Abstract
A large carbon sink in northern land surfaces inferred from global carbon cycle inversion models led to concerns during Kyoto Protocol negotiations that countries might be able to avoid efforts to reduce fossil fuel emissions by claiming large sinks in their managed forests. The greenhouse gas balance of Canada's managed forest is strongly affected by naturally occurring fire with high interannual variability in the area burned and by cyclical insect outbreaks. Taking these stochastic future disturbances into account, we used the Carbon Budget Model of the Canadian Forest Sector (CBM-CFS3) to project that the managed forests of Canada could be a source of between 30 and 245 Mt CO2e yr−1 during the first Kyoto Protocol commitment period (2008–2012). The recent transition from sink to source is the result of large insect outbreaks. The wide range in the predicted greenhouse gas balance (215 Mt CO2e yr−1) is equivalent to nearly 30% of Canada's emissions in 2005. The increasing impact of natural disturbances, the two major insect outbreaks, and the Kyoto Protocol accounting rules all contributed to Canada's decision not to elect forest management. In Canada, future efforts to influence the carbon balance through forest management could be overwhelmed by natural disturbances. Similar circumstances may arise elsewhere if global change increases natural disturbance rates. Future climate mitigation agreements that do not account for and protect against the impacts of natural disturbances, for example, by accounting for forest management benefits relative to baselines, will fail to encourage changes in forest management aimed at mitigating climate change.
Keywords: greenhouse gases, factoring out, mitigation options, forest management, Kyoto Protocol
Forests and forest management can contribute toward reducing future atmospheric greenhouse gas (GHG) concentrations (1). The Kyoto Protocol to the United Nations Framework Convention on Climate Change (UNFCCC) seeks to reduce emissions (sources) and increase removals (sinks) in the land use, land-use change, and forestry (LULUCF) sector. A large carbon sink in northern land surfaces, inferred from global carbon cycle inversion models (2–5), and to a lesser degree by forest inventory-based analyses (6), led to concerns during international negotiations leading up to the seventh Conference of the Parties (COP7) to the UNFCCC in 2001 that some countries might be able to avoid efforts to reduce fossil fuel emissions by claiming large sinks in the LULUCF sector. New findings have since indicated that northern terrestrial ecosystems are taking up less C than thought (7) and that terrestrial C sinks are weakening (8, 9), but these findings had not yet emerged at the time of the COP7 negotiations. Negotiators addressed their concern about windfall forest C sinks through country-specific caps on accountable emissions and removals from forest management for the first commitment period of the Kyoto Protocol (2008–2012) (10). Moreover, because countries were uncertain about the contribution to their national GHG balances of various land management activities under Article 3.4 of the Protocol, the negotiators also agreed that countries could wait until the end of 2006 to decide whether to include these activities in their GHG accounting, thereby giving them time to assess the likely contributions.
The net contribution of any forest to the global atmospheric GHG balance is a relatively small difference between several large fluxes: uptake of CO2 by photosynthesis (gross primary production), release of CO2 by autotrophic and heterotrophic respiration, release of CO2, CH4, and N2O by disturbance, and transfer of carbon to the forest products sector, treated as an emission under the current accounting rules of the Intergovernmental Panel on Climate Change (11). Changes in one or more of these fluxes can shift the forest toward being a net sink or source of GHGs. For example, emissions from a small proportion of Canadian forests during major disturbance episodes can exceed CO2 uptake through growth in the rest of the country's forests (12, 13). In addition, the legacy of past natural disturbances and management has a strong influence on future forest GHG budgets because it affects the forest age–class distribution (14, 15). Because stand age is a key factor influencing ecosystem productivity (16–18), changes in forest age–class structure can result in significant change to the rates of C uptake and release from the forest landscape (12). All forest ecosystem GHG fluxes are small, however, relative to the size of the stocks of carbon stored in forest biomass, dead organic matter, and soils (19).
Historical, natural, and anthropogenic factors will influence the future net GHG balance in the managed forests of any country. The degree to which these factors will exert influence over the future forest GHG balance will differ between biomes and between countries. Global change factors, including CO2 fertilization, atmospheric N deposition, and climate change may already be influencing forest productivity (20–22). Whereas increases in net primary productivity (NPP) have been inferred from satellite observations and attributed to these factors, the impact of these factors on net ecosystem productivity (NEP) in boreal forests remains a subject of debate (15, 23). Atmospheric N deposition, which has been presented by some as the principal factor driving European forest GHG sinks, is far less important in boreal North America (24, 25). CO2 fertilization saturates under certain conditions (26), and the positive impacts of beneficial climatic changes (e.g., longer growing seasons) could be negated by other, less beneficial changes (e.g., summer drought) in the same ecosystems (27). Recent results from inversion modeling suggest that northern forests may not be contributing as strong a GHG sink as thought (7), narrowing the gap between top-down and bottom-up estimates for these forests (6).
More than 7% of the world's forests, including 20% of the world's boreal forests, are located in Canada (28, 29). Here, we report on analyses conducted to project the GHG balance of Canada's managed forest (a 240 million ha subset of Canada's 310 million ha total forest area (30)) for the first commitment period of the Kyoto Protocol and to 2022. We estimated the annual forest GHG balance for 2000–2022 by using the National Forest Carbon Monitoring Accounting and Reporting System (NFCMARS) (31) to simulate forest growth and decay and extending natural disturbance monitoring statistics with projections of future disturbance rates. Using a Monte Carlo simulation approach, we generated a probability distribution of projected future annual average GHG balances and identified a large range of possible outcomes. Simulations were conducted by using the Carbon Budget Model of the Canadian Forest Sector (CBM-CFS3), an empirical stand- and landscape-level forest ecosystem simulation model that is driven by forest inventory and empirical forest growth data compiled from across Canada (12, 32, 33). The CBM-CFS3 does not take into account any influence on forest growth or decomposition of increased atmospheric CO2 concentrations, enhanced atmospheric N deposition, or climate changes that are not already accounted for in the empirical growth and yield data used to drive the model. These factors, however, have less impact on the forest carbon budget in Canada than do disturbances (34).
Results
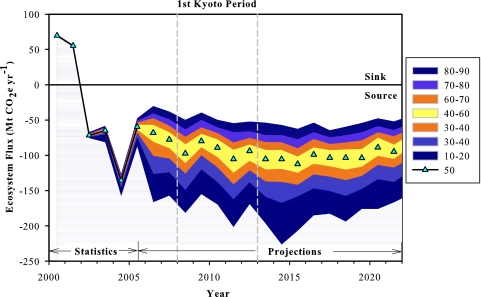
Canada's managed forest was estimated to have acted as net GHG sink for two years in 2000 and 2001 (70 Mt CO2e and 55 Mt CO2e, respectively) before becoming a net source in 2002 (−72 Mt CO2e), and remained a net GHG source through 2022 (Fig. 1) [1 Mt C is equivalent to 3.67 Mt CO2e; emissions of CH4 and N2O are converted to units of CO2-equivalent according to their global warming potentials (11)]. The wide range of possible outcomes was a reflection of the large variation in potential area burned or infested by insects. By 2002, the mountain pine beetle infestation began to severely impact the western Canadian forest, infesting a cumulative area of >100,000 km2 by 2006 (35). We projected the mountain pine beetle infestation to reach its peak in 2009, although its influence on GHG emissions was projected to continue through 2022. Salvage logging to recover beetle-killed wood resulted in increased total wood volume harvested during 2006–2016. Simulation of the anticipated major spruce budworm outbreak in the spruce-fir forests of eastern Canada further contributed to the GHG source of the managed forest. Monte Carlo simulations having the largest annual area burned resulted in extreme emissions, exceeding 400 Mt CO2e in single years (low probability), whereas simulations having the smallest areas disturbed resulted in the best-case scenario of 3 Mt CO2e of emissions in a single year. The combined risk of large-scale wildfires, western mountain pine beetle infestation, increased salvage logging, and the projection of an anticipated severe eastern spruce budworm outbreak (starting sometime between 2008 and 2011) contributed to the forecast of a net GHG source from Canada's managed forests in 2002–2022.
Fig. 1.
Annual net GHG balance (ecosystem flux) for Canada's managed forests. The model results are based on disturbance and management statistics for 2000–2005 and projections for 2006–2022. A small range in the estimates for 2003–2005 resulted from the need to fill some gaps in the available disturbance data with Monte Carlo projections. Monte Carlo simulations were used to project ecosystem GHG balance for future years, in which the area disturbed by fire and insects is not yet known, resulting in the wide range of projected estimates. The 50th percentile estimate for each year is indicated with a cyan triangle, and colors indicate the areas representing the range of estimates between the 10th and 90th percentiles. Negative GHG balance represents a net flux from the forest to the atmosphere (net GHG source).
During the first commitment period of the Kyoto Protocol, the managed forests of Canada were predicted to be a source of GHGs between 30 and 245 Mt CO2e yr−1 (Fig. 2). The Monte Carlo projections indicated a wide range (215 Mt CO2e) of potential average annual GHG emissions. This 215 Mt CO2e yr−1 range is equivalent to nearly 30% of the total CO2e emissions in Canada in 2005 (36). The actual emissions in the period will be a single number and will be determined once the actual area disturbed is known.
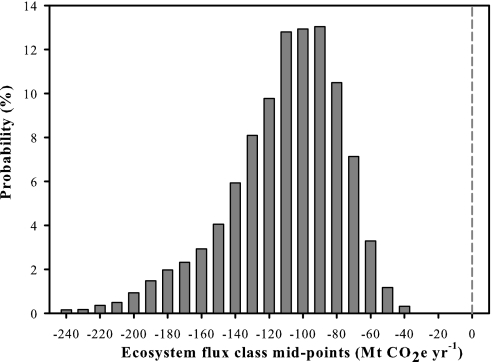
Fig. 2.
Probability distribution of Canada's managed forest ecosystem GHG balance during the first Kyoto Protocol commitment period (average annual ecosystem GHG balance during 2008–2012). The distribution fell entirely below 0, indicating a projected 100% probability that Canada's managed forest will be a net source of GHG emissions to the atmosphere during this period. The estimates ranged from emissions of 30 to >245 Mt CO2e yr−1, mostly because of the risk of natural disturbance. The long tail on the left side of the distribution indicates the low probability of very large emissions resulting from extremely severe natural disturbances during the period. Note that some of the projected emissions are in fact transfers of carbon to the forest product sector. Carbon residence time in wood products and landfills can be several decades.
Discussion
There is a large range in the risk of future GHG emissions from the managed forests of Canada because of the inherent unpredictability of natural disturbances. The asymmetrical shape of the probability distribution (Fig. 2) is a reflection of the disturbance impact risks; the long tail on the left side reflects the low probability of very severe disturbances during the commitment period, whereas the tail on the right side is shorter because there is a limit to how well forests can grow in the absence of disturbance.
Three different types of disturbance influence the shape, width, and position of the GHG probability distribution (Fig. 2): fire, insects, and harvesting. Fire affects the shape and width of the probability distribution but not its location along the x axis given our assumption that the fire regime, as characterized by the fire probability distribution functions, is unchanged during the projection period. The total area burned by forest fire in Canada varies widely from year to year (37) and, consequently, so does the impact of fire on the forest GHG budget. Annual area burned, however, cannot be deterministically predicted because of the influence of unpredictable large weather systems (38, 39). Our approach, using regionally calibrated probability density functions of annual area burned and area affected by forest insect disturbances, provides a mechanism for estimating both the range and likelihood of future forest GHG emissions and removals. Trends in the area annually burned in response to rising temperatures can already be detected (40) and further increases are predicted over the course of this century (41). Over short time horizons, however, these trends are masked by high interannual variability and were therefore not taken into account in this study.
Insect outbreak dynamics are cyclical (42), and consequently, so are insect impacts on the forest GHG balance. During outbreak periods, the entire probability distribution of the 5-year average GHG balance (Fig. 2) is shifted to the left, that is, toward a greater source. Canada's 2007 submission to the UNFCCC (based on the same modeling framework) reported a net GHG sink in the managed forest during most years in the 1990s, a decade with relatively little insect outbreak activity, and reported net sources in more recent years (36), coincident with the increase in insect outbreak activity. The two insects that have the greatest impact on Canada's forests, spruce budworm and mountain pine beetle, had cyclical outbreak dynamics during the twentieth century (43–45) and dendrochronological reconstructions indicate that there have been cyclical spruce budworm outbreak dynamics for several centuries in eastern Canada (46), following a ≈35-year cycle (43). Insect outbreaks reduce growth rates and kill trees, resulting in large transfers of carbon from biomass to dead organic matter and subsequent decomposition (12). Here, we projected that the current mountain pine beetle outbreak will peak soon in British Columbia, but did not project any expansion of the outbreak across the historical eastern range limit, the Rocky Mountain geoclimatic barrier (47). Our projections of the anticipated eastern spruce budworm outbreak are based on information from the Spruce Budworm Decision Support System (48) and assumed that the same budworm population control measures will be taken in this outbreak as were taken in the previous outbreak.
The timing of insect outbreak cycles is a key factor influencing the apparent shift from sink to source after 2001 (Fig. 1). During the 1990s insects had relatively small impacts in Canada's forests, but after 2001 the impacts of the mountain pine beetle outbreak in western Canada started to affect the national forest C budget and continue to do so. Although there have been large mountain pine beetle outbreaks in the recorded past, the current outbreak is the most widespread and severe bark beetle epidemic ever recorded in Canada (45). The carbon impacts of this outbreak will continue for decades until carbon uptake by forest regeneration exceeds carbon release from decomposition of beetle-killed tree biomass and the removal of carbon by salvage logging. Around the time when the impacts of this outbreak are diminishing, the impacts of the anticipated eastern spruce budworm outbreak will start to affect Canada's national forest carbon budget. Both of these insects affect forest productivity in such a way that their impacts cannot easily be isolated. Unlike fires, which cause large direct emissions, the insects' most important impacts are indirect—transfers of carbon from living biomass to dead organic matter with subsequent increases in heterotrophic respiration and changes to forest succession and age–class structures. In the modeling approach used here, heterotrophic respiration from insect-killed organic matter cannot be distinguished from other heterotrophic respiration. To fully isolate and quantify the insect impacts, model runs with and without insects would be required for each of several insect species and for all Monte Carlo runs.
Timber harvesting is a planned activity and harvest rates in Canada are set with the goal of achieving a sustained, even flow of harvested wood volume to meet society's demands and maintain stable employment in the forestry sector. Harvest rates do change in response to disturbance and associated salvage opportunities or disruptions to planned harvest schedules, but harvest impacts tend to remain relatively stable compared with natural disturbance impacts. For this study we assumed that harvested carbon that leaves the ecosystem is emitted immediately to the atmosphere. This assumption conforms to the accounting rules of the Kyoto Protocol but is known to be inaccurate because a large proportion of the biomass C harvested in Canada is accumulating in long-lived wood products and landfills (49).
One possible strategy for reducing GHG emissions from the managed forest is to engage in more aggressive management of natural disturbances. It is, however, not possible to fully control natural disturbances because of the scale and remoteness of Canada's forests and the characteristics of the disturbance agents involved. The largest 3% of fires in Canada contribute 97% of total area burned (37). These fires typically burn during severe fire weather and are typically extinguished by changes in weather, not by fire suppression. Average public expenditures for fighting forest fires in Canada are $500 to $600 million yr−1 (50); these efforts are largely aimed at protecting human life, property, and forest resources. Insect outbreaks have proven equally difficult to contain; bark beetles such as the mountain pine beetle burrow under the bark to feed on tree phloem, making it difficult to apply insecticides, particularly over large infested areas (51). Concerns about insecticide use further reduce the attractiveness of such control options, even where they may be effective (52).
This analysis demonstrates that Canada's managed forest GHG balance is highly influenced by naturally occurring fires and insect outbreaks. The disturbance rates in Canada are not unique; natural disturbances are an integral part of the ecology of northern forests. Disturbances are increasingly being recognized as the predominant drivers of forest carbon dynamics, particularly for northern high-latitude ecosystems (12, 18, 34, 53). It is therefore not surprising that disturbances are projected here to drive the future carbon budget of Canada's forests. Although our projections suggest that Canada's managed forests will continue to act as a net carbon source in the coming decades, largely because of the direct and residual impacts of insect outbreaks, it should be noted that any further increases in disturbance rates would result in even larger carbon losses from these ecosystems. A doubling in average area burned by wildfire is projected for the end of this century (41), and this could result in an increase in emissions up to 100 Mt CO2e yr−1 for the managed forest of Canada (54)—a doubling of our projected carbon losses. Conversely, if there is a period in the future with no major ongoing insect infestations (as during the 1990s), then carbon losses during that period will be lower than carbon losses projected here for 2002–2022, where we have a major mountain pine beetle outbreak immediately followed by a major eastern spruce budworm outbreak.
Disturbances are, of course, not the only processes influencing forest C dynamics. Other global change factors, including increased atmospheric CO2, N deposition, and climate changes may also be affecting forest C dynamics. Having not accounted explicitly for these factors in this analysis, we have likely underestimated the true risk of C loss, but we will also have underestimated the C sink potential of Canada's managed forest lands. Had we been able to take these non-disturbance-related factors into account, this analysis would have produced a probability distribution similar to that presented in Fig. 2, but shifted to the right if the net effect of global change factors is sink enhancing, or to the left if their net effect reduces sinks. The essence of our findings of a wide range of possible outcomes due to natural disturbance impacts would not change.
North America is estimated to contribute 50–90% of the estimated global terrestrial carbon sink (19). Canada's managed forest constitutes 13% of the land area in North America and in the 1990s contributed only 2% of the sink (36). If these estimates are correct, then the remaining land areas must be taking up disproportionately more carbon. If Canada's managed forest transitions to a carbon source, other parts of the global carbon cycle will have to compensate or the atmosphere will accumulate GHGs faster than predicted by using the current estimates of the terrestrial sink strength. Other forested areas, currently estimated to be carbon sinks, may also transition to sources if the impacts of disturbances increase.
In Canada, efforts to influence the carbon balance through forest management, such as increasing harvest rotation lengths, reducing regeneration delays, or increasing stocking densities, may be overwhelmed by natural disturbances. Similar circumstances may arise elsewhere in the future as disturbance rates increase as a result of global change or introduction of invasive species. For example, in countries where fire and insects are easier to manage and control, drought or windthrow could cause significant forest growth losses (55) or dieback (56). An increase in the frequency or intensity of extreme weather events is an anticipated outcome of climate change (57). Extreme weather events and disturbances both pose a risk to terrestrial GHG sinks, and there is already emerging evidence of reduced efficiency of natural sinks (9).
Differing national circumstances will be considered during international negotiations on how to treat forests and forest management in a post-2012 climate mitigation agreement (58). Although forests and forest management can contribute to national and global mitigation portfolios (1), current international GHG accounting rules fail to encourage changes in forest management for the benefit of the atmosphere in countries with the potential for large emissions from natural disturbances, such as wildfire, insect outbreaks, or extreme weather. In Canada, there is a high risk that emissions from natural disturbances might completely negate efforts to affect the GHG balance through forest management because the current accounting rules do not factor out natural and indirect human effects from direct human effects. Because of the risk of natural disturbance impacts and the accounting rules that require that emissions resulting from both human activities and natural events have to be reported, Canada has decided not to elect forest management for its Kyoto Protocol accounting (59).
Research is ongoing to develop approaches to factor out direct human effects from natural and indirect human effects (15) and account for harvested carbon in the forest product sector (49, 60). Future climate mitigation agreements that have successfully negotiated solutions to these issues are a prerequisite to encouraging increased involvement of the forest sector in climate mitigation. For example, greenhouse gas mitigation efforts could be evaluated against a dynamic baseline that could address the impacts of age–class structures or cyclical insect outbreaks. Natural disturbances could be factored out from the accounting by using ex post analyses of expected area burned given fire weather conditions, thus accounting for the benefits of fire suppression efforts (15). Accounting of mitigation activities must, however, also address leakage—increased emissions of GHGs elsewhere resulting from changes in activities. Leakage erodes GHG benefits of mitigation activities if, for example, reduced harvest rates cause replacement of construction lumber with concrete or metal materials with higher net GHG emissions. Accounting systems should provide incentives for land managers to choose activities that increase sinks or reduce sources relative to dynamic baselines that are calculated taking into consideration forest dynamics (age–class structure effects) and natural disturbance events.
Materials and Methods
The geographic scope of this study was the managed forest of Canada (240 million ha) [see supporting information (SI) Text for details]. Provincial and territorial forest resource management agencies provided forest inventory and growth and yield information for this study either directly, or via the Canadian Forest Inventory (CanFI 2001), a national compilation of inventory data for Canada (30). We used national datasets for fire (61) and harvest (62) as input to the model for simulating disturbances during 2000–2005. Provincial and territorial forest management agencies provided insect-monitoring data, additional data on fire and harvesting, and information used to formulate simulation rules. Data describing the area infested by mountain pine beetle were available for 2000–2006, and data for other insects were available for 2000–2005.
We used a Monte Carlo risk assessment approach to project future GHG emissions and removals from Canada's managed forest. This enabled us to evaluate both the range of potential future outcomes and their probabilities. Six disturbance agents—(i) fire, (ii) spruce budworm (Choristoneura fumiferana Clemens), (iii) mountain pine beetle (Dendroctonus ponderosae Hopkins), (iv) aspen defoliators (Malacosoma disstria Hübner, Choristoneura conflictana Walker, and Operophtera bruceata Hulst), (v) jack pine budworm (Choristoneura pinus Freeman), and (vi) hemlock looper (Lambdina fiscellaria fiscellaria Guenée)—have historically had the greatest impact on forest dynamics in Canada (46, 63–66). We developed separate regional probability density functions (PDFs) for each of these disturbance agents based on 1959–2000 natural disturbance statistics. These PDFs were used to generate time series of future area disturbed for each of 24 modeling regions (see SI Text for details). We used projections of future harvest levels from provincial and territorial timber supply planning processes to estimate future harvest rates. We then estimated the managed forest GHG balance distribution with use of a Monte Carlo approach by generating 100 model outputs using combined disturbance time series for fire and insects as input to the Carbon Budget Model of the Canadian Forest Sector (CBM-CFS3) (see SI Fig. 3 for details). Last, we resampled combinations of the regional results to generate 500 national estimates per time step for annual distributions (Fig. 1) and 5,000 national estimates for the 2008–2012 average annual distribution (Fig. 2).
Supplementary Material
ACKNOWLEDGMENTS.
This study was a collaborative effort involving the Canadian Forest Service of Natural Resources Canada and forest resource management agencies from all Canadian provinces and territories (except Nunavut, where there are no managed forests) and their representatives on the National Forest Sinks Committee. We thank provincial and territorial government collaborators for providing data and detailed reviews: D. Draper, G. Lawrence, M. Boyce, D. Spittlehouse, C. Fletcher and J. Parminter (British Columbia); D. Price, D. Aitkin, and E. Wrangler (Alberta); M. Johnston and B. Wynes (Saskatchewan); G. Carlson, J. Lui, W. Xu, and S. Warrington (Manitoba); M. Ter-Mikaelian, S. Colombo, and P. Gray (Ontario); M. Campagna and B. Levesque (Quebec); R. Dick and D. Mason (New Brunswick); J. Hutchinson and G. Williams (PEI); J. Beyeler, K. Snow and R. O'Keefe (Nova Scotia); B. Pittman and I. Downton (Newfoundland and Labrador); T. Lakusta, L. Smith, and S. Corey (Northwest Territories); C. Burgess, P. MacDonell, K. Price, and A. Ogden (Yukon Territory). We thank K. Power for her assistance with inventory data, and P. Boudewyn and A. Song for their assistance with biomass estimation models. Fire and insect projections were developed with input provided by J. Metsaranta, S. Magnussen, B. Stocks, B. Amiro, M. Flannigan, R. Landry, B. de Groot, K. Anderson, S. Taylor, R. Simpson, K. Porter, D. MacLean, D. Gray, J. Volney, T. Hogg, A. Carroll, L. Safranyik, T. Ebata, T. Shore, R. Fleming, and L. Royer. We thank T. Trofymow, C. Smyth, C. Shaw, E. Banfield, B. Simpson. K. Belanger, M. Magnan, and G. Zhang for CBM-CFS3 parameter calibration and coding. We also thank T. Lemprière, D. Booth, P. Graham, and J. Wood for helping to provide direction for this study and T. Lemprière, M. Flannigan, S. Glover, and two anonymous reviewers for helpful comments on an earlier draft. This work was supported by Canada's Climate Change Action Plan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708133105/DC1.
References
- 1.Nabuurs GJ, Masera O, Andrasko K, Benitez-Ponce P, Boer R, Dutschke M, Elsiddig E, Ford-Robertson J, Frumhoff P, Karjalainen T, et al. In: Climate Change 2007: Mitigation. Contribution of Working group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA, editors. Cambridge: Cambridge Univ Press; 2007. pp. 541–584. [Google Scholar]
- 2.Tans PP, Fung IY, Takahashi T. Science. 1990;247:1431–1438. doi: 10.1126/science.247.4949.1431. [DOI] [PubMed] [Google Scholar]
- 3.Denning AS, Fung IY, Randall DA. Nature. 1995;376:240–243. [Google Scholar]
- 4.Fan S, Gloor M, Mahlman J, Pacala S, Sarmiento J, Takahashi T, Tans P. Science. 1998;282:442–446. doi: 10.1126/science.282.5388.442. [DOI] [PubMed] [Google Scholar]
- 5.Peylin P, Baker D, Sarmiento J, Ciais P, Bousquet P. J Geophys Res Atmos. 2002;107:5.1–5.25. ACH. [Google Scholar]
- 6.Goodale CL, Apps MJ, Birdsey RA, Field CB, Heath LS, Houghton RA, Jenkins JC, Kohlmaier GH, Kurz W, Liu S, et al. Ecol Appl. 2002;12:891–899. [Google Scholar]
- 7.Stephens BB, Gurney KR, Tans PP, Sweeney C, Peters W, Bruhwiler L, Ciais P, Ramonet M, Bousquet P, Nakazawa T, et al. Science. 2007;316:1732–1735. doi: 10.1126/science.1137004. [DOI] [PubMed] [Google Scholar]
- 8.Fung IY, Doney SC, Lindsay K, John J. Proc Natl Acad Sci USA. 2005;102:11201–11206. doi: 10.1073/pnas.0504949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canadell JG, Le Quéré C, Raupach MR, Field CB, Buitenhuis ET, Ciais P, Conway TJ, Gillett NP, Houghton RA, Marland G. Proc Natl Acad Sci USA. 2007;104:18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United Nations Framework Convention on Climate Change (UNFCCC) Report of the Conference of the Parties on its Seventh Session. 2002 FCCC/CP/2001/13/Add. 1. Available at: http://unfccc.int/resource/docs/cop7/13a01.pdf.
- 11.Houghton JT, Meira Fihlo LG, Lim B, Treanton K, Manaty I, Bonduki Y, Griggs DJ, Callender BA, editors. Intergovernmental Panel on Climate Change (IPCC) Revised 1996 IPCC Guidelines for National Greenhouse Inventories. Bracknell, UK: IPCC/Organisation for Economic Co-operation and Development/International Energy Association; 1997. [Google Scholar]
- 12.Kurz WA, Apps MJ. Ecol Appl. 1999;9:526–547. [Google Scholar]
- 13.Amiro BD, Todd JB, Wotton BM, Logan KA, Flannigan MD, Stocks BJ, Mason JA, Martell DL, Hirsch KG. Can J For Res. 2001;31:512–525. [Google Scholar]
- 14.Caspersen JP, Pacala SW, Jenkins JC, Hurtt GC, Moorcroft PR, Birdsey RA. Science. 2000;290:1148–1151. doi: 10.1126/science.290.5494.1148. [DOI] [PubMed] [Google Scholar]
- 15.Canadell JG, Kirschbaum MUF, Kurz WA, Sanz M-J, Schlamadinger B, Yamagata Y. Environ Sci Pol. 2007;10:370–384. [Google Scholar]
- 16.Litvak M, Miller S, Wofsy SC, Goulden M. J Geophys Res Atmos. 2003;108:WFX 6.1–6.11. [Google Scholar]
- 17.Pregitzer KS, Euskirchen ES. Global Change Bio. 2004;10:2052–2077. [Google Scholar]
- 18.Luyssaert S, Inglima I, Jung M, Richardson AD, Reichstein M, Papale D, Piao SL, Schulze ED, Wingate L, Matteucci M, et al. Global Change Biol. 2007;13:1–29. [Google Scholar]
- 19.Denman KL, Brasseur G, Chidthaisong A, Ciais P, Cox PM, Dickinson RE, Hauglustaine D, Heinze C, Holland E, Jacob D, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Cambridge: Cambridge Univ Press; 2007. pp. 499–587. [Google Scholar]
- 20.Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Nature. 1997;386:698–702. [Google Scholar]
- 21.Nemani RR, Keeling CD, Hashimoto H, Jolly WM, Piper SC, Tucker CJ, Myneni RB, Running SW. Science. 2003;300:1560–1563. doi: 10.1126/science.1082750. [DOI] [PubMed] [Google Scholar]
- 22.Slayback DA, Pinzon JE, Los SO, Tucker CJ. Global Change Biol. 2003;9:1–15. [Google Scholar]
- 23.Euskirchen ES, McGuire AD, Kicklighter DW, Zhuang Q, Clein JS, Dargaville RJ, Dye DG, Kimball JS, McDonald KC, Melillo JM, et al. Global Change Biol. 2006;12:731–750. [Google Scholar]
- 24.de Vries W, Reinds GJ, Gundersen P, Sterba H. Global Change Biol. 2006;12:1151–1173. [Google Scholar]
- 25.Magnani F, Mencuccini M, Borghetti M, Berbigier P, Berninger F, Delzon S, Grelle A, Hari P, Jarvis PG, Kolari P, et al. Nature. 2007;447:848–850. doi: 10.1038/nature05847. [DOI] [PubMed] [Google Scholar]
- 26.Canadell JG, Pataki DE, Gifford R, Houghton RA, Luo Y, Raupach MR, Smith P, Steffen W. In: Terrestrial Ecosystems in a Changing World, The IGBP Series. Canadell JG, Pataki DE, Pitelka LF, editors. Heidelberg: Springer; 2007. pp. 59–78. [Google Scholar]
- 27.Barber VA, Juday GP, Finney BP. Nature. 2000;405:668–673. doi: 10.1038/35015049. [DOI] [PubMed] [Google Scholar]
- 28.Food and Agriculture Organization (FAO) Global Forest Resources Assessment 2005: Progress Towards Sustainable Forest Management. Rome: FAO; 2006. FAO Forestry Paper 147. Available at: http://www.fao.org/forestry/site/1191/en/ [Google Scholar]
- 29.Wulder MA, Campbell C, White JC, Flannigan M, Campbell ID. Forestry Chron. 2007;83:539–556. [Google Scholar]
- 30.Power K, Gillis M. Canada's Forest Inventory 2001. Victoria: Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre; 2006. BC-X-408. [Google Scholar]
- 31.Kurz WA, Apps MJ. Mitig Adapt Strat Global Change. 2006;11:33–43. [Google Scholar]
- 32.Kurz WA, Apps MJ, Webb TM, McNamee PJ. Carbon Budget of the Canadian Forest Sector Phase I. Edmonton: Forestry Canada, Northwest Region, Northern Forestry Centre; 1992. [Google Scholar]
- 33.Kull S, Kurz WA, Rampley GJ, Banfield GE, Schivatcheva RK, Apps MJ. Operational-Scale Carbon Budget Model of the Canadian Forest Sector (CBM-CFS3) Version 1.0: User's Guide. Edmonton: Natural Resources Canada; 2006. [Google Scholar]
- 34.Bond-Lamberty B, Peckham SD, Ahl DE, Gower ST. Nature. 2007;450:89–92. doi: 10.1038/nature06272. [DOI] [PubMed] [Google Scholar]
- 35.Westfall J. 2006 Summary of Forest Health Conditions in British Columbia. Victoria: British Columbia Ministry of Forests and Range; 2007. Pest Management Report No. 15. [Google Scholar]
- 36.Environment Canada. National Inventory Report: Greenhouse Gas Sources and Sinks in Canada 1990–2005. Gatineau, Quebec: Governement of Canada; 2007. [Google Scholar]
- 37.Stocks BJ, Mason JA, Todd JB, Bosch EM, Wotton BM, Amiro BD, Flannigan MD, Hirsch KG, Logan KA, Martel DL, et al. J Geophys Res Atmos. 2002;108:FFR5.1–FFR5.12. [Google Scholar]
- 38.Johnson EA. Fire and Vegetation Dynamics: Studies from the North American Boreal Forest. New York: Cambridge Univ Press; 1992. [Google Scholar]
- 39.Smith LA. Proc Natl Acad Sci USA. 2002;99:2487–2492. doi: 10.1073/pnas.012580599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillett NP, Weaver AJ, Zwiers FW, Flannigan MD. Geophys Res Lett. 2004;31:18211. [Google Scholar]
- 41.Flannigan MD, Logan KA, Amiro BD, Skinner WR, Stocks BJ. Climatic Change. 2005;72:1–16. [Google Scholar]
- 42.Anderson RM, May RM. Science. 1980;210:658–661. doi: 10.1126/science.210.4470.658. [DOI] [PubMed] [Google Scholar]
- 43.Royama T. Ecol Monogr. 1984;54:429–462. [Google Scholar]
- 44.Royama T, MacKinnon WE, Kettela EG, Carter NE, Hartling LK. Ecology. 2005;86:1212–1224. [Google Scholar]
- 45.Taylor SW, Carroll AL, Alfaro RI, Safranyik L. In: The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts in Lodgepole Pine. Safranyik L, Wilson WR, editors. Victoria: Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre; 2006. pp. 67–94. [Google Scholar]
- 46.Blais JR. Can J For Res. 1983;13:539–547. [Google Scholar]
- 47.Carroll AL, Taylor SW, Régniere J, Safranyik L. Mountain Pine Beetle Symposium: Challenges and Solutions. Victoria, BC: Natural Resources Canada; 2004. pp. 223–232. [Google Scholar]
- 48.MacLean DA, Erdle TA, MacKinnon WE, Porter KB, Beaton KP, Cormier G, Morehouse S, Budd M. Can J For Res. 2001;31:1742–1757. [Google Scholar]
- 49.Apps MJ, Kurz WA, Beukema SJ, Bhatti JS. Environ Sci Pol. 1999;2:25–41. [Google Scholar]
- 50.Hirsch KG, Fuglem P. Canadian Wildland Fire Strategy: Background Syntheses, Analyses, and Perspectives. Edmonton, Alberta, Ottawa, Ontario: Natural Resources Canada, Canadian Forest Service, Northern Forestry Centre; 2006. [Google Scholar]
- 51.Carroll AL, Shore TL, Safranyik L. In: The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine. Safranyik L, Wilson WR, editors. Victoria: Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre; 2006. pp. 155–172. [Google Scholar]
- 52.Pimentel D, Acquay H, Biltonen M, Rice P, Silva M, Nelson J, Lipner V, Giordano S, Horowitz A, D'Amore M. BioScience. 1992;42:750–760. [Google Scholar]
- 53.Balshi MS, McGuire AD, Zhuang Q, Melillo J, Kicklighter DW, Kasischke E, Wirth C, Flannigan M, Harden J, Clein JS, et al. J Geophys Res. 2007 doi: 10.1029/2006JG000380. [DOI] [Google Scholar]
- 54.Thornley JHM, Cannell MGR. Tree Phys. 2004;24:765–773. doi: 10.1093/treephys/24.7.765. [DOI] [PubMed] [Google Scholar]
- 55.Granier A, Reichstein M, Breda N, Janssens IA, Falge E, Ciais P, Grunwald T, Aubinet M, Berbigier P, Bernhofer C, et al. Agric For Meteor. 2007;143:123–145. [Google Scholar]
- 56.Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG, Romme WH, Kastens JH, Floyd ML, Belnap J, et al. Proc Natl Acad Sci USA. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trenberth KE, Jones PD, Ambenje P, Bojariu R, Easterling D, Klein Tank A, Parker D, Rahimzadeh F, Remwick JA, Rusticucci M, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Cambridge: Cambridge Univ Press; 2007. pp. 235–336. [Google Scholar]
- 58.Schlamadinger B, Johns T, Ciccarese L, Braun M, Sato A, Senyaz A, Stephens P, Takahashi M, Zhang X. Environ Sci Pol. 2007;10:295–305. [Google Scholar]
- 59.Government of Canada. Canada's Initial Report Under the Kyoto Protocol. 2007 Available at: http://unfccc.int/files/national_reports/initial_reports_under_the_kyoto_protocol/application/pdf/initial_report_of_canada.pdf.
- 60.Eriksson E, Gillespie AR, Gustavsson L, Langvall O, Olsson M, Sathre R, Stendahl J. Can J For Res. 2007;37:671–681. [Google Scholar]
- 61.Lee J, Alexander ME, Hawkes BC, Lynham TJ, Stocks BJ, Englefield P. Comput Electron Agric. 2002;37:185–198. [Google Scholar]
- 62.Canadian Council of Forest Ministers. Compendium of Canadian Forestry Statistics. 2005 Available at: http://nfdp.ccfm.org.
- 63.Johnson EA. Fire and Vegetation Dynamics: Studies from the North American Boreal Forest. New York: Cambridge Univ Press; 1992. [Google Scholar]
- 64.Otvos IS, Clarke LJ, Durling DS. A History of Recorded Eastern Hemlock Looper Outbreaks in Newfoundland. St. John's: Environment Canada, Newfoundland Forest Research Centre; 1979. [Google Scholar]
- 65.Volney WJA, Fleming RA. Agric Ecosys Environ. 2000;82:283–294. [Google Scholar]
- 66.Volney WJA, Hirsch KG. Forestry Chron. 2005;81:662–668. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.