East Smithfield cemetery in London is one of a small number of excavated cemeteries with unambiguous documentary and archaeological evidence linking it to the Black Death of 1349–1350 (23, 24). For example, coins dating to the time of the epidemic or the years leading up to it were discovered in the burials; no later material was found (24). More importantly, a cartulary of 1348 from the Church of the Holy Trinity just outside London's walls provides the exact dimensions and location of the cemetery, verifies that it was founded specifically in response to the Black Death, and notes that the land had been used for agricultural purposes up to that time (23). More than 1,000 skeletons were excavated at East Smithfield; many of these, however, were badly degraded by corrosive chemicals used at the Royal Mint, which occupied part of the site from 1806 to 1967 (16, 24). A total of 490 skeletons of all ages were well enough preserved to be scored and used in the present analysis.
The Danish pre-Black Death control skeletons were selected from the urban parish cemeteries of St. Albani Church (Odense) and St. Mikkel Church (Viborg). The skeletons from these sites form part of the Anthropological Database (Odense University, Odense, Denmark), a skeletal collection well known for its size and the quality of its chronological control (32). St. Mikkel Church is believed to date from before 1129, and it was first mentioned in mid-12th century written sources (there is evidence that some burials on this site may predate construction of the church itself). The city of Viborg converted to Protestantism in 1529, and by April of that year, all churches associated with the city were demolished, including St. Mikkel. St. Mikkel was a peri-urban parish with a congregation made up mostly of people of lower socioeconomic status (52). St. Albani Church in Odense was founded in the early 11th century and is first mentioned in written records in 1086 (53). St. Albani served an urban congregation until 1529, and the church was demolished in 1542 (53). Although the St. Albani and St. Mikkel cemeteries were both in use from the 1100s until the early 1500s (54, 55), individual burials can be dated with reasonable accuracy on the basis of arm positions (38, 39). Examination of medieval and early modern Danish cemeteries has revealed a series of rapid changes in the predominant arm position of interred individuals. Kieffer-Olsen (38) analyzed medieval cemeteries in Denmark and found that dating by arm position is more reliable than radiocarbon dating, as the margin of error associated with arm position is much narrower. For this study, only individuals with arms positions that were used exclusively or predominately before the Black Death in Denmark (a total of 291 skeletons) were included in the sample. Individuals from the St. Albani Church, St. Mikkel Church, and East Smithfield cemeteries were included in the samples only if they were preserved well enough to score for age, sex, and the presence of skeletal lesions.
For this study, it was assumed that all individuals in the East Smithfield sample were victims of the Black Death. This is not an unreasonable assumption given that Black Death mortality overwhelmed normal mortality during the epidemic (21). However, it is possible that a few individuals in the East Smithfield cemetery died from other causes. It was also assumed that the Danish cemetery sample contains only individuals who died before the Black Death, although it is possible that some individuals in the Danish samples were victims of the Black Death or died after the epidemic. According to Kieffer-Olsen (38) and Jantzen et al. (39), one of the arm positions included in the sample was still being used, albeit rarely, for a short time during and after the Black Death in Denmark. If East Smithfield includes non-Black Death individuals and the Danish sample includes victims of the Black Death, apparent differences between the two cemeteries should be reduced. However, this study suggests a consistent and large difference in the degree of selectivity with respect to frailty between the two cemeteries. Therefore, the possibility that East Smithfield does not purely reflect Black Death mortality and that Denmark does not purely reflect normal mortality only strengthens the conclusions made here about differences in selective mortality.
For this study, it was also assumed that the Danish and East Smithfield populations were stable, i.e., closed to migration, with constant age-specific fertility and mortality rates and stable age distributions. This assumption is generally reasonable in paleodemographic studies because the mathematical property of “weak ergodicity” ensures that most populations closely approximate a stable age distribution even in the face of migration and changing vital rates (2, 3). However, because the catastrophic mortality levels of the Black Death would perturb a population's age distribution away from its stable form, the assumption of stability would be problematic in the East Smithfield case unless the Black Death ran its course very rapidly. If the epidemic lasted a long time in any given locale, mortality would be acting on a very different age distribution at the end of the epidemic than it was at the initial outbreak of the disease. Recent estimates of the time course of the Black Death show that it swept through specific localities in a matter of a few weeks (21), suggesting that the age distribution of the local population early in the epidemic was unlikely to have differed much from that in its later stages.
Skeletal Lesions.
The following bony lesions or stress markers were scored as proxy measures of frailty for all of the skeletons included in the East Smithfield and Danish samples: porotic hyperostosis, cribra orbitalia, linear enamel hypoplasia, periosteal lesions of the tibia, and short femur length (indicative of short stature and possible juvenile growth faltering). All lesions were scored by the first author (S.N.D.) by using standard protocols (56), thus eliminating interobserver error and ensuring comparability.
Porotic hyperostosis, cribra orbitalia, linear enamel hypoplasia, and short femur length in adulthood are generally attributed to episodes of disease or malnutrition during childhood (1). Periosteal lesions can occur because of infection or trauma throughout life (1, 14). Periosteal lesions were scored on the tibia, a robust bone that is often well preserved in our samples. Individuals of all ages were scored for the presence of porotic hyperostosis, cribra orbitalia, linear enamel hypoplasia, and periosteal lesions; only adults were included in the analysis of femur length. Femur lengths ≤ −1 SD of the mean for the corresponding sex were classified as short; those individuals with femur lengths > −1 SD of the mean for their sex were considered to be normal with respect to stature.
Age-at-Death Estimation.
Ages at death for juveniles were estimated from dental development/eruption and epiphyseal union by using established methods (56–59). Traditional methods of adult age-at-death estimation, unfortunately, have been shown to be biased toward the age composition of whatever modern known-age reference sample is used as a standard (60). Recently, researchers have developed statistical methods to correct this bias (5–8); for example, the so-called Rostock protocol uses maximum likelihood estimation and Bayesian inversion to produce unbiased age estimates (7, 8). Previous studies of the East Smithfield cemetery have estimated age-at-death by using traditional methods (16, 17) or Bayesian inversion assuming (as opposed to estimating) a model prior distribution (18). This study is the first application to our knowledge of the complete Rostock protocol to the East Smithfield adult skeletons. The same protocol was also applied to adult skeletons from the two Danish cemeteries.
This study uses empirical weight functions (age-specific probabilities of observing particular skeletal age indicators in a known age-at-death reference sample) estimated by Boldsen and Milner (61) using the Terry reference collection. All adults in the Danish and East Smithfield samples were scored for the 19 skeletal indicators of age examined by Boldsen and Milner (61) by using their methods; these indicators included cranial suture closure and various features of the pubic symphysis and iliac auricular surface. The Gompertz-Makeham mortality function was used as a parametric model for the prior adult age-at-death distribution (62), and its parameters were estimated by maximum likelihood for each of the two sets of skeletons. Bayesian inversion was then performed using the parameter estimates to provide point estimates of individual ages at death.
The specific version of the Rostock protocol used in this study is the multivariate latent-trait method (40). In this method, multiple age indicators are used to estimate age at death, but the indicators are not assumed to be independent of each other. Instead the method assumes that all age indicators are correlated with the same latent “biological age” trait z. Although the value of z for each individual is unmeasurable, the entire distribution of z among individuals (assumed to follow a gamma density function) and the correlation of z with each age indicator can be estimated and converted into a posterior estimate of age at death for each skeleton using Bayes's theorem (40). This method has several virtues: It uses multivariate data, it does not assume that age indicators are independent, it allows skeletons with some missing age-indicator data to be included, and it has a reasonable number of parameters to estimate.
Model Estimation.
All models were estimated by maximum likelihood procedures by using Holman's special-purpose program mle (63). The global peak of the likelihood surface was found by simulated annealing using multiple start values to avoid local maxima. The program mle routinely gives standard errors for all parameter estimates. For the parameter values in Table 1, however, these standard errors are almost certainly underestimates because they do not incorporate the (possibly large) errors involved in estimating the ages of individual skeletons. By the same logic, the likelihood value associated with the peak of the surface is likely to be misestimated by an unknown amount. For this reason, probability (“significance”) levels are not reported in Table 1. We feel compelled to point out that we are not adopting this approach because we failed to get any significant results; indeed, most of our effects and differences appeared to be significant by using conventional cut-off points and taking our standard errors and likelihood ratios at face value. We just do not believe that the purported P values (or the conventional cut-off points) are meaningful. In our judgment, the standard errors and likelihood ratios in Table 1 should be used solely as loose, informal guides to model assessment.
ACKNOWLEDGMENTS.
We are indebted to Bill White and his associates, Jelena Bekvalac, Lynne Cowal, Tania Kausmally, and Richard Mikulski, at the Museum of London Centre for Human Bioarchaeology for providing access to the East Smithfield skeletons, and to Jesper Boldsen for allowing us to examination the Danish skeletal collections at the Anthropological Database (Odense University, Odense, Denmark). Both Drs. White and Boldsen provided physical facilities and useful advice as well as access to skeletons. We thank Ulla Freund for her technical support and other help in Odense. Bethany Usher and Darryl Holman provided invaluable analytical and computational guidance, for which we are grateful. We also thank Tim Gage, Ken Weiss, Ottar Bjornstad, Jeffrey Kurland, Pat Johnson, and two anonymous reviewers for critically reading and commenting on the paper. Funding for the research was provided by the National Science Foundation (Grant BCS-0406252), the Wenner-Gren Foundation for Anthropological Research (Grant 7142), the American-Scandinavian Foundation, and Pennsylvania State University's Research and Graduate Studies Office.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Larsen CS. Bioarchaeology. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 2.Wood JW. In: Encyclopedia of Population. Demeny P, McNicoll G, editors. New York: Macmillan; 2003. pp. 717–720. [Google Scholar]
- 3.Milner GR, Wood JW, Boldsen JL. In: Skeletal Biology of Past Peoples. Saunders S, Katzenberg M, editors. New York: Wiley-Liss; 2000. pp. 467–497. [Google Scholar]
- 4.Wood JW, Milner GR, Harpending HC, Weiss KM. Curr Anthropol. 1992;33:343–370. [Google Scholar]
- 5.Konigsberg LW, Frankenberg SR. Am J Phys Anthropol. 1992;89:235–256. doi: 10.1002/ajpa.1330890208. [DOI] [PubMed] [Google Scholar]
- 6.Konigsberg LW, Frankenberg SR. Evol Anthropol. 1994;3:92–105. [Google Scholar]
- 7.Müller HG, Love B, Hoppa RD. Am J Phys Anthropol. 2002;117:1–14. doi: 10.1002/ajpa.10000. [DOI] [PubMed] [Google Scholar]
- 8.Hoppa RD, Vaupel JW, editors. Paleodemography. Cambridge, UK: Cambridge Univ Press; 2002. [Google Scholar]
- 9.Wright LE, Yoder CJ. J Archaeol Res. 2003;11:43–70. [Google Scholar]
- 10.Usher BM. University Park, PA: Pennsylvania State Univ; 2000. PhD dissertation. [Google Scholar]
- 11.Ferrell Thomas RJ. University Park, PA: Pennsylvania State Univ; 2003. PhD dissertation. [Google Scholar]
- 12.Jackes D, Lubell D, Meiklejon C. Antiquity. 1997;71:639–658. [Google Scholar]
- 13.Hoppa RD. Homo. 1999;50:228–243. [Google Scholar]
- 14.Ortner DJ. Identification of Pathological Conditions in Human Skeletal Remains. Amsterdam: Academic; 2003. [Google Scholar]
- 15.Chamberlain A. Demography in Archaeology. Cambridge, UK: Cambridge Univ Press; 2006. [Google Scholar]
- 16.Margerison BJ, Knusel CJ. Am J Phys Anthropol. 2002;119:134–143. doi: 10.1002/ajpa.10082. [DOI] [PubMed] [Google Scholar]
- 17.Waldron HA. Int J Epidemiol. 2001;30:104–108. doi: 10.1093/ije/30.1.104. [DOI] [PubMed] [Google Scholar]
- 18.Gowland RL, Chamberlain AT. Antiquity. 2005;79:146–157. [Google Scholar]
- 19.Horrox R, trans, editors. The Black Death. Manchester, UK: Manchester Univ Press; 1994. [Google Scholar]
- 20.Hinde A. England's Population. London: Hodder and Arnold; 2003. [Google Scholar]
- 21.Wood JW, Ferrell RJ, DeWitte-Aviña SN. Hum Biol. 2003;75:427–448. doi: 10.1353/hub.2003.0067. [DOI] [PubMed] [Google Scholar]
- 22.Benedictow OJ. The Black Death 1346–1353: The Complete History. Rochester, NY: Boydell & Brewer; 2004. [Google Scholar]
- 23.Hawkins D. Antiquity. 1990;64:637–642. [Google Scholar]
- 24.Grainger I, Hawkins D. London Achaeol. 1988;5:429–436. [Google Scholar]
- 25.Vaupel JW, Johnson TE, Lithgow GJ. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- 26.Hatcher J. Plague, Population and the English Economy 1348–1530. London: Macmillan; 1977. [Google Scholar]
- 27.Bean JMW. In: The Black Death. Williman D, Sirasis N, editors. Binghamton, NY: Center for Medieval and Early Renaissance Studies; 1982. pp. 23–38. [Google Scholar]
- 28.Bolton J. In: The Black Death in England. Ormrod WM, Lindley PG, editors. Stamford, UK: Paul Watkins; 1996. pp. 17–78. [Google Scholar]
- 29.Platt C. King Death. Toronto: Univ of Toronto Press; 1996. [Google Scholar]
- 30.Herlihy D. The Black Death and the Transformation of the West. Cambridge, MA: Harvard Univ Press; 1997. [Google Scholar]
- 31.Paine RR. Am J Phys Anthropol. 2000;112:181–190. doi: 10.1002/(SICI)1096-8644(2000)112:2<181::AID-AJPA5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Boldsen JL. Humaniora. 1998;3:1325–1329. [Google Scholar]
- 33.Benedictow OJ. Plague in the Late Medieval Nordic Countries. Oslo: Middelalderforlaget; 1993. [Google Scholar]
- 34.Sawyer B, Sawyer PH. Medieval Scandinavia. Minneapolis: Univ of Minnesota Press; 1993. [Google Scholar]
- 35.Poulsen B. In: Medieval Farming and Technology. Astill GG, Langdon J, editors. The Netherlands: Brill, Leiden; 1997. pp. 115–146. [Google Scholar]
- 36.Roesdahl E, editor. Dagligliv i Danmarks Middelalder. Copenhagen: Nordisk Forlag; 1999. [Google Scholar]
- 37.Capelli C, Redhead N, Abernethy JK, Gratix F, Wilson JF, Moen T, Hervig T, Richards M, Stumpf MPH, Underhill PA, et al. Curr Biol. 2003;13:979–984. doi: 10.1016/s0960-9822(03)00373-7. [DOI] [PubMed] [Google Scholar]
- 38.Kieffer-Olsen J. Århus, Denmark: Århus Univ; 1993. PhD dissertation. [Google Scholar]
- 39.Jantzen C, Kieffer-Olsen J, Madsen PK. Mark og Montre. 1994:26–36. [Google Scholar]
- 40.Holman DJ, Wood JW, O'Connor KA. In: Paleodemography. Hoppa RD, Vaupel JW, editors. Cambridge, UK: Cambridge Univ Press; 2002. pp. 193–201. [Google Scholar]
- 41.Siler W. Ecology. 1979;60:750–757. [Google Scholar]
- 42.Siler W. Stats Med. 1983;2:373–380. doi: 10.1002/sim.4780020309. [DOI] [PubMed] [Google Scholar]
- 43.Gage TB. Am J Phys Anthropol. 1988;76:429–441. doi: 10.1002/ajpa.1330760403. [DOI] [PubMed] [Google Scholar]
- 44.Doocy S, Rofi A, Moodie C, Spring E, Bradley S, Burnham G, Robinson C. Bull WHO. 2007;85:273–278. doi: 10.2471/BLT.06.033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan CC, Lin YP, Chen HH, Chang TY, Cheng TJ, Chen LS. Ann Epidemiol. 2003;13:502–508. doi: 10.1016/s1047-2797(03)00040-1. [DOI] [PubMed] [Google Scholar]
- 46.Ellidokuz H, Ucku R, Aydin UY, Ellidokuz E. Croat Med J. 2005;46:613–618. [PubMed] [Google Scholar]
- 47.Osaki Y, Minowa M. Am J Epidemiol. 2001;153:153–156. doi: 10.1093/aje/153.2.153. [DOI] [PubMed] [Google Scholar]
- 48.Luongo G, Perrotta A, Scarpati C, De Carolis E, Patricelli G, Ciarallo A. J Volcanol Geothermal Res. 2003;126:169–200. [Google Scholar]
- 49.Milner GR, Anderson E, Smith VG. Am Antiq. 1991;56:581–603. [Google Scholar]
- 50.Willey P, Emerson TE. Plains Anthropol. 1993;38:227–269. [Google Scholar]
- 51.Maharatna A. The Demography of Famines. New Delhi, India: Oxford Univ Press; 1996. [Google Scholar]
- 52.Petersen HC, Boldsen JL, Paine RR. In: Urbanism in the Preindustrial World. Storey GR, editor. Tuscaloosa: Univ of Alabama Press; 2004. [Google Scholar]
- 53.Boldsen JL, MØllerup L. Am J Phys Anthropol. 2006;130:344–351. doi: 10.1002/ajpa.20363. [DOI] [PubMed] [Google Scholar]
- 54.Kristensen HK. Middelalderbyen Viborg. Århus, Denmark: Statens Humanistiske Forskningoraad; 1987. [Google Scholar]
- 55.Christensen SB. Middelalderbyen Odense. Århus, Denmark: Århus Univ; 1988. [Google Scholar]
- 56.Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains. Fayetteville, AK: Arkansas Archeological Survey; 1994. [Google Scholar]
- 57.Bass WM. Human Osteology. Columbia, MO: Missouri Archaeological Soc; 1987. [Google Scholar]
- 58.Scheuer L, Black S. The Juvenile Skeleton. London: Elsevier; 2004. [Google Scholar]
- 59.Baker BJ, Dupras TL, Tocheri MW. The Osteology of Infants and Children. College Station, TX: Texas A&M Univ Press; 2005. [Google Scholar]
- 60.Bocquet-Appel JP, Masset C. J Hum Evol. 1982;11:321–333. [Google Scholar]
- 61.Boldsen JL, Milner GR, Konigsberg LW, Wood JW. In: Paleodemography. Hoppa RD, Vaupel JW, editors. Cambridge, UK: Cambridge Univ Press; 2002. pp. 73–106. [Google Scholar]
- 62.Wood JW, Holman DJ, O'Connor KA, Ferrell RJ. In: Paleodemography. Hoppa RD, Vaupel JW, editors. Cambridge, UK: Cambridge Univ Press; 2002. pp. 129–168. [Google Scholar]
- 63.Holman DJ. mle. 2002 Version 2.0 available at http://faculty.washington.edu/∼djholman/mle. [Google Scholar]
- 64.Lang JM, Rothman KJ, Cann CI. Epidemiology. 1998;9:7–8. doi: 10.1097/00001648-199801000-00004. [DOI] [PubMed] [Google Scholar]