Abstract
The myocardin family proteins (myocardin, MRTF-A, and MRTF-B) are serum response factor (SRF) cofactors and potent transcription activators. Gene-ablation studies have indicated important developmental functions for myocardin family proteins primarily in regulation of cardiac and smooth muscle development. Using Xenopus genome and cDNA databases, we identified a myocardin-related transcription factor expressed specifically in the skeletal muscle lineage. Synteny and sequence alignments indicate that this gene is the frog orthologue of mouse MASTR [Creemers EE, Sutherland LB, Oh J, Barbosa AC, Olson EN (2006) Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell 23:83–96]. Inhibition of MASTR function in the Xenopus embryo by using dominant-negative constructions or morpholino knockdown results in a dramatic reduction in expression of skeletal muscle marker genes. Overexpression of MASTR in whole embryos or embryonic tissue explants induces ectopic expression of muscle marker genes. Furthermore, MASTR cooperates with the myogenic regulatory factors MyoD and Myf5 to activate transcription of skeletal muscle genes. An essential function for MASTR in regulation of myogenic development in the vertebrate embryo has not been previously indicated.
Keywords: Myf5, serum response factor, Xenopus
The interplay of several different families of transcription factors is required for regulation of muscle development in the vertebrate embryo. The myogenic regulatory factors (MRFs), MyoD, Myf5, myogenin, and MRF4 comprise a closely related family of regulatory proteins that are essential for normal muscle development (1, 2). MRFs bind to the E-box target sequence (CANNTG) located in the regulatory regions of many skeletal muscle genes. MyoD and Myf5 function as specification factors during the earliest stages of the myogenic program and both of these factors are capable of strong activation of muscle gene expression in nonmuscle cells (3–6). MRFs interact with serum response factor (SRF) to regulate skeletal muscle gene expression (7). SRF binds to the CArG box DNA element CC(A/T)6GG, which is essential for expression of many skeletal, cardiac, and smooth muscle genes (8, 9). SRF-binding sites are frequently located near E boxes in the regulatory regions of skeletal muscle genes, and MyoD and SRF can cooperate to activate muscle gene expression (10, 11).
The myocardin family of SRF cofactors (myocardin, MRTF-A and B) do not appear to possess DNA-binding activity but locate to the regulatory regions of muscle genes through physical interaction with SRF (12). Knockout studies in mouse have demonstrated that each of the myocardin family proteins function to regulate muscle gene expression. Mice lacking myocardin function die at embryonic day (E)10.5 because of defective vascular smooth muscle development (13). MRTF-A knockout animals are viable, but myoepithelial cells of the mammary glands fail to differentiate (14, 15), and ablation of MRTF-B function results in embryonic death at E13.5–14.5 because of a range of defects in cardiac and smooth muscle development (16).
In Xenopus, the myocardin-related transcription factor, MASTR, is expressed specifically in skeletal muscle tissue from the earliest stages of development. Our studies demonstrate that MASTR synergizes with the myogenic factors, MyoD and Myf5 to activate expression of skeletal muscle genes in the embryo. We conclude that MASTR is an important component of the myogenic program in vertebrate embryos.
Results
The Xenopus Myocardin-Related Gene, MASTR, Is Expressed in Skeletal Muscle.
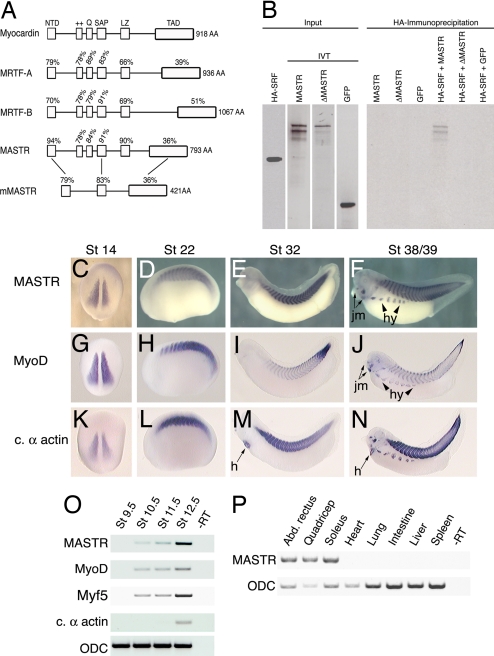
Searches of the Xenopus tropicalis genome and EST databases identified a previously uncharacterized member of the myocardin gene family. Alignment with Xenopus myocardin and MRTF-A and B proteins reveals a high degree of sequence identity within the previously characterized functional domains [Fig. 1A and supporting information (SI) Fig. 5]. Examination of synteny (SI Fig. 6) showed that the gene is the Xenopus orthologue of the recently described mouse MASTR sequence (17). We note that Xenopus and zebrafish MASTR proteins (Fig. 1A and data not shown) exhibit significant differences from mouse and human MASTR proteins in domain structure. Most notably, mammalian MASTR lacks the glutamine-rich and positively charged domains required for SRF binding and the leucine-zipper dimerization domain (Fig. 1A). In view of the differences between the mouse and frog proteins, we tested the ability of Xenopus MASTR to interact with SRF. Coimmunoprecipitation (Co-IP) experiments demonstrate that frog MASTR forms a complex with SRF and that mutation of the SRF binding domains within the MASTR protein (12) eliminates this interaction (Fig. 1B). Furthermore, SRF and frog MASTR form a ternary complex with DNA containing the CArG element (SI Fig. 7). Based on primary sequence conservation and the ability of MASTR to complex with SRF, we conclude that MASTR is a distinct fourth member of the myocardin family of transcription factors.
Fig. 1.
MASTR is a previously uncharacterized member of the myocardin-related family of proteins. (A) Alignment illustrating the domains of the four known Xenopus myocardin family proteins and also mouse MASTR. Percentages indicate identity within each domain relative to myocardin or between Xenopus and mouse MASTR. (B) Co-IP experiments demonstrate that MASTR forms a physical complex with SRF. HA-tagged SRF and radiolabeled in vitro-translated MASTR, ΔMASTR, and EGFP are shown (Left). Only full-length MASTR protein coprecipitated with SRF (Right). (C–F). In situ hybridization analysis of MASTR expression during Xenopus development. MASTR transcripts are detected specifically in skeletal muscle tissues at all stages. Expression in hypaxial muscle (hy) and jaw muscle (jm) is indicated. (G–J) Transcriptional expression of MyoD. Intensity of MyoD expression differs from MASTR, but the location of transcripts is identical. (K–N) Expression of the striated muscle marker, cardiac α-actin. In addition to marking all tissues expressing MASTR, cardiac α-actin is expressed in the heart (h). (O) RT-PCR analysis showing MASTR expression is first detected in the gastrula (St 10.5) coincident with the appearance of MyoD and Myf5 mRNA. The muscle differentiation marker cardiac α-actin is not detected until St 12.5. Ornithine decarboxylase (ODC) was used as a loading control. (P) RT-PCR analysis of MASTR transcripts in adult frog tissues. MASTR is expressed in representative skeletal muscles (abdominal rectus, quadriceps, and soleus) but not in heart, smooth muscle (intestine), or other tissues.
By using whole-mount in situ hybridization, MASTR transcripts were first detected in the presomitic mesoderm of the early neurula embryo (Fig. 1C). Levels of MASTR transcripts increased at later stages and remained restricted to skeletal muscle tissues throughout embryonic development (Fig. 1 D–F). The distribution of MASTR expression was equivalent to MyoD at all stages examined (Fig. 1 G–J). In the tadpole (St 39), MASTR transcripts were also detected in the developing jaw muscles and in the hypaxial muscles that migrate ventrally and fuse to form muscles of the body wall (Fig. 1F). Unlike the striated muscle marker cardiac α-actin, MASTR transcripts were never observed in the developing heart (compare Fig. 1 E and F and Fig. 1 M and N).
RT-PCR analysis (Fig. 1O) indicated that expression of MASTR and the myogenic transcription factors MyoD and Myf5 occurred approximately simultaneously during early gastrulation (St 10.5). Analysis of adult tissues showed that MASTR expression was restricted to skeletal muscle (represented by abdominal rectus, quadriceps, and soleus) and was not detected in adult cardiac or smooth muscle (intestine) or other nonmuscle tissues (Fig. 1P).
Loss-of-Function Studies Demonstrate a Role for MASTR in Embryonic Muscle Gene Expression.
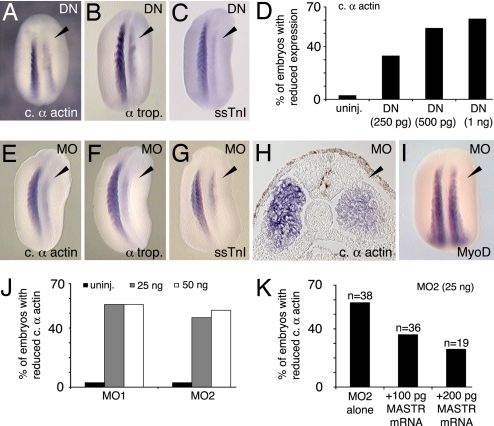
To address the function of MASTR during skeletal muscle development, we first used a dominant-negative form of the protein (DN-MASTR), which lacks the transcription-activating domain and is structurally equivalent to previously characterized dominant-negative myocardin and MRTF-A proteins (12, 18). This construction effectively blocks MASTR function in control experiments (SI Fig. 8). None of the other myocardin family members is expressed in somite tissues during early Xenopus development (19), and therefore DN-MASTR should interfere specifically with MASTR function. The side of the embryo expressing DN-MASTR exhibited a significant reduction in expression of muscle markers including cardiac α-actin, α-tropomyosin, and slow skeletal troponin I (ssTnI) (Fig. 2 A–C and Table 1). The inhibitory effect was dose-dependent, causing a reduction of cardiac α-actin expression in ≈60% of embryos when 1 ng of mRNA was injected (Fig. 2D). Although DN-MASTR reduced levels of muscle marker transcripts, complete elimination of skeletal muscle marker expression was never observed.
Fig. 2.
Inhibition of MASTR function reduces expression of skeletal muscle markers. (A–C) Embryos expressing a dominant-negative form of the MASTR protein (DN-MASTR) were assayed at St 22/23 for muscle markers as shown. Injected sides are indicated by arrowheads. (D) Dose-dependent reduction in cardiac α-actin expression after expression of DN-MASTR. (E–G) MO knockdown of MASTR expression results in reduced muscle marker expression as indicated. MO-treated sides are indicated by the arrowheads. (H) Transverse section through the trunk of St 22 embryo treated with MO on the right-hand side and assayed for cardiac α-actin expression. The general structure of the somite is normal, but marker expression is reduced on the treated side. (I) MO-treated embryo shows no reduction in MyoD expression. (J) Two nonoverlapping MO sequences directed against MASTR mRNA (MO1 and MO2) were equally effective in reducing expression of cardiac α-actin. (K) Rescue of the MO-induced phenotype. Embryos were injected with MO2 alone or with MO2 plus MASTR mRNA. Addition of MASTR mRNA achieved partial rescue of MO inhibition in a dose-dependent manner.
Table 1.
Inhibition of MASTR function
| Marker | Treatment | Normal gene expression, n (%) | Reduced gene expression, n (%) | No. of embryos |
|---|---|---|---|---|
| Cardiac α-actin | Uninjected | 74 (97) | 2 (3) | 76 |
| DN-MASTR (250 pg) | 14 (67) | 7 (33) | 21 | |
| DN-MASTR (500 pg) | 6 (46) | 7 (54) | 13 | |
| DN-MASTR (1 ng) | 20 (39) | 31 (61) | 51 | |
| MO1 (25 ng) | 10 (44) | 13 (56) | 23 | |
| MO1 (50 ng) | 11 (44) | 14 (56) | 25 | |
| MO2 (25 ng) | 17 (53) | 15 (47) | 32 | |
| MO2 (50 ng) | 38 (48) | 42 (52) | 80 | |
| Control MO (50 ng) | 22 (96) | 1 (4) | 23 | |
| Control MO (25 ng) | 37 (95) | 2 (5) | 43 | |
| α-Tropomyosin | Uninjected | 30 (97) | 1 (3) | 31 |
| DN-MASTR (1 ng) | 17 (68) | 8 (42) | 25 | |
| MO2 (50 ng) | 10 (40) | 15 (60) | 25 | |
| ssTnI | Uninjected | 30 (94) | 2 (6) | 32 |
| DN-MASTR (1 ng) | 13 (59) | 9 (41) | 22 | |
| MO2 (50 ng) | 22 (39) | 34 (61) | 56 | |
| MyoD | Uninjected | 29 (100) | 0 (0) | 29 |
| MO2 (50 ng) | 34 (92) | 3 (8) | 37 |
To complement the dominant-negative studies, antisense morpholino oligomers (MOs) were used to specifically inhibit MASTR expression in the embryo. Two distinct, nonoverlapping MOs (MO1 and MO2) were directed against the 5′ UTR of Xenopus MASTR mRNA and both effectively blocked translation from a test construction in control experiments (SI Fig. 9). As shown in Fig. 2 E–G, MO1 inhibited expression of muscle markers on the injected side of the embryo, and the overall appearance of the MO-treated embryos was extremely similar to those expressing DN-MASTR (compare Fig. 2 A–C with Fig. 2 E–G; see Table 1). Similar levels of inhibition of muscle marker expression were observed by using MO2 (Fig. 2J. Table 1), whereas injection of control MO produced no significant effect (Table 1). Histological sections showed that the general epithelialized structure of the somite was retained on the MO-treated side of the embryo, but muscle marker expression was reduced throughout the tissue (Fig. 2H). Similar to the dominant-negative studies, MO knockdown of MASTR activity caused a marked reduction in muscle gene expression but was never observed to eliminate expression entirely. The disruption of muscle differentiation marker expression was not due to nonspecific toxicity, because the MOs caused no noticeable effect on MyoD expression (Fig. 2I; Table 1). Rescue of the MO phenotype was achieved in a dose-dependent manner by injection of MASTR mRNA lacking the MO target sequence (Fig. 2K). Taken together, the loss of function experiments demonstrated that MASTR is required for normal muscle gene expression in the Xenopus embryo.
MASTR Activates Expression of Skeletal Muscle Genes.
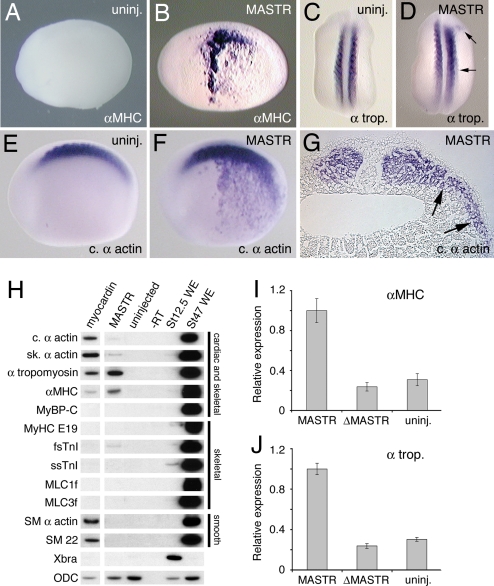
Previous studies showed that the cardiac and smooth muscle transcription factor, myocardin, was capable of activating ectopic expression of cardiac and smooth muscle genes in the Xenopus embryo (19). Injection of MASTR mRNA also caused ectopic expression of muscle markers in the Xenopus embryo. This was observed for α-Myosin Heavy Chain (αMHC), α-tropomyosin, and cardiac α-actin but not for some other markers including Myosin Binding Protein-C (MyBP-C) (Fig. 3B, D, and F and Table 2). Whereas ectopic expression of αMHC and cardiac α-actin was broad and robust, α-tropomyosin expression was weak and limited to areas immediately adjacent to the somites. Histological sections showed that ectopic expression was present in the mesoderm, but not in adjacent ectoderm or endoderm tissue (Fig. 3G). These results indicated that MASTR can activate ectopic expression of muscle differentiation markers in the embryo and that the response varies for individual muscle markers.
Fig. 3.
MASTR induces ectopic expression of muscle markers. (A) Uninjected control embryo at St 22 (lateral view) shows no expression of αMHC marker. (B) Embryo injected with 500 pg of MASTR mRNA shows extensive ectopic expression of αMHC. (C) Control embryo (dorsal view) showing expression of the muscle marker, α-tropomyosin in the somites. (D) Embryo injected with 500 pg of MASTR mRNA shows limited ectopic expression of α-tropomyosin immediately lateral to the somites (arrows). (E and F) Uninjected control embryo (E) and embryo injected with 500 pg of MASTR mRNA (F) assayed for expression of cardiac α-actin. Injected embryo shows extensive ectopic expression of cardiac α-actin. (G) Transverse section through trunk of St 22 embryo injected with MASTR mRNA and assayed for cardiac α-actin transcripts. Arrows indicate that ectopic marker expression is limited to the lateral mesoderm tissue and is absent from epidermis and endodermal tissue. (H) MASTR activates expression of muscle markers in nonmuscle (animal cap) tissue. Embryos were injected with 500 pg of mRNA encoding either myocardin or MASTR, and animal cap explants were isolated and then analyzed by RT-PCR at St 12.5. Myocardin serves as a positive control. Muscle markers are indicated on the left-hand side of the figure. Muscle tissues expressing the various markers are indicated at the right. Lanes labeled St 12.5 WE and St 47 WE are positive control samples from whole embryos at St 12.5 and 47 respectively. ODC transcripts serve as a loading control. (I and J) Mutant MASTR lacking SRF interaction domains (ΔMASTR) fails to activate muscle marker expression. Experiments were conducted exactly as described for H above but analyzed by quantitative PCR. In the case of both αMHC and α-tropomyosin, a low level of transcript is detected in the untreated animal cap. Results are expressed relative to marker transcripts induced by MASTR.
Table 2.
Ectopic marker expression in response to MASTR
| Marker | mRNA | Normal gene expression,n (%) | Ectopic gene expression,n (%) | No. of embryos |
|---|---|---|---|---|
| αMHC | Uninjected | 49 (100) | 0 (0) | 49 |
| MASTR (500 pg) | 47 (64) | 26 (36) | 73 | |
| α-Tropomyosin | Uninjected | 55 (100) | 0 (0) | 55 |
| MASTR (500 pg) | 22 (79) | 6 (21) | 28 | |
| Cardiac α-actin | Uninjected | 28 (100) | 0 (0) | 28 |
| MASTR (500 pg) | 2 (6) | 34 (94) | 36 | |
| MyBP-C | Uninjected | 41 (100) | 0 (0) | 41 |
| MASTR (500 pg) | 9 (100) | 0 (0) | 9 |
Myocardin is capable of activating expression of cardiac and smooth muscle genes in nonmuscle tissues and cell lines (12, 19–22). We have carried out experiments using Xenopus embryonic ectoderm explants (animal caps) to determine the range of muscle markers that may be activated by MASTR in nonmuscle tissue. Note that animal caps contain SRF (19) but do not express muscle markers under normal culture conditions (23, 24). Myocardin was included in these studies to act as a positive control and to determine possible differences in the transcription activation properties of different myocardin family members. Consistent with previous studies (19), myocardin activates transcription of a number of cardiac and smooth muscle markers (Fig. 3H, lane 1), but it does not activate transcription of any of the skeletal muscle-specific markers examined. MASTR activates transcription of α-tropomyosin and, to a lesser extent, αMHC, skeletal α-actin, cardiac α-actin, and fast skeletal troponin I (fsTnI), but exhibits negligible activity for any of the other genes examined (Fig. 3H, lane 2). Of the markers that are skeletal muscle-specific, only fast skeletal troponin I (fsTnI) shows any significant response to MASTR. Unlike myocardin, MASTR showed no ability to activate expression of smooth muscle markers. It appears that the levels of SRF present in animal caps were sufficient to cooperate with MASTR to activate muscle gene expression. These experiments indicate that myocardin and MASTR regulate an overlapping but distinct set of downstream targets. Muscle marker activation by MASTR depended on the ability to interact with SRF, because a mutant form of MASTR lacking SRF binding domains failed to induce expression (Fig. 3 I and J). Activation of muscle marker expression was independent of mesoderm induction, as indicated by the absence of brachyury (Xbra) expression in the explants. Furthermore, we can exclude the possibility that skeletal muscle genes were activated indirectly via expression of myogenic regulatory factors, because no transcripts for MyoD, Myf5, MRF4, or myogenin were detected in the ectoderm explants expressing MASTR (SI Fig. 10).
MASTR Cooperates with MyoD and Myf5 to Promote Skeletal Muscle Gene Expression.
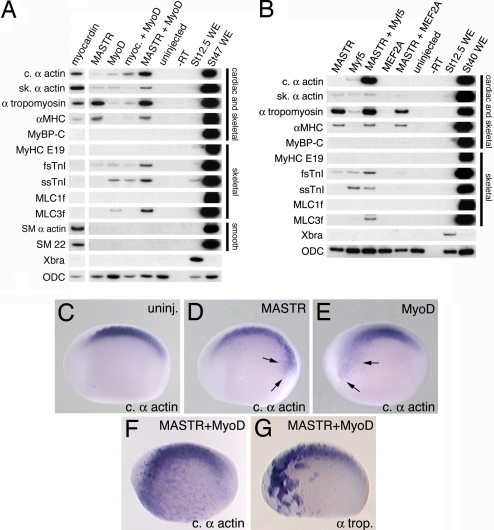
Because MASTR is coexpressed with MyoD and Myf5 in myogenic tissues in the embryo, we tested whether these factors might cooperate to regulate muscle gene expression. As expected (25, 26), MyoD alone is sufficient to activate transcription of numerous (but not all) skeletal muscle markers in animal cap explants (Fig. 4A, lane 3). However, coexpression of both MyoD and MASTR results in effective activation of a number of muscle genes (Fig. 4A, lane 5). This is most evident for cardiac α-actin, fsTnI, and Myosin Light Chain-3f (MLC3f) and, to a lesser extent, skeletal α-actin and ssTnI. In the case of α-tropomyosin and αMHC, coexpression with MyoD does not increase transcript levels above those observed with MASTR alone. Quantitation of transcript levels indicated that MASTR and MyoD synergized in the activation of some muscle markers, with cardiac α-actin and MLC3f showing particularly conspicuous responses (SI Fig. 11). Note that myocardin did not cooperate with MyoD for any of the markers examined. Protein-binding experiments suggest that frog MASTR and MyoD do not physically associate (SI Fig. 12), consistent with results reported for mouse MASTR (17). Similar to MyoD, expression of Myf5 alone is capable of activating transcription of a range of muscle genes (Fig. 4B, lane 2), and a combination of Myf5 and MASTR synergistically activated expression of cardiac α-actin, fsTnI, and MLC3f (Fig. 4B, lane 3). Unlike MyoD, Myf5 does not synergize with MASTR to activate expression of skeletal α-actin and ssTnI, indicating that at least some differences exist in the ability of different MRFs to cooperate with MASTR. We conclude that MASTR can cooperate with both of the MRFs present in the early Xenopus embryo to promote skeletal muscle gene expression. The continued requirement for SRF to mediate efficient MASTR activity was demonstrated in an assay using exogenous reporter plasmid DNA containing a muscle promoter (SI Fig. 13). The ability of MASTR to cooperate does not extend to every skeletal muscle transcription factor. For example, we find no evidence that MASTR can cooperate with MEF proteins (MEF2A and MEF2D), to activate muscle marker transcription (Fig. 4B, lane 5, and data not shown).
Fig. 4.
MASTR cooperates with MyoD or Myf5 to activate expression of muscle markers. Embryos were injected with mRNA encoding either myocardin or MASTR alone or together with mRNA encoding MyoD or Myf5. Animal cap explants were harvested at St 12.5 for RT-PCR analysis. Muscle markers are indicated on the left-hand side of the figure. Muscle tissues expressing the various markers are indicated at the right. ODC served as a loading control. (A) Coexpression of MASTR and MyoD results in increased transcription of muscle markers over either factor alone. This is particularly evident for cardiac α-actin, fsTnI, and MLC3f. Coexpression of myocardin with MyoD does not result in an equivalent activation of marker expression. (B) Coexpression of MASTR with Myf5 also activates transcription of muscle markers. Methods are identical to those described in A. No cooperation is observed between MASTR and the MADS factor, MEF2A. (C–G) Embryos were injected with mRNA encoding MASTR or MyoD alone or in combination and then assayed at St 22/23 for expression of muscle markers. Control (C), MASTR mRNA-injected, 100 pg (D), MyoD mRNA-injected, 100 pg (E), and MASTR plus MyoD mRNA-injected (F) embryos (100 pg of each) assayed for cardiac α-actin expression. Minor activation of cardiac α-actin expression is indicated by arrows in D and E. As shown in F, coexpression of MASTR and MyoD resulted in high levels of ectopic expression of cardiac α-actin D. (G) Coexpression of MASTR and MyoD results in high levels of ectopic expression of α-tropomyosin. Quantitation of the results of whole-embryo coexpression studies is presented in SI Table 3.
Additional experiments have been performed to determine whether the synergy of MASTR and MyoD observed in animal caps also applies to the whole embryo. In these studies, relatively small amounts of mRNA encoding MASTR or MyoD were injected either alone or in combination, and embryos were assayed for ectopic expression of muscle markers. MASTR mRNA (100 pg) or MyoD mRNA (100 pg) alone produced weak ectopic cardiac α-actin expression at rather low frequency (Fig. 4 D and E and SI Table 3). In contrast, when embryos were coinjected with MASTR and MyoD mRNA (100 pg of each) 81% of embryos (30 of 37) showed high levels of ectopic cardiac α-actin expression (Fig. 4F and SI Table 3). As shown (Fig. 3D), MASTR was not very effective at activating α-tropomyosin expression in the whole embryo, but coexpression of MASTR and MyoD activated strong ectopic expression of α-tropomyosin in the majority of embryos (Fig. 4G and SI Table 3). These studies suggest that MASTR cooperates with MRF proteins to regulate skeletal muscle gene expression during Xenopus embryonic development.
Discussion
MASTR Is Required for Normal Skeletal Muscle Gene Expression in the Frog Embryo.
We have identified a member of the Xenopus myocardin family of transcription factors not previously identified (Fig. 1A). Despite rather low conservation of primary sequence, synteny analysis indicates that the Xenopus gene is the orthologue of mouse MASTR (17). The most striking difference between the Xenopus and mouse MASTR proteins is the absence of SRF-interacting domains and the leucine zipper dimerization module from the mouse sequence (Fig. 1A). This observation strongly suggests that the mouse MASTR protein does not require interaction with SRF for function. Whereas mouse MASTR is expressed at high levels in skeletal muscle and at lower levels in a number of other tissues (17), Xenopus MASTR is skeletal muscle-specific (Fig. 1 C–F and P). Both dominant-negative and antisense MO strategies demonstrated that MASTR activity was required for normal expression of skeletal muscle genes during developmental myogenesis in Xenopus (Fig. 2). Although both DN-MASTR and MO knockdown effectively reduced muscle gene expression, complete elimination of marker expression was never observed. As discussed below, this result is consistent with a mechanism where MyoD and Myf5 activate muscle gene expression in the embryo, and MASTR positively coregulates transcript levels.
MASTR Activates Expression of Muscle Markers in Nonmuscle Tissue.
Mis-expression of MASTR in frog embryos was sufficient to activate ectopic expression of muscle markers (Fig. 3). Therefore, MASTR joins a small group of proteins, including MyoD, Myf5, MRF4, MEF2D, and myocardin, which possess the ability to induce muscle gene expression in nonmuscle tissues of the frog embryo (6, 19, 25, 27, 28). It is noteworthy that each of these factors plays an essential role in regulation of muscle development and physiology (28–31). The transcription-activating properties of MASTR were further examined in Xenopus ectoderm explants. These studies demonstrated that MASTR alone was a rather poor activator of skeletal muscle gene expression (Fig. 3H). Of the 12 muscle markers tested, MASTR induced strong transcription of only two genes, α-tropomyosin and αMHC. Weak activation was observed for cardiac α-actin, skeletal α-actin, and fsTnI. This study also revealed distinct differences in the gene-activation profiles of the Xenopus myocardin and MASTR proteins (Fig. 3H), indicating that despite the overall similarity of protein domain structure (Fig. 1A), the proteins possess distinct transcriptional specificities. Inspection of Xenopus genome sequences indicates that muscle genes that respond strongly to MASTR alone, or synergize with MyoD and Myf5, contain consensus SRF-binding sites (CArG elements) in their promoter regions.
Myogenic Factors Cooperate with MASTR to Boost Skeletal Muscle Gene Expression.
Coexpression of MASTR with MyoD or Myf5 resulted in the synergistic activation of a number of muscle differentiation markers (Fig. 4 and SI Fig. 11). No such synergistic activation was observed when myocardin was coexpressed with MyoD, indicating that the ability to cooperate with MRFs is not a general property of the myocardin family of proteins. In several cases the major consequence of coexpressing MASTR with MyoD or Myf5 was to increase the expression level observed with the MRF alone. For example, MyoD activates low levels of cardiac α-actin, skeletal α-actin, α-tropomyosin, fsTnI, ssTnI, and MLC3f, and coexpression of MyoD and MASTR results in a significant increase in transcription of these markers. However, this effect is not universal because coexpression of MASTR with MyoD or Myf5 did not increase αMHC and α-tropomyosin transcription over the fairly high levels generated by MASTR alone. The observation that MASTR acts to increase the levels of gene expression above that produced by MRFs is consistent with the MASTR knockdown phenotype, where marker expression was reduced but not eliminated (Fig. 2). Our results are in general agreement with those reported for mouse MASTR, which enhanced the ability of MyoD to activate muscle marker expression in 10T1/2 cells (17). Mouse MASTR also cooperated with MEF2 family proteins to stimulate transcription from MEF2 reporter plasmids in COS cells. In contrast to these results, we observed no detectable cooperation between Xenopus MASTR and MEF2 proteins for muscle gene expression (Fig. 4B).
Previous studies have demonstrated protein–protein interactions between MRFs and SRF and have shown that MRFs and SRF cooperate to regulate expression of some skeletal muscle genes (10, 11, 32–34). These studies support a model in which MRFs assemble in a protein complex with SRF to regulate muscle gene expression. Because Xenopus MASTR is capable of binding SRF (Fig. 1B), it seems likely that MASTR will assemble into the SRF/MRF complex regulating the muscle promoter. Mouse MASTR protein lacks the SRF-interacting regions, and so assembly into a transcription regulatory complex may rely on interactions with other factors (e.g., MEF2 proteins).
Regulation of MASTR Target Specificity.
Myocardin family proteins activate different target genes depending on the cell type or tissue in which they are expressed (12, 20, 22). It seems probable, therefore, that additional regulatory mechanisms work to modulate target specificity of the myocardin family proteins. Our studies suggest that MASTR target specificity is similarly dependent on additional regulatory mechanisms. For example, MASTR weakly activates cardiac α-actin in animal caps, but strongly activates ectopic expression in the whole embryo. Because MyoD and Myf5 are only expressed in developing muscle tissues (1, 28), it is possible that additional factors cooperate with MASTR to activate marker expression in nonmuscle tissue. Alternatively, factors inhibiting MASTR function may be present in the ectodermal explant tissue. Also, our experiments show that MASTR strongly activates expression of the αMHC gene at ectopic locations in the whole embryo and in explant tissue (Figs. 3B, H). This is surprising because, during normal development, αMHC is not expressed in the somites (35), even though MASTR is expressed at high levels in this tissue (Fig. 1 C–F). During later development however, it seems likely that MASTR regulates αMHC expression in developing jaw muscles (Fig. 1F). In summary, our studies indicate that MASTR is an important regulator of skeletal muscle development in the embryo and raise the possibility that disruption of MASTR function may also be involved in muscle disease.
Materials and Methods
Cloning of Xenopus laevis MASTR, MyoD, and Myf5.
Searching of Xenopus EST databases revealed the presence of a previously uncharacterized myocardin-related sequence. By using EST data, primers were designed to cover the putative start and stop codons and the coding region was amplified by RT-PCR using Pfu polymerase and cDNA template prepared from X. laevis embryonic RNA. The resulting product was inserted into the vector pT7TS and the MASTR sequence determined (GenBank accession no. EU379568). Dominant negative MASTR (DN-MASTR), encoded the first 492 aa of MASTR but lacked the C-terminal transcription activating domain. Control experiments demonstrated that this construction efficiently inhibited transcription activation by the full-length MASTR construction (SI Fig. 7). A mutant form of MASTR (ΔMASTR) lacking the SRF interaction domains was generated by inverse PCR. The coding regions of X. laevis Myf5 and MyoD were amplified by RT-PCR, inserted into pT7TS and sequence verified.
Co-IP Experiments.
HA-tagged human SRF (38) was generated by transfection into COS-7 cells. Full-length MASTR, ΔMASTR and EGFP proteins were radiolabeled by translation in the wheat germ cell free translation system (Promega). After mixing of COS cell extracts containing HA-SRF with in vitro translation products, immunoprecipitations were carried out by using standard procedures (39) and anti-HA antibody (Roche). Bound proteins were fractionated on a 10% SDS/PAGE gel and visualized by autoradiography. HA-SRF protein was verified by Western blotting and detection by using chemiluminescent solution (Supersignal kit, West Dura kit).
Microinjection and Embryological Manipulations.
RNAs encoding MASTR, DN-MASTR, MyoD or Myf5 for microinjection were prepared by using the T7 Message Machine kit (Ambion). Dose curves were carried out for all transcripts. For marker expression studies, mRNAs were microinjected into one cell of two-cell-stage embryos in 0.4× MMR/4%Ficoll, cultured thereafter in 0.2× MMR and processed at neurula stages. For animal cap explant studies, embryos were injected at the one-cell stage, caps were isolated at St 9 and cultured in 0.75X MMR until sibling control embryos reached St 12.5. Preparation and microinjection of MOs was performed as described (36). MASTR antisense morpholinos (MO1 5′ AGGCACAGTGACGGACGTAATGGTT 3′ and MO2 5′ GGTCCCAAAGGACTGAGGGAG 3′) were targeted to sequences in the 5′ UTR of the MASTR transcript. Both MO1 and MO2 effectively blocked translation from a MASTR test transcript containing 5′ UTR of MASTR mRNA sequence fused to the coding region of EGFP (SI Fig. 8). For in vivo experiments, 25 ng or 50 ng of MO1 or MO2 was microinjected into the mediolateral region of one cell of two-cell staged embryos. For MO control experiments, we used an MO that blocks expression of Wnt11-R (36) but which has no other effects on early embryonic development. Whole-mount in situ hybridization was carried out by using digoxigenin-labeled probes and standard conditions (37). For RT-PCR analysis, total RNA was isolated from 20 animal caps per treatment by using TRIzol (Invitrogen). RT-PCR was carried out including 0.3 μCi of α-32P-dATP in a standard reaction, products were fractionated on a 5% nondenaturing polyacrylamide gel and visualized by autoradiography. Primers, annealing temperatures, and cycle numbers for RT-PCR analysis are presented in SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Carol Gregorio for assistance with the Co-IP experiments, Dha-Zhi Wang for the SRF construction, and Tom Drysdale for the MyBP-C plasmid. S.M.M. was supported in part by Training Grant GM08659 from the Department of Molecular and Cellular Biology, and A.S.W. was supported by a postdoctoral fellowship from the American Heart Association. P.A.K. is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine and is supported by the Sarver Heart Center and by National Heart, Lung, and Blood Institute of the National Institutes of Health Grants HL63926 and HL74184.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank databank (accession no. EU379568).
This article contains supporting information online at www.pnas.org/cgi/content/full/0703918105/DC1.
References
- 1.Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol. 2005;16:585–595. doi: 10.1016/j.semcdb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M. Skeletal muscle formation in vertebrates. Curr Opin Genet Dev. 2001;11:440–448. doi: 10.1016/s0959-437x(00)00215-x. [DOI] [PubMed] [Google Scholar]
- 3.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub H, et al. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA. 1989;86:5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J, et al. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigment epithelial cells into striated, mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci USA. 1990;87:7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hopwood ND, Pluck A, Gurdon JB. Xenopus Myf-5 marks early muscle cells and can activate muscle genes ectopically in early embryos. Development. 1991;111:551–560. doi: 10.1242/dev.111.2.551. [DOI] [PubMed] [Google Scholar]
- 7.Puri PL, Sartorelli V. Regulation of muscle regulatory factors by DNA-binding interacting proteins and post-transcriptional modifications. J Cell Physiol. 2000;185:155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Reecy J, Beaguli NS, Schwartz RJ. In: SRF/Homeobox Protein Interactions in Heart Development. Harvey RP, Rosenthal N, editors. San Diego: Academic; 1998. pp. 273–290. [Google Scholar]
- 9.Shore P, Sharrocks AD. The MADS-box family of transcription factors. Eur J Biochem. 1995;229:1–13. doi: 10.1111/j.1432-1033.1995.tb20430.x. [DOI] [PubMed] [Google Scholar]
- 10.Sartorelli V, Webster KA, Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990;4:1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- 11.Catala F, et al. A skeletal muscle-specific enhancer regulated by factors binding to E, CArG boxes is present in the promoter of the mouse myosin light-chain 1A gene. Mol Cell Biol. 1995;15:4585–4596. doi: 10.1128/mcb.15.8.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, et al. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Wang DZ, Wang Z, Richardson JA, Olson EN. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA. 2003;100:9366–9370. doi: 10.1073/pnas.1233635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Chang S, Qi X, Richardson JA, Olson EN. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol Cell Biol. 2006;26:5797–5808. doi: 10.1128/MCB.00211-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, et al. Acute myeloid leukemia-associated Mlk1 is a key regulator of mammary gland function. Mol Cell Biol. 2006;26:5809–5826. doi: 10.1128/MCB.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh J, Richardson JA, Olson EN. Requirement for myocardin-related transcription factor-B for remodeling of branchial arch arteries and smooth muscle differentiation. Proc Natl Acad Sci USA. 2005;102:15122–15127. doi: 10.1073/pnas.0507346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creemers EE, Sutherland LB, Oh J, Barbosa AC, Olson EN. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell. 2006;23:83–96. doi: 10.1016/j.molcel.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Li S, et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc Natl Acad Sci USA. 2005;102:1082–1087. doi: 10.1073/pnas.0409103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small EM, et al. Myocardin is sufficient and necessary for cardiac gene expression in Xenopus. Development. 2005;132:987–997. doi: 10.1242/dev.01684. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du KL, et al. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Tuyn J, et al. Activation of cardiac and smooth muscle-specific genes in primary human cells after forced expression of human myocardin. Cardiovasc Res. 2005;67:245–255. doi: 10.1016/j.cardiores.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Smith JC. A mesoderm-inducing factor is produced by Xenopus cell line. Development. 1987;99:3–14. doi: 10.1242/dev.99.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- 25.Hopwood ND, Gurdon JB. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990;347:197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- 26.Gaillard C, et al. α-tropomyosin gene expression in Xenopus laevis: differential promoter usage during development and controlled expression by myogenic factors. Dev Genes Evol. 1998;207:435–445. doi: 10.1007/s004270050134. [DOI] [PubMed] [Google Scholar]
- 27.Chambers AE, et al. The RSRF/MEF2 protein SL1 regulates cardiac muscle-specific transcription of a myosin light-chain gene in Xenopus embryos. Genes Dev. 1994;8:1324–1334. doi: 10.1101/gad.8.11.1324. [DOI] [PubMed] [Google Scholar]
- 28.Chanoine C, Della Gaspera B, Charbonnier F. Myogenic regulatory factors: Redundant or specific functions? Lessons from Xenopus. Dev Dyn. 2004;231:662–670. doi: 10.1002/dvdy.20174. [DOI] [PubMed] [Google Scholar]
- 29.Black BL, Olson EN. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu Rev Cell Dev Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Chanoine C, Hardy S. Xenopus muscle development: From primary to secondary myogenesis. Dev Dyn. 2003;226:12–23. doi: 10.1002/dvdy.10206. [DOI] [PubMed] [Google Scholar]
- 31.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 32.Groisman R, et al. Physical interaction between the mitogen-responsive serum response factor and myogenic basic helix–loop–helix proteins. J Biol Chem. 1996;271:5258–5264. doi: 10.1074/jbc.271.9.5258. [DOI] [PubMed] [Google Scholar]
- 33.Biesiada E, Hamamori Y, Kedes L, Sartorelli V. Myogenic basic helix–loop–helix proteins and Sp1 interact as components of a multiprotein transcriptional complex required for activity of the human cardiac a-actin promoter. Mol Cell Biol. 1999;19:2577–2584. doi: 10.1128/mcb.19.4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latinkic BV, et al. Distinct enhancers regulate skeletal and cardiac muscle-specific expression programs of the cardiac alpha-actin gene in Xenopus embryos. Dev Biol. 2002;245:57–70. doi: 10.1006/dbio.2002.0639. [DOI] [PubMed] [Google Scholar]
- 35.Garriock RJ, Meadows SM, Krieg PA. Developmental expression and comparative genomic analysis of Xenopus cardiac myosin heavy chain genes. Dev Dyn. 2005;233:1287–1293. doi: 10.1002/dvdy.20460. [DOI] [PubMed] [Google Scholar]
- 36.Garriock RJ, D'Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryo. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- 38.Chen CY, Schwartz RJ. Recruitment of the Tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol. 1996;16:6372–6384. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregorio CC, Fowler VM. Mechanisms of thin filament assembly in embryonic chick cardiac myocytes: Tropomodulin requires tropomyosin for assembly. J Cell Biol. 1995;129:683–695. doi: 10.1083/jcb.129.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.