Abstract
CD8+ T cells recognize peptide fragments of endogenously synthesized antigens of cancers or viruses, presented by MHC I molecules. Such antigen presentation requires the generation of peptides in the cytosol, their passage to the endoplasmic reticulum, loading of MHC I with peptides, and transport of MHC I–peptide complexes to the cell surface. Heat-shock protein (hsp) 90 is a cytosolic chaperone known to associate with peptide and peptide precursors of MHC I epitopes. We report here that treatment of cells with hsp90 inhibitors leads to generation of “empty” MHC I caused by inhibited loading of MHC I with peptides. Inhibition of hsp90 does not inhibit synthesis of MHC I, nor does it affect the activity of proteasomes. Hsp90-inhibited cells, such as proteasome-inhibited cells, are poor stimulators of T lymphocytes. The role of hsp90 in presentation of an ovalbumin epitope is shown to be at a postproteasomal step: hsp90 associates with N-terminally extended precursors of the SIINFEHL epitope, and such peptides are depleted from hsp90 preparations in hsp90-inhibited cells. Inhibition of hsp90 in the antigen donor cell compromises their ability to cross-prime. Conversely, stressed cells expressing elevated hsp90 levels show a heat-shock factor-dependent, enhanced ability to cross-prime. These results demonstrate a substantial role for hsp90 in chaperoning of antigenic peptides in direct and indirect presentation. The introduction of a stress-inducible component in these pathways has significant implications for their modulation during fever and infection.
Keywords: chaperones, cross-presentation, cross-priming, MHC I
CD8+ T cells play a crucial role in the immune response to cancers and infections. These T cells recognize noncovalent complexes of MHC I and 8-aa peptides generated by degradation of endogenous antigens. The degradation occurs primarily in the cytosol, leading to generation of peptides that enter the endoplasmic reticulum (ER) through the transporter associated with antigen processing (TAP) (1). Within the ER, the peptides are loaded onto MHC I and trimmed to size (2, 3), and the MHC I–β2-microglobulin–peptide complex is transported to the cell surface. Of the three components of this trimolecular complex, the peptide remains mysterious in terms of the mechanisms of its journey from the point of its generation to its tryst with the MHC I. Clearly, the peptides do not diffuse freely; a biochemical analysis does not reveal the presence of free peptides in cellular extracts (4, 5). The idea that peptides are accompanied from the point of their generation to their entry into TAP and then within the ER by a “relay line” of chaperones was proposed early (6, 7). Biochemical analyses have long shown the presence of hsp70 and hsp90–peptide complexes in the cytosol and glycoprotein of Mr 96,000 (gp96) and calreticulin–peptide complexes in the ER (8–15). Binder et al. (16) showed that antigenic peptides chaperoned by heat-shock proteins (HSPs) were more efficient by orders of magnitude than free peptides in being presented by MHC I and that treatment of cells with deoxyspergualin, an hsp70 inhibitor, leads to a reduced surface expression of folded MHC I. Ishii et al. (11) noted that hsp70, hsp90, and gp96 isolated from murine leukemia cells were associated with a precise Ld-restricted epitope and its precursors. Direct evidence for an obligate association of peptides with chaperones for antigen presentation came from Shastri and colleagues, who developed an elegant method to detect precursors of a modified ovalbumin (KOVAK)-derived antigenic peptide SIINFEHL (5). Kunisawa and Shastri (17, 18) showed that association of SIINFEHL precursors with TRiC and hsp90α in the cytosol was necessary for presentation of SIINFEHL by Kb.
Here, we explore mechanistically the involvement of hsp90 in antigen presentation and cross-priming, and we show that (i) hsp90 plays a global role in direct presentation; (ii) this role is specific to chaperoning of peptides by hsp90 and does not affect synthesis of MHC I or the activity of proteasomes; (iii) hsp90 is involved postproteasomally in chaperoning of endogenously generated N-terminally extended peptides; and (iv) hsp90 plays a significant role in cross-priming.
Results
Hsp90 Inhibitors Reduce Expression of Folded Cell Surface MHC I.
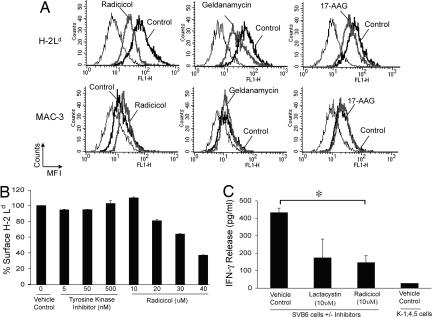
Stable cell surface expression of MHC I requires it to be peptide-loaded. We have treated cultured murine cells with radicicol, geldanamycin, or a geldanamycin derivative, 17-AAG, all specific inhibitors of hsp90 that block its N-terminal ATP/ADP-binding domain. Expression of surface-folded MHC I on untreated and inhibitor-treated cells was assessed by staining with conformation-specific antibodies against the appropriate MHC I. The cell line 4T1 (H-2d) was treated for 12 h with titrated concentrations of inhibitors indicated or DMSO as a control and surface-stained with the antibody 30-5-7S specific for folded H-2Ld (19), or as a negative control, with an antibody to the integral membrane protein MAC-3. Expression of folded H-2Ld is significantly diminished in radicicol- (46% reduction), geldanamycin- (59% reduction), or 17-AAG- (48% reduction) treated cells compared with untreated cells (Fig. 1A). Treatment with either inhibitor had no effect on expression of MAC-3. Surface expression of other control proteins CD71 and ICAM-1 showed no inhibition (data not shown). Treatment of the T antigen-transformed line SVB6 (H-2b) with radicicol or 17-AAG followed by staining for folded Kb using the antibody Y3 showed a reduction in expression of Kb but not control proteins MAC-3 and ICAM1 [supporting information (SI) Fig. 6]. Similar data were obtained with Ld, Dd, Kb, and Db (data not shown). The effect of radicicol was shown to be time-dependent (SI Fig. 7).
Fig. 1.
Inhibition of hsp90 results in decreased levels of folded MHC class I. (A) 4T1 cells were treated for 12 h with the indicated inhibitor: 30 μM radicicol (Left), 25 μM geldanamycin (Center), 50 μM 17-AAG (Right), or with DMSO as a control. Cells were stained with an antibody specific for conformational Ld (Upper) or for MAC-3 (Lower) or isotype control antibodies. Staining of inhibitor-treated cells (bold gray) is compared with control cells (bold black) and isotype control stained cells (thin black). (B) 4T1 cells were treated for 12 h with titrated doses of SU11652, a tyrosine kinase inhibitor, or radicicol and then stained with antibody specific for conformational Ld or an isotype control antibody. Results are plotted as percentage surface Ld. Error bars represent the standard deviation of triplicate samples for a single experiment. (C) T antigen-expressing SVB6 cells were treated with the indicated doses of lactacystin, radicicol, or a vehicle control for 12 h. The T antigen epitope I-deficient K-1, 4, 5 cells were used as a negative control. Treated cells were added as stimulators of K11 cells (a T antigen epitope I-specific T cell clone) for 20 h, and supernatant was assayed for IFN-γ. Results of three independent experiments (each performed in duplicate) are plotted, with error bars representing the standard deviation between averages of two experiments.
Tyrosine kinases constitute a substantial proportion of the many client proteins of hsp90 (20). The possibility that the effects of hsp90 inhibitors resulted from downstream effects on tyrosine kinases was considered. 4T1 cells were exposed to titrated quantities of the potent tyrosine kinase inhibitor SU11652 (21) in parallel with radicicol, and expression of folded Ld was monitored. Fig. 1B shows that SU11652 had no effect on surface expression of folded Ld, whereas radicicol did.
The ability of untreated and radicicol-treated cells to stimulate T cells was tested. SVB6 cells that express the SV40 large T antigen stimulate the antigen-specific CTL clone K11 (22) to secrete IFN-γ. K11 recognizes the T antigen epitope I (amino acids 206–215, SAINNYAQKL) presented by SVB6; this epitope is deleted in the T antigen-transformed cell line K-1, 4, 5. Untreated SVB6 cells, cells treated with radicicol, and as a positive control, cells treated with the proteasome inhibitor lactacystin were incubated for 20 h with K11 T cells, and the culture supernatant was assayed for IFN-γ. As seen in Fig. 1C, untreated SVB6 cells were able to stimulate K11 cells robustly, whereas the K-1, 4, 5 cells lacking a proper epitope were unable to do so. Cells treated with lactacystin showed a significantly diminished ability to stimulate K11 cells, as did the radicicol-treated cells. Thus, inhibition of hsp90 diminishes the antigen presenting capacity of cells to a level comparable with the effects of proteasome inhibition.
Inhibition of Hsp90 Affects Charging of MHC I with Peptides.
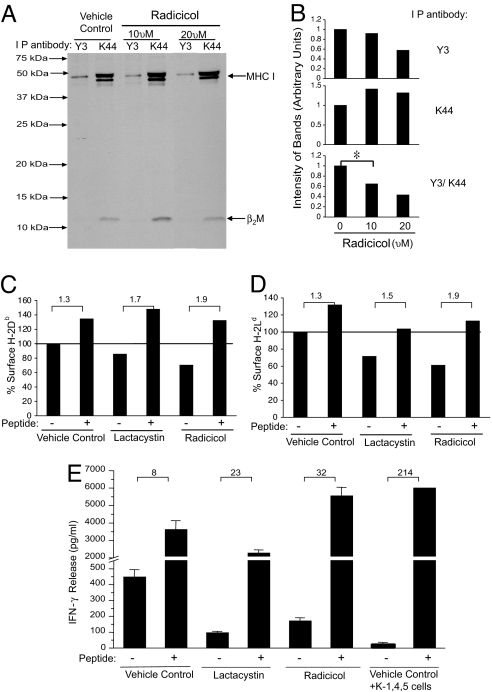
Untreated or radicicol- (10 μM or 20 μM) treated EL4 cells were labeled with [35S]methionine for 3 h, and cell lysates were immunoprecipitated with the antibody K44 (23), which recognizes a monomorphic epitope on all murine MHC I, or with Y3, which recognizes peptide-loaded Kb (24). The immunoprecipitate was resolved by SDS/PAGE and autoradiographed. In parallel, the surface expression of folded Kb and Db was analyzed by FACS (as in Fig. 1). The results of the surface staining were consistent with the data shown in Fig. 1, such that radicicol-treated cells had a significantly reduced expression of folded MHC I (data not shown). Immunoprecipitation experiments demonstrated (Fig. 2A) that treatment of cells with radicicol (at each concentration) resulted in a slight increase of K44-precipitated MHC I. However, treatment with radicicol resulted in reduced immunoprecipitation of Y3-reactive Kb. The bands in the gel were quantified in arbitrary units; cells treated with radicicol (10 or 20 μM) contained 142% and 132%, respectively, of the K44-precipitated material compared with the untreated control. In contrast, cells treated with radicicol (10 or 20 μM) contained 92% and 57%, respectively, of the Y3-precipitated material compared with the untreated control (Fig. 2B). The ratio of Y3- to K44-precipitated MHC I was calculated as an index of peptide-loaded Kb, assigning as 1 the ratio of the two values in untreated cells: treatment with radicicol led to a titrated decrease (64.7% with 10 μM radicicol and 43.1% with 20 μM radicicol) in peptide-loaded Kb (Fig. 2B), although synthesis of MHC I is unaffected. Analysis of total synthesized proteins in radicicol-treated cells showed no influence of radicicol on protein synthesis (data not shown).
Fig. 2.
Inhibition of hsp90 results in appearance of empty surface MHC I. (A) EL4 cells were treated with the indicated dose of radicicol or DMSO for 12 h. Cells were metabolically labeled with 35S for 3 h and lysed for immunoprecipitation with Y3 (specific for conformational H-2Kb) or K44 (recognizing total mouse MHC I). An autoradiograph representative of two independent experiments is shown. (B) The intensity of the Y3 (conformation-dependent) or K44 (conformation-independent) immunoprecipitated MHC class I bands from autoradiograph (A) was quantitated in arbitrary units (Top and Middle). The ratio the intensity of Y3 (conformation-dependent H-2Kb) to K44 (total conformation-independent MHC I) was calculated in arbitrary units (Bottom). The reduction in the Y3/K44 ratio seen in presence of 10 μM radicicol is statistically significant (P = 0.008, two-tailed test). (C) SVB6 cells were treated with 10 μM lactacystin, 10 μM radicicol, or DMSO for 12 h in the presence or absence of the Db-restricted T antigen epitope II peptide (CKGVNKEYL). Cells were stained with a Db conformation-specific antibody or isotype control and analyzed as in Fig. 1B. (D) 4T1 cells were treated with 20 μM lactacystin, 30 μM radicicol, or DMSO for 12 h in the presence or absence of the Ld-restricted LCMV-NP peptide (RPQASGVYM). Cells were stained and analyzed as in Fig. 1B. (E) T antigen-expressing SVB6 cells were treated with the indicated doses of lactacystin, radicicol, or a vehicle for 12 h in the presence or absence of the T antigen epitope I peptide (SAINNYAQKL). The T antigen epitope I-deficient K-1, 4, 5 cell line was used as a negative control. Treated cells were added as stimulators of K11 cells (a T antigen epitope I-specific T cell clone) for 20 h, and supernatant was assayed for IFN-γ. Results of three independent experiments (each in duplicate) are plotted, with error bars representing the standard deviation between the averages of duplicate samples.
As another approach to measure empty MHC I, the ability of exogenous peptides to “rescue” the surface expression of MHC I was tested (25). SVB6 cells were treated with radicicol, lactacystin, or control for 12 h. Each group was incubated in the presence or absence of the Db T antigen epitope II peptide (CKGVNKEYL) at 1 μM, and all cells were stained with the B22-249.R1 antibody specific for folded Db. Untreated cells show a modest 1.3-fold increase in folded Db upon pulsing with the exogenous peptide CKGVNKEYL (Fig. 2C), consistent with a proportion of MHC I on the surface being empty. Lactacystin- or radicicol-treated cells show lower levels of Db than untreated cells, as in Fig. 1. However, lactacystin-treated and peptide-pulsed cells show a significantly higher increment (1.7-fold) in Db staining than lactacystin-treated unpulsed cells, as expected from the fact that lactacystin-treated cells have fewer peptides and more empty Db capable of being rescued. Similarly, radicicol-treated and peptide-pulsed cells show an even higher increment (1.9-fold) in Db staining than lactacystin-treated unpulsed cells. Experiments for folded Ld with antibody 30-5-7S showed similar results (Fig. 2D): the ratio of folded Ld between peptide-pulsed and unpulsed cells in untreated, lactacystin-treated, and radicicol-treated cells was 1.3, 1.5, and 1.9, respectively.
A similar experiment was carried out quantifying MHC I–peptide complexes via T cell stimulation (Fig. 2E). As a positive control, the ratio of IFN-γ release between peptide-pulsed and unpulsed cells in case of K-1, 4, 5 cells that lack epitope I was an enormous 214-fold. Untreated, or lactacystin-treated or radicicol-treated SVB6 cells were unpulsed or pulsed with T antigen epitope I (SAINNYAQKL) and were used to stimulate K11 cells. The ratio of IFN-γ release between peptide-pulsed and unpulsed cells in untreated, lactacystin-treated, and radicicol-treated cells was 8-fold, 23-fold, and 32-fold, respectively. Thus, inhibition of hsp90 leads to an increased proportion of empty MHC I, comparable with or greater than that observed with proteasome inhibition.
Inhibition of Hsp90 Is Not a Preproteasomal or a Proteasomal Step.
Charging of MHC I with peptides involves triaging of proteins for degradation or refolding with the help of hsp90/hsp70 and their co-chaperones (26), ubiquitination of proteins, generation of peptides by proteasomes, and postproteasomal transport of peptides. We sought to determine systematically which of these step(s) is inhibited by inhibition of hsp90.
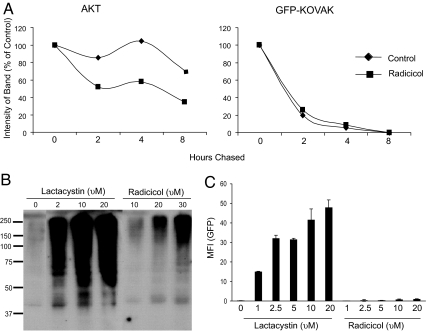
Inhibition of hsp90, such as by radicicol, inhibits refolding of client proteins and sends more molecules to degradation by proteasomes. We sought to test whether KOVAK is a client protein of hsp90. If it were to be one, inhibition of hsp90 would lead to a more rapid degradation of KOVAK. The protein kinase Akt (27) is a known client protein of hsp90 and was used as a positive control. PIK23 cells that express Akt and a GFP-KOVAK fusion protein (see Materials and Methods) were labeled with [35S]methionine for 1 h, and the pulse was chased for 2, 4, and 8 h, followed by immunoprecipitation of Akt and GFP-KOVAK. The precipitates were resolved on SDS/PAGE, autoradiographed, and quantitated (Fig. 3A). The results show that degradation of Akt is significantly enhanced by inhibition of hsp90, whereas degradation of GFP-KOVAK is unaffected under the same conditions. Thus, GFP-KOVAK is not a client protein of hsp90, and inhibition of hsp90 has no influence on its degradation. Next, the influence of radicicol on ubiquitination was tested. 4T1 cells were treated with titrated doses of the lactacystin (as a control) or radicicol for 12 h and evaluated for accumulation of ubiquitinated proteins by immunoblotting of cell lysates with anti-ubiquitin antibody (Fig. 3B). Although lactacystin-treated cells show a massive accumulation of ubiquitinated proteins in a dose-dependent manner, radicicol-treated cells show a very modest accumulation and certainly no inhibition of ubiquitination. Modest enhancement in the quantity of ubiquitinated proteins by inhibition of hsp90 has been reported (28). Our observations in Fig. 3B indicate that inhibition of hsp90 is not influencing ubiquitination of proteins in a manner that may explain its activity of inhibiting charging of MHC I with peptides.
Fig. 3.
Radicicol treatment does not act at preproteasomal or proteasomal steps. (A) PIK23 cells were treated with 10 μM radicicol or DMSO for 7 h. Cells were metabolically labeled with 35S for 1 h and chased for time points as indicated. Cells were lysed for immunoprecipitation with anti-Akt (Left) or anti-GFP (Right) antibody. Immunoprecipitated material was resolved by SDS/PAGE and autoradiographed. The band intensity was measured and plotted taking the initial time point (0 h) as 100%. (B) 4T1 cells were treated with the indicated doses of lactacystin, radicicol, or DMSO for 12 h and lysed, resolved by SDS/PAGE, and immunoblotted for ubiquitin. (C) 4T1-proSensor-76B, a clone stably expressing the pZsProSensor-1, was treated with the indicated doses of lactacystin or radicicol for 12 h and analyzed for expression of GFP by FACS. Error bars in C represent standard deviation of a single experiment performed in triplicate. Results are representative of three independent experiments.
The possibility that radicicol inhibits proteasomes was investigated. The reporter construct pZsProsensor-1 (Clontech) encodes a form of GFP with a murine ornithine decarboxylase domain that targets the GFP for rapid degradation by the proteasome, without ubiquitination. In cells with normal proteasome function, the turnover of this GFP is too rapid for GFP to accumulate; thus, GFP is detectable in these cells only if proteasomes are inhibited. A stable clone of 4T1 cells expressing the plasmid (4T1-proSensor-76B) was isolated and treated with titrated concentrations of lactacystin (as a positive control) or radicicol for 12 h, and cells were evaluated for expression of GFP. Cells treated with lactacystin show a titratable increase in GFP accumulation; in contrast, radicicol-treated 4T1-proSensor-76B cells show no accumulation of GFP at any dose tested (Fig. 3C). Thus, radicicol does not inhibit proteasome activity.
Radicicol Inhibits Association of Hsp90 with N-Terminal Extended Precursors.
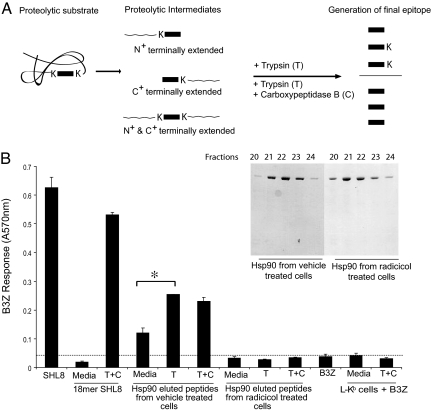
Considering that inhibition of hsp90 had no preproteasomal consequence, we investigated whether inhibition of hsp90 was influencing association of peptides with hsp90. Untreated or radicicol-treated PIK23 cells were used to isolate hsp90 to homogeneity, and associated peptides were eluted as described (18, 29). These peptides were used undigested, or they were treated with trypsin to release any N-terminal extensions or with trypsin + carboxypeptidase B (18) to release N- and C-terminal extensions (Fig. 4A). Each of these peptide mixtures was pulsed onto L-Kb cells, which were used to stimulate B3Z cells as described in Materials and Methods (30). The results (Fig. 4B) show that unstimulated B3Z have minimal activity, whereas B3Z cells stimulated with the precise SIINFEHL epitope are powerfully stimulated. The synthetic N- and C-terminal extended precursor of SIINFEHL in an undigested form has no activity. Peptides eluted from hsp90 from untreated PIK23 cells show significant stimulation of B3Z, and this stimulation is enhanced further by digestion of peptides with trypsin, indicating N-terminal extended precursors. Further digestion of these peptides with carboxypeptidase B shows no further enhancement of stimulation, indicating that the mixture does not contain C-terminal extended precursors. This pattern of stimulation indicates that hsp90 from uninhibited PIK23 cells is associated with SIINFEHL and its N-terminal extended precursors. Interestingly, peptides eluted from hsp90 (undigested or digested) from radicicol-treated PIK23 cells did not elicit any B3Z-stimulating activity. Because proteasome activity is essential for generation of peptides with the precise C terminus, our observations indicate that radicicol inhibits association of hsp90 with peptides generated by proteasomes and that this association is required for further transport of peptides in the antigen presentation pathway.
Fig. 4.
Hsp90 associates with N-terminally extended peptides. (A) The KOVAK model system expresses antigenic peptide (rectangular box) that is flanked at its N and C termini by lysine (K). After proteolysis, intermediates are generated that could have N-, C-, or both N- and C-terminal extensions. Trypsin digestion generates the exact epitope from N-terminally extended precursors. Release of the exact epitope from C- or both N- and C-terminal extended precursor requires both carboxypeptidase B and trypsin. (Adapted from ref. 18.) (B) PIK23 cells were treated with 10 μM radicicol or DMSO for 7 h. After treatment, cells were washed, hsp90 was purified (see Inset), and peptides eluted as described in Materials and Methods. Eluted peptides were digested with trypsin (T) alone or trypsin and carboxypeptidase B (C) or undigested for 4 h at 37°C. After digestion, peptides were pulsed onto L-Kb cells and incubated with B3Z cells for 20 h as described in Materials and Methods.
Hsp90 Is Required for Cross-Priming of Cellular Antigen.
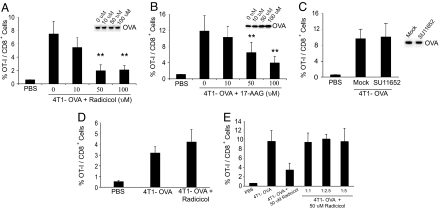
Previous studies have suggested that HSP-chaperoned peptides generated in an antigen-expressing cell can act as the substrate for transfer to antigen-presenting cells (APCs) during cross-priming (31, 32) and indeed may be necessary and sufficient for cross-priming (14); a requirement for hsp90 in cross-priming of antigen from intact cells has been hinted but not tested rigorously (33). The role of hsp90 in cross-priming was tested by using radicicol and geldanamycin. 4T1 cells of the d haplotype, untreated or treated with radicicol or geldanamycin, were electroporated with ovalbumin (OVA) and used to immunize C57BL/6 mice of the b haplotype, which had received OT-I cells. Expansion of the OT-I population was the endpoint for cross-priming. As seen in Fig. 5 A and B, immunization with OVA-containing uninhibited 4T1 cells led to a significant and vigorous expansion of OT-I cells; however, pretreatment of 4T1 cells with radicicol (Fig. 5A) or geldanamycin (Fig. 5B), caused a significant, dose-dependent, and near complete inhibition of cross-priming (respectively, P < 0.0075, P < 0.0073 at highest doses). Inhibition of hsp90 did not affect the OVA content of the immunizing cells at any concentration of inhibitor (see Fig. 5 A and B, Insets).
Fig. 5.
Inhibition of Hsp90 inhibits cross-priming. (A) 4T1 cells were treated with the indicated doses of radicicol or DMSO for 6 h and electroporated with purified, LPS-free, soluble OVA protein. Cells were washed and irradiated with 7,500 Gray before being used to immunize OT-I recipients. Expansion of OT-I cells in the draining lymph nodes was quantified and plotted as the percentage of the total CD8+ population. (Inset) At the time of immunization, a portion of each immunizing population was analyzed by immunoblotting for OVA. (B) 4T1 cells were treated with the indicated doses of 17-AAG or DMSO as a control for 6 h and used for immunization as in A. (C) 4T1 cells were treated with the indicated doses of SU11652 or DMSO as a control for 6 h and used for immunization and analysis as in A. (D) 4T1 cells were electroporated with purified, LPS-free, soluble OVA and cultured for 12 h before treating with the indicated doses of radicicol or DMSO as a control for 6 h. Cells were washed and irradiated with 7,500 Gray before being used to immunize OT-I recipients. Expansion of OT-I cells in the draining lymph nodes was plotted as the percentage of the total CD8+ population. (E) 4T1 cells treated with 50 μM radicicol (as in A) were mixed at the indicated ratios with untreated cells and used for immunization and analysis as in A.
To discriminate between specific effects of hsp90 in peptide trafficking from nonspecific effects, 4T1-OVA cells were treated with SU11652 instead of radicicol or geldanamycin and used for immunization and measurement as in Fig. 5 A and B. Treatment with SU11652 had no influence on cross-priming (Fig. 5C) or on OVA content in the immunizing cells (Fig. 5C, Inset). To test further for nonspecific effects, the sequence of the introduction of OVA and treatment with radicicol was reversed before immunization of OT-I recipients. Reversal of order did not lead to abrogation of cross-priming (Fig. 5D), indicating that the deficit in cross-priming elicited by treatment with radicicol was specific to some aspect of hsp90–OVA interaction. We considered the possibility that radicicol-treated 4T1 cells are toxic to the host APCs, thus causing inhibition of cross-priming. Untreated or radicicol-treated 4T1 cells were electroporated with OVA; and, as seen in Fig. 5A, 4T1-OVA cells treated with radicicol are compromised in their ability to cross-prime. If radicicol-treated 4T1-OVA cells were toxic to cross-priming APCs, then they should abrogate cross-priming of antigen from untreated 4T1-OVA cells in a dominant fashion. Thus, we immunized OT-I recipient mice with titrated mixtures of untreated and radicicol-treated 4T1-OVA cells, as indicated in Fig. 5E. It is observed that upon immunization with mixtures of radicicol-treated and untreated cells (in any ratio), the inhibition of cross-priming is never the dominant phenotype.
The possibility that heat-shocked cells, which express higher levels of most HSPs including hsp90, show enhanced cross-priming, was tested. OT-I recipient mice were immunized with OVA-electroporated or mock-electroporated cells that were or were not heat-shocked at 42°C, and expansion of OT-I cells was monitored. OVA-electroporated heat-shocked cells were significantly better agents of cross-priming than corresponding non-heat-shocked cells (SI Fig. 8A, P < 0.01). Cells not containing OVA showed no cross-priming activity. Treatment of heat-shocked cells with KNK437, an agent that inhibits trimerization of the heat shock factor (34), inhibited the heat shock-enhanced cross-priming (SI Fig. 8B).
Discussion
A peptide-chaperoning property was attributed to hsp90 family members in the early 1990s as a possible explanation for the specific immunogenicity of tumor-derived HSP preparations (see refs. 6 and 7). Our results here support that explanation. Our results are threefold. First, we show that sequestration of hsp90 leads to a specific inhibition of loading of a variety of MHC I alleles with cognate peptides and consequent abrogation of expression of folded MHC I. Inhibition of hsp90 does not affect generation of peptides or MHC I synthesis, but only MHC I loading. Second, our results show that hsp90 associates with N-terminal extended peptides; this observation places the role of hsp90 in direct presentation specifically at a postproteasomal step, as envisaged by us (7). This result differs from that obtained from Kunisawa and Shastri (18), who noted that hsp90 was associated with N- and C-terminal extended peptides. This discrepancy may arise from the fact that Kunisawa and Shastri used immunoprecipitated hsp90 that may include, adventitiously, hsp90-associated large KOVAK fragments, including the whole protein. In the studies here, we used instead biochemically homogeneous preparations of hsp90 (see Fig. 4B, Inset). Third, we present evidence for a role for hsp90 in cross-priming, as envisaged by us on theoretical grounds (7). Inhibition of hsp90 in the antigen donor cell inhibits cross-priming, whereas heat shock conditions enhance cross-priming. Clearly, complexes of any of the chaperones (gp96, hsp70, calreticulin) with peptides can mediate cross-priming (14). However, the present data suggest that association of hsp90 with peptides is upstream of association of other chaperones with peptides, such that blocking hsp90 inhibits the association of all other chaperones with peptides.
These observations firmly place the chaperoning of peptides by hsp90 in direct and indirect antigen presentation and set the stage for a closer scrutiny of the trafficking of peptides in the two pathways. The introduction of a stress-inducible component in the two antigen presentation pathways has significant implications for of modulation of these pathways during fever and infection.
Materials and Methods
Cells, Antibodies, Peptides, and Chemicals.
4T1 (H-2d), EL4 (H-2b), and P815 (H-2d) cells were purchased from American Type Culture Collection (ATCC). PIK23 cells are P815 cells stably transfected with a gene encoding a fused GFP-KOVAK protein, under an ecdysone-inducible promoter. The T antigen-transformed cell line SVB6 (H-2b), K-1, 4, 5 cells, and K11 cells (a T antigen epitope I-specific T cell clone) were provided by Satvir Tevethia and Todd Schell (both from Pennsylvania State University, Hershey, PA). L-Kb cells (used as APCs) and B3Z T cell hybridomas were provided by David Williams (University of California, Berkeley, CA) and Nilabh Shastri (University of Toronto, Toronto, ON, Canada). Conformational specific antibodies for H-2Ld (30-5-7S; Cedar Lane), H-2Db (B22-249.R1; Cedar Lane), H-2Dd (34-5-8S; BD PharMingen), H-2Kb (purified from Y3 hybridoma from ATCC) were used according to the manufacturers' instructions. ICAM-1 (3E2; BD PharMingen), Mac-3 (M3/84; ebiosciences), K44 (23), ubiquitin (Calbiochem), GFP (Calbiochem), and Akt (Cell Signaling) were also used. H-2Ld-restricted epitope from the LCMV-NP (118–126; RPQASGVYM), H-2Db-restricted T antigen epitope I (206–215; SAINNYAQKL), epitope II (223–231; CKGVNKEYL), SHL8 (SIINFEHL), and 18-mer SHL8 (LEQLKSIINFEHLKEWTS) were synthesized by Genemed Synthesis and were >95% pure. Lactacystin, 17-AAG, AdaAhx3L3VS, epoxomicin, and SU11652 were purchased from Calbiochem. All chemicals were purchased from Sigma unless otherwise specified.
Mice.
C57BL/6 mice were purchased from the Jackson Laboratory. OT-I T cell receptor transgenic RAG2−/− (Taconic) mice were used to isolate TcR transgenic cells. All animal work was approved by the Center for Laboratory Animal Care of our school.
Purification of Hsp90 and Extraction of Chaperoned Peptides.
Hsp90 was purified as described in ref. 29. PIK23 (2 × 108) cells were Dounce-homogenized in hypotonic buffer, and supernatant was collected after a 100,000 × g centrifugation. The buffer was exchanged to phosphate buffer (20 mM sodium phosphate, pH 7.4, 1 mM EDTA, with 200 mM NaCl), and the sample was applied to a Mono Q (Amersham Biosciences) column. Bound proteins were eluted by a linear salt gradient 200–600 mM sodium chloride. Hsp90 (≈2 mg) eluted in five fractions at ≈500 mM NaCl. These fractions were pooled and boiled in 10% formic acid for 10 min. Eluted peptides were lyophilized and desalted with a C18 column (Gracevydac). Peptides were lyophilized and used in B3Z cell activation assay (described in SI Methods).
Supplementary Material
ACKNOWLEDGMENTS.
Matthew Buckwalter and John Kelly read the manuscript critically. This work was supported by National Institutes of Health Grant CA 084479 to P.K.S., who is also supported by a Physicians Health Services Chair. M.K.C. is supported in part by a National Institutes of Health Medical Scientist Training Program grant.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711365105/DC1.
References
- 1.Androlewicz MJ, Cresswell P. How selective is the transporter associated with antigen processing? Immunity. 1996;5:1–5. doi: 10.1016/s1074-7613(00)80304-0. [DOI] [PubMed] [Google Scholar]
- 2.Cox JH, Yewdell JW, Eisenlohr LC, Johnson PR, Bennink JR. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 1990;247:715–718. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- 3.Serwold T, Shastri N. Specific proteolytic cleavages limit the diversity of the pool of peptides available to MHC class I molecules in living cells. J Immunol. 1999;162:4712–4719. [PubMed] [Google Scholar]
- 4.Menoret A, Peng P, Srivastava PK. Association of peptides with heat shock protein gp96 occurs in vivo and not after cell lysis. Biochem Biophys Res Commun. 1999;262:813–818. doi: 10.1006/bbrc.1999.1306. [DOI] [PubMed] [Google Scholar]
- 5.Paz P, Brouwenstijn N, Perry R, Shastri N. Discrete proteolytic intermediates in the MHC class I antigen processing pathway and MHC I-dependent peptide trimming in the ER. Immunity. 1999;11:241–251. doi: 10.1016/s1074-7613(00)80099-0. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava PK, Udono H, Blachere NE, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 8.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. [PubMed] [Google Scholar]
- 10.Lammert E, et al. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol. 1997;27:923–927. doi: 10.1002/eji.1830270418. [DOI] [PubMed] [Google Scholar]
- 11.Ishii T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–1309. [PubMed] [Google Scholar]
- 12.Grossmann ME, et al. Proteomics shows Hsp70 does not bind peptide sequences indiscriminately in vivo. Exp Cell Res. 2004;297:108–117. doi: 10.1016/j.yexcr.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Demine R, Walden P. Testing the role of gp96 as peptide chaperone in antigen processing. J Biol Chem. 2005;280:17573–17578. doi: 10.1074/jbc.M501233200. [DOI] [PubMed] [Google Scholar]
- 14.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 15.Binder RJ, Kelly JB, Vatner RE, Srivastava PK. Specific Immunogenicity of heat shock protein gp96 derives from chaperoned antigenic peptides and not from contaminating proteins. J Immunol. 2007;179:7254–7261. doi: 10.4049/jimmunol.179.11.7254. [DOI] [PubMed] [Google Scholar]
- 16.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163–17171. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 17.Kunisawa J, Shastri N. The group II chaperonin TRiC protects proteolytic intermediates from degradation in the MHC class I antigen processing pathway. Mol Cell. 2003;12:565–576. doi: 10.1016/j.molcel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Kunisawa J, Shastri N. Hsp90α chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Ozato K, Hansen TH, Sachs DH. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980;125:2473–2477. [PubMed] [Google Scholar]
- 20.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao AT, et al. Inhibition of constitutively active forms of mutant kit by multitargeted indolinone tyrosine kinase inhibitors. Blood. 2002;100:585–593. doi: 10.1182/blood-2001-12-0350. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AE, Foley FL, Tevethia SS. Demonstration of multiple antigenic sites of the SV40 transplantation rejection antigen by using cytotoxic T lymphocyte clones. J Immunol. 1983;130:490–492. [PubMed] [Google Scholar]
- 23.Ozato K, Wan YJ, Orrison BM. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci USA. 1985;82:2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammerling GJ, Rusch E, Tada N, Kimura S, Hammerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci USA. 1982;79:4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljunggren HG, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 26.Schneider C, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA. 1996;93:14536–14541. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso AD, et al. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 28.Mimnaugh EG, et al. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- 29.Srivastava PK. Purification of heat shock protein–peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- 30.Karttunen J, Sanderson S, Shastri N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc Natl Acad Sci USA. 1992;89:6020–6024. doi: 10.1073/pnas.89.13.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 32.Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basta S, Stoessel R, Basler M, van den Broek M, Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J Immunol. 2005;175:796–805. doi: 10.4049/jimmunol.175.2.796. [DOI] [PubMed] [Google Scholar]
- 34.Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942–2948. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.