Abstract
Human beings exhibit wide variation in their timing of daily behavior. We and others have suggested previously that such differences might arise because of alterations in the period length of the endogenous human circadian oscillator. Using dermal fibroblast cells from skin biopsies of 28 subjects of early and late chronotype (11 “larks” and 17 “owls”), we have studied the circadian period lengths of these two groups, as well as their ability to phase-shift and entrain to environmental and chemical signals. We find not only period length differences between the two classes, but also significant changes in the amplitude and phase-shifting properties of the circadian oscillator among individuals with identical “normal” period lengths. Mathematical modeling shows that these alterations could also account for the extreme behavioral phenotypes of these subjects. We conclude that human chronotype may be influenced not only by the period length of the circadian oscillator, but also by cellular components that affect its amplitude and phase. In many instances, these changes can be studied at the molecular level in primary dermal cells.
Keywords: chronotype, circadian, fibroblast, genetics
From photosynthetic bacteria to man, organisms throughout evolution have evolved biological clocks to adapt better to the 24-h period of the solar day. These so-called “circadian” clocks (from the Latin circa diem, “about a day”) permit both the anticipation of daily environmental changes and the segregation to different time periods of antagonistic biological processes. In mammals, many aspects of physiology show circadian regulation: sleep–wake cycles and cognitive performance, cardiac function (heartbeat and blood pressure), renal function, and most aspects of digestion (enzyme secretion, gastric throughput, and detoxification). In total, ≈10% of genes have circadian patterns of expression (1).
In addition to its roles in regulating physiology, the circadian oscillator is believed to control behavior: for example, daytime preference (“chronotype”) in human beings. It has been suggested that people with longer free-running circadian periods, i.e., the time taken for one circadian cycle under constant environmental conditions, have later phases of behavior under normal day–night conditions, and people with shorter periods have earlier phases. The validity of this model within the human population has been challenging to assess because subjects must be kept for prolonged intervals in a controlled laboratory environment. Under such conditions, a loose correlation has been observed between human circadian period and chronotype (2).
Twin studies show that daily behavior has a significant genetic component (3). Syndromes associated with extreme chronotype (Familial Advanced Sleep Phase or Delayed Sleep Phase Syndrome) have been mapped to mutations in circadian clock genes (4, 5), and in one case have been specifically tied to shortened period length in affected individuals (6). Nevertheless, the separation of environmental factors from genetic ones in the study of behavior remains a formidable obstacle in the identification of biological factors that affect human chronotype. In this study we have taken a cellular approach to this question.
The circadian clock is organized in a hierarchical fashion. A master clock tissue in the suprachiasmatic nucleus of the brain hypothalamus receives light input via the retinohypothalamic tract, and peripheral “slave” oscillators in the cells of most other tissues receive entrainment signals from the SCN. These signals are probably redundant, and include indirect cues such as body temperature and feeding time, and direct ones such as hormone secretion and sympathetic enervation (7). The clock mechanism itself is cell-autonomous, and in mammals is probably driven by an interlocked network of transcriptional feedback loops. These loops involve gene products that act both positively (CLOCK, BMAL1) and negatively (CRY1-2, PER1-3, REV-ERBα) upon cis-acting elements (E-boxes and ROR elements) (8).
Such elements have been used separately in the context of luciferase fusion constructs to permit bioluminescent measurement of circadian clock phase and period, both in transfected cells and in explanted tissues from transgenic reporter animals (9, 10). Because the fundamental nature of the circadian “clockwork” in the SCN and peripheral tissues is highly similar and based upon identical components, mutations in circadian clock genes affect clock function comparably both in the SCN and in isolated peripheral tissues (11, 12). Phenotypes of circadian mutations are often exaggerated in explanted peripheral tissues, probably because circadian phase among these cells is less tightly coupled than in neurons (13, 14).
Previously, we have used a lentivirally delivered reporter system to measure circadian period length in fibroblasts isolated from mice and from human subjects. Fibroblasts furnish an excellent model system because they are easy to obtain and cultivate, and in explants from transgenic Per2:luc mice, their period closely matched that of the SCN (9). In our studies of mice and humans of different genetic background, fibroblast period length seemed to be a property of the individual from whom they were taken. In mice, it was similar to the period of wheel-running in the same animals: mutations in clock genes that shorten, lengthen, or abolish the circadian period of wheel-running had like effects upon circadian clocks in fibroblasts taken from these animals (15).
In this article, we have used a fibroblast-based assay to address the contribution of circadian clock properties to human daily behavior. Biopsies from subjects of early and late behavioral phase (as measured by a chronotype questionnaire) were cultivated, and the clock properties of fibroblast pools were measured. Overall, a correlation was seen between fibroblast period length and subject behavioral phase. This correlation was especially striking in individuals whose fibroblasts had extreme period lengths. Among individuals whose fibroblasts had “normal” period length, further analyses of some samples demonstrated either an abnormal magnitude of circadian gene transcription (resulting in a lower oscillator amplitude), or abnormal phase-shifting properties in response to a chemical phase-shifting agent. We show by simple mathematical models that each of these differences would be able to change the phase of the circadian clock without affecting period length. Thus, our results are consistent with the hypothesis that human daytime preferences within the general population can be influenced by multiple clock properties that can be studied in primary dermal cells.
Results
There Is a Significant Correlation Between Human Chronotype and Dermal Fibroblast Period Length.
A questionnaire about daytime preference [the Horne–Ostberg Chronotype Questionnaire, HOQ (16)] was administered to subjects responding to newspaper and television advertisements or personalized recruitment efforts seeking individuals of extreme chronotype. Eleven individuals of early type (“larks”) and 17 of late type (“owls”) were chosen for participation. The larks possessed a HOQ score of >60, and the owls had a HOQ score of <40. These scores reflect responses to questions in which subjects are asked to characterize their preferred waking time and sleeping times, alertness at different times of day, and vacation habits.
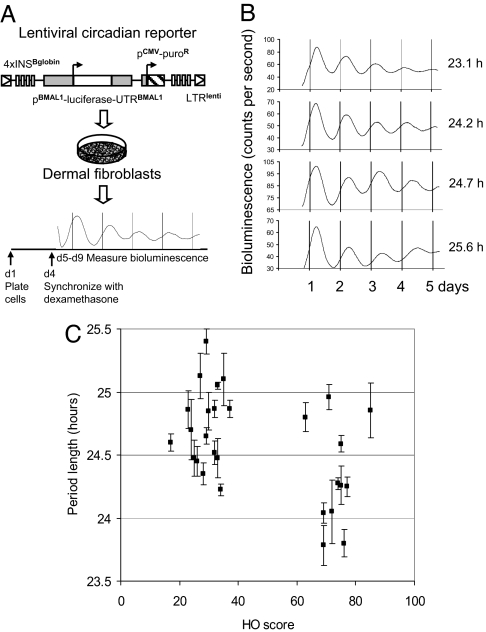
From these individuals, two 2-mm dermal punch biopsies were taken and separately cultivated. Fibroblasts from these biopsies were infected with a lentivirus harboring a circadian reporter construct with the mouse Bmal1 promoter driving expression of the firefly luciferase gene (15). Circadian rhythms in infected fibroblast cultures were synchronized with dexamethasone (17), and circadian bioluminescence, corresponding to Bmal1 promoter activity, was measured for 5 days under constant conditions in a cell culture incubator. Four to eight independent measurements were taken for each subject. (This protocol is outlined in Fig. 1A, and representative measurements from individuals of long and short period are shown adjacently in Fig. 1B.)
Fig. 1.
Comparison of subject chronotype with fibroblast period length. (A) Schematic diagram of the experimental procedure. (B) Representative raw data (un-normalized, with background, smoothened by means of a running average over 10-minute intervals) from four subjects. Period lengths were, from Top to Bottom, 23.1 h, 24.2 h, 24.7 h, 25.6 h. (C) Graph showing average period length versus Horne–Ostberg score for all subjects. Period values shown are average ± SEM from two different biopsies, each measured twice. The Wilcoxon test suggests that the difference between lark and owl period lengths taken collectively is highly significant (P = 0.000027). Nevertheless, a Spearman rank test to compare Horne–Ostberg Questionnaire score and fibroblast period length shows only moderate correlation (ρ = −0.36, P = 0.04), implying the existence of factors additional to period length that affect Horne–Ostberg score.
Subjects of early chronotype in general displayed a shorter fibroblast period (τ = 24.33 ± 0.41 h) than those of late chronotype (τ = 24.74 ± 0.32 h). This tendency can be seen in Fig. 1C, in which Horne–Ostberg Questionnaire score is plotted vs. fibroblast period length for all subjects (statistical analyses in figure legend). A good correlation is seen between behavior and period length in subjects with fibroblasts of extreme period length. This result confirms existing models about the relationship between circadian period and behavioral phase. Nevertheless, approximately half of both lark and owl subject populations showed periods of “normal” length. [In our previous study of individuals of normal chronotype, τ = 24.5 ± 0.75 (15).] Also seen were three subjects of longer period length but early behavioral phase, all of whom were subsequently determined to exhibit Seasonal Affective Disorder (SAD) (see Discussion). Thus, although these data show a correlation between period length and behavioral phase in individuals of extreme cellular period, they also imply that behavioral phase in individuals of normal period is influenced by other factors.
Fibroblast Period Length and Fibroblast Transcriptional Phase Correlate Under Entrained Conditions.
In the experiments above, subject behavioral phase was measured by means of questionnaires and period length was measured by means of reporter gene expression. To test the cellular origin of the correlation between these two values, we wished to test circadian phase in fibroblasts entrained to a 24-h circadian cycle. In vivo, it has been shown that Per1−/− fibroblasts with a 20-h free-running period in culture adopt the 24-h period of a WT host animal when encapsulated and subcutaneously implanted (12). One powerful signal by which this entrainment is accomplished in mammals is by means of circadian modulation of body temperature (18). Hence, we decided to entrain subject fibroblasts to a 24-h circadian regime of varying incubator temperature (16 h at 37°C, 8 h at 33°C), and examine the phase of reporter gene expression within this temperature regimen. Previously, we have used this method to confirm the phenotype of cells expressing a mutation in the Per2 gene that is responsible for Familial Advanced Sleep Phase Syndrome (19).
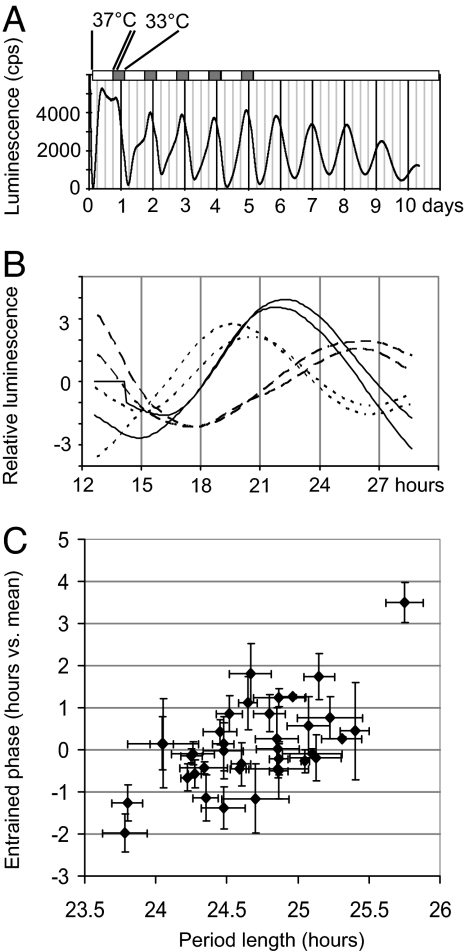
To test the entrainment procedure, we used mouse NIH 3T3 fibroblasts (period length of 24.5 h) robustly expressing a stably integrated circadian E-box-luciferase reporter (19). Cells were synchronized with fresh medium, entrained to a 24-h temperature cycle of 37°C:33°C for 6 days, and then kept at constant temperature for the next 6 days. When the cells were maintained in the temperature regime, the peak of reporter gene expression occurred at the same time each day. During the constant-temperature portion of this experiment, the peak was later each day, consistent with the free-running period of these cells (Fig. 2A).
Fig. 2.
Comparison of fibroblast period length with phase of reporter expression. (A) Expression of an E-box-luciferase reporter transgene in NIH 3T3 cells maintained under conditions of temperature entrainment. Days 0–4, 16 h 37°C and 8 h 33°C per day. Days 5–10, constant 37°C. (B) Sample human data from two different biopsies of three subjects (one each of early, intermediate, and late phase). Cells were phase-entrained for 6 days in a cycle identical to A, and then transferred to a multichannel measurement device at constant 37°C for 1.5 days of measurement. Graphed bioluminescence levels were normalized for ease of viewing to compensate for different degrees of infection. (C) Graph showing average period length versus entrained phase of reporter expression for fibroblasts from all subjects. Values are average ± SEM from two biopsies, each measured twice. x axis, period length in hours. y axis, phase in hours, relative to the mean of all subjects. Pearson Correlation Coefficient R = 0.6133 suggests moderate correlation, again indicating that factors in addition to period can influence phase. (t test for coefficient significance = 4.53, P = 0.0001.)
We next performed a similar protocol on fibroblasts from each of our subjects. Cells were incubated in the 37°C:33°C temperature regime for 6 days, and the phase of the peak of viral Bmal1-luc reporter transcription was measured during the 37°C period of the seventh day. (Sample human data are shown in Fig. 2.B.) Among individuals of extreme period length there was a correlation between fibroblast period length and transcriptional phase, confirming that period length can influence entrained phase (Fig. 2C). Interestingly, however, individuals of average period length also exhibited widely differing phases, suggesting that other factors might affect human clock phase at the cellular level. In fact, the correlation of Horne–Ostberg score with fibroblast transcriptional phase was weaker than with fibroblast period length (Spearman's ρ = −0.26, negative because higher HOQ score correlates with earlier phase), reflecting (i) that other factors could account for a significant part of human circadian behavioral variation, (ii) that measurement of phase in this cellular assay is subject to larger experimental variations than that of period, and (iii) that entrainment by temperature is probably a separate pathway from that of the entrainment by light that is crucial to behavior (20, 21).
Mathematical Modeling Predicts That Oscillator Amplitude and Circadian Input Intensity Can also Affect Circadian Phase.
It has been demonstrated previously in rodent models that the magnitude of circadian phase shifting can be affected by the amplitude of the circadian oscillator, i.e., the difference between trough and peak levels of circadian clock transcripts and proteins (22).
To determine whether these basic clock properties other than circadian period length might also affect circadian phase under entrained conditions, we modeled the circadian clock mathematically. We were interested in general clock properties that might affect phase rather than in specific genes, so we modeled the clock as simply as possible based upon the Goodwin model of a limit cycle oscillator (23). Light-driven input to the clock was depicted as a stimulus that directly affects the concentration of a single component (for example light-mediated induction of Per mRNA in mammals.) We then looked at what basic clock properties could affect the phase of this system under “entrained” conditions with an environmental cycle of fixed period length. [The equations governing this clock model system are shown in supporting information (SI) Fig. 5.]
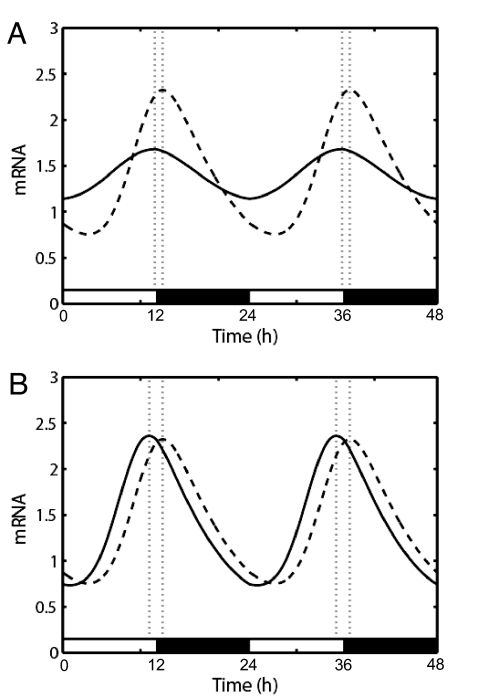
As shown by others, changes in the period length of this system result in changes in entrained phase, with longer periods producing later phases (24). Reduction in the general amplitude of the oscillator (by increasing the trough levels and decreasing the peak levels in the abundance of a state variable) makes the clock more sensitive to phase-shifting stimuli, and this change in sensitivity can shift it to an earlier entrained phase (Fig. 3A). Finally, varying the strength of input pathways without changing the oscillator itself also changed clock phase (Fig. 3B). Each of these changes results in a change in the phase response curve (PRC, a measure of how much the clock responds to light at different times of day) that under normal day/night conditions changes chronotype.
Fig. 3.
Theoretical modeling of the effects of clock properties upon entrained phase. (A) Graph of the circadian oscillations of mRNA in a 12 h:12 h light:dark cycle for two hypothetical oscillators in which the amplitude of transcription varies 2-fold. Dashed line, high-amplitude oscillator; solid line, low-amplitude oscillator. The resultant difference in entrained phase (dotted lines) is 1 h. In this scenario, the high-amplitude oscillator was created by setting h = 14, and the low-amplitude by setting h = 11 (see SI Materials and Methods for a description of mathematical methodology and variables used). (B) Similar graph for two hypothetical oscillators in which the amplitude of perceived phase shifting (i.e., light) varies 2-fold. Dashed line, lesser perceived light; solid line, more perceived light. The resultant difference in entrained phase (dotted lines) is 1.7 h. The curve from lesser perceived light was obtained with a = 0.015, and that from more perceived light used a = 0.03.
The Level of Transcription of Clock Genes Varies in Fibroblasts from Different Individuals.
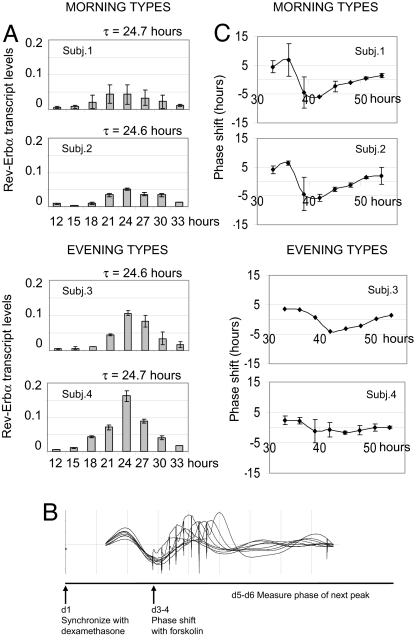
To test whether differences in transcriptional amplitude might account for phase differences among subjects, we next tried to quantify this amplitude in subjects of normal period length. One way of accomplishing this task would be to measure the amplitude of bioluminescent oscillation, but this method is sensitive to the degree of viral infection. Because this property varies among subjects, we decided instead to measure RNA levels of multiple clock genes by means of quantitative real-time PCR (qPCR). We synchronized multiple identical plates of fibroblasts from 11 subjects of approximately equivalent, “normal” fibroblast period length but different behavioral phase using the hormone dexamethasone (17), and then harvested these plates every 3 h throughout the day. We determined transcript levels of the clock genes Per2, Bmal1, Rev-Erbα, and Cry1 in each plate by means of qPCR. Most individuals showed similar levels of circadian gene transcription (SI Fig. 6). Extremes within this subject pool showed differences of up to 3-fold in transcription of the gene of highest amplitude, Rev-Erbα, but most showed intermediate levels. Oscillations of Rev-Erbα mRNA from fibroblasts of four subjects, two early-types and two late-types, with nearly identical periods but opposite behavioral phenotypes are shown in Fig. 4A. In these examples, morning types show low-amplitude Rev-Erbα rhythms, whereas evening types have high-amplitude rhythms. In general, the phases of analyzed transcripts correlated superbly among all genes and subjects, but amplitude did not (SI Fig. 6). In other words, individuals with abnormally high or low amplitudes of circadian Rev-Erbα transcription did not necessarily show higher or lower amplitudes in the transcription of other circadian genes, and vice versa. We therefore postulate that different genetic factors may influence the regulation of different clock genes. In extreme cases, overall clock amplitude is affected, but in most cases compensation by other clock genes probably eliminates systematic effects.
Fig. 4.
Comparison of fibroblast clock transcriptional amplitude with phase-shifting behavior. (A) Eight identical plates of fibroblasts from four subjects, two morning types and two evening types of identical period, were synchronized with dexamethasone, then harvested every 3 h. Graphs show the relative amount of Rev-Erbα RNA determined by qPCR. x axis, time of harvest relative to dexamethasone synchronization. y axis, relative RNA levels in arbitrary units (see Materials and Methods). (B) Schematic of phase-shifting protocol, with resulting Bmal1-luc bioluminescence levels from one subject. (C) Phase response curves in response to a stimulus with 0.3 μM forskolin. x axis, time of stimulation relative to dexamethasone synchronization. y axis, phase shift in hours.
Fibroblasts from Different Individuals of the Same Period Length Show Different Sensitivities to Phase-Shifting Agents.
Among the four individuals of identical period who showed differences in Rev-Erbα expression, those with lower amplitude were morning-types and those with higher amplitude were evening-types. Our model predicts a causal relationship among these factors: differences in amplitude causes changes in sensitivity to phase shifts, which in turn alters the phase of entrainment in normal day–night conditions. Phase-shifting signals would have greater effects in the cells of lower circadian amplitude. We decided to test this prediction with the chemical phase shifting agent forskolin, which activates adenyl cyclase similarly to normal circadian phototransduction through melanopsin (25).
To do this experiment, we began by synchronizing multiple identical plates of cells from different subjects with dexamethasone. After a full day of oscillation, we then added forskolin to a different plate every 3 h, and measured the phase of the next peak of transcription. This peak was compared with that of a control plate in which vehicle alone was added. (This protocol is diagrammed in Fig. 4B.) From each of these phase shifts, a phase response curve (PRC) was calculated by plotting the magnitude of the induced phase shift as a function of the time at which forskolin was added. As predicted, larger phase shifts were seen in the fibroblasts with lower circadian amplitude (Fig. 4C). When stronger phase-shifting conditions were used, each PRC became more extreme, but the relative hierarchy among subjects was preserved (SI Fig. 7). Thus, our results are consistent with the hypothesis that differences in the amplitude of human circadian transcription can lead to differences in entrained phase and chronotype.
Interestingly, two other subjects who had similar period and clock gene transcriptional amplitude but different chronotype also demonstrated different phase response curves in this assay (data not shown). We postulate that these individuals might have differences in the input pathways that transduce phase shifting signals such as forskolin to the circadian oscillator.
Discussion
Wide differences in human chronotype are easily apparent. Individuals of extreme chronotype (medically designated as suffering from Advanced or Delayed Sleep Phase Syndrome) have serious difficulties in normal society, and these problems translate directly to psychiatric morbidity (26). Numerous genetic correlations have been established between bipolar disorder, major depression, SAD, and circadian rhythm abnormalities, and effective treatment of these disorders can target the circadian oscillator directly or indirectly (27). Our observations in fibroblasts suggest a strong genetic contribution of an endogenous circadian clock to human daily behavior, and show that the specific properties of this clock can be measured in peripheral cells at a molecular level. The differences that we measure probably arose from a range of mechanistically different causes, with the period length of the oscillator and circadian amplitude of core clock gene transcription, as well as input pathway strength, all playing key roles. Potentially, these differences could form a basis for classification of chronobiological disorders and more targeted treatment strategies.
The molecular and behavioral phenotypes of the subjects that we analyzed are shown in SI Fig. 8. Of 28 subjects, 12 showed period lengths that were significantly different from those exhibited by individuals of opposite chronotype (Wilcoxon P < 0.001). Relative to the average fibroblast period length of 24.5 h that we have determined in earlier studies (15), individuals of early behavioral phase had shorter or normal periods, and individuals of late behavioral phase had longer or normal periods. We noticed three exceptions to this statement, individuals of longer fibroblast period reporting an early behavioral phase. All three of these individuals exhibited significant SAD, as determined by means of the Seasonal Pattern Assessment Questionnaire (28). In a previous study, SAD patients were characterized as late types (29). We are currently using a fibroblast-based system to investigate seasonal disorders in greater detail, to look for changes in clock properties in these individuals without confounding effects of seasonal lighting and mood.
The remaining subjects had fibroblasts whose periods were of average length, 24.2–24.8 h. It is possible that these individuals were misclassified by the Horne–Ostberg Questionnaire as early and late chronotypes. We recontacted subjects to request that another chronotype probe, the Munich Chronotype Questionnaire (30), be completed. The Munich Questionnaire proved to be a more stringent selection, i.e., some of the individuals classified as “extreme chronotypes” by means of the Horne–Ostberg Questionnaire and included in our study fall within the “normal” range by means of the MCTQ (SI Fig. 9A). With either questionnaire, however, many subjects of extreme chronotype showed normal period length. (SI Fig. 9B). Because fibroblasts from these subjects nevertheless displayed a wide variety of different phases when entrained to a 24-h day, our result suggested that some differences in phase could originate from factors other than period, even at the cellular level.
Mathematical modeling suggests that general properties like period length, oscillator amplitude, and input pathway strength influence phase. At the transcriptional level, circadian phase is probably determined by the combinatorial effects of cis-acting promoter elements and the time constant of RNA processing. Because our system uses a mouse promoter and a heterologous reporter construct, it is likely that the differences we observe are mediated by generally conserved elements within the clockwork itself. For example, Vitaterna et al. have shown that mice containing a mutation in the CLOCK protein that activates transcription of evening-transcribed genes show reduced amplitude of circadian transcription, and this property leads to an increased sensitivity to phase shifts (22).
The same mechanism may also affect human chronotype. Among our subjects, we were able to identify two pairs of individuals with opposite chronotype, identical, normal fibroblast period length, and and 2- to 3-fold differences in the amplitude of transcription of the clock gene Rev-Erbα. Fibroblasts from these subjects also showed considerable differences in phase-shifting behavior, supporting a possible role for circadian oscillator amplitude in determining human chronotype.
The same differences in phase-shifting sensitivity would also be expected if signaling pathways that input to the oscillator exhibited different strengths, even if the oscillator itself were “normal.” These pathways, for example, the immediate-early induction of the Per1 and Per2 genes in response to light and serum, are conserved at a cellular level (17, 31, 32), so it is plausible that interindividual differences herein might also modify chronotype. A comparison of two subjects in our study hints at a role for such factors.
By studying fibroblast circadian clocks, we have found differences in circadian clock properties that both reflect and explain the daily behavior of more than half the human subjects from whom they were taken. Other subjects had no detectable phenotype at the fibroblast molecular level, suggesting a role for psychological or neuronal influences. The wide range of alterations that we did find, in amplitude and period length of the core oscillator, as well as in input pathways, suggests that human chronotype might be influenced separately by a variety of factors at a cellular level. At the same time, it establishes a relatively simple screen for these human phenotypes.
Materials and Methods
Subject Recruitment Criteria.
Eleven individuals of early type (“larks,” 5 male and 6 female, average age 52 ± 14 years) and 17 of late type (“owls,” 7 male and 10 female, average age 36 ± 16 years) were chosen for participation based upon responses to the Horne–Ostberg Morningness-Eveningness Questionnaire (16): owls had scores <40, and larks >60. Because older individuals in general display an earlier behavioral chronotype and higher HOQ score, it is possible that a small part of the difference that we observe in our subjects is tied to subject age. For example, in two independent studies, Horne–Ostberg scores differed by 4–7 points among individuals with average ages of 30 and 50 (33, 34).
Tissue Isolation, Fibroblast Culture, and Viral Infection.
This procedure is identical to what we have used previously (15).
Synchronization and Measurement of Circadian Rhythms.
Four days or more after infection or cell passage, circadian rhythms in identically grown plates of cells were synchronized with dexamethasone (17). After washing with PBS, cells were incubated in medium without phenol red supplemented with 0.1 mM luciferin, and light emission was measured in a Lumicycle photomultiplier device (Actimetrics) for 5 days. Alternatively, cells in luciferin-supplemented medium were synchronized by incubation for 6 days in a temperature controlled incubator under a 16 h:8 h 37°C:33°C daily temperature cycle. On the seventh day, cells were transferred to the Lumicycle device at 37°C, and bioluminescence was measured for 16 h.
Measurement of Circadian Transcript Levels.
Eight identical plates of fibroblasts from each subject were cultivated 4 days, and then synchronized with dexamethasone as above. A single plate of cells from each subject was harvested every 3 h starting at 15 h after dexamethasone synchronization, rinsed with PBS, and frozen at −70°C. Later, total RNA from all plates was prepared by using a commercial kit (Qiagen), and used as a substrate for cDNA synthesis and quantitative real-time PCR using the reagents and instructions of the PCR machine manufacturer (Applied Biosystems).
Phase Response Experiments.
Eight identical plates of fibroblasts from each subject were prepared and synchronized as above, placed in luciferin-containing medium, and their bioluminescence was measured for 1.5 days. Beginning 33 h after dexamethasone synchronization, 0.3 μM forskolin (final concentration) was added in 100 μl of medium to one plate every 3 h without removing plates from the incubator, and measurement of bioluminescence was continued for another 36 h.
Mathematical Modeling.
For an explanation of our model, see SI Materials and Methods. Consider a circadian oscillator with a free-running period τ and phase θ, entrained to a zeitgeber of period T. The entrainment phase ψent is the stable solution of the equation
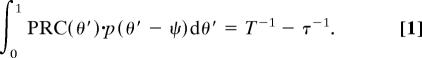 |
It depends on three factors: (i) the period mismatch τ − T, characterizing the chronotype; (ii) the magnitude and shape of the PRC, and (iii) the magnitude of the external phase-shifting signal p(θ − ψ). Points ii and iii may help explain the large phase spread for subjects with an intermediate τ.
For fuller explanations, see SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank T. Roenneberg (Ludwig-Maximilians-Universita̋t, Munich, Germany) for supplying the Munich Chronotype Questionnaire and the algorithms used for its analysis; and J. Ripperger and E. Kowalska for a critical reading of the manuscript. This work was supported by grants from the Humboldt Research Foundation, the Swiss National Science Foundation, Deutsche Forschungsgemeinschaft Grant SFB 618, and the 6th European Union Framework program EUCLOCK.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707772105/DC1.
References
- 1.Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma. 2004;113:103–112. doi: 10.1007/s00412-004-0296-2. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 3.Koskenvuo M, Hublin C, Partinen M, Heikkila K, Kaprio J. Heritability of diurnal type: A nationwide study of 8753 adult twin pairs. J Sleep Res. 2007;16:156–162. doi: 10.1111/j.1365-2869.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 4.Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 6.Jones CR, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999;5:1062–1065. doi: 10.1038/12502. [DOI] [PubMed] [Google Scholar]
- 7.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- 8.Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo SH, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 11.Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- 12.Pando MP, Morse D, Cermakian N, Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect by means of SCN hierarchical dominance. Cell. 2002;110:107–117. doi: 10.1016/s0092-8674(02)00803-6. [DOI] [PubMed] [Google Scholar]
- 13.Liu AC, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Brown SA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 17.Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 18.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- 19.Vanselow K, et al. Differential effects of PER2 phosphorylation: Molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heintzen C, Loros JJ, Dunlap JC. The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell. 2001;104:453–464. doi: 10.1016/s0092-8674(01)00232-x. [DOI] [PubMed] [Google Scholar]
- 21.Boothroyd CE, Wijnen H, Naef F, Saez L, Young MW. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genet. 2007;3:e54. doi: 10.1371/journal.pgen.0030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitaterna MH, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci USA. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin BC. Oscillatory behavior in enzymatic control processes. Adv Enzyme Regul. 1965;3:425–438. doi: 10.1016/0065-2571(65)90067-1. [DOI] [PubMed] [Google Scholar]
- 24.Johnson CH, Elliott J, Foster R, Honma K, Kronauer R. In: Chronobiology: Biological Timekeeping. Dunlap JC, Loros JJ, DeCoursey PJ, editors. Sunderland, MA: Sinauer; 2004. pp. 67–105. [Google Scholar]
- 25.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 26.Haba-Rubio J. Psychiatric aspects of organic sleep disorders. Dialogues Clin Neurosci. 2005;7:335–346. doi: 10.31887/DCNS.2005.7.4/jhabarubio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal NE, Sack DA, Skwerer RG, Wehr TA. In: The Psychobiology of Bulimia. Hudson JL, Pope HG, editors. Washington, DC: Am Psychiatr Press; 1987. pp. 205–228. [Google Scholar]
- 29.Murray G, Allen NB, Trinder J. Seasonality and circadian phase delay: Prospective evidence that winter lowering of mood is associated with a shift towards Eveningness. J Affect Disord. 2003;76:15–22. doi: 10.1016/s0165-0327(02)00059-9. [DOI] [PubMed] [Google Scholar]
- 30.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 32.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 33.Zavada A, Gordijn MC, Beersma DG, Daan S, Roenneberg T. Comparison of the Munich Chronotype Questionnaire with the Horne–Ostberg's Morningness-Eveningness Score. Chronobiol Int. 2005;22:267–278. doi: 10.1081/cbi-200053536. [DOI] [PubMed] [Google Scholar]
- 34.Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: Influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years). J Biol Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.