Abstract
The M2 protein of influenza A virus forms a transmembrane proton channel important for viral infection and replication. Amantadine blocks this channel, thus inhibiting viral replication. Elucidating the high-resolution structure of the M2 protein and its change upon amantadine binding is crucial for designing antiviral drugs to combat the growing resistance of influenza A viruses against amantadine. We used magic-angle-spinning solid-state NMR to determine the conformation and dynamics of the transmembrane domain of the protein M2TMP in the apo- and amantadine-bound states in lipid bilayers. 13C chemical shifts and torsion angles of the protein in 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine (DLPC) bilayers indicate that M2TMP is α-helical in both states, but the average conformation differs subtly, especially at the G34–I35 linkage and V27 side chain. In the liquid-crystalline membrane, the complexed M2TMP shows dramatically narrower lines than the apo peptide. Analysis of the homogeneous and inhomogeneous line widths indicates that the apo-M2TMP undergoes significant microsecond-time scale motion, and amantadine binding alters the motional rates, causing line-narrowing. Amantadine also reduces the conformational heterogeneity of specific residues, including the G34/I35 pair and several side chains. Finally, amantadine causes the helical segment N-terminal to G34 to increase its tilt angle by 3°, and the G34–I35 torsion angles cause a kink of 5° in the amantadine-bound helix. These data indicate that amantadine affects the M2 proton channel mainly by changing the distribution and exchange rates among multiple low-energy conformations and only subtly alters the average conformation and orientation. Amantadine-resistant mutations thus may arise from binding-incompetent changes in the conformational equilibrium.
Keywords: high-resolution structure, membrane protein, solid-state NMR, conformational heterogeneity, chemical-shift perturbation
The M2 protein of the influenza A virus forms a membrane-bound proton channel that acidifies the endosomally trapped virus, which triggers the release of the viral RNA into the infected cell, initiating viral replication (1, 2). The cationic amine amantadine inhibits viral replication by blocking this proton channel and thus has been used for the prophylaxis and treatment of influenza A infections (3, 4). However, in the last few years, amantadine resistance has skyrocketed among influenza A viruses in Asia and North America (5), making it imperative to develop alternative antiviral drugs.
The M2 protein contains a transmembrane α-helical domain (6) that has the essential amantadine-sensitive proton channel activity of the intact protein (7). Mutagenesis and electrophysiological experiments showed that the residues important for proton conduction and amantadine interaction lie on one face of the helix, namely, V27, A30, S31, and G34 (8, 9). Neutron diffraction data indicated that the amantadine ring is localized at ≈6 Å from the center of dioleoylphosphatidylcholine (DOPC) bilayers, close to V27 (10). Fourier analysis of the periodic oscillations in the channel reversal potential, pH-sensitive current, and amantadine resistance of cysteine mutants of the M2 protein yielded a functional structure of the channel (11). The active form of the channel is a tetramer for the intact protein (12, 13) and the transmembrane peptide (M2TMP) (14), as shown by 19F solid-state NMR (SSNMR) of the membrane-bound peptide.
The most extensive molecular-level structural information of M2TMP came from static 15N SSNMR data of Cross and coworkers (15, 16). Using uniaxially aligned lipid membranes, they determined the orientation of M2TMP in the apo (15) and complexed (16) states from 15N chemical shift and N H dipolar couplings. The apo peptide is tilted by 38° from the bilayer normal (15), whereas the amantadine-complexed peptide exhibits a kink with 31° and 20° tilt angles (16). However, sample preparation conditions such as solvents, membrane composition, and peptide concentration varied greatly in these studies, which may contribute to the observed orientation difference. From the 15N orientational data, no direct information on the backbone and side chain conformations can be extracted. The side chain conformation may be especially sensitive to amantadine binding, yet so far only one 13C
H dipolar couplings. The apo peptide is tilted by 38° from the bilayer normal (15), whereas the amantadine-complexed peptide exhibits a kink with 31° and 20° tilt angles (16). However, sample preparation conditions such as solvents, membrane composition, and peptide concentration varied greatly in these studies, which may contribute to the observed orientation difference. From the 15N orientational data, no direct information on the backbone and side chain conformations can be extracted. The side chain conformation may be especially sensitive to amantadine binding, yet so far only one 13C 15N distance (17) and four 19F
15N distance (17) and four 19F 19F distances (14, 18) have been reported. Recently, amantadine was found to cause substantial narrowing of the 15N NMR spectra (19, 20), suggesting that it either reduces the conformational heterogeneity or changes the dynamics of the protein, but which factor dominates is unknown.
19F distances (14, 18) have been reported. Recently, amantadine was found to cause substantial narrowing of the 15N NMR spectra (19, 20), suggesting that it either reduces the conformational heterogeneity or changes the dynamics of the protein, but which factor dominates is unknown.
To elucidate the atomic-resolution conformation and dynamics of the backbone and side chains of this important proton channel with and without amantadine, we have used magic-angle-spinning (MAS) 13C and 15N NMR techniques on M2TMP bound to 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine (DLPC) bilayers. To identify sites of structural perturbation, we measured and compared the 13C and 15N isotropic shifts and torsion angles of eight residues spread throughout the peptide. The data indicate that amantadine most significantly perturbs the backbone of G34 and I35 and the side chain of V27. Analysis of the homogeneous and inhomogeneous 13C line widths in the gel and liquid-crystalline (LC) phases of the membrane indicates that the apo peptide backbone undergoes significant motion on the microsecond time scale. Amantadine binding alters the motional rates and reduces the conformational distribution. Thus, the central feature of M2TMP structure appears to be the presence of multiple low-energy conformations, which are readily modified and selected by amantadine.
Results and Discussion
M2TMP Conformation With and Without Amantadine.
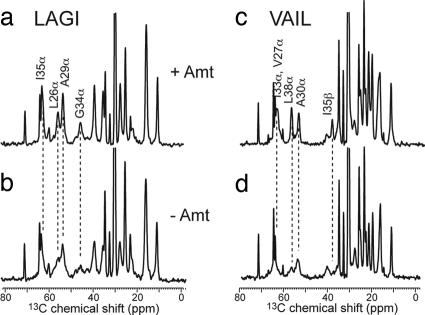
We chose eight residues in M2TMP for 13C- and 15N-labeling. Based on the approximate seven-residue periodicity of the protein (11), these sites cover channel-lining positions (V27, A30, and G34), helix–helix interfaces (L26, I33, and L38), and lipid-facing positions (A29 and I35). In this way, we assess the impact of amantadine binding to M2TMP structure from all regions of the tetrameric bundle. Two peptides were synthesized that each contained four uniformly 13C, 15N-labeled residues. The “LAGI” sample contained labeled L26, A29, G34, and I35, and the “VAIL” sample contained labeled V27, A30, I33, and L38. Fig. 1 shows representative 13C cross-polarization (CP) MAS spectra of the peptide in DLPC bilayers with (red) and without (black) amantadine at 303 K. The 13C isotropic line widths narrow substantially upon amantadine binding. In the apo peptide, many backbone signals such as G34 Cα are broad and poorly defined, whereas with amantadine, all Cα resonances narrow and increase in intensity. Side chain signals also are narrowed but less dramatically. This 13C line-narrowing is similar to that seen in 15N spectra of the protein (16, 19). In the next section, we investigate the origin of this line-narrowing.
Fig. 1.
13C CP-MAS spectra of M2TMP at 303 K with (a and c) and without (b and d) amantadine. (a and b) LAGI. (c and d) VAIL. Note the significant line-narrowing and intensity increase in the presence of amantadine.
To determine the M2TMP conformation and its perturbation by amantadine, we measured the 13C and 15N isotropic chemical shifts of the peptide without and with amantadine. 13C 13C 2D double-quantum (DQ)-filtered correlation spectra and 15N
13C 2D double-quantum (DQ)-filtered correlation spectra and 15N 13C correlation spectra were measured at 243 K where the peptide motion is frozen. Both spectra remove all lipid natural-abundance 13C signals, thus simplifying resonance assignment. Fig. 2 shows the 2D 13C
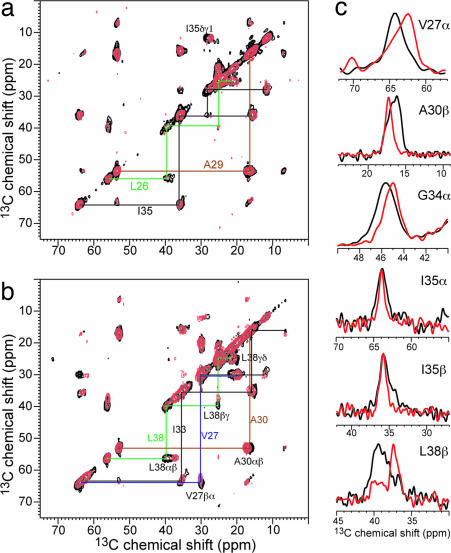
13C correlation spectra were measured at 243 K where the peptide motion is frozen. Both spectra remove all lipid natural-abundance 13C signals, thus simplifying resonance assignment. Fig. 2 shows the 2D 13C 13C correlation spectra of LAGI and VAIL with (red) and without (black) amantadine. The spectra are readily assigned based on the connectivity patterns. Various chemical-shift changes are observed, for example, at V27α, A30β, I35γ1, and L38β. The largest Cα shift change occurs at V27, which exhibits a 1.0-ppm upfield shift in the complex. G34 Cα is not detected in the 2D 13C
13C correlation spectra of LAGI and VAIL with (red) and without (black) amantadine. The spectra are readily assigned based on the connectivity patterns. Various chemical-shift changes are observed, for example, at V27α, A30β, I35γ1, and L38β. The largest Cα shift change occurs at V27, which exhibits a 1.0-ppm upfield shift in the complex. G34 Cα is not detected in the 2D 13C 13C correlation spectrum because of the DQ excitation condition, but its signal is visible in the 2D 15N
13C correlation spectrum because of the DQ excitation condition, but its signal is visible in the 2D 15N 13C spectra [supporting information (SI) Fig. 7] and shows a downfield 15N chemical-shift change of 2.5 ppm. Interestingly, the two Ile residues flanking G34 also exhibit 15N chemical-shift changes but in opposite directions, causing their amantadine-bound 15N shifts to differ by 5.8 ppm. SI Table 2 lists the isotropic shifts of the apo and complexed M2TMP.
13C spectra [supporting information (SI) Fig. 7] and shows a downfield 15N chemical-shift change of 2.5 ppm. Interestingly, the two Ile residues flanking G34 also exhibit 15N chemical-shift changes but in opposite directions, causing their amantadine-bound 15N shifts to differ by 5.8 ppm. SI Table 2 lists the isotropic shifts of the apo and complexed M2TMP.
Fig. 2.
2D 13C 13C DQ-filtered spectra of M2TMP in DLPC bilayers without (black) and with (red) amantadine at 243 K. Intraresidue connectivities and cross-peaks with chemical-shift changes are indicated. (a) LAGI. (b) VAIL. (c) Selected 1D cross-sections that exhibit line-narrowing and chemical-shift changes upon amantadine binding. The G34α trace was extracted from 1D CP spectra.
13C DQ-filtered spectra of M2TMP in DLPC bilayers without (black) and with (red) amantadine at 243 K. Intraresidue connectivities and cross-peaks with chemical-shift changes are indicated. (a) LAGI. (b) VAIL. (c) Selected 1D cross-sections that exhibit line-narrowing and chemical-shift changes upon amantadine binding. The G34α trace was extracted from 1D CP spectra.
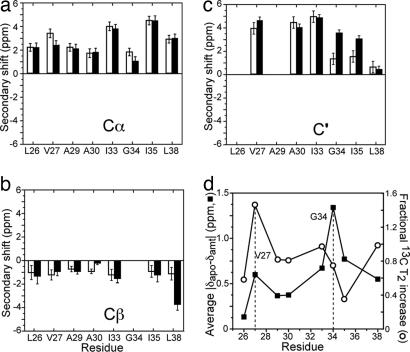
Despite the chemical-shift perturbations by amantadine, no change is large enough to indicate a nonhelical structure (21), which is reflected by the positive Cα and C′ secondary shifts and negative Cβ secondary shifts (Fig. 3 a–c) for all eight labeled residues. Fig. 3d plots the amantadine-induced average absolute chemical-shift changes of each residue. The maximum perturbation occurs at the channel-lining G34, followed by its adjacent I35 and I33. A second local maximum is seen at V27, consistent with its proximity to amantadine (10). In terms of residue location, the channel-lining residues, the interfacial residues, and the lipid-facing residues except for the G34-neighboring I35 have average chemical-shift changes of 0.79 ppm, 0.46 ppm, and 0.33 ppm, respectively.
Fig. 3.
Amantadine-induced isotropic shift and T2 changes of M2TMP. (a–c) Secondary shifts are plotted for Cα (a), Cβ (b), and C′ (c). Open and filled bars correspond to the apo and complexed M2TMP, respectively. The average chemical-shift uncertainty is 0.35 ppm, estimated from the intrinsic line widths of the spectra. (d) Average absolute chemical-shift changes (filled squares) and fractional 13C T2 increase at 303 K (open circles). Local maxima of chemical shift and T2 perturbation occur at V27 and G34.
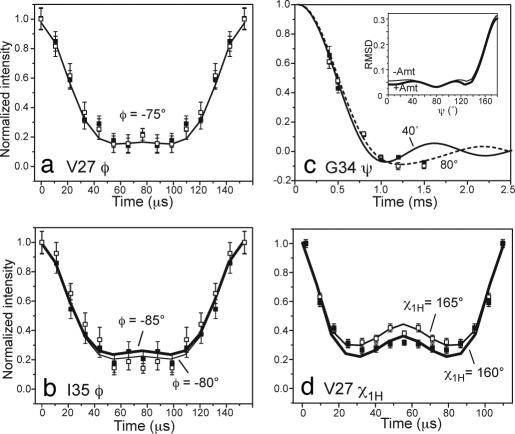
To obtain more quantitative conformational constraints, we measured φ, ψ, and χ1H torsion angles by using dipolar correlation techniques. The φ angles of all labeled residues except for G34 were measured by using the HNCH technique (22), which correlates the N H and Cα
H and Cα Hα bond orientations of each residue. Most φ angles fall between −45° and −85°, with no large difference between the apo and complexed peptide within the angular resolution of the technique (Fig. 4a, Table 1). When taking into account the significant 15N, 13Cα, and 13C′ shift changes at G34–I35, the observed I35 φ angles of −80° for the apo-M2TMP and −85° for the complex (Fig. 4b) may reflect a real torsion angle difference. The ψ angle of G34 was measured by using the NCCN technique (23). The data (Fig. 4c) yielded a best-fit ψ angle of −80° at 243 K with and without amantadine. However, the angular resolution of the technique is limited in the α-helical region (23), as shown by the shallow rmsd minimum. Thus, we used the TALOS program (24) to predict the G34 torsion angles based on the experimental chemical shifts of the I33/G34/I35 triplet, yielding G34 (φ, ψ) of (−66°, −37°) and (−67°, −42) for the apo and complexed peptide, respectively (Table 1). Indeed, the calculated −40° NCCN curve agrees well with the experimental data.
Hα bond orientations of each residue. Most φ angles fall between −45° and −85°, with no large difference between the apo and complexed peptide within the angular resolution of the technique (Fig. 4a, Table 1). When taking into account the significant 15N, 13Cα, and 13C′ shift changes at G34–I35, the observed I35 φ angles of −80° for the apo-M2TMP and −85° for the complex (Fig. 4b) may reflect a real torsion angle difference. The ψ angle of G34 was measured by using the NCCN technique (23). The data (Fig. 4c) yielded a best-fit ψ angle of −80° at 243 K with and without amantadine. However, the angular resolution of the technique is limited in the α-helical region (23), as shown by the shallow rmsd minimum. Thus, we used the TALOS program (24) to predict the G34 torsion angles based on the experimental chemical shifts of the I33/G34/I35 triplet, yielding G34 (φ, ψ) of (−66°, −37°) and (−67°, −42) for the apo and complexed peptide, respectively (Table 1). Indeed, the calculated −40° NCCN curve agrees well with the experimental data.
Fig. 4.
Selected torsion angle data of M2TMP without (open squares) and with (filled squares) amantadine, along with their rmsd-quantified best-fit simulations (lines). Thick lines indicate best-fit curves for the complexed M2TMP when different from the apo peptide. (a) V27 φ. (b) I35 φ. (c) G34 ψ. (Inset) The rmsd between the simulation and the experiment. (d) V27 χ1H.
Table 1.
(φ, ψ, χ1) torsion angles of eight residues in M2TMP in DLPC bilayers with and without amantadine
| Residue | Torsion angle | − Amantadine | + Amantadine |
|---|---|---|---|
| L26 | φ | −80 ± 5° | −80 ± 5° |
| V27 | φ | −75 ± 5° | −75 ± 5° |
| χ1H | 165 ± 3° | 160 ± 3° | |
| A29 | φ | −75 ± 15° | −65 ± 10° |
| A30 | φ | −83 ± 10° | −87 ± 10° |
| I33 | φ | −74 ± 10° | −74 ± 10° |
| χ1H | 157 ± 3° | 157 ± 3° | |
| G34 | φ* | −66 ± 7° | −67 ± 7° |
| ψ* | −37 ± 6° | −42 ± 5° | |
| I35 | φ | −80 ± 5° | −85 ± 10° |
| χ1H | 163 ± 3° | 165 ± 3° | |
| L38 | φ | −45 ± 10° | −45 ± 10° |
Angles that change between the two states are in bold italic type.
*These torsion angles were obtained from TALOS calculations.
For the β-branched Val and Ile residues, the χ1H torsion angle were obtained by correlating the Cα Hα and Cβ
Hα and Cβ Hβ bond orientations (25). Here, the angular resolution is much higher, ±3°, because of the nearly trans nature of the measured conformation. I33 and I35 show no χ1H change, consistent with their lack of Cα/Cβ chemical-shift perturbation. In contrast, the channel-lining V27 exhibits a significant χ1H difference of 5° (Fig. 4d), consistent with the 1.0-ppm Cα and Cγ1 chemical-shift change at this residue (SI Table 2).
Hβ bond orientations (25). Here, the angular resolution is much higher, ±3°, because of the nearly trans nature of the measured conformation. I33 and I35 show no χ1H change, consistent with their lack of Cα/Cβ chemical-shift perturbation. In contrast, the channel-lining V27 exhibits a significant χ1H difference of 5° (Fig. 4d), consistent with the 1.0-ppm Cα and Cγ1 chemical-shift change at this residue (SI Table 2).
Overall, the chemical shifts and torsion angle data indicate that amantadine induces only small conformational changes in M2TMP, with the main sites of perturbation being the G34–I35 pair and V27. This small conformational change contrasts with the large and extensive dynamic changes shown below.
M2TMP Dynamics and Conformational Heterogeneity.
The 1D 13C MAS spectra show dramatic differences in the line widths of the apo- and amantadine-complexed M2TMP. In general, NMR line widths have two contributions: inhomogeneous line widths mainly attributable to conformational heterogeneity and homogeneous line widths attributable to relaxation induced by stochastically fluctuating local fields on the microsecond time scale and residual dipolar couplings (26). Homogeneous broadening is not refocused in a spin-echo experiment, whereas inhomogeneous broadening is. Thus, to distinguish conformational heterogeneity from microsecond-time scale dynamics and to compare them between the apo- and complexed M2TMP, we measured the 13C T2 relaxation times by using a Hahn-echo experiment (27). The echo-derived T2 is related to the homogeneous line width Δ by T2 = 1/πΔ (28). Because residual dipolar couplings resulting from imperfect 1H decoupling or insufficiently fast MAS are the same between the apo and complexed peptide, any T2 or Δ differences should mainly result from dynamic differences of the two states. SI Fig. 8 shows representative T2 decay curves at 303 K. All resolved sites exhibit longer T2 curves with amantadine than without; this is true for both the backbone and side chains and for both channel-lining residues and other residues (SI Table 3). For backbone Cα, side chain carbons, and methyl carbons, the average T2 increases by 1.5 ms, 2.1 ms, and 2.5 ms, respectively. The T2 increase also varies with the residue location with respect to the channel: the three channel-lining residues experience the largest average T2 increase of 2.0 ms, followed by T2 increases of 1.9 ms and 1.4 ms for the interfacial and lipid-facing residues, respectively. Fig. 3d plots the fractional 13C T2 increase of the complexed peptide over the apo-M2TMP as a function of residues, calculated as , where n is the number of sites measured in each residue. The largest T2 increase is seen at V27, which again correlates with its purported close distance to the fused ring of amantadine (10, 29).
In 1H-decoupled solids, 13C T2 relaxation times increase when motional correlation times either decrease or increase beyond the microsecond time window (26). Thus, both fast motions in the extreme narrowing limit and slow motions in rigid solids give rise to long T2 curves. Comparison of the 13C T2 at 303 K, 243 K, and other temperatures (SI Table 4) suggests that the motional rates of Cα sites in the apo peptide are near the characteristic frequency of the T2 minimum (≈2π·70 kHz) in the LC phase, and that amantadine binding increases the motional rates. This finding suggests that amantadine may widen the pore slightly, reducing steric hindrance and facilitating motion (30). Indeed, most side chain methyl groups show an increase in the motional rates in the amantadine-bound state based on the temperature-dependent T2 curves, consistent with their motion being facilitated by a widening of the pore.
To assess the conformational heterogeneity of the protein, we compare the homogeneous line widths Δ derived from the T2 with the apparent line widths Δ* measured from the spectra. In the LC phase, the Cα line width of apo-M2TMP is almost completely homogeneously broadened by motion, as seen by the similar Δ and Δ* (SI Fig. 9). Amantadine binding reduces Δ by a factor of two. For the side chains, conformational heterogeneity is detectable in both the apo and complexed peptide because of narrower intrinsic line widths. To evaluate the conformational heterogeneity without different homogeneous line widths between the apo and complexed peptide, we froze the DLPC-bound M2TMP to 243 K, where the homogeneous line widths become similar between the two states (SI Table 5). Under this condition, most sites show similar Δ* and hence similar conformational heterogeneity between the apo and complexed peptide. The exceptions are G34, I35, and the side chains of L26, A30, and L38, where the complexed peptide has significantly narrower lines, indicating reduced conformational heterogeneity; this also is seen in the cross-sections of the 13C 2D spectra (Fig. 2c).
In summary, in the LC phase of the lipid bilayer, the apo-M2TMP backbone undergoes large-amplitude microsecond-time scale motion that causes significant homogeneous broadening of the 13C spectra and consequent loss of intensity. Amantadine binding increases the T2 relaxation times for all sites by changing the motional rates, thus narrowing the intrinsic line widths. When the motion is frozen, the conformational distribution of the peptide is revealed to be reduced by amantadine at specific residues, including the G34–I35 junction and several methyl-rich side chains.
Amantadine-Induced M2TMP Orientation Change.
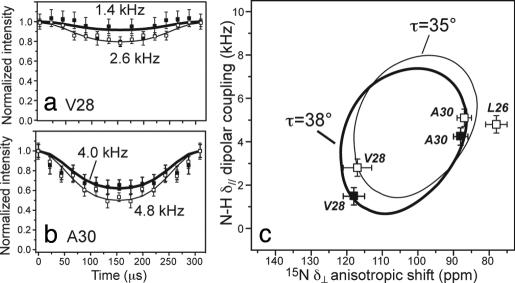
We recently measured the orientation of the apo-M2TMP by using a powder-sample approach that exploits fast rigid-body uniaxial diffusion of the peptide backbone around the bilayer normal (31). Under this condition, motionally averaged powder spectra are obtained that indicate the peptide orientation from the bilayer normal (32). We now use this approach to determine the orientation of M2TMP in complex with amantadine. 15N 1H dipolar couplings and 15N chemical-shift anisotropies (CSA), which are extremely sensitive to the helix orientation, were measured. Fig. 5 a and b shows the N
1H dipolar couplings and 15N chemical-shift anisotropies (CSA), which are extremely sensitive to the helix orientation, were measured. Fig. 5 a and b shows the N H dipolar-shift (DIPSHIFT) curves of V28 and A30 at 313 K where the peptide is uniaxially mobile. Amantadine binding decreases the N
H dipolar-shift (DIPSHIFT) curves of V28 and A30 at 313 K where the peptide is uniaxially mobile. Amantadine binding decreases the N H dipolar coupling of both residues. Correlating the motionally averaged δ// N
H dipolar coupling of both residues. Correlating the motionally averaged δ// N H dipolar coupling with the δ⊥ edge of the 15N CSA obtained from static 1D spectra (data not shown), we obtain 2D “PISA wheels” (33, 34) (Fig. 5c). For the apo peptide in DLPC bilayers, previous data yielded a tilt angle τ of 35° (31), whereas the current amantadine-bound M2TMP has a slightly larger τ of 38°. The rotation angle of the wheel is unchanged. The 3° increase, although small, is consistent with amantadine binding at the N terminus of the helix, pushing it open slightly. The orientation of the segment C-terminal to G34 is not probed here because no 15N labels are used in that region.
H dipolar coupling with the δ⊥ edge of the 15N CSA obtained from static 1D spectra (data not shown), we obtain 2D “PISA wheels” (33, 34) (Fig. 5c). For the apo peptide in DLPC bilayers, previous data yielded a tilt angle τ of 35° (31), whereas the current amantadine-bound M2TMP has a slightly larger τ of 38°. The rotation angle of the wheel is unchanged. The 3° increase, although small, is consistent with amantadine binding at the N terminus of the helix, pushing it open slightly. The orientation of the segment C-terminal to G34 is not probed here because no 15N labels are used in that region.
Fig. 5.
Orientation of amantadine-bound M2TMP. (a and b) 15N 1H dipolar coupling of unoriented M2TMP in DLPC bilayers with amantadine (filled squares, thick line). For comparison, the apo peptide data published recently are superimposed (open squares, thin line) (31). (a) V28. (b) A30. (c) PISA wheels of M2TMP constructed from the δ// N
1H dipolar coupling of unoriented M2TMP in DLPC bilayers with amantadine (filled squares, thick line). For comparison, the apo peptide data published recently are superimposed (open squares, thin line) (31). (a) V28. (b) A30. (c) PISA wheels of M2TMP constructed from the δ// N H dipolar couplings and δ⊥ 15N anisotropic shifts. The data fit to a wheel with a tilt angle τ of 38° (thick line). The apo peptide shows a τ = 35° (open symbols, thin line) (31).
H dipolar couplings and δ⊥ 15N anisotropic shifts. The data fit to a wheel with a tilt angle τ of 38° (thick line). The apo peptide shows a τ = 35° (open symbols, thin line) (31).
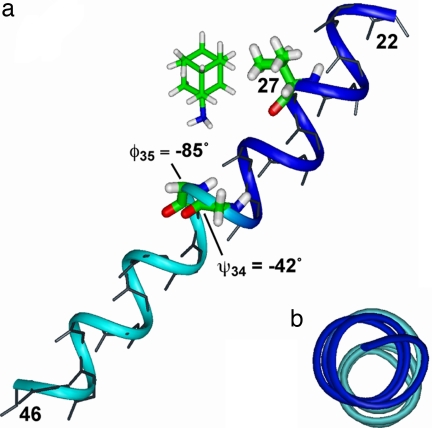
Fig. 6 shows the chemical shift and torsion-angle constrained structure of M2TMP in the presence of amantadine, refined from the 15N NMR-derived model 1NYJ (15). At the G34–I35 junction, a G34 ψ angle of −42° and I35 φ of −85° were used. The resulting helix shows a small kink of 5° between the segments N-terminal and C-terminal to G34, visible in the top view (Fig. 6b). The kink is defined as the angle between the average N H bond orientation for residues 27–33 and for residues 37–43. This kink is reminiscent of the recent 15N NMR data of dimyristoylphosphatidylcholine (DMPC)-bound M2TMP, which showed a bend of 11° at G34 (16). We found that the exact value of the kink is sensitive to the G34/I35 torsion angles. With ψ34 = −60°, the kink increases to ≈16°, whereas with a more ideal φ35 of −60°, the kink is almost completely removed.
H bond orientation for residues 27–33 and for residues 37–43. This kink is reminiscent of the recent 15N NMR data of dimyristoylphosphatidylcholine (DMPC)-bound M2TMP, which showed a bend of 11° at G34 (16). We found that the exact value of the kink is sensitive to the G34/I35 torsion angles. With ψ34 = −60°, the kink increases to ≈16°, whereas with a more ideal φ35 of −60°, the kink is almost completely removed.
Fig. 6.
Chemical shift and torsion-angle restrained backbone and partial side chain structure of amantadine-bound M2TMP. (a) Side view. (b) Top view. The exact position and orientation of amantadine is unknown and is shown here only as a reference to the peptide. The G34 ψ and I35 φ angles create a helix kink of 5°, highlighted by the blue N-terminal and the cyan C-terminal segments.
Conclusion
The NMR data here provide an extensive set of high-resolution conformational and dynamical constraints of the backbone and side chains of M2TMP in lipid bilayers without and with amantadine and elucidate the nature of the spectral line-narrowing caused by amantadine. The data indicate that amantadine binding to M2TMP exerts the largest effect on the dynamics and conformational heterogeneity of the protein, affects to a lesser extent the average backbone and side chain conformations, and only subtly affects the helix orientation. The apo peptide exhibits large-amplitude microsecond-time scale motion that homogeneously broadens the NMR lines. Amantadine increases the motional rates of most backbone Cα sites, causing substantial line-narrowing. It also reduces the conformational heterogeneity of certain residues, including G34, I35, and the side chains of L26, A30, and L38. Perturbation of the average conformation occurs mainly at G34–I35 and at the V27 side chain. Combined, the data strongly suggest that conformational plasticity is essential to proton conduction and gating of the apo channel, and at least part of amantadine's mechanism of action is to modify and select among the multiple low-energy conformations of M2TMP. This finding is consistent with energy surface mapping (35) and analytical ultracentrifugation data of M2TMP and its mutants (36, 37). It is possible, then, that amantadine resistance can arise from mutations that alter the protein conformational distribution and dynamics, thus preventing amantadine binding.
The observed large V27 chemical-shift and T2 changes are in excellent agreement with mutagenesis data indicating strong interaction of this residue with amantadine. Mutation of V27 to Ala, Ser, Ile, and Thr caused either complete or partial resistance to amantadine in various viral strains (3, 9). Thus, the interaction of amantadine with the channel is exquisitely sensitive to the size and hydrophobicity of the side chain at this position.
Complexed with amantadine, the M2TMP helix shows a small degree of nonideality in the backbone torsion angles. In particular, the deviation of the G34–I35 conformation from the ideal helix geometry causes a helix kink of 5°, which may have an effect on the interhelical interaction of H37 imidazole rings downstream (19). The exact value of the kink and the exact orientation of the helix in the DLPC membrane differ slightly from those found in the DMPC membrane. Given the differences in sample preparation conditions, such as membrane thickness and the state of alignment (19, 20), these differences further underscore the structural plasticity of the peptide. The spectra of the amantadine-bound M2TMP show a single signal for each label; thus, the four helices of the tetramer are rotationally symmetric and chemically identical. This conclusion implies that, in the LC phase, not only does amantadine have the same uniaxial mobility as the tetrameric bundle, but it also exchanges among the four helices on a time scale faster than the nuclear spin interactions (<10−5 s) (19).
Materials and Methods
Peptides and Lipids.
Fmoc-protected uniformly 13C, 15N-labeled amino acids were either prepared in-house (38) or purchased from Sigma and Cambridge Isotope Laboratories. The transmembrane peptide of the M2 protein of the Udorn strain (residues 22–46) (39) was synthesized by PrimmBiotech (Cambridge, MA) and purified to >95% purity. The amino acid sequence is SSDPLVVAASIIGILHLILWILDRL. In addition to peptides containing multiple uniformly 13C, 15N-labeled residues, two peptides each containing a single 15N label at V28 and A30 were synthesized for orientation measurements.
Membrane Sample Preparation.
M2TMP was reconstituted into lipid vesicles by detergent dialysis (18). DLPC lipids were chosen because of the favorable dynamics of the protein in this membrane (31) and the similar phase transition temperature (−2°C) of this bilayer to biological membranes. The vesicle solution was prepared by suspending dry DLPC powder (Avanti Polar Lipids) in 1 ml of phosphate buffer (10 mM Na2HPO4/NaH2PO4, 1 mM EDTA, and 0.1 mM NaN3) at pH 7.5, vortexing and freeze-thawing six to eight times to create uniform vesicles of ≈200-nm diameter (40). M2TMP powder was codissolved with the detergent octyl-β-d-glucopyranoside (OG) in 2 ml of phosphate buffer to reach an OG concentration of 30 mg/ml. The M2TMP/OG solution then was mixed with the DLPC vesicle solution, giving a final OG concentration of 15 mg/ml. The mixture was vortexed for 1 h, allowed to stand for 6–8 h at room temperature, and then dialyzed with a 3.5-kDa cutoff against 1 liter of phosphate buffer at 4°C for 3 days with buffer changes every 8–12 h to ensure complete removal of the detergent. The dialyzed M2TMP/DLPC solution was centrifuged at 150,000 × g for 3 h at 10°C to give a wet pellet with ≈50 wt % water. The final peptide/lipid (P/L) molar ratio is 1:15. UV-visible spectrum of the supernatant indicated ≈98% binding of the peptide to the membrane. For amantadine-bound samples, 10 mM amantadine hydrochloride was added to the phosphate buffer throughout the lipid vesicle formation and peptide assembly process.
For orientation measurements, 15N-labeled M2TMP was codissolved with DLPC lipids in trifluoroethanol at a P/L of 1:20, lyophilized, and then rehydrated to 50 wt % water with a pH 8.1 phosphate buffer. For amantadine-bound samples, 2 mmol of amantadine hydrochloride was added to the dry M2TMP/lipid mixture before dissolution in trifluoroethanol.
SSNMR Spectroscopy.
Most NMR experiments were carried out on a Bruker (Karlsruhe, Germany) AVANCE-600 (14.1-T) spectrometer by using a 4-mm triple-resonance MAS probe. 13C 13C and 15N
13C and 15N 13C 2D correlation and torsion angle experiments were conducted at 243 K to freeze peptide motion. All other parameters, including 15N CSA, 15N
13C 2D correlation and torsion angle experiments were conducted at 243 K to freeze peptide motion. All other parameters, including 15N CSA, 15N 1H dipolar coupling, and 13C T2 relaxation times, were measured at 303 K or 313 K where the peptide is uniaxially mobile in the LC phase of the DLPC bilayer. Typical radiofrequency pulse lengths were 5 μs for 13C and 3.5–4.0 μs for 1H. 1H TPPM (41) or SPINAL (42) decoupling of 60–70 kHz were applied. 13C chemical shifts were referenced to the α-Gly C′ signal at 176.49 ppm on the TMS scale, and 15N chemical shifts were referenced to the 15N signal of N-acetyl-valine at 122 ppm on the liquid ammonia scale. For G34 torsion angles extraction from TALOS, the 13C chemical shifts were converted to the 3-(trimethylsilyl)-propionate scale by adding 1.82 ppm to the measured shifts.
1H dipolar coupling, and 13C T2 relaxation times, were measured at 303 K or 313 K where the peptide is uniaxially mobile in the LC phase of the DLPC bilayer. Typical radiofrequency pulse lengths were 5 μs for 13C and 3.5–4.0 μs for 1H. 1H TPPM (41) or SPINAL (42) decoupling of 60–70 kHz were applied. 13C chemical shifts were referenced to the α-Gly C′ signal at 176.49 ppm on the TMS scale, and 15N chemical shifts were referenced to the 15N signal of N-acetyl-valine at 122 ppm on the liquid ammonia scale. For G34 torsion angles extraction from TALOS, the 13C chemical shifts were converted to the 3-(trimethylsilyl)-propionate scale by adding 1.82 ppm to the measured shifts.
2D DQ-filtered 13C 13C correlation spectra were measured by using a SPC5 sequence (43) <7-kHz MAS. DQ filtration removes lipid background 13C signals, thus simplifying assignment of the protein signals. 2D 15N
13C correlation spectra were measured by using a SPC5 sequence (43) <7-kHz MAS. DQ filtration removes lipid background 13C signals, thus simplifying assignment of the protein signals. 2D 15N 13C correlation spectra were measured by using a REDOR pulse train (44) of 0.7–2.1 ms for 13C
13C correlation spectra were measured by using a REDOR pulse train (44) of 0.7–2.1 ms for 13C 15N coherence transfer (45).
15N coherence transfer (45).
φ angles were measured under 6.5-kHz MAS by using the HNCH technique, with doubling of the N H dipolar coupling to enhance the angular resolution (22, 46). 1H
H dipolar coupling to enhance the angular resolution (22, 46). 1H 1H homonuclear coupling was removed by an FSLG sequence (47). The HNCH data were simulated by using a doubled N
1H homonuclear coupling was removed by an FSLG sequence (47). The HNCH data were simulated by using a doubled N H coupling of 12.0 kHz and a C
H coupling of 12.0 kHz and a C H dipolar coupling of 12.5 kHz, both scaled by the FSLG scaling factor of 0.577. These values were directly measured by C
H dipolar coupling of 12.5 kHz, both scaled by the FSLG scaling factor of 0.577. These values were directly measured by C H and N
H and N H DIPSHIFT correlation experiments on the protein at 243 K. ψ angle was measured with the NCCN experiment (23) correlating the Ni
H DIPSHIFT correlation experiments on the protein at 243 K. ψ angle was measured with the NCCN experiment (23) correlating the Ni Cαi and C′i
Cαi and C′i Ni+1 bond orientations. Spinning speeds of 4 and 5 kHz were used to obtain multiple time points on the angle-dependent curve. χ1H torsion angles (Hα
Ni+1 bond orientations. Spinning speeds of 4 and 5 kHz were used to obtain multiple time points on the angle-dependent curve. χ1H torsion angles (Hα Cα
Cα Cβ
Cβ Hβ) were measured by correlating the Cα
Hβ) were measured by correlating the Cα Hα and Cβ
Hα and Cβ Hβ bond orientations by using a modified HCCH technique (25) under 9-kHz MAS. A HORROR sequence with a resonance condition of ω1 = ωr/2 (48) was used to selectively excite the Cα
Hβ bond orientations by using a modified HCCH technique (25) under 9-kHz MAS. A HORROR sequence with a resonance condition of ω1 = ωr/2 (48) was used to selectively excite the Cα Cβ DQ coherence, followed by a dipolar-doubled C
Cβ DQ coherence, followed by a dipolar-doubled C H DIPSHIFT period. A doubled and FSLG-scaled C
H DIPSHIFT period. A doubled and FSLG-scaled C H dipolar coupling of 26.0 kHz was used to simulate the angle-dependent curves. All these torsion angles have an inherent double degeneracy caused by the uniaxial nature of the dipolar coupling. The wrong angle is readily identified by the fact that it falls into either unpopulated regions of the Ramachandran diagram or the β-sheet region, which contradicts NMR chemical shifts.
H dipolar coupling of 26.0 kHz was used to simulate the angle-dependent curves. All these torsion angles have an inherent double degeneracy caused by the uniaxial nature of the dipolar coupling. The wrong angle is readily identified by the fact that it falls into either unpopulated regions of the Ramachandran diagram or the β-sheet region, which contradicts NMR chemical shifts.
15N 1H dipolar couplings for orientation determination were obtained from a dipolar-doubled DIPSHIFT experiment (46, 49, 50) under 7-kHz MAS. An FSLG sequence with an effective field of 76.5 kHz was used for 1H homonuclear decoupling.
1H dipolar couplings for orientation determination were obtained from a dipolar-doubled DIPSHIFT experiment (46, 49, 50) under 7-kHz MAS. An FSLG sequence with an effective field of 76.5 kHz was used for 1H homonuclear decoupling.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Prof. Yoshitaka Ishii for help with the TALOS simulation. This work is supported by National Science Foundation Grants MCB-0543473 and DBI-0421374.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711500105/DC1.
References
- 1.Pinto LH, Holsinger LJ, Lamb RA. Influenza virus M2 protein has ion channel activity. Cell. 1992;69:517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- 2.Pinto LH, Lamb RA. Controlling influenza virus replication by inhibiting its proton flow. Mol Bio Syst. 2007;3:18–23. doi: 10.1039/b611613m. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb RA, Holsinger KJ, Pinto LH. In: Cellular Receptors of Animal Viruses. Wemmer E, editor. Plainview, NY: Cold Spring Harbor Lab Press; 1994. pp. 303–321. [Google Scholar]
- 5.Bright RA, et al. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- 6.Duff KC, Kelly SM, Price NC, Bradshaw JP. The secondary structure of influenza A M2 transmembrane domain: A circular dichroism study. FEBS Lett. 1992;311:256–258. doi: 10.1016/0014-5793(92)81114-2. [DOI] [PubMed] [Google Scholar]
- 7.Duff KC, Ashley RH. The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers. Virology. 1992;190:485–489. doi: 10.1016/0042-6822(92)91239-q. [DOI] [PubMed] [Google Scholar]
- 8.Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsinger LJ, Nichani D, Pinto LH, Lamb RA. Influenza A virus M2 ion channel protein: A structure-function analysis. J Virol. 1994;68:1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duff KC, Gilchrist PJ, Saxena AM, Bradshaw JP. Neutron diffraction reveals the site of amantadine blockade in the influenza A M2 ion channel. Virology. 1994;202:287–293. doi: 10.1006/viro.1994.1345. [DOI] [PubMed] [Google Scholar]
- 11.Pinto LH, et al. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci USA. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi T, Tu Q, Pinto LH, Lamb RA. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc Natl Acad Sci USA. 1997;94:5000–5005. doi: 10.1073/pnas.94.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salom D, Hill BR, Lear JD, DeGrado WF. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 2000;39:14160–14170. doi: 10.1021/bi001799u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo W, Hong M. Determination of the oligomeric number and intermolecular distances of membrane protein assemblies by anisotropic (1)h-driven spin diffusion NMR spectroscopy. J Am Chem Soc. 2006;128:7242–7251. doi: 10.1021/ja0603406. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Kim S, Kovacs F, Cross TA. Structure of the transmembrane region of the M2 protein H (+) channel. Protein Sci. 2001;10:2241–2250. doi: 10.1110/ps.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, et al. Backbone stucture of the amantadine-block trans-membrane domain M2 proton channel from influenza A virus. Biophys J. 2007;92:4335–4343. doi: 10.1529/biophysj.106.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura K, Kim S, Zhang L, Cross TA. The closed state of a H+ channel helical bundle combining precise orientational and distance restraints from solid state NMR. Biochemistry. 2002;41:13170–13177. doi: 10.1021/bi0262799. [DOI] [PubMed] [Google Scholar]
- 18.Luo W, Mani R, Hong M. Side chain conformation and gating of the M2 transmembrane peptide proton channel of influenza A virus from solid-state NMR. J Phys Chem. 2007;111:10825–10832. doi: 10.1021/jp073823k. [DOI] [PubMed] [Google Scholar]
- 19.Hu J, Riqiang F, Cross TA. The chemical and dynamical influence of the anti-viral drug amantadine on the M2 proton channel transmembrane domain. Biophys J. 2007;93:276–283. doi: 10.1529/biophysj.106.102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Qin H, Gao FP, Cross TA. Solid-state NMR characterization of conformational plasticity within the transmembrane domain of the influenza A M2 proton channel. Biochim Biophys Acta. 2007;1768:3162–3170. doi: 10.1016/j.bbamem.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Neal S, Wishart DS. RefDB: A database of uniformly referenced protein chemical shifts. J Biomol NMR. 2003;25:173–195. doi: 10.1023/a:1022836027055. [DOI] [PubMed] [Google Scholar]
-
22.Hong M, Gross JD, Griffin RG. Site-resolved determination of peptide torsion angle φ from the relative orientations of backbone N
 H and C
H and C H bonds by solid-state NMR. J Phys Chem B. 1997;101:5869–5874. [Google Scholar]
H bonds by solid-state NMR. J Phys Chem B. 1997;101:5869–5874. [Google Scholar] - 23.Costa PR, Gross JD, Hong M, Griffin RG. Solid-state NMR measurement of ψ in peptides: A NCCN 2Q-heteronuclear local field experiment. Chem Phys Lett. 1997;280:95–103. [Google Scholar]
- 24.Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 25.Feng X, et al. Direct determination of a molecular torsional angle by solid-state NMR. Chem Phys Lett. 1996;257:314–320. [Google Scholar]
- 26.Rothwell WP, Waugh JS. Tranverse relaxation of dipolar coupled spin systems under RF irradiation: Detecting motions in solids. J Chem Phys. 1981;74:2721–2732. [Google Scholar]
- 27.Hahn EL. Spin echoes. Phys Rev. 1950;80:580–594. [Google Scholar]
- 28.Lesage A, Bardet M, Emsley L. Through-bond carbon–carbon connectivities in disordered solids by NMR. J Am Chem Soc. 1999;121:10987–10993. [Google Scholar]
- 29.Sansom MS, Kerr ID. Influenza virus M2 protein: A molecular modelling study of the ion channel. Protein Eng. 1993;6:65–74. doi: 10.1093/protein/6.1.65. [DOI] [PubMed] [Google Scholar]
- 30.Astrahan P, Kass I, Cooper MA, Arkin IT. A novel method of resistance for influenza against a channel-blocking antiviral drug. Proteins Struct Funct Bioinf. 2004;55:251–257. doi: 10.1002/prot.20018. [DOI] [PubMed] [Google Scholar]
- 31.Cady SD, Goodman C, Tatko CD, DeGrado WF, Hong M. Determining the orientation of uniaxially rotating membrane proteins using unoriented samples: A 2H, 13C, and 15N solid-state NMR investigation of the dynamics and orientation of a transmembrane helical bundle. J Am Chem Soc. 2007;129:5719–5729. doi: 10.1021/ja070305e. [DOI] [PubMed] [Google Scholar]
- 32.Hong M, Doherty T. Orientation determination of membrane-disruptive proteins using powder samples and rotational diffusion: A simple solid-state NMR approach. Chem Phys Lett. 2006;432:296–300. doi: 10.1016/j.cplett.2006.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marassi FM, Opella SJ. A solid-state NMR index of helical membrane protein structure and topology. J Magn Reson. 2000;144:150–155. doi: 10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, et al. Imaging membrane protein helical wheels. J Magn Reson. 2000;144:162–167. doi: 10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]
- 35.Torres J, Kukol A, Arkin IT. Mapping the energy surface of transmembrane helix–helix interactions. Biophys J. 2001;81:2681–2692. doi: 10.1016/S0006-3495(01)75911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stouffer AL, Nanda V, Lear JD, DeGrado WF. Sequence determinants of a transmembrane proton channel: An inverse relationship between stability and function. J Mol Biol. 2005;347:169–179. doi: 10.1016/j.jmb.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 37.Howard KP, Lear JD, DeGrado WF. Sequence determinants of the energetics of folding of a transmembrane four-helix-bundle protein. Proc Natl Acad Sci USA. 2002;99:8568–8572. doi: 10.1073/pnas.132266099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpino LA, Han GY. 9-Fluorenylmethoxycarbonyl amino-protecting group. J Org Chem. 1972;37:3404–3409. [Google Scholar]
- 39.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–5498. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traikia M, Warschawski DE, Recouvreur M, Cartaud J, Devaux PF. Formation of unilamellar vesicles by repetitive freeze-thaw cycles: Characterization by electron microscopy and 31P-nuclear magnetic resonance. Eur Biophys J. 2000;29:184–195. doi: 10.1007/s002490000077. [DOI] [PubMed] [Google Scholar]
- 41.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J Chem Phys. 1995;103:6951–6958. [Google Scholar]
- 42.Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 43.Hohwy M, Rienstra CM, Jaroniec CP, Griffin RG. Fivefold symmetric homonuclear dipolar recoupling in rotating solids: Application to double-quantum spectroscopy. J Chem Phys. 1999;110:7983–7992. [Google Scholar]
- 44.Gullion T, Schaefer J. Rotational echo double resonance NMR. J Magn Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Hong M, Griffin RG. Resonance assignment for solid peptides by dipolar-mediated 13C/15N correlation solid-state NMR. J Am Chem Soc. 1998;120:7113–7114. [Google Scholar]
- 46.Hong M, et al. Coupling amplification in 2D MAS NMR and its application to torsion angle determination in peptides. J Magn Reson. 1997;129:85–92. doi: 10.1006/jmre.1997.1242. [DOI] [PubMed] [Google Scholar]
- 47.Bielecki A, Kolbert AC, de Groot HJM, Griffin RG, Levitt MH. Frequency-switched Lee-Goldburg sequences in solids. Adv Magn Reson. 1990;14:111–124. [Google Scholar]
- 48.Nielsen NC, Bildsoe H, Jakobsen HJ, Levitt MH. Double-quantum homonuclear rotary resonance: Efficient dipolar recovery in magic-angle spinning nuclear magnetic resonance. J Chem Phys. 1994;101:1805–1812. [Google Scholar]
- 49.Huster D, Yamaguchi S, Hong M. Efficient β-sheet identification in proteins by solid-state NMR spectroscopy. J Am Chem Soc. 2000;122:11320–11327. [Google Scholar]
- 50.Munowitz MG, Griffin RG, Bodenhausen G, Huang TH. Two-dimensional rotational spin-echo nuclear magnetic resonance in solids: Correlation of chemical shift and dipolar interactions. J Am Chem Soc. 1981;103:2529–2533. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.