Abstract
Understanding the mechanisms and processes that generate biological diversity is a fundamental problem in evolution and ecology. In the past decade, the theory of evolutionary branching and adaptive diversification has provided new perspectives for understanding the evolution of diversity caused by ecological interactions. In models of adaptive diversification, the fitness landscapes change dynamically, so that the likelihood of diversification into different phenotypic clusters increases over time. In contrast, in models with static fitness landscapes, the likelihood of diversification decreases as populations climb fitness peaks, because crossing maladaptive fitness valleys becomes increasingly difficult. We used experimental evolution in bacteria to test how the likelihood of diversification changes over time in a bacterial lineage that has diversified in sympatry from a single ancestral strain. By analyzing the “fossil” record of this lineage, and restarting the lineage from different time points in the evolutionary past, we demonstrate that: (i) the lineage has initially undergone a phase of directional adaptation to the competitive environment, and (ii) during this phase, the likelihood of diversification increases significantly over time. These results suggest evolutionary branching caused by frequency-dependent competition as the main mechanism of diversification in our experimental populations.
Keywords: Escherichia coli, evolutionary branching, experimental evolution, frequency dependence
Diversification is thought to occur when descendent populations come to occupy different ecological niches. This situation is formalized by adaptive landscapes, in which different peaks correspond to different niches (1–5). When fitness landscapes are static and fixed over time, diversification can occur when an ancestral population is near a trough in such a landscape, and different subpopulations evolve toward different fitness peaks. In this case, it becomes increasingly difficult to cross the fitness valleys if the ancestral population evolves up a fitness peak. As a consequence, diversification becomes less likely with increasing adaptation (1, 6–8).
In contrast, if the fitness landscape depends on the current position of the population in phenotype space, i.e., if selection is frequency-dependent, the landscape changes as the population evolves. In this case, new niches (i.e., fitness peaks) may only open up as the population progresses along its evolutionary trajectory, and hence diversification may become more likely over time. An elegant framework for describing diversification when selection is frequency-dependent is provided by the theory of adaptive dynamics (9–11), in which adaptive diversification is described by the mathematical phenomenon of evolutionary branching (10). When a population undergoes evolutionary branching, it first converges under directional selection toward a point in phenotype space at which selection turns disruptive because of frequency-dependent interactions. From this evolutionary branching point the population subsequently diverges into two phenotypic clusters. Evolutionary convergence to fitness minima and subsequent evolutionary branching has been found in mathematical models describing many different ecological scenarios (12–14).
We investigated whether the temporal patterns of the propensity to diversify predicted by adaptive diversification and evolutionary branching can be observed in experimental bacterial lineages that have undergone sympatric diversification. In our lineages, single genotypes were used to found populations of Escherichia coli that were propagated in serial batch culture for many generations. In a single batch (≈6.7 generations), populations are inoculated into a mixture of glucose and acetate, a preferred and a nonpreferred carbon source, respectively. In this environment, the bacteria exhibit diauxic growth because genetic regulation represses the consumption of nonpreferred resources, thereby forcing the bacteria to consume glucose before acetate. Once the glucose is exhausted, subsequent expression of previously repressed genes allows E. coli to consume the nonpreferred carbon source acetate (15). Once both resources have been exhausted, the bacteria enter stationary phase, after which they are transferred into new medium to initiate a new batch.
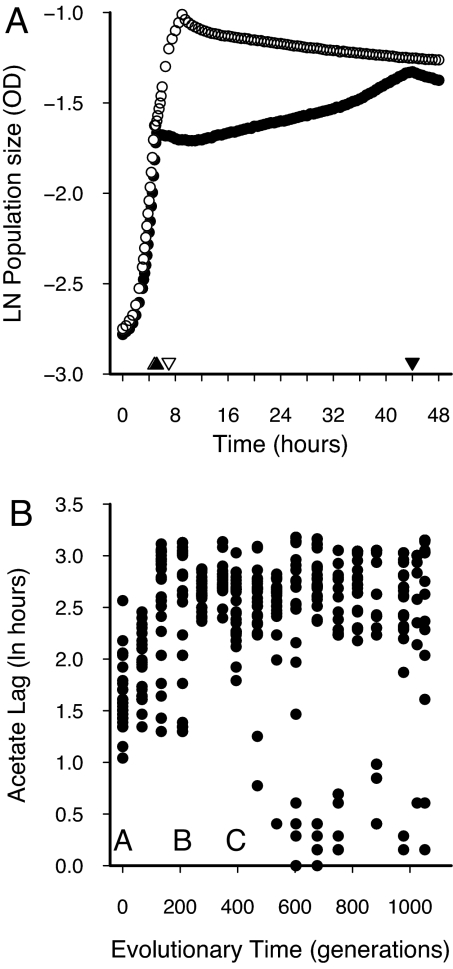
Corroborating previous experiments (18), replicate E. coli populations diversified into two coexisting ecotypes after 1,000 generations of evolution in serial batch culture in the glucose-acetate environment. These ecotypes were named “slow switchers” (SS) and “fast switchers” (FS) according to how fast they were able to make the transition from glucose to acetate consumption during the diauxic shift (Fig. 1A). The ecotypes also differ in their growth rates and competitive ability on the two resources, with SS the better competitor on glucose and FS the better competitor on acetate (unpublished work). Previous work in this system provides clear evidence that the sympatric diversification into the SS and FS ecotypes is caused by frequency-dependent interactions caused by a tradeoff in the ability to use the two resources (17, 18).
Fig. 1.
Population growth profiles for FS and SS ecotypes on glucose and acetate and evolutionary change in acetate lag in an E. coli population. (A) FS (open symbols) and SS (filled symbols) ecotypes sampled after 1,000 generations of evolution consume glucose during the first stage of growth, then switch to acetate for the remainder of each 24-h batch culture. Acetate lag is the difference in hours between the switching point, or diauxic shift, between the two resources (triangles) and the maximum growth rate on acetate (inverted triangles). (B) The time from the switching point to the maximum growth rate on acetate (see A), or acetate lag, increases from the ancestral state (generation 0) to the branching point (generation 395), where the population splits into two characteristic ecotypes, SSs with a long acetate lag and FSrs with a short acetate lag. Data points represent randomly selected isolated genotypes from the frozen evolutionary record of the population. Letters on the x axis represent the ancestral (A), midpoint (B), and MRCA (C) subpopulations used in the rediversification experiment.
Microbial evolution experiments offer the opportunity to analyze the evolutionary record of a lineage and restart the evolutionary dynamics from strains frozen during the evolutionary history. This process allowed us to investigate changes in the propensity to diversify by sampling individual genotypes from three different time points during the prediversification history of a single evolutionary lineage. We then evolved these genotypes anew to determine how frequently resource-use polymorphisms arose from each genotype.
Results
We first established when the original lineage diversified into FS and SS by sampling isolated genotypes from the frozen stocks at approximately every 70 generations (about every 10–11 days in the frozen “fossil” record; see Methods). We grew each genotype in the glucose/acetate mixture in which the strains were evolved and measured the acetate lag, or the time lapsed between the end of glucose use and the maximum growth rate on acetate (Fig. 1A). We used the presence of a low mode in the empirical acetate lag distributions to assess when diversification had occurred in our experimental lineage. In our frozen samples, the acetate lag distribution lacked a low mode from generations 0 to 395. During that time, acetate lag increased in the population at an average rate of 0.019 per generation (linear regression on untransformed prebranching data: ln acetate lag = 7.771 + 0.019 generation, F1,197 = 56.89, P < 0.0001; Fig. 1B). The next sample taken after generation 395 was taken at generation 469, which was the first frozen population to contain not only the SS ecotype, but also a second ecotype that had a very short acetate lag, i.e., an FS type (Fig. 1A). Pooled acetate lags from generations 469 to the endpoint fall into two distinct clusters (randomization test, P < 0.0001). Because we did not detect the FS type at the time points preceding or including generation 395, we assumed that the frozen population at generation 395 represented one of the last undiversified populations in the frozen record, and that it contained the most recent common ancestor (MRCA) of the ultimate FS and SS ecotypes present after 1,000 generations. We defined generation 395 as the MRCA population and selected generation 208 as the midpoint population between the MRCA and the ancestral population (A, B, and C in Fig. 1B). We sampled 30 isolated SS genotypes at random from each of these three time points and established new, isolated lineages, one corresponding to each isolated genotype [supporting information (SI) Fig. 4]. Although it is possible that FS types existed before the putative branching point at a frequency lower than our detection threshold, we only selected SS types to evolve for the rediversification experiment. Because differential acetate use underscores the critical difference between the two derived ecotypes, we skewed the resource conditions heavily toward acetate to detect different ecotypes so that any growth advantage of acetate should be detected as a deviation from ancestral or SS growth profiles (Fig. 1A and SI Fig. 4). This assay ensured that the genotypes that we used as founders for the rediversification experiments were indeed true SS types. The SS-founded lineages were evolved for ≈140 generations (21 days) under conditions similar to the original evolution experiment to generate replicate MRCA-derived, midpoint-derived, and ancestor-derived populations. The derived populations were then assessed for the emergence of diversity in acetate usage patterns.
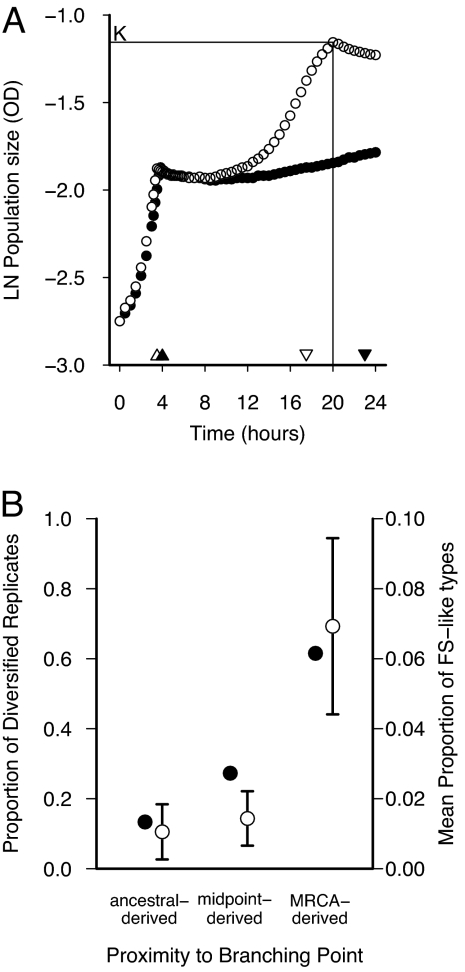
All derived populations contained SS types. However, some derived populations also contained genotypes exhibiting diauxic growth patterns resembling the FS type and showing a higher growth rate in the acetate phase (Fig. 2A). To emphasize differential acetate use, we assessed derived populations for diversity by using the acetate-skewed resource ratio. We also shortened our assay to 24 h to decrease sample processing time. With the shorter growth profile assay, acetate lag measurements are truncated at 24 h, which would lead to underestimates of the acetate lag for the SS types. However, as Fig. 2A illustrates, under the new assay conditions an increase to a maximum population size (carrying capacity) provides a reliable method to detect FS-like types. In all derived populations in which FS-like types occurred, these types could easily be distinguished from the SS types because of their high final population density after growth (in isolation) in a single batch culture. FS-like types typically reached a much higher density than the SS types (Fig. 2A). Based on this distinction, we defined a derived population to be diversified if it contained both SS- and FS-like types. We found that 62% of the MRCA-derived replicates, 26% of the midpoint-derived replicates, and 13% of the ancestral-derived replicates had diversified (Fig. 2B, ●). Populations that were derived from single genotypes of the MRCA were significantly more likely to show diversification than ancestral-derived populations [Kruskal–Wallis χ2 = 6.956, P = 0.031; W*(AC) = 5.21, P = 0.001], and marginally significantly more likely to show diversification than midpoint-derived populations [W*(BC) = 3.29, P = 0.049]. We also calculated the frequency of FS-like types within each replicate population. In the replicates derived from the midpoint and ancestor, the mean frequency of FS types was significantly lower than in MRCA-derived replicates [○ in Fig. 2B; Kruskal–Wallis χ2 = 8.613, P = 0.014, W*(AC) = 5.29, P = 0.001, W*(BC) = 4.08, P = 0.011]. These results demonstrate that genotypes that are closer to the branching point have an increased propensity to diversify.
Fig. 2.
Growth profiles reveal that replicate populations far from the branching point are less likely to rediversify. (A) Curves represent growth of ecotypes from an MRCA-derived population. Growth occurred for 24 h in an acetate-enriched resource ratio relative to the evolutionary environment to magnify differences in growth on acetate (see Methods). FS-like types (○) consume all of the acetate to reach carrying capacity (K) within 24 h, whereas SS-like types (●) do not reach carrying capacity. On average, for all types screened from the rediversified replicate populations, FS-like types had a growth rate on acetate (slope at inverted triangle) of 0.140 nm/h that significantly exceeded the rate of 0.035 nm/h for SS-like types (t66 = 12.02; P < 0.0001). (B) Replicate populations reevolved from the ancestral population are less likely to contain FS ecotypes than populations reevolved from the MRCA. Diversity is shown as the proportion of diversified replicates (●, left axis) and the mean ± 1 SE of the proportion of FS-like types within replicate (○, right axis). Ancestor-, midpoint-, and MRCA-derived treatments contained 15, 11, and 13 replicates, respectively.
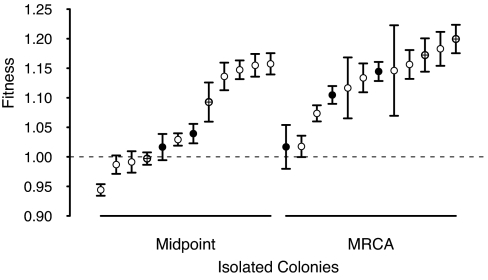
Further to the rediversification experiment, we assessed fitness, relative to the ancestor, for a subset of the founder genotypes for the midpoint and MRCA populations (Fig. 3). The most fit genotypes from each population do not significantly differ (randomization test: P = 0.093), although the distribution of mean fitness is shifted slightly higher in the MRCA (randomization test: P = 0.020), so that types with higher fitness are more common in MRCA populations than in midpoint populations. We have both relative fitness estimates and probability of rediversification data for only nine genotypes, four from the midpoint and five from the MRCA. Of these, the two most fit genotypes founded populations that did not rediversify, whereas two types with fitness similar to the ancestor did rediversify.
Fig. 3.
Fitness varies similarly in midpoint and MRCA populations. Mean fitness (±1 SEM) is shown relative to the ancestor for 12 genotypes isolated from the midpoint and the MCRA populations, shown in rank order for each population. ● represent genotypes that founded strains that rediversified; ⊕ represent genotypes that founded strains that did not rediversify, and ○ represent founding genotypes for which rediversification was not assayed. Each point represents three replicate competition assays.
Discussion
We used a bacterial lineage that is known to have diversified because of frequency-dependent competition for limiting carbon resources to investigate the temporal dynamics of the propensity for diversification during the lineage's evolutionary history. The mathematical theory of adaptive diversification through evolutionary branching (11, 19–21) predicts that during the early stages of the lineage's history, the lineage should undergo directional adaptation, and the propensity to diversity should increase over time. Our results reproduce these patterns (11, 19–21): before diversification occurs, the population shows a uniform increase in the mean acetate lag, indicating a prolonged phase of directional adaptation; as this directional phase progresses, the evolving population becomes more likely to diversify. In as much as these results confirm the temporal patterns of the propensity to diversify predicted by theory, they support the notion that this bacterial lineage has undergone evolutionary branching.
Naively, one might expect that because the evolution environment consists of a mixture of glucose and acetate, evolutionary branching would consist of first evolving into a glucose/acetate generalist and then diversifying into two specialists consuming only glucose or acetate, respectively. In contrast, in our bacterial lineages populations first become more specialized on glucose, before they diversify into a glucose specialist and a glucose/acetate generalist. Given the asymmetry in the value of the resource (E. coli generally much prefer glucose over acetate), this finding is entirely consistent with models of evolutionary branching. Both general models of asymmetric competition (13, 22) and specific models for bacterial evolution in glucose/acetate mixtures (17) predict such asymmetric patterns of evolutionary branching. What is common to all models of evolutionary branching is that the different niches allowing diversification are not present at the outset, but instead are generated through evolutionary change in the ancestral population. Thus, in our bacterial lineage the ecological conditions prevalent in ancestral and midpoint populations could be unfavorable for FS-like types, even if such types were possible genetically. A new niche for FS ecotypes that can efficiently consume both glucose and acetate (albeit glucose less efficiently than the glucose specialist) would only open up once the populations had moved far enough along the directional adaptive path toward glucose specialization. This scenario is the essence of frequency dependence: as the populations adapt and exhibit different growth dynamics in batch culture, the ecological environment encountered by newly arising mutants changes, until eventually the environment generated by the resident population favors the coexistence of FS-like types with SS types.
Incidentally, this scenario seems to be different from the perhaps best-known example of diversification in bacterial microcosms, in which Pseudomonas fluorescens populations diversify in spatially heterogeneous environments (8). In that case, the different niches are essentially present from the very beginning, because they are given by different spatial areas in the heterogeneous environment. In particular, existence of these niches does not require prior evolutionary change in the ancestral population, in which diversification can occur already through single mutations enabling colonization of a different spatial niche (23). This prerequisite may explain why diversification is so much faster in the P. fluorescens system, in which it typically occurs after only a few generations (8), compared with the system considered here, in which diversification is established only after hundreds of generations of evolution in the ancestral population.
As an alternative explanation for the patterns we observed, one could envisage that purely genetic constraints would imply that a mutation causing FS-like growth patterns can only arise after a number of other mutations have occurred. Thus, emergence of FS-like types would first require evolution of the appropriate genetic background. If, by coincidence, evolution in glucose/acetate batch cultures generated such genetic backgrounds, then the emergence of FS-like types would be more likely in the MRCA populations than in the midpoint and the ancestral populations because the MRCA populations have a longer evolutionary history in this environmental regime. However, it seems unlikely that such genetic constraints are solely responsible for an increase in the rate of diversification over time. First, during the directional phase of evolution to the MRCA population the bacteria evolve a longer acetate lag (Fig. 1B) and hence away from the FS type, at least phenotypically. Second, prototype FSs can already arise through only a few genetic changes, because in our rediversification experiments we observed them, although rarely, evolving from the ancestral genotype within 140 generations. Indeed, previous work in a similar system demonstrated that large physiological changes in acetate metabolism can be caused by single mutations in regulatory genes (24).
Clonal interference (25, 26) provides another alternative explanation for the observed increase in rates of diversification. Even if FS-like types were able to invade the resident at any point in time, such invasion attempts could be thwarted during the early evolutionary stages by mutations that are better adapted than both the current resident and the rare FS-like invaders. Thus, general adaptations to the glucose/acetate environment could sweep through populations that are in the process of diversifying and replace both the current resident and the FS-like invader. Because such sweeps are more likely to occur in the common SS-like resident, FS-like types would be continually lost (even if they were viable when they arose) until the population reached a fitness plateau where adaptive sweeps are rare. If such clonal interference was responsible for low rates of diversification early on, one would expect that, as a result of successive sweeps, the genotype with maximum fitness in the MRCA population would have significantly greater fitness than the most fit genotype in the midpoint population, while the variance in fitness would be low for both populations. This is not the case when fitness is measured as competitive ability against the ancestral strain (Fig. 3). Instead, the most fit genotypes from each population are quite similar, and the ranges of fitness are wide in both populations, suggesting that selective sweeps are already rare at intermediate time points during the prediversification history. However, the MRCA population does have a higher mean fitness than the midpoint population because frequencies of different fitness types shift, so that types with higher fitness are more common in MRCA populations than in midpoint populations. The increase in fitness against the ancestor and the shift in frequencies of different fitness types constitute a change in the competitive environment for newly arising mutants that may ultimately have enabled invasion of FS types.
Finally, it is worth pointing out that one could envision a direct test of how fitness landscapes change over time by performing invasion experiments of endpoint FS types into populations from different time points along the evolutionary trajectory. One would then predict that these FS types could not invade early populations, but could invade later populations because of changes in the fitness landscape. Unfortunately, this procedure is not feasible because late-stage FS types have not only acquired the FS characteristics that distinguish them from the SS types, but also various other adaptations to the glucose/acetate environment that they have in common with SS types. Thus, later occurring types generally outcompete earlier types, independent of whether they are SS or FS, a fact that would introduce an uncontrolled bias into experiments for invasion of FS types into early populations. Such invasion experiments would only be informative if one could isolate the genetic changes that are responsible for diversification between SS and FS from the changes reflecting local adaptation to the competitive environment. In fact, a recent global gene expression study (27) revealed that most of the genetic changes found between evolved populations and ancestral populations are common to both SS and FS types and likely reflect adaptation to the competitive environment before diversification. Furthermore, relatively few changes in the derived populations are responsible for the distinction between SS and FS types. These findings again support the notion that the ancestral population first underwent a period of directional adaptation to the environment before undergoing adaptive diversification.
In sum, in stark contrast to earlier empirical work (7) and classical theory based on static fitness landscape concepts (1, 6), we show that adaptation to novel environmental conditions can significantly enhance the ability of bacterial genotypes to diversify. We found that as a bacterial population becomes more adapted to its competitive environment, it becomes more likely to generate diverse ecotypes. This study provides empirical support for models of evolutionary branching, in which an initial directional phase of evolution is followed by diversification caused by frequency-dependent ecological interactions.
Methods
Strain Evolution.
The focal population dst1018 was one of 10 replicate populations of E. coli B that were initiated from an isolated genotype from one of two isogenic lines, REL606 and REL607 [provided by R. Lenski, Michigan State University, East Lansing (28)]; these two ancestral strains perform equivalently in the resource environments described below and can be used to confirm that the serial transfer procedure created independently evolved lineages with no cross-contamination between populations. Even-numbered strains were initiated with REL606, odd with REL607. We evolved the strains for 1,000 generations in batch culture. Batch culture evolution via 1:101 dilution every 24 ± 2 h was conducted in 18-mm-diameter test tubes supplied with 10 ml of sterile Davis Minimal medium (DM) supplemented with 0.25 mg/ml glucose and 1.3225 mg/ml sodium acetate trihydrate as the sole carbon sources (GA medium). We selected the acetate concentration to yield a similar carrying capacity to glucose when consumed by REL606. Cultures were incubated at 37°C and vigorously shaken (0.88 × g) for 24 h. Cultures were frozen at −80°C every 3–4 days in 20% glycerol and screened for cross-contamination between lines every 7 days by plating on tetrazolium arabinose agar plates (28). One day of batch culture was equivalent to 6.7 bacterial doublings or generations.
Branching Point.
We sampled the evolutionary fossil record at intervals of ≈67 generations, grew bacterial genotypes (isolated colonies) on tryptone agar plates, and randomly selected 30 isolated genotypes from each time interval. We cultured each genotype in 5 ml of GA medium for 24 h and diluted this at 1:101 into 150 μl of GA medium in a Bioscreen C culture plate. Bacteria were grown in a Bioscreen C (ThermoLabsystems) at 37°C with continuous shaking, and the OD was measured at 600 nm and recorded every 10 min for 48 h. From this series of ODs, we used C++ code to calculate the acetate lag or the time lapsed between the end of glucose use and the maximum growth rate on acetate (Fig. 1B). We plotted empirical density distributions in R (29) and categorized a population as diversified if its R-generated density distribution contained an acetate lag mode <1.0. For generations 0–395, no such low mode in acetate lag existed, and the mean acetate lag showed a steady increase with evolutionary time. Beginning at generation 469, a low acetate lag mode emerged in each population (with the exception of generation 817) with FSs present in frequencies >3.3%. All data from seven populations sampled between generations 0 and 395 were included in a linear regression to determine whether acetate lag increased significantly over evolutionary time before branching.
For the time points that represented >1,000 generations of evolution from the ancestor, we also extracted the maximum growth rate on acetate for each genotype by using a sliding window of five 10-min time units, starting at the point at which glucose was exhausted, to calculate the slope for the growth curve. Individuals identified as FSs from their short acetate lags had a mean acetate growth rate of 0.245 nm/h, whereas the SS individuals grew at an average rate of 0.036 nm/h (t56 = 28.94, P < 0.0001).
Rediversification.
We subsampled the original population at generations 0, 208, and 395 onto tryptone agar plates to isolate 30 randomly selected and independent genotypes from each time point. Each of these 90 genotypes was subjected to 21 days (≈140 generations) of daily dilutions (1:101) in 1 ml of GA medium and shaking at 0.88 × g at 37°C. From replicate populations in each treatment, we randomly subsampled 19 genotypes from populations after the 21 days of evolution. These genotypes were cultured to examine their growth profiles in the Bioscreen by using assay conditions that favored the acetate growth phase. We supplemented DM with 0.175 mg/ml d-glucose and 1.719 mg/ml sodium acetate trihydrate, which resulted in a shift to 70% of the glucose and 130% of the acetate available in the evolutionary environment and allowed for easier identification of FS-like growth patterns. We also truncated the assay from 48 to 24 h to decrease sample processing time. Growth profiles were variable in most populations, but among the sampled genotypes we could conservatively define an FS-like genotype, characterized by a growth curve that reached carrying capacity during the second phase of diauxic growth (Fig. 2A, ○). Proportion of diversified replicates was calculated as the proportion of populations that contained FS-like genotypes. Mean proportion of FS-like types within each replicate population was the average across replicates of the proportion of FS-like genotypes among the 19 sampled genotypes from each population. Using the C++ code described above, we extracted the maximum growth rate on acetate for each genotype. FS-like individuals had a mean acetate growth rate of 0.140 nm/h, whereas the remaining individuals grew at an average rate of 0.035 nm/h (t66 = 12.02; P < 0.0001).
Fitness Characterization of Isolated Genotypes.
For 11 and 13 genotypes from the midpoint and MRCA populations, respectively, we assessed relative fitness against the ancestor using competition experiments (28). We inoculated mixtures of each evolved genotype in a 1:1 ratio (by volume) with ancestor REL607, which bears the opposite arabinose marker from our population. We grew the cultures for 48 h in test tubes containing 10 ml of GA at 37°C and 0.88 × g, with a serial transfer after 24 h. We plated the initial mixture at inoculation (T0) and the mixture after 2 days of competition (T2) to determine changes in densities of the focal genotype relative to the ancestor. Each genotype was competed in three replicate tubes. We then calculated relative fitness after Lenski et al. (28). The resulting fitness scores indicate the amount of variation in fitness present in the population at the midpoint and the MRCA.
Characterization of FS-Like Types.
New FS-like genotypes were characterized by a growth curve that reached carrying capacity during the second phase of diauxic growth. The FS-like types identified in the rediversification experiment bore some of the trademark resource use aspects of the FSs found at the endpoint of the original experiment and in previous evolution experiments (17). In particular, the qualitative increase in maximum acetate growth rate of the FS-like relative to the SS-like types that evolved in the rediversification experiment was reminiscent of the difference in average acetate growth rates in the FS (0.245 nm/h) and SS (0.036 nm/h) from the time points sampled after generation 1,000 (Fig. 2A). However, the FS-like types that arose from the ancestor, midpoint, and MRCA in the rediversification experiments did not exhibit the large decrease in acetate lag of the endpoint FS strains (Fig. 2A). Even though we stopped our rediversification experiments after 140 generations, it is likely that the endpoint FS strains present after 1,000 generations in the original evolution experiments did arise from evolutionary precursors similar to the FS-like types described here.
Statistical Analysis.
We used the density function in R (29) to assess branching patterns by using empirical distribution functions for up to 30 isolated genotypes from each sampled generation. A low acetate lag mode emerged at generation 469, which we assigned to the occurrence of the FS. For assay generations before this split, we determined whether selection was directional by fitting a linear model to ln acetate lag data from isolated genotypes (Fig. 1B) for generations 0–395 and conducted an F test in JMPIN4 (30). To estimate the nonzero rate of directional selection, we repeated the linear regression analysis on untransformed acetate lag. For assay generations 469–1,052 (the final generation assayed), we assigned any genotype with ln acetate lag <1.4 a FS moniker. We randomized the data in R with respect to assigned type for 10,000 iterations to show that the FS group switched significantly faster than the SS group. We performed a Kruskal–Wallis test in JMPIN4 (30) to detect differences in the diversity between each group by using angularly transformed proportional data. We compared the differences between each pairwise combination of the time points (ancestor, midpoint, and MRCA) by using a nonparametric Steel-Dwass-Critchlow-Fligner W* test statistic (16, 31) with a probability of detecting treatment differences of 0.05/3 = 0.0166. The distributions of fitnesses from the midpoint and the MRCA were compared by using 10,000 iterations of data randomization in R.
Supplementary Material
ACKNOWLEDGMENTS.
We thank R. McBride and M. Yang for data collection; J. Briscoe, N. Havard, R. Suprun, and G. Khaira for assistance in the laboratory; and M. Ackerman, A. Albert, A. Agrawal, T. Day, L. Harmon, L. Rowe, and three anonymous reviewers for comments. Funding was provided by the Natural Sciences and Engineering Research Council (Canada) and the James S. McDonnell Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708504105/DC1.
References
- 1.Wright S. The roles of mutation, inbreeding, crossbreeding, and selection in evolution.. In: Jones DF, editor. Proceedings of the Sixth International Congress of Genetics; Brooklyn, NY: Brooklyn Botanic Garden; 1932. pp. 356–366. [Google Scholar]
- 2.Dobzhansky T. New York: Columbia Univ Press; 1937. Genetics and the Origin of Species. [Google Scholar]
- 3.Levene H. Genetic equilibrium when more than one ecological niche is available. Am Nat. 1953;87:331–333. [Google Scholar]
- 4.Simpson GG. New York: Columbia Univ Press; 1953. The Major Features of Evolution. [Google Scholar]
- 5.Gavrilets S. Princeton: Princeton Univ Press; 2004. Fitness Landscapes and the Origin of Species. [Google Scholar]
- 6.Wright S. Surfaces of selective value revisited. Am Nat. 1988;131:115–123. [Google Scholar]
- 7.Buckling A, Wills MA, Colegrave N. Adaptation limits diversification of experimental bacterial populations. Science. 2003;302:2107–2109. doi: 10.1126/science.1088848. [DOI] [PubMed] [Google Scholar]
- 8.Rainey PB, Travisano M. Adaptive radiation in a heterogeneous environment. Nature. 1998;394:69–72. doi: 10.1038/27900. [DOI] [PubMed] [Google Scholar]
- 9.Dieckmann U, Law R. The dynamical theory of coevolution: A derivation from stochastic ecological processes. J Math Biol. 1996;34:579–612. doi: 10.1007/BF02409751. [DOI] [PubMed] [Google Scholar]
- 10.Geritz SAH, Kisdi E, Meszena G, Metz JAJ. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol Res. 1998;12:35–57. [Google Scholar]
- 11.Metz JAJ, Geritz SAH, Meszena G, Jacobs FJA, van Heerwaarden JS. Adaptive dynamics: A geometrical study of the consequences of nearly faithful reproduction. In: van Strien SJ, Verduyn Lunel SM, editors. Stochastic and Spatial Structure of Dynamical Systems. Dordrecht, The Netherlands: KNAW Verhandelingen; 1996. pp. 183–231. [Google Scholar]
- 12.Christiansen FB. On conditions for evolutionary stability for a continuously varying character. Am Nat. 1991;138:37–50. [Google Scholar]
- 13.Doebeli M, Dieckmann U. Evolutionary branching and sympatric speciation caused by different types of ecological interactions. Am Nat. 2000;156:S77–S101. doi: 10.1086/303417. [DOI] [PubMed] [Google Scholar]
- 14.Kisdi E, Gyllenberg M. Adaptive dynamics and the paradigm of diversity. J Evol Biol. 2005;18:1170–1173. doi: 10.1111/j.1420-9101.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- 15.O'Beirne D, Hamer G. The utilisation of glucose/acetate mixtures by Escherichia coli W3110 under aerobic growth conditions. Bioprocess Eng. 2000;23:375–380. [Google Scholar]
- 16.Hollander M, Wolfe DA. New York: Wiley; 1999. Nonparametric Statistical Methods. [Google Scholar]
- 17.Friesen ML, Saxer G, Travisano M, Doebeli M. Experimental evidence for sympatric ecological diversification due to frequency-dependent competition in Escherichia coli. Evolution. 2004;58:245–260. [PubMed] [Google Scholar]
- 18.Tyerman J, Havard N, Saxer G, Travisano M, Doebeli M. Unparallel diversification in bacterial microcosms. Proc R Soc Ser B. 2005;272:1393–1398. doi: 10.1098/rspb.2005.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geritz SAH, Kisdi E, Meszena G, Metz JAJ. Evolutionarily singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol. 1998;12:35–57. [Google Scholar]
- 20.Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. [DOI] [PubMed] [Google Scholar]
- 21.Dieckmann U, Doebeli M, Metz JAJ, Tautz D. Cambridge: Cambridge Univ Press; 2004. Adaptive Speciation. [Google Scholar]
- 22.Kisdi E. Evolutionary branching under asymmetric competition. J Theor Biol. 1999;197:149–162. doi: 10.1006/jtbi.1998.0864. [DOI] [PubMed] [Google Scholar]
- 23.Goymer P, et al. Adaptive divergence in experimental populations of Pseudomonas fluorescens. II Role of the GGDEF regulator WspR in evolution and development of the wrinkly spreader phenotype. Genetics. 2006;173:515–526. doi: 10.1534/genetics.106.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer CC, Bertrand M, Travisano M, Doebeli M. Adaptive diversification in genes regulating resource use in Escherichia coli. PLoS Genet. 2007 doi: 10.1371/journal.pgen.0030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerrish PJ, Lenski RE. The fate of competing beneficial mutations in an asexual population. Genetica. 1998;103:127–144. [PubMed] [Google Scholar]
- 26.Rozen DE, de Visser J, Gerrish PJ. Fitness effects of fixed beneficial mutations in microbial populations. Curr Biol. 2002;12:1040–1045. doi: 10.1016/s0960-9822(02)00896-5. [DOI] [PubMed] [Google Scholar]
- 27.Le Gac M, et al. Metabolic changes associated with adaptive diversification in Escherichia coli. Genetics. 2008 doi: 10.1534/genetics.107.082040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenski RE, Rose MR, Simpson SC, Tadler SC. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat. 1991;138:1315–1341. [Google Scholar]
- 29.R Development Core Team. Vienna: R Foundation for Statistical Computing; 2007. R. [Google Scholar]
- 30.SAS Institute. Cary, NC: SAS Institute; 2001. JMPIN4. [Google Scholar]
- 31.Critchlow DE, Fligner MA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Methods. 1991;20:127–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.