Abstract
Natural killer (NK) cells are important early mediators of host immunity to viral infections. The NK activatory receptors NKG2D and NKp80, both C-type lectin-like homodimeric receptors, stimulate NK cell cytotoxicity toward target cells. Like other herpesviruses, Kaposi's sarcoma-associated herpesvirus (KSHV) down-regulates MHC class I molecules to avoid detection by cytotoxic T lymphocytes but renders cells susceptible to NK cell cytotoxicity. We now show that the KSHV immune evasion gene, K5, reduces cell surface expression of the NKG2D ligands MHC class I-related chain A (MICA), MICB, and the newly defined ligand for NKp80, activation-induced C-type lectin (AICL). Down-regulation of both MICA and AICL requires the ubiquitin E3 ligase activity of K5 to target substrate cytoplasmic tail lysine residues. The common MICA *008 allele has a frameshift mutation leading to a premature stop codon and is resistant to down-regulation because of the loss of lysine residues. K5-mediated ubiquitylation signals internalization but not degradation of MICA and causes a potent reduction in NK cell-mediated cytotoxicity. The down-regulation of ligands for both the NKG2D and NKp80 activation pathways provides KSHV with a powerful mechanism for evasion of NK cell antiviral functions.
Keywords: immune evasion, ubiquitin, E3 ligase
Herpesviruses establish lifelong residence, despite the presence of an active host immune system. Infection with Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also known as human herpesvirus 8, can be contained by the immune system in healthy individuals but causes severe disease in immunocompromised transplant recipients or patients with AIDS. Natural killer (NK) cells are critical in defense against viral infections, where they provide protection by releasing cytokines such as IFN-γ and by direct lysis of infected targets. Restoration of NK cell activity correlates with resolution of KS in AIDS patients, suggesting an important role for NK cells in the control of KS development (1). NK cell activity is controlled by a balance of inhibitory and activatory signals received through cell surface receptors. KSHV down-regulates surface MHC class I molecules via the K3 and K5 immune evasion genes, which encode viral E3 ligases that target MHC class I alleles for ubiquitylation and lysosomal degradation (2–4). This reduction in MHC class I expression may render KSHV-infected cells susceptible to NK cell killing.
NK cells express a range of activating receptors, including NKG2D, 2B4, NKp80, and the natural cytotoxicity receptors NKp30, NKp44, and NKp46. NKG2D recognizes MHC class I-related chains (MIC) A and B as well as members of the UL16-binding protein (ULBP) family, and it plays a central role in innate immunity (5). Cell surface MIC expression can be induced by heat shock or infection and signals to the immune system that a cell is stressed or abnormal (6, 7). The importance of the NKG2D activatory pathway is highlighted by the down-regulation of its ligands by multiple viruses, including human cytomegalovirus (HCMV) (8, 9), mouse CMV (10–13), zoonotic orthopoxviruses (14), and HIV (15).
Recent work suggests that synergistic interactions between activatory receptors promote the induction of cytotoxicity in resting NK cells (16). Like NKG2D, NKp80 is a C-type lectin-like homodimeric receptor that stimulates NK cell cytoxicity but is expressed on NK cells alone (17). It was recently shown to recognize the activation-induced C-type lectin (AICL; also known as CLEC2B), a receptor found at the surface of myeloid cells (18). KSHV productively infects monocytes within KS lesions and can undergo lytic replication in these cells (19). No virus has yet been described to target the NKp80 pathway for evasion of NK cell cytotoxicity. Given the importance of NK cell evasion to members of the herpesvirus family, we set out to determine whether KSHV genes modulate cell surface expression of ligands for the activatory NK receptors NKG2D and NKp80.
Results
KSHV K5 Down-Regulates NK-Activating Ligands MICA, MICB, and AICL.
To investigate whether the KSHV immune evasion gene K5 affects expression of ligands for activating NK receptors, a variety of cell lines expressing endogenous MICA were transduced with K5 and MICA expression analyzed by flow cytometry. In the presence of K5 (gray shading in Fig. 1), a marked decrease in cell surface MICA was seen in some cells (e.g., U373; Fig. 1A), whereas an intermediate decrease (e.g., Jurkat cells; Fig. 1A) or no effect (e.g., HeLa cells; Fig. 1A) was observed in others. In contrast, little variation was seen in the ability of K5 to down-regulate MHC class I in the same cells (Fig. 1A). MICB, the highly related homolog to MICA, is also a ligand for NKG2D and has a conserved cytoplasmic tail (Fig. 1C). Expression of K5 decreased cell surface MICB levels in C1R-MICB cells (Fig. 1B). In contrast, K3 affected neither cell surface MICA nor MICB expression in C1R cells (data not shown). To understand the variation in the effect of K5 on MICA expression, we compared the protein sequences of the known MICA alleles. One common MICA allele, *008, has a premature stop codon, resulting in a truncated transmembrane and absent cytoplasmic tail (Fig. 1C). Indeed, this is the predominant allele in several populations (20). Other common MICA alleles contain the three conserved lysine residues close to the transmembrane region. MICB is less polymorphic than MICA, and all described alleles have a full-length cytoplasmic tail. Genotyping the endogenous MICA alleles in the cell lines tested with K5 showed a clear correlation between the MICA genotype and the ability of K5 to affect MICA surface expression (Table 1). K5 decreased cell surface expression of all MICA alleles with a wild-type cytoplasmic tail but had no effect on cell lines homozygous for the truncated MICA allele *008. Those cell lines where the effect of MICA expression by K5 was intermediate were heterozygous for the MICA *008 allele. In these cases, we predict that only one allele is targeted by K5, whereas surface expression of the truncated *008 is unaffected.
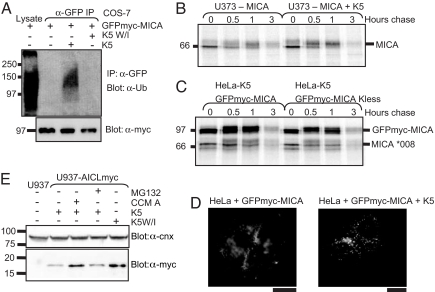
Fig. 1.
K5 down-regulates specific MICA alleles, MICB and AICL. (A) U373, Jurkat, and HeLa cells were stained with anti-MICA (2C10), anti-MHC I (w6/32) and isotype control (dotted lines), after transduction with K5 GFP lentivirus (gray) or GFP lentivirus (black). (B) C1R-MICB stable cell lines were transduced as above and stained with anti-MICA/B (6D4). (C) Sequence alignment of part of the transmembrane (gray) and cytoplasmic tails of MICA alleles, MICB, and AICL. (D) U937 AICL-myc stable cell lines were transduced as above and stained with anti-c-myc (9E10) and anti-MHC I (w6/32) mAb.
Table 1.
Variation in the down-regulation of MICA alleles by K5
| Cell line, origin | Down-regulation† | Allele 1/Allele 2‡ |
|---|---|---|
| U373, astrocytoma | +++ | 001/001 |
| HMEC-1, endothelial carcinoma | +++ | 00201–052/00201–052 |
| Caco-2, colon carcinoma | +++ | 010/010 |
| HT29, colon carcinoma | +++ | 004/016 |
| HepG2, liver carcinoma | +++ | 00201–052/00901–049 |
| Jurkat, T cell leukaemia | + | 00801–00804/00901–049 |
| BJAB, B cell lymphoma | + | 00801–00804/011–047 |
| HeLa, cervical carcinoma | − | 00801–00804/00801–00804 |
| 293T, kidney carcinoma | − | 00801–00804/00801–008 |
†Down-regulation: +++, 50–100%; +, 10–50%; −, 0–10%.
‡MICA allele genotype follows the nomenclature at www.anthonynolan.org.uk. Ambiguous allele assignment, e.g., 00804 not distinguished from 00801, is given as 00801–00804.
Other ligands recognized by NKG2D include the ULBP/retinoic acid early transcript-1 (RAET-1) family. ULBP1–3 are GPI-linked proteins and therefore lack cytoplasmic tails and are unlikely to be targeted by K5. RAET-1E has no lysines in its short cytoplasmic tail, whereas the single lysine in the cytoplasmic tail of RAET-1G is not targeted by K5 (data not shown).
Activatory receptors act cooperatively to induce cytotoxicity in resting NK cells (16). We therefore wanted to determine whether K5 targets ligands of other NK activatory receptors. KSHV infects monocytes (19), and a monocyte-specific activating receptor, AICL, was recently identified as a ligand for the activating NK receptor NKp80 (18). AICL is a type II transmembrane protein with a short cytoplasmic tail of 7 aa, 3 of which are lysine residues (Fig. 1C). Because no commercial antibody to AICL is available, we added a luminal (C-terminal) c-myc tag to allow visualization of AICL-myc at the cell surface of the monocytic cell line, U937 (Fig. 1D). In the presence of K5 (gray shading), AICL-myc cell surface expression was reduced to isotype control levels (Fig. 1D), and MHC I expression was also down-regulated (Fig. 1D). As with MICA and MICB, K3 was unable to down-regulate AICL-myc (data not shown).
Down-Regulation of MICA and AICL by K5 Is RING- and Lysine-Dependent.
The ubiquitin ligase function of both K3 and K5 is absolutely dependent on the integrity of the RING-CH domain (2, 4), which recruits host E2 enzymes. Our structural analysis of the K3 RING-CH domain identified residues (tryptophan-41 and isoleucine-11) necessary for recruitment of the E2 enzymes (21). Mutation of the tryptophan residue leaves a K3 mutant unable to ubiquitylate or degrade MHC I (4). Mutation of the equivalent residues in the K5 RING (tryptophan-47 and isoleucine-17 to alanines) generated a K5 mutant (K5W/I) that is stably expressed but unable to ubiquitylate or down-regulate MHC I (Fig. 2A and data not shown). No down-regulation of cell surface MICA and AICL is seen with the K5W/I mutant (Fig. 2A), indicating that the ubiquitin E3 ligase activity of K5 is required.
Fig. 2.
Down-regulation of MICA and AICL by K5 is RING- and lysine-dependent. (A) C1R-MICA (Left and Center) or U937 AICL-myc cells (Right) were stained with anti-MHC I (w6/32), anti-MICA (2C10), anti-c-myc (9E10), and isotype control (dotted lines), after transduction with K5 W/I GFP (gray) or control GFP lentivirus (black). (B) MICA cytoplasmic tail mutants stably expressed in C1R cells were transduced with K5 GFP lentivirus to give ≈50% transduction levels. Cells were stained with anti-MICA (2C10) mAb. Sequences show cysteine and lysine residues of the MICA cytoplasmic tail, with mutations underlined. Lysine (K) was mutated to arginine (R); cysteine (C) to serine (S). (C) U937 expressing AICL lacking cytoplasmic lysines (AICL-Kless-myc) were stained with anti-c-myc (9E10) and anti-MHC I (w6/32) mAb after transduction with K5 GFP (gray) or control GFP lentivirus (black).
The requirement for the K5 RING-CH domain for MICA and AICL down-regulation suggested that K5 was targeting cytoplasmic tail residues for ubiquitylation. Sequence alignment of MICA alleles, MICB, and AICL identified three lysine residues close to the membrane in all three targets (Fig. 1C). To determine the lysine requirements of K5 for MICA down-regulation, we created a series of MICA mutants. The cytoplasmic tail lysine residues were individually mutated to arginine residues and the resultant constructs transduced into C1R cells to generate stable cell lines, which were subsequently transduced with K5 in a GFP-expressing construct. Wild-type and mutant MICA down-regulation by K5 could then be compared. Mutation of all three lysine residues to arginines completely abrogates down-regulation of MICA by K5 (Fig. 2 B and C). MICA is recruited to membrane subdomains by acylation of the cysteine residues (Fig. 1C) (23). Mutation of the two cysteine residues (to serines) had no effect on the down-regulation of MICA by K5 (Fig. 2B), suggesting that recruitment of MICA to a lipid subdomain is not required for K5 function. Analysis of lysine residue mutations showed that the single, membrane-proximal lysine residue (KRR) promoted MICA down-regulation as efficiently as wild-type MICA (Fig. 2B). However, movement of this single lysine residue distal to the membrane (RKR) caused a less significant down-regulation. MICA down-regulation by K5 is abolished when the lysine is two positions away (RRK). A single lysine is therefore sufficient for K5-mediated down-regulation of MICA, and the position of this lysine relative to the membrane is critical. The presence of more than one lysine residue does not improve the down-regulation of MICA (Fig. 2B) but confirms the importance of the membrane proximal lysine for the down-regulation of MICA by K5.
We also created a tagged AICL construct with all three lysine residues in the cytoplasmic tail mutated to arginines (AICL-Kless-myc) (Fig. 2C). K5 was unable to down-regulate AICL-Kless-myc, but in the same cells, K5 decreased MHC I expression (Fig. 2C). These results suggest that K5 directly targets AICL through its cytoplasmic tail lysine residues.
MICA Is Ubiquitylated and Targeted to an Intracellular Compartment by K5.
Having shown that down-regulation of MICA by K5 depends on the presence of a single lysine residue in the MICA cytoplasmic tail and a functional K5 RING-CH, we predicted that K5 acts as an E3 ligase to ubiquitylate MICA, as seen with its other substrates (2, 24, 25). To confirm this prediction, we immunoprecipitated GFP-myc-tagged MICA from COS-7 cells in the presence of K5 or the inactive K5W/I mutant and probed for ubiquitin expression. In the presence of wild-type but not mutant K5, a ubiquitylation band is seen in MICA-expressing cells (Fig. 3A). Transfection controls show equivalent MICA expression for all cell lines (Fig. 3A).
Fig. 3.
K5 ubiquitylates MICA and targets AICL for endolysosomal degradation. (A) K5 promotes ubiquitylation of MICA. The indicated constructs were transiently expressed in COS-7 cells. Cell lysates were immunoprecipitated with anti-GFP mAb, proteins were separated, and membranes were probed with anti-ubiquitin (P4D1) mAb. First lane is 2% of cell lysate from MICA transfection. Ten percent of cell lysates were run on SDS/PAGE and probed for GFP-myc-MICA (9E10) mAb. (B and C) Pulse–chase analysis of MICA in the presence of K5. Cells were radiolabeled for 30 min and chased for the indicated time periods. MICA protein (B) or tagged and untagged MICA proteins (C) were immunoprecipitated with anti-MICA (2C10) mAb from 1% Triton X-100 lysates and resolved by SDS/PAGE. (D) Immunofluoresence of GFP-MICA in HeLa cells in the presence or absence of K5. (Scale bar: 10 μm.) (E) Myc-tagged AICL-expressing U937 cells (U937-AlCL-myc) were transduced with lentiviral K5 or K5W/I, and 72 h later they were treated with 6 μM MG132 or 50 nM concanamycin A (CCM A). Solubilized lysates were PNGase F-treated, and separated proteins were probed with anti-c-myc (8E10) or anti-calnexin (CNX) (AF8) mAb.
We wanted to determine the fate of ubiquitylated MICA because the K5-mediated ubiquitylation of MHC class I (26) but not CD1d (25) leads to enhanced lysosomal degradation. Pulse–chase analysis of MICA immunoprecipitates from [35S]methionine-radiolabeled U373 cells showed that K5 does not significantly increase the rate of MICA degradation (Fig. 3B). This experiment was repeated in K5-expressing HeLa cells transfected with GFP-myc-MICA or GFP-myc-MICA-Kless. Because HeLa cells express the MICA *008, which is unaffected by K5, these cells provide an internal control and allowed us to compare the fate of the “K5-sensitive” and “K5-insensitive” MICA alleles under identical conditions (Fig. 3C). The degradation of GFP-myc-MICA is not significantly increased compared with the truncated, endogenous MICA allele *008 (Fig. 3C).
Because K5 does not affect MICA degradation, we determined how K5 affects MICA redistribution from the plasma membrane. Immunofluorescence of HeLa cells showed GFP-myc-MICA predominantly at the plasma membrane (Fig. 3D). In the presence of K5, GFP-myc-MICA is redistributed to punctate, intracellular vesicles (Fig. 3D), which do not colocalize with markers for budding vesicles (clathrin), early endosomes (EEA-1, Vps-26), lysosomes (LAMP-1), or TGN (TGN-46) (data not shown). Thus, K5 expression leads to MICA ubiquitylation and redistribution to an intracellular compartment, without enhancing degradation.
AICL Is Targeted for Endolysosomal Degradation by K5.
To determine how K5 affects AICL, we compared wild-type with mutant (K5W/I) on U937 cells stably expressing myc-tagged AICL (U937-AICL-myc) in the presence or absence of proteasomal (MG132) or lysosomal (concanamycin A) inhibitors. In the presence of K5, AICL-myc was degraded in a concanamycin A-sensitive compartment (Fig. 3E). MG132 treatment did not rescue AICL-myc degradation, suggesting that, as seen with class I, K5 targets AICL for lysosomal and not proteasomal degradation.
Induction of Lytic Cycle KSHV Genes Leads to a Decrease in Cell Surface MICA Expression.
To study the effect of KSHV infection on MICA expression, we analyzed cell surface levels on the primary effusion lymphoma cell line, BC3, which is latently infected with KSHV. Untreated BC3 cells express only a subset of latent viral genes. Lytic gene expression is induced by sodium butyrate (27), which increases the proportion of cells undergoing lytic replication from <5% to 87% live cells, as measured by expression of the ORF-59 early lytic gene (Fig. 4A). In agreement, a rapid induction of K5 was seen from mRNA isolated after sodium butyrate-treated BC3 cells (Fig. 4A). A full-length MICA allele was genotyped in BC3 cells, but because no surface MICA was detected either before or after sodium butyrate treatment (data not shown), BC3 cells were stably transduced with MICA (Fig. 4B). Induction of lytic KSHV replication decreased cell surface MICA expression to ≈40% of untreated levels (Fig. 4B), comparable to MHC class I (≈50% untreated) and ICAM-1 (≈15% untreated) (Fig. 4B). Expression of lysineless MICA (MICA-Kless) (Fig. 4C) was unaffected by lytic gene expression, whereas down-regulation of MHC class I and ICAM-1 was apparent (Fig. 4C). To ensure that receptor down-regulation in BC3 depended on the induction of KSHV and not a toxic effect, the C1R B cell lymphoma line was treated with sodium butyrate. C1R does not express MICA, but ICAM-1 and MHC class I were both unaffected by sodium butyrate (Fig. 4D). Thus, induction of lytic cycle KSHV genes by sodium butyrate in BC3 cells decreases cell surface expression of wild-type, but not lysineless, MICA.
Fig. 4.
Lytic KSHV infection decreases cell surface MICA levels in BC3 cells. (A) Induction of lytic KSHV replication. (Left) RT-PCR of KSHV K5 expression after 2 mM sodium butyrate (NaBu) treatment of BC3 cells. (Right) Acetone/methanol-permeabilized BC3 cells treated as in the Left were stained with anti-ORF-59 mAb. (B and C) BC3 stably transudced with lentiviral wild-type MICA (B) or MICA lacking cytoplasmic tail lysine residues (MICA-Kless) (C) were stained with anti-MICA (2C10), anti-MHC I (w6/32), anti-ICAM-1 (15.2), and isotype control (dotted lines) before (solid lines) or after (gray) NaBu treatment. (D) Cytofluorometric analysis of MHC I and ICAM-1 after sodium butyrate treatment of the KSHV negative C1R cells, treated as in A.
Down-Regulation of MICA and AICL by K5 Protects Cells from NK Cell Cytotoxicity.
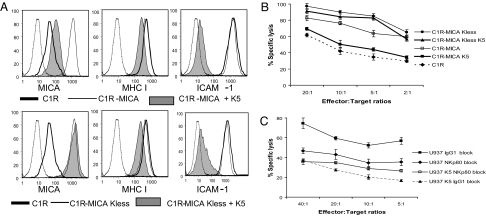
To study the functional significance of MICA down-regulation by K5, we tested whether the K5-mediated down-regulation of MICA protected target cells from killing by primary NK cell lines in 51chromium cytotoxicity assays. Because K5 also down-regulates cell surface MHC class I molecules, we needed a target cell line that allowed us to differentiate the effect of K5 on MICA from its effect on MHC class I. We therefore chose the C1R B cell line, which expresses low cell surface MICA and relatively low MHC class I (Fig. 5A). The low expression of MHC class I molecules renders C1R sensitive to background NK killing (Fig. 5B). C1R cell lines stably expressing MICA and lysineless MICA were examined for (i) expression of MICA, MHC class I, and ICAM-1 (Fig. 5A) and (ii) killing by primary human NK cell lines (Fig. 5B) in the presence and absence of K5. The basal level of NK-mediated killing of C1R targets was increased after expression of MICA (Fig. 5B). K5 expression reduced the killing of C1R-MICA K5 cells to background levels, an effect not seen on C1R-MICA-Kless K5-expressing target cells. These results, seen in primary human NK cell lines from three independent donors, suggest that K5 protects cells from NK killing and that this effect is absolutely dependent on the down-regulation of cell surface MICA and not on other cell surface receptors whose expression may also be affected by K5.
Fig. 5.
K5 protects cells from NK cytotoxicity by down-regulation of MICA and AICL. (A) Cytofluorometric analysis of C1R clones used in NK cytotoxicity assays. Cell surface staining (as Fig. 1) of C1R (bold line), stable C1R clones expressing MICA or MICA lacking cytoplasmic tail lysine residues (MICA-Kless) transduced with K5 GFP (gray) or GFP lentivirus (black). Dotted lines show isotype control. (B) 51Cr release assay (percentage specific lysis) with polyclonal NK cells against C1R clones. Error bars give the SE of the six replicates at each point. The results are representative of three independent experiments with polyclonal NK cells from unrelated donors. (C) 51Cr release assay using IgG1- or NKp80-specific mAb blocked NK cell line against control or K5-expressing U937 cells. Error bars give the SE of the six replicates at each point.
U937 cells express AICL, but not MICA, and are sensitive to NK cell lysis in an NKp80-dependent manner (18). Therefore, we examined the effect of K5 on NK cell killing of U937 cells. A primary NK cell line expressing NKp80 was used in cytotoxicity assays. In the absence of endogenous AICL-specific antibody, the contribution of AICL to NKp80-mediated killing cannot be directly determined. However, an NKp80-blocking antibody allowed us to assess the contribution of NKp80 to the killing of U937 (Fig. 5C) and provides an indirect assessment of the effect of AICL. It makes the assumption that no additional NKp80 ligands are expressed on U937 cells. As shown (Fig. 5C), K5 expression reduced the specific lysis of U937 cells compared with control cells. This protection from NK-mediated lysis was similar to that seen with NKp80-blocking antibody, and no additional protection was seen in the presence of K5 when NK cells were NKp80-blocked (Fig. 5C). Taken together, these results suggest that the protection of U937-K5 cells compared with U937 cells (Fig. 5C) is NKp80-dependent and likely results from the down-regulation of AICL by K5.
Discussion
NK cell killing is regulated by a balance of signals received through activatory and inhibitory receptors. The down-regulation of MHC class I molecules by the KSHV-encoded K3 and K5 genes, together with the up-regulation of activating receptors induced by viral infection, may shift this balance to make KSHV-infected cells susceptible to NK cell killing. Here, we show that K5 decreases cell surface expression of MICA/MICB and AICL, ligands of the NK activatory receptors NKG2D and NKp80. K5 ubiquitylates lysine residues in the cytoplasmic tail of MICA, and receptor down-regulation occurs during lytic viral replication and protects cells from NKG2D-mediated NK cytotoxicity. K5 is the first viral protein found to down-regulate AICL, allowing KSHV to evade NKp80-mediated cytotoxicity.
Previous experiments to assess the potential role of K5 in protecting cells from NK-mediated cytotoxicity led to contradictory results, due in part to the use of NK cell lines that may not be representative of killing by primary human NK cells (3, 28). By identifying MICA/B as targets of K5, we provide evidence that K5 expression can protect cells from NKG2D-mediated NK cytotoxicity, independent of its effect on other surface receptors such as ICAM-1. The down-regulation of MICA by K5 showed ≈30% change in protection from NK cytotoxicity, but taken in combination with the K5 down-regulation of MICB, the effect is likely to be greater.
K5 down-regulates AICL, a monocyte-specific C-type lectin (18), and protected U937 cells from NK cell lysis by an NKp80-positive polyclonal NK line. K5 may play a role in KSHV productive infection of monocytes, which provide a reservoir for viral transmission, allowing the increase and maintenance of viral load during the late stages of infection (19). The down-regulation of AICL may also suppress TNF production by infected monocytes (18). Because AICL could not be expressed in BC3 cells, a B cell line, it was difficult to analyze the effect of lytic KSHV replication on cell surface expression of AICL.
Although it is always difficult to extrapolate in vitro results to viral infection in vivo, recent data emphasize the importance of K5 as one of a cluster of lytic cycle viral genes expressed in the early stages of KSHV infection in both endothelial cells and fibroblasts (29, 30). De novo KSHV infection strongly activates the K5 promoter and correlates with down-regulation of other K5 targets (MHC class I and ICAM-1) (30). Loss of cell surface MICA may therefore help KSHV evade NK cell surveillance in vivo. It may be important to avoid NK detection in the early phase of KSHV infection, before establishing latency, when KSHV infects endothelial and epithelial cells that express cell surface MICA, as well as later during lytic cycle reactivation and viral replication.
Both AICL and MICA are directly targeted by K5. The MICA allele sensitivity to down-regulation by K5 and the absolute requirement for lysine residues in the MICA cytoplasmic tail are of particular significance, given the high gene frequency of the K5-resistant *008 allele. Whether the presence of this allele affects the clinical outcome of KSHV infection remains to be determined and will be of particular interest in the HIV-infected population where coinfection with KSHV carries a high morbidity and mortality (31). The *008 MICA allele is also resistant to down-regulation by HCMV (8), and it will be important to determine whether viral infection has driven selection for this allele.
The lysine requirements of K5 on MICA down-regulation demonstrate the positional constraints for ubiquitylation by this E3 ligase. Although down-regulation by K5 requires only a single lysine residue in the tail of MICA, the distance of this residue from the membrane is critical. At +4 residues from the membrane, K5 shows full activity, but transfer to +6 residues leads to a complete loss of MICA down-regulation. AICL also has a lysine at position +4 from the membrane, as well as two lysines abutting the membrane. Although the majority of cellular E3 ligases are cytosolic, as an integral membrane protein the ability of K5 to target the more distal lysine residues for ubiquitylation may be more constrained.
K5 is remarkable for its ability to target so many different immunoreceptors, including MHC class I, ICAM-1, CD86, CD1d as well as PECAM, ALCAM, the IFN receptor, AICL and MICA/B (24, 25, 28, 32–34). By down-regulating these receptors, K5 is effectively preventing recognition by cells of the adaptive (CD8, CD4, and CD1d-restricted T cells), as well as the innate (NKG2D- and NKp80-specific NK cells and IFN receptor) immune system. Thus, a single viral gene is able to inhibit multiple cellular recognition pathways and help KSHV to evade the host immune response and establish permanent infection. The down-regulation of AICL by K5 may contribute to the ability of the virus to maintain an infectious reservoir in monocytes within a lesion. Further experiments will help determine the importance of MICA down-regulation in the evasion of CD8+ T cells, γδ T cells, and in KSHV tumor formation.
Materials and Methods
Cell Lines and Culture.
The cell lines BC3, C1R, U373, HeLa, BJAB, Jurkat, U937, HT29, HepG2, Caco-2, and 293T were grown in RPMI medium 1640 supplemented with 10% FCS. HMEC-1 were grown in supplemented MCDB 131 medium. Stable cell lines (C1R-MICA, C1R MICA-mutants, C1R MICA-Kless ± K5, U373-MICA, HeLa-K5-GFP-MICA-Kless) were created by lentiviral transduction of parent cells. For C1R-MICB, MICB was amplified from the HT29 colon cancer line (American Type Culture Collection) and transfected into C1R cells.
Antibodies.
Primary antibodies and their sources were as follows: 2C10 α-MICA, α-myc mAb 9E10, and α-Ub P4D1 (Santa Cruz Biotechnology); α-human MHC I W6/32 and α-MICA/B 6D4 (BD Biosciences); α-ICAM (clone 15.2; Serotec); α-GFP mAb 1218 (AbCam); α-ORF-59 (ABI); α-calnexin (mAb AF8; a gift from M. Brenner, Harvard Medical School); and α-NKp80 (MA152 mAb; a gift from A. Moretta, University of Genova). Secondary antibodies were from Jackson ImmunoResearch.
Constructs.
The lentiviral expression plasmids pHRSin and pHRSin UbEm (control GFP lentivirus), based on a pHR vector from A. Thrasher (35), were used in combination with the envelope plasmid pMD.G, and packaging plasmid pCMVR8.91. K5 was subcloned into pHRSin UbEm to give pHRSin K5 UbEm (K5 GFP lentivirus).
The MICA IMAGE clone [National Center for Biotechnology Information (NCBI) accession no. BC016929; Geneservice] was amplified as a BamHI/NotI fragment and cloned into pCR4-TOPO (Invitrogen) to give pCR4-TOPO MICA. MICA was subcloned into pHRSin. GFP-myc-tagged MICA constructs were made in PK-1, which contains a generic N-terminal signal sequence, GFP, a myc tag, and the insertion site. MICA or MICA Kless (from exon 2) were amplified from pCR4-TOPO MICA/MICA-Kless as XhoI/XbaI fragments and subcloned into PK-1. The AICL IMAGE clone (Geneservice) (NCBI accession no. NM_005127) was amplified as a BamHI/AgeI fragment and cloned into pCANT-myc-ZZ. AICL-myc and AICL-Kless-myc were subcloned into pHRSin as BamHI/NotI fragments.
Transfection and Lentiviral Transduction.
Packaging, envelope, and vector plasmids were cotransfected into 293T cells. Viral supernatants harvested at 48 and 72 h and concentrated by ultracentrifugation, and the high-titer virus was incubated with cells for 1 h at 37°C for transduction.
Oligonucleotides.
The following oligonucleotides were used for cloning: MICA Kless, ctatgtccgttgttgtaGAaGAaGaacatcagctgcagag; MICA Cless, gttattattattttctatgtccgttCttCtaag; MICA 1K 332, ctatgtccgttgttgtaAaagaagaacatcagctgcagag; MICA 1K 333, ctatgtccgttgttgtagaaAaagaacatcagctgcagag; MICA 1K 334, ctatgtccgttgttgtagaagaaAaacatcagctgcagag; MICA 2K 332–334, ctatgtccgttgttgtaAaagaaAaacatcagctgcagag; MICA 2K 332–333, ctatgtccgttgttgtaAaaAaagaacatcagctgcagag; MICA 2K 333–334, ctatgtccgttgttgtagaaAaaAaacatcagctgcagag; MICA XhoI F, ctcgagggagctggtgcaccccacagtcttcg; MICA XbaI R, tctagactaggtgccctcag; MICA BamHI F, ggatccgccaccatggggctgggc; MICA NotI R, gcggccgcctaggtgccctca; K5 BamHI F, ggatccaccatggcgtccaaggacgtagaag; K5 NotI R, atgcggccgctcaaccgttgttttttggatgattt; GAPDH F, gcaggggggagccaaaaggg; GAPDH R, tgccagccccagcgtcaaag; AICL BamHI F, ggatccgccaccatgatgaccaaacat; AICL AgeI R, accggttcctccacttagtgtattctt; AICL Kless, gatgaccaGacataGaaGgtgttttaaattg; AICL-myc NotI, ggacttgtccggaggtacctgagcggccgcgggtgctagc.
Sequencing-Based Typing of the MICA Gene.
Genotyping of the repeat polymorphism in the MICA exon 5 was done by PCR followed by capillary electrophoresis. Further genotyping involved sequencing of MICA exons 2, 3, and 4. Sequences were aligned with the Gap4 program from the Staden package (www.mrc-lmb.cam.ac.uk/pubseq). MICA genotypes were reconstructed by matching the observed data to MICA alleles (S. Field, S. Nejentsev, N. Walker, J. A. Todd, unpublished data).
Flow Cytometry.
Flow cytometry was performed as described in ref. 4.
Immunoprecipitation and Immunoblotting.
Cells were lysed in RIPA buffer (0.1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl, 0.1% SDS, 50 mM Tris·HCl, pH 7.4) with 0.5 mM PMSF, 5 mM indoleacetic acid, 25 mM N-ethylmaleimide, and Complete protease inhibitors (Roche) for 30 min on ice. For AICL-myc blots, samples were treated with peptide:N-glycosidase (PNGase F)(New England Biolabs) for 1 h at 37°C before loading. For immunoblots, lysates were heated in SDS sample buffer, separated by SDS/PAGE, transferred to PVDF membranes (Millipore), and probed with the indicated antibodies. Reactive bands were detected by ECL. For immunoprecipitations, lysates were precleared with Sepharose CL-4B and protein A and GFP-myc-MICA immunoprecipitated with anti-GFP, eluted in SDS reducing sample buffer, separated by SDS/PAGE, and transferred to PVDF. The membrane was dentatured in 6 M guanidium chloride, blocked, and probed with anti-Ub mAb P4D1.
Metabolic Labeling and Immunoprecipitation.
Radiolabelling was performed as described in ref. 4, and cells were lysed in 1% Triton X-100 in Tris-buffered saline (TBS) with inhibitors as described for the immunoblot protocol. Lysates were precleared with Sepharose CL-4B and protein A, incubated with primary antibody (2C10 or anti-GFP) and CL-4B/protein A overnight, washed in 0.1% Triton X-100/TBS, and proteins were dissociated at 70°C in SDS reducing sample buffer. Samples were separated by SDS/PAGE and processed for autoradiography with STORM scanner (Molecular Dynamics).
NK Killing Assays.
Primary polyclonal NK cell lines were established from PBMC of HCMV-seropositive and -seronegative donors, and NK killing assays were performed with the target cells: C1R, C1R-MICA K5, C1R-MICA-Kless K5, C1R-MICA, C1R-MICA-Kless, U937, and U937-K5 as described in ref. 36.
RNA Extraction and RT-PCR.
BC3 RNA was extracted (TRIzol), reverse-transcribed with random hexamers, and treated with RNase. K5 was amplified by PCR with the primers K5 BamHI forward and K5 NotI reverse. GAPDH was amplified by using GAPDH forward and reverse.
Immunofluorescence.
Cells were viewed with a Axiophot fluorescence microscope (Zeiss).
ACKNOWLEDGMENTS.
We thank Marco Londei, Yasuhiro Ikeda, Rob Eagle, Louise Boyle, Sara Sigismund, A. Moretta, and all members of the Lehner and Wills laboratories. S.N. is a Diabetes Research and Wellness Foundation Non-Clinical Fellow. P.J.L. holds a Lister Institute Research Prize. This work was supported by sponsorship from the Wellcome Trust (to P.J.L.), Medical Research Council (to V.C.), and Cancer Research U.K. Program Grant C399/A6199 (to V.C.),
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sirianni MC, Vincenzi L, Topino S, Giovannetti A, Mazzetta F, Libi F, Scaramuzzi D, Andreoni M, Pinter E, Baccarini S, et al. Eur J Immunol. 2002;32:2711–2720. doi: 10.1002/1521-4141(2002010)32:10<2711::AID-IMMU2711>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Coscoy L, Sanchez DJ, Ganem D. J Cell Biol. 2001;155:1265–1273. doi: 10.1083/jcb.200111010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishido S, Choi JK, Lee BS, Wang C, DeMaria M, Johnson RP, Cohen GB, Jung JU. Immunity. 2000;13:365–374. doi: 10.1016/s1074-7613(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt EW, Duncan L, Mufti D, Baker J, Stevenson PG, Lehner PJ. EMBO J. 2002;21:2418–2429. doi: 10.1093/emboj/21.10.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodoen MB, Lanier LL. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 7.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Proc Natl Acad Sci USA. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou Y, Bresnahan W, Taylor RT, Stastny P. J Immunol. 2005;174:3098–3104. doi: 10.4049/jimmunol.174.5.3098. [DOI] [PubMed] [Google Scholar]
- 9.Chalupny NJ, Rein-Weston A, Dosch S, Cosman D. Biochem Biophys Res Commun. 2006;346:175–181. doi: 10.1016/j.bbrc.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 10.Hasan M, Krmpotic A, Ruzsics Z, Bubic I, Lenac T, Halenius A, Loewendorf A, Messerle M, Hengel H, Jonjic S, et al. J Virol. 2005;79:2920–2930. doi: 10.1128/JVI.79.5.2920-2930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krmpotic A, Hasan M, Loewendorf A, Saulig T, Halenius A, Lenac T, Polic B, Bubic I, Kriegeskorte A, Pernjak-Pugel E, et al. J Exp Med. 2005;201:211–220. doi: 10.1084/jem.20041617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenac T, Budt M, Arapovic J, Hasan M, Zimmermann A, Simic H, Krmpotic A, Messerle M, Ruzsics Z, Koszinowski UH, et al. J Exp Med. 2006;203:1843–1850. doi: 10.1084/jem.20060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krmpotic A, Busch DH, Bubic I, Gebhardt F, Hengel H, Hasan M, Scalzo AA, Koszinowski UH, Jonjic S. Nat Immunol. 2002;3:529–535. doi: 10.1038/ni799. [DOI] [PubMed] [Google Scholar]
- 14.Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN. J Exp Med. 2007;204:1311–1317. doi: 10.1084/jem.20062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, Santoni A, Doria M. J Gen Virol. 2007;88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 16.Bryceson YT, March ME, Ljunggren HG, Long EO. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitale M, Falco M, Castriconi R, Parolini S, Zambello R, Semenzato G, Biassoni R, Bottino C, Moretta L, Moretta A. Eur J Immunol. 2001;31:233–242. doi: 10.1002/1521-4141(200101)31:1<233::AID-IMMU233>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Welte S, Kuttruff S, Waldhauer I, Steinle A. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 19.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Sturzl M. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersdorf EW, Shuler KB, Longton GM, Spies T, Hansen JA. Immunogenetics. 1999;49:605–612. doi: 10.1007/s002510050655. [DOI] [PubMed] [Google Scholar]
- 21.Dodd RB, Allen MD, Brown SE, Sanderson CM, Duncan LM, Lehner PJ, Bycroft M, Read RJ. J Biol Chem. 2004;279:53840–53847. doi: 10.1074/jbc.M409662200. [DOI] [PubMed] [Google Scholar]
- 22.Cadwell K, Coscoy L. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- 23.Eleme K, Taner SB, Onfelt B, Collinson LM, McCann FE, Chalupny NJ, Cosman D, Hopkins C, Magee AI, Davis DM. J Exp Med. 2004;199:1005–1010. doi: 10.1084/jem.20032194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansouri M, Douglas J, Rose PP, Gouveia K, Thomas G, Means RE, Moses AV, Fruh K. Blood. 2006;108:1932–1940. doi: 10.1182/blood-2005-11-4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez DJ, Gumperz JE, Ganem D. J Clin Invest. 2005;115:1369–1378. doi: 10.1172/JCI24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coscoy L, Ganem D. Proc Natl Acad Sci USA. 2000;97:8051–8056. doi: 10.1073/pnas.140129797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Alcendor DJ, Zong JC, Ambinder RF, Hayward GS. J Virol. 2002;76:3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coscoy L, Ganem D. J Clin Invest. 2001;107:1599–1606. doi: 10.1172/JCI12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. J Virol. 2004;78:3601–3620. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adang LA, Tomescu C, Law WK, Kedes DH. J Virol. 2007;81:5079–5090. doi: 10.1128/JVI.02738-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holkova B, Takeshita K, Cheng DM, Volm M, Wasserheit C, Demopoulos R, Chanan-Khan A. J Clin Oncol. 2001;19:3848–3851. doi: 10.1200/JCO.2001.19.18.3848. [DOI] [PubMed] [Google Scholar]
- 32.Bartee E, McCormack A, Fruh K. PLoS Pathogens. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehner PJ, Hoer S, Dodd R, Duncan LM. Immunol Rev. 2005;207:112–125. doi: 10.1111/j.0105-2896.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Means R, Lang S, Jung JU. J Virol. 2007;81:2117–2127. doi: 10.1128/JVI.01961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 36.Wills MR, Ashiru O, Reeves MB, Okecha G, Trowsdale J, Tomasec P, Wilkinson GW, Sinclair J, Sissons JG. J Immunol. 2005;175:7457–7465. doi: 10.4049/jimmunol.175.11.7457. [DOI] [PubMed] [Google Scholar]