Abstract
During sustained viewing of an ambiguous stimulus, an individual's perceptual experience will generally switch between the different possible alternatives rather than stay fixed on one interpretation (perceptual rivalry). Here, we measured pupil diameter while subjects viewed different ambiguous visual and auditory stimuli. For all stimuli tested, pupil diameter increased just before the reported perceptual switch and the relative amount of dilation before this switch was a significant predictor of the subsequent duration of perceptual stability. These results could not be explained by blink or eye-movement effects, the motor response or stimulus driven changes in retinal input. Because pupil dilation reflects levels of norepinephrine (NE) released from the locus coeruleus (LC), we interpret these results as suggestive that the LC–NE complex may play the same role in perceptual selection as in behavioral decision making.
Keywords: attention, norepinephrine, vision, decision making
One pervading mystery in neuroscience is how the brain can generate an “internal” perceptual experience from the available “external” sensory information. Ambiguous stimuli, like the Necker cube, offer a unique means to investigate this process because observers generally experience changes between multiple perceptual states without corresponding changes in the stimulus (1). This phenomenon (“perceptual rivalry”) has been suggested to reflect a general strategy that balances a need for a decisive stable percept for action planning (2), against the need for rapid reinterpretation of sensory information that is often ambiguous or impoverished (3, 4). Neuroimaging and electrophysiological studies are beginning to tease apart the different aspects of neural activity that correlate with the perception or suppression of alternative perceptual states (for review see refs. 1 and 5). However, the mechanisms driving the switch in perception are less clear. To date, no physiological marker has been identified that shows any predictive relationship to the duration of stability between successive switch events.
Here we turn to pupil diameter, a physiological measure used frequently half a century ago, but generally disregarded in modern eye-tracking and imaging studies. A number of these older studies identified differential pupillary response to flashed lights in the dominant or suppressed eye during binocular rivalry (6–8). These findings remain intriguing; however, they are tangential to our current focus on the relationship between pupil diameter and the timing of perceptual rivalry switch events.
Results
Pupil diameter of the right eye in six naïve observers was recorded at 1 kHz during exposure to four different types of rivalry stimuli: a Necker cube, structure from motion, visual plaid, and auditory streaming. All measurements were recorded in the dark. After familiarization with the stimuli, subjects were presented each stimulus for 5 min and instructed to immediately report any perceptual switch by pressing one of two keys (“immediate report condition”).
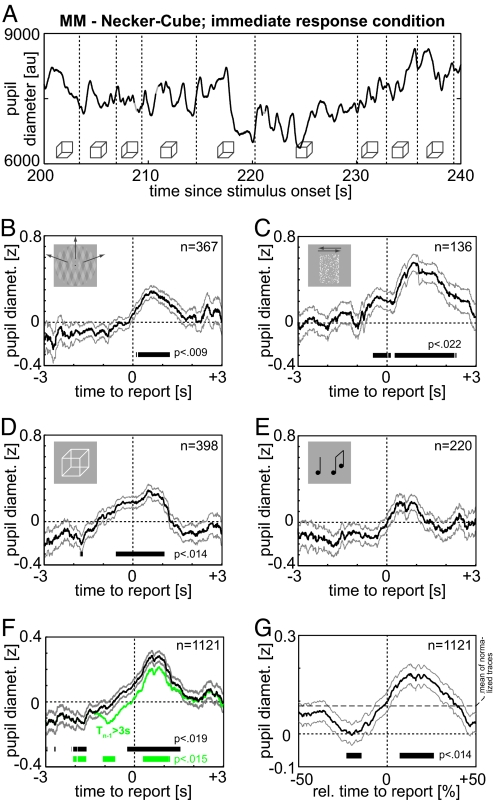
In each individual, pupil diameter varies considerably during constant presentation of an ambiguous visual stimulus (Fig. 1A). However, after aligning pupil diameter traces to the time of reported perceptual switches and pooling the data, one observes a sharp increase around the time of perceptual switching. This time course was qualitatively similar for all rivalry stimuli tested (Fig. 1 B–E), and for all individuals [supporting information (SI) Fig. 4]. A t test was used to compare pupil diameter to the 5-min mean (0 z score) at each of the 6,001 time points spanning ± 3 s from the reported switch. To correct for multiple comparisons, significance is asserted only for time points with a P value below that corresponding to an expected false discovery rate (FDR) of 0.05. Under this criterion, pupil diameter is significantly increased around the time of the perceptual switch in three of four stimuli (black indicators in Fig. 1 B–E) and for five of six subjects (SI Fig. 4 and SI Table 1). When pooling over all subjects and stimuli (Fig. 1F), the period of significance for the dilation response extends from 244 ms before to 1,552 ms after the reported switch (peaking at + 602 ms: P = 1.5 × 10−18). Surrogate analysis rules out statistical artifacts (uncorrected P > 0.12 for all time points). These findings provide evidence of a relationship between pupil dilation and perceptual switch events.
Fig. 1.
Time course of pupil response. (A) Pupil diameter during Necker cube presentation (40 s from 5 min total) in subject MM. Horizontal lines indicate times of button presses, Necker cube symbols the corresponding percept. Pupil diameter is in arbitrary units (AU) as recorded by the eye-tracker, which are linear in true diameter. For display purposes, diameter is interpolated during blinks (gray). (B–E) Pupil diameter normalized to zero mean and unit standard deviation (z score) and aligned to time of reported switch; mean and SEM. pooled across all switches of all subjects. Black lines denote periods significantly different from 0, at an expected FDR of 0.05 (t test, p < pFDR=0.05, threshold given in the figure). Insets are visualization aids only and not to scale (B, plaid; c, Structure from Motion (SfM); D, Necker cube; and E, auditory rivalry). (F) Black, All stimuli, representation as in B–E. Green, all stimuli but excluding preswitch durations <3 s (green and black trace overlap exactly for t < −1.5 s, as all analysis truncates traces at the midpoints between switches). (G) All data in normalized time frame (switch at 0, midpoints of dominance durations at ±50%). As z normalization uses absolute time, marker denotes significant difference from the mean of all normalized traces (dashed line).
Because the pupil response spans nearly 2 s, pupil dilation at any time point may be affected by either the preceding or the following switch. Indeed, if we minimize the influence of increased dilation stemming from the previous switch by excluding all short (<3 s) preswitch durations, the calculated preswitch diameter is systematically reduced, although the overall pattern of pupil dilation is conserved (Fig. 1F, green). Because the dominance durations exhibit large variability within and across subjects (means ranging from 2–155 s, SI Table 2), we reanalyzed pupil dilation with respect to a normalized time frame (9). This maps each dominance period to a unit interval, aligning the switches at time t = 0 and the midpoints of each dominance duration at ±50%. This makes it possible to measure the phase-shift of pupil-modulation relative to the surrounding switches. In this time frame, pupil dilation has a trough at −20%, i.e., a fifth cycle, before the switch (or four fifth cycles after the preceding one), and peaks at +13% after (= −87% before) each switch (Fig. 1G). The switch coincides with the strongest slope of pupil dilation, whereas the mid-point of a dominance period (±50%) is associated with relatively stable pupil diameters. This analysis shows that the rate of pupil dilation increase is maximal around the time of perceptual switching, and starts just before its report.
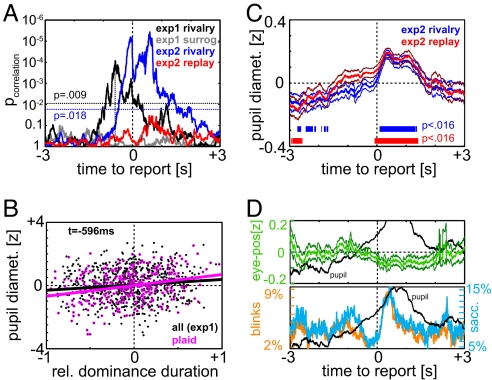
The timing of perceptual rivalry transitions is characteristically unpredictable. We were, therefore, interested to assess whether pupil dilation shows any correspondence with inter-switch interval (duration of sustained dominance). Because dominance durations vary considerably between subjects/conditions and may show systematic fluctuations over the course of the trial, we calculate a normalized measure of “postswitch” duration relative to the corresponding “preswitch” duration (for detailed results on absolute durations and the contribution of normalization, see SI Text). The relative measure is, by definition, 0 if both intervals have the same duration, positive (between 0 and +1) if the postswitch interval is longer than the preswitch interval, and negative (between −1 and 0) if it is shorter. Pooling across all subjects and rivalry types, there were a total of 1,121 postswitch durations calculated. To identify at which time point pupil diameter was most correlated with duration of postswitch stability, we correlated these 1,121 postswitch durations with the corresponding 1,121 pupil diameter values, for each time point within ±3 s of the switch (Fig. 2A black). Correlations were significantly different from 0 (at a threshold of pthresh,FDR=0.05 = 0.009, i.e., 0.05 FDR) for all time points between 745 ms before to 78 ms after the report of the switch, and peaked 0.596 s before (r = 0.13; P = 8.5 × 10−5, Fig. 2B). This shows that already 600 ms before the begin of a new dominance interval, pupil diameter is correlated to its duration. Surrogate control analysis rules out statistical artifacts (P > 0.09 for all time points, Fig. 2A gray). In short, the larger the pupil dilation around the time of perceptual selection, the more stable the subsequent percept will be.
Fig. 2.
Prediction of dominance durations and control conditions. (A) Significance of the correlation between relative postswitch dominance duration and pupil diameter plotted for each time point ± 3 s around the switch. The logarithmic scale indicates higher significance (lower P values) toward the top. Black, all data from experiment 1; pupil dilation offers the greatest prediction of subsequent perceptual stability 596 ms before the reported switch (r = 0.13, P = 8.5 × 10−5). Blue, all rivalry data from experiment 2. Red, replay data from experiment 2. The lack of significance in the replay condition shows that the prediction effect is not an artifact of analysis. For experiment 1, where no replay condition exists, this verification is done through surrogate analysis (gray). Horizontal lines indicate the P value thresholds corresponding to an expected FDR of 0.05. (B) Plot of correlation for data of experiment 1 at the time point of peak significance (t = −596 ms). Pink circles mark data from plaid stimulus, which are significant on their own right. (C) Experiment 2 pooled over subjects and stimuli. Red, replay; blue, rivalry. Significance markers for individual thresholds (FDR = 0.05) analogous to Fig. 1. Between the two traces there is no significant difference at any time-point up to an FDR of 0.63; no point after the switch exhibits significance even at an uncorrected 5% level (P > 0.12, for all two-sample t tests). (D) (Upper) Green, z normalized eye-position (distance from center) analyzed analogously to pupil dilation. Black, Pupil dilation trace in same scale for comparison. (Lower) Blink (red) and saccade (blue) frequency compared with pupil diameter trace (black from Fig. 1F). Traces are normalized to the same dynamic range, individual scales are given in the respective color. Data of experiment 1 is used here, but experiment 2 yields comparable results.
Although we find time points that are significant at an uncorrected level of 0.05, for all stimulus types, the effect is not robust enough to be seen for each individual subject and stimulus type. Only the plaid withstands the FDR correction [pmin = p(t = −584 ms) = 9.8 × 10−7 < pthreshFDR=0.05 = 0.009, Fig. 2B magenta]. Similarly, the effect was only large enough to reach corrected significance in one subject (MM) (pmin = 9.1 × 10−6 < pFDR=0.05 = 0.032). Notwithstanding possible differences between subjects and/or stimuli, this likely reflects the noisiness of the correlation measure (Fig. 2B), which demands a large number of measurements to exhibit significance.
To further test this prediction effect, six additional observers performed the original rivalry paradigm for two of the four stimuli (plaid and structure from motion, “experiment 2”) in a fixation and a free viewing condition. Each rivalry block was immediately followed by a “replay” block during which one of two strongly biased versions of the ambiguous stimulus were presented alternately at time intervals, matching the preceding pattern of reported rivalrous switches. Observers were asked to immediately report the stimulus-induced switch. After removing incorrect button-presses and unrealistically large reaction times (>2 s, this affects only eight of 1,161), the mean latency between stimulus switch and button response was 524 ms (std = 229 ms). Consistent with our initial findings we replicated the observed prediction effect in the 6 new subjects during rivalry (Fig. 2A blue). Importantly, however, no predictive relationship between pupil dilation and subsequent dominance duration was observed in replay (Fig. 2A red), despite the fact that the overall magnitude of the pupil response was similar in both conditions (Fig. 2C). Together, these results verify the correlation between pupil dilation and relative dominance duration as a true and robust effect, not simply a consequence of analysis method or the natural variability in the duration of consecutive dominance periods.
Eye position, blinks, and saccades have previously been reported to be related to rivalry switching (10, 11). Regarding eye position, we compared the time-course of pupil dilation in the fixation conditions of experiment 2 to those of the corresponding free-viewing data. There was no difference in the observed pupil response (SI Fig. 5), despite reductions in eye movements (SI Table 3). In addition, eye position (measured as distance to center) shows a much weaker modulation than pupil dilation when analyzed in the same z-normalized frame (Fig. 2D Upper), and did not reach significance at any time point. Furthermore, the relation between eye position and switching is, if existent, inconsistent between observers and stimuli (SI Fig. 6), in sharp contrast to the consistent increase of pupil dilation (SI Fig. 4). The same holds for all measures of eye position tested, including absolute positions and projections on the cardinal axes (data not shown). Therefore, we can rule out any confounding effect of eye position in the observed pupil dilation effects. Saccades and blinks do show modulation; however, their frequency dips after pupil dilation starts rising (257 ms and 290 ms before the switch, respectively). The frequency of saccades and blinks then rises rapidly to their peak (at 476 ms and 435 ms, respectively) but diminishes again before the pupil dilation response subsides (Fig. 2D Lower). This difference in time course, which is also observed in normalized time (not shown), illustrates clearly that pupil dilation exhibits the earliest and most robust effect. Although this does not exclude an important role for eye position, saccades, blinks, and other factors like attention in perceptual rivalry, it rules out eye movements and blinks as the cause of the observed pupil dilation effects.
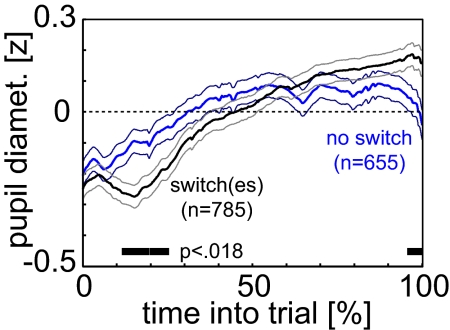
In the “counting” condition of experiment 1, we control whether the relation between pupil diameter and switching is contingent on an immediate overt motor response. Each subject performed 60 trials for each stimulus, whose durations matched the median dominance period of the corresponding “immediate report” condition. Subjects did not press any key during presentation, but silently counted and reported the number of switches after each trial. For about half of the trials, at least one switch was reported (785/1,440 = 55%). On average, pupil diameter at the beginning of the no-switch trials was larger than in trials for which at least one switch was reported (Fig. 3). Toward the end of the trial, this relation is reversed. Although the difference fails to reach significance at an FDR of 0.05, a trend is visible. At an FDR of 0.1 (corresponding to a P value threshold of 0.018), both the initial and the final differences are significant. This result is consistent with the immediate report data: smaller pupil diameter is followed by shorter dominance durations, thereby increasing switch frequency over a subsequent fixed interval. After a switch there is a rapid increase in pupil dilation, consistent with a larger endpoint if a switch is reported. Hence, data in the absence of an overt motor response is consistent with the immediate report data. Our pupil dilation response is, therefore, unlikely to be an effect of the motor response itself.
Fig. 3.
In the absence of a motor response, average pupil diameter in “counting” is clearly distinguishable for trials with a switch (black) and without switch (cyan). Thin lines denote SEM over trials. Marker denotes periods in which mean traces are significantly different from another at an FDR of 0.1 (no significant points for FDR of 0.05) using two-sample t tests.
Discussion
Our data demonstrate a clear link between pupil dilation and perceptual switches induced by ambiguous stimuli. For the three visual and one auditory rivalry stimuli tested, pupil diameter was found to increase at the time of a perceptual transition. The magnitude of dilation around the time of the switch was a significant predictor of the subsequent duration of perceptual stability. Analysis showed that observed pupil dilation could not be explained as a consequence of blinks, saccades, eye position, or the manual report.
We interpret these results as evidence that norepinephrine (NE) released from the locus coeruleus (LC) plays a critical role in perceptual rivalry. We base this conclusion on the fact that pupil dilation is mediated almost exclusively via NE released from the LC (through stimulation of α-adrenoceptors on the iris dilator muscle and postsynaptic α2-adrenoceptors within the neighboring Edinger–Westphal nucleus, which projects to the ciliary ganglion controlling the dilation of the iris) (12, 13). This dilation response is distinct from the strong contractions exhibited during the pupillary light-reflex mediated by acetylcholine (via the iris sphincter muscle) (13). Therefore, in conditions of constant low light levels, pupil diameter is a reliable and accessible measure of NE levels (13–15). Although other neurotransmitters, such as serotonin, are known to influence dilation, these effects are similarly known to be mediated via the LC–NE complex (16).
Current evidence suggests that the LC–NE complex is involved in optimizing the balance between exploitation (continue what you are doing) and exploration (disengage and choose between one of the alternative possibilities) (14, 17, 18). This model of behavioral selection has a number of striking similarities with current models of perceptual rivalry (1, 2), although this is yet to be recognized in the literature. In both cases, representations of all available alternatives are believed to compete (outside of awareness) through an interaction of mutually inhibitory neural connections or feedback mechanisms (19). As soon as one of the alternatives begins to dominate and reaches an activation threshold, “phasic” activation of LC neurons is triggered resulting in rapid bursts of NE release (17). NE enhances the evoked excitatory or inhibitory responses, while simultaneously reducing the spontaneous activity of individual neurons (20, 21). This increases the gain within the competing neural populations rapidly “tipping the balance” in favor of the dominant option while suppressing the alternatives. In short, LC–NE activity consolidates the behavioral decision. In respect to perceptual rivalry, therefore, the predictions made by the LC–NE model are very explicit: the first evidence of pupil dilation should be seen (i) immediately after the resolution of perceptual competition (Fig. 1 B–F), (ii) before the execution of a manual report (Fig. 1G), and (iii) in the absence of a behavioral response (Fig. 3). All three requirements of this model are consistent with the timing of results presented here. An additional important feature of the LC-NE model is that each activation is followed by a transient inhibition of firing (14, 17). Therefore, the duration of perceptual “stability” should be correlated with size of the phasic NE burst (degree of pupil dilation), before the perceptual switch—the exact relationship seen in our results.
Although the current experiments used a specific set of ambiguous stimuli, we assume that the general findings extend to natural visual and auditory perception, where available sensory information is often ambiguous or impoverished (for a detailed account of the similarities between normal and ambiguous viewing conditions, see ref. 3). It should also be noted that the role of NE proposed here does not preclude the existence of hierarchical, mutually inhibitory networks that are thought to underlie rivalry (1). Rather the most parsimonious interpretation of our results with the LC–NE model of behavioral optimization, presupposes the existence of underlying competing neural populations. The bursts of cortex-wide release of NE provide a mechanism by which the outcome of competitive interactions can be rapidly coordinated across distributed neural populations (22). Small individual differences in the LC–NE system would explain the fact that individual variation in perceptual switch rates are consistent across different rivalry types (23, 24), whereas the distributed release of NE would account for instances of perceptual entrainment arising when multiple ambiguous stimuli are temporally interleaved (25). Given that the LC–NE system is believed to be primarily involved in behavioral orienting (14) and cognitive engagement (13, 26), this model can also account for results from a variety of recent studies demonstrating clear links between the selection of dominance during rivalry and the mechanisms driving switches in attentional focus (9, 27–30). Even Levelt's paradoxical observation that increases in the salience of one rivalry target will lead to an increase in its overall predominance, through reduced suppression durations rather than prolonged dominance durations, can be explained by the fact that NE can lead to increases in responsiveness to subthreshold (nondominant) stimuli, without a corresponding increase in responsiveness to suprathreshold (dominant) stimuli (20).
Perceptual alternations during rivalry, follow a stochastic time course (1). It is worth considering, therefore, whether the firing pattern of LC neurons can solely account for the characteristic timing of rivalry transitions. Although it is generally agreed that NE plays a role in consolidating the new posttransition state, it remains debated whether NE also plays a role in triggering a new transition event (for review see refs. 14 and 22). Aston-Jones and Cohen (14) propose that increased baseline “tonic” NE release leads to greater nonselective neural responsivity and connectivity. This, in turn, effectively destabilizes the system and increases the chance that a new “task-irrelevant” event will reach threshold. This model is difficult to reconcile with the stochastic time course of rivalry, as it necessitates interdependence between the magnitude of each phasic LC–NE response and the timing of the preceding and subsequent LC-NE responses. If, however, we adopt the simplified model by Bouret and Sara (22), phasic NE release is only implicated in consolidating new state transitions, effectively resetting the dynamic interactions within the newly configured network, while playing no role in triggering or facilitating the subsequent transition event. Because the stimulus and tasks requirements remain constant during rivalry, this reset model of NE could, at least partially, account for the stochastic time course of perceptual switches. In this case, each burst of NE release is essentially triggered independently, although the magnitude of release (and the associated period of perceptual dominance) may vary on each occasion, depending on any number of factors such as those associated with changes in cognitive, neurochemical, or cortical function. This model (22) is also more consistent with our finding that pupil responses are basically identical for the endogenously driven perceptual switches of rivalry and the stimulus-induced visual transients of the replay condition (Fig. 2C). Although an accurate interpretation of our results clearly depends on the validity of different LC–NE models, comparing their predictions with these types of human data may serve to test and further refine animal-based models of NE function.
The data and the theory both suggest that the relevant events are happening within a rather small time window between perceptual selection and response initiation. Given that neuromodulators have a reputation for being relatively slow acting, it is important to consider whether the proposed involvement of the LC–NE system is physiologically plausible. In monkey, LC phasic response is found to occur ≈100 ms after a relevant event (31). It then takes an additional 60–70 ms for the activity within the LC nucleus to be conducted along the LC projections through to frontal cortex (≈100 ms for occipital cortex). Therefore, the delay from the triggering event to NE release at the site of neural competition would be ≈150–200 ms (31). This time course is well within the range required if NE is having a functional influence at the original site of neural competition, before the manual report of the perceptual transition.
All of the results reported indicate that pupil diameter increases around the time of a perceptual switch. We interpret this as evidence for a role of NE in consolidating the perceptual transition by aiding rapid reconfiguration of the neural networks underlying the perceptual representation. However, based on our results, it is impossible to speculate about the exact point in the transition process in which NE release is involved. At one extreme, the model proposed by Aston-Jones and Cohen (14) suggests a role for NE in driving the switch by promoting the dominance of the previously suppressed alternative. At the other extreme, it cannot be ruled out that NE is released simply as a consequence of a change in arousal/attention triggered by the arrival of a newly dominant percept. Indeed, there is a lot of evidence linking NE and attention (32). In addition, it is known that there are projections between the LC and regions of the frontal cortex (33), although electrophysiological data suggests that LC activity reliably precedes associated responses in frontal cortex (34). Future research is needed, therefore, before any firm conclusions can be drawn as to the exact time course of the response and any causal relationship between shifts in attentional focus and pupil dilation in perceptual rivalry.
The interpretation of our results, not the data themselves, depends on pupil dilation being a faithful measure of LC activity and associated NE release. Although there is strong physiological evidence for this relation under constant illumination (13–15), further direct measures are needed to confirm the suggested role of NE in perceptual rivalry and its connection to other neurotransmitter systems previously implicated in rivalry (28).
To our knowledge, this is the first study to identify a physiological measure that shows any relation to the duration of perceptual stability and is one of the few studies to identify physiological changes that can be linked specifically to perceptual switch events. Despite their surprising nature, our results do not conflict with any evidence from rivalry research. Instead, they are not too dissimilar from recent speculations that extend the relevance of rivalry well beyond sensory processing and visual attention (2–4). Similarly, the interpretation of our findings does not contradict any current evidence about the function of the LC–NE system in the consolidation of cognitive and behavioral state-transitions. Instead, the contribution of the current study is that it provides the first evidence that LC–NE system may play a similar role in perception. Given that pupil diameter is easily measured by standard eye-tracking technologies, it is hoped that this study will motivate others to consider these, previously disregarded, data in a new light.
Materials and Methods
Observers.
Twelve volunteers from the Caltech community (age: 18–33; nine male, three female) participated, six in experiment 1 and six in experiment 2. All had uncorrected normal vision, were naïve to the purpose of the study, and gave informed written consent. All experiments conformed to Institutional Guidelines for experiments with human subjects and to the Declaration of Helsinki.
Stimuli.
The plaid stimulus was composed of two superimposed square-wave gratings (dark phase, 7 cd/m2; bright phase, 13 cd/m2) of wavelength 33 pixels (0.9°). The first grating was rotated by 20° clockwise, the second one by 20° counterclockwise relative to the vertical axis. Both gratings drifted upward at 1/30 cycles per frame (8.3 ms), i.e., 132 pixels/s or 3.7°/s, and were linearly averaged. The complete stimulus was contained in a disk of radius 100 pixels (2.8°), which was surrounded by a 10 cd/m2 gray background. A central disk of radius 15 pixels (0.4°) was filled with background color and a central black dot. Observers were asked to report only switches between coherent (“plaid”) and component (“gratings”) motion, and not to report switches from leftward to rightward motion.
The lines of the Necker cube were 20 cd/m2 in luminance, 1 pixel wide and presented on a 10 cd/m2 background. The length of the cube's faces was 100 × 100 pixels (2.8° × 2.8°). To avoid biasing subjects as to which cue to use for the percept, they were instructed on the two different percepts by using a biased version of the cube.
The “structure from motion” (SfM) stimulus represented a cylinder 150 pixels (4.2°) by 100 pixels (2.8°). Each horizontal line contained two dots (3 × 3 pixel, 20 cd/m2), with an average speed of 100 pixels/s (2.8°/s), which varied sinusoidally across the stimulus to evoke the impression of a rotating cylinder cycling at 0.5 Hz.
The auditory rivalry stimulus is described in detail in ref. 35. In brief, two tones “A” and “B” of different pitch (A, 500 Hz; B, 700 Hz) were presented for 50 ms, in a repeating cycle of A–B–A–silence. The duration between consecutive A tones was 240 ms, with a B tone presented 120 ms after every second A tone. This stimulus evokes two distinct percepts: either a coherent A–B–A “galloping” sound, or two clearly isolated “streams” of A and B tones. During the auditory stimulus, the screen presented a homogeneous 10 cd/m2 gray.
Setup.
The study was conducted in a dark room with black walls, resulting in ambient light levels below 0.001 cd/m2. Visual stimuli (plaid, Necker, SfM) were presented on a 20-inch CRT monitor located 80 cm from the subjects; the auditory stimulus was presented through two speakers located adjacent to the monitor. Pupil diameter was measured by using a noninvasive infrared “Eyelink-1000” (SR Research, Osgoode, ON, Canada) eye tracker at a rate of 1,000 Hz. All presentation used Matlab (Mathworks, Natick, MA) and its psychophysics and eyelink toolbox extensions (http://psychtoolbox.org) (36).
Experimental Design.
Experiment 1 was subdivided into four sessions, one for each stimulus. The order of the visual stimuli was balanced across observers, and the auditory stimulus was used in the last session for all subjects. Before each session the eye-tracker was calibrated and validated by using the procedures recommended by the manufacturer. Following calibration, observers were given 3 min to passively observe the stimulus. After this familiarization period, observers were asked whether they had perceived the rivalry and to assign keys to the two distinct percepts. In the “immediate report” condition, the rivalry stimulus was presented continuously for 5 min, and observers pressed the respective key whenever their percept switched. The “counting” condition consisted of 60 trials. In each trial, the stimulus was presented for the median inter-switch interval of the preceding block, but at least 2 s and maximally 10 s. Observers were asked to count the number of switches during each trial and reported the number at the end of the trial. In all blocks, observers were free to move their eyes; in the auditory conditions, they were additionally reminded to look at the screen.
Experiment 2 replicated the immediate report condition for plaid and SfM stimuli. In addition, both stimuli were used in a “fixation” condition, in which observers were instructed to fixate a central black cross on the stimulus. After each 5-min rivalry presentation, a replay condition was shown by using biased variants of the same stimulus. For plaid, coherent motion was induced by a wide angle (±70°), component motion by a narrow angle (±10°) of the gratings relative to the vertical. For SfM, only the “front” surface was displayed, i.e., dots moving either to the left or to the right. In all other respects (contrast, speed, dot density, etc.) stimuli were identical to the ambiguous versions.
Data Analysis.
Adjustment for multiple comparisons.
Throughout the paper, we base our analysis on statistical tests at each given time point. Because 6,001 data points (±3 s around the switch, recording at 1 kHz) or 10,000 (for resampled traces in counting and normalized analysis) are tested, a correction for multiple comparisons is required. Although most of the pooled results would withstand even a Bonferroni adjustment (e.g., to alpha = 0.05/6001 = 8.3 × 10−6, e.g., right column of SI Table 1), Bonferroni is overly conservative. In particular, Bonferroni adjustment gets stricter with higher sampling rate and our data are already densely sampled (samples neighboring in time are highly correlated). The issue of appropriate alpha-level adjustment for our time series is analogous to thresholding significance in activity maps. We thus employ a method widely used in fMRI data analysis (37), by constraining an expected “false discovery rate” (FDR). Across the paper, we assign “significance” to a time point if its P value is below the threshold resulting from the FDR procedure (using the Benjamini and Hochberg method; ref. 38) at an expected FDR of 0.05. The thus adjusted alpha-level depends on the distribution of P values, but always falls between the uncorrected level (P = 0.05) and the Bonferoni correction (P = 0.05/6001). The former would result, if all points are below 0.05; the latter, if exactly one point is below 0.05.
Notation.
Because the threshold obtained from the FDR method depends on the distribution of P values, the threshold for significance will vary between different datasets. Therefore, all P values are reported as uncorrected values, but are called “significant” only if they are below the threshold corresponding to an expected FDR of 0.05 (this is always stricter than a threshold of uncorrected 0.05). In the text, we report this threshold as pthresh,FDR=0.05 along with the P value. For pupil dilation traces, we denote the threshold at the significance indicator in the corresponding color (Figs. 1, 2C, 2D, and 3, and SI Fig. 5) or use a horizontal line to mark significance of correlations (Fig. 2A). In Fig. 3, we use an FDR of 0.1, which is less strict than FDR = 0.05, but still stricter than no correction. The P value threshold for asserting significance by the FDR procedure is always lower than 0.05. Evidence against a significant effect (or more precisely: no evidence for a significant effect) is therefore stronger, if P values not only are above this threshold, but also remain above uncorrected 0.05 at all time points. This is particularly the case for all surrogate controls.
Normalizing pupil diameter and alignment of switches.
To make data comparable across observers, we normalized the pupil diameters to z scores. In the “immediate report” condition, mean and SD were computed across the whole 5-min block (excluding blinks). Pupil diameter was then aligned to times of button-presses. To avoid using the same data point multiple times, we truncated each trace at the midpoints between switches: For each button press tn, we compute the distance to the preceding button press (dominance duration) Tn = tn − tn−1 and only used the data up to half this interval for the preceding switch (i.e., t > tn−1 + Tn/2) and the succeeding switch (i.e., t < tn + Tn+1/2), when analyzing the perceptual switch reported at tn.
Normalized time axis.
To generate a normalized representation of the relative phase at which pupil modulation occurs during the dominance-transition cycle, we adopt a technique proposed earlier for eye position (9): rather than averaging in absolute time, each dominance period is normalized to unit time. Technically, for a switch at tn with preceding dominance duration Tn = tn − tn−1 the trace between tn − Tn/2 and tn is remapped to the interval [−50%, 0]. Similarly, the interval [tn, tn + Tn+1/2] was mapped to [0, +50%]. After resampling each trace to have equal resolution of 10,000 data points (by spline interpolation) in the [−50%, +50%] interval, traces can be averaged akin to the absolute time analysis. In the normalized representation, switches occur at t = 0, halfway points between switches correspond to t = ±50%, and the time frame is periodic at length 1 (50% before the next equals 50% after the previous switch).
Surrogate analysis.
To control our analyses for statistical artifacts, we generated surrogate data: within one block, i.e., within the same subject and rivalry type, we randomly reshuffled the times of switching, while keeping the number of switches and the distribution of inter-switch intervals identical to the original data. The pupil diameter trace remained unchanged and all analysis was identical to the original data. Finding no evidence for significance in surrogate data, therefore, ensures that the effects found in the main analyses do not result from the distribution of interswitch intervals, from normalization, averaging, or any other analysis artifacts.
Eyeblinks and saccades.
For the main analysis, we excluded periods around eye blinks. We treated the time during which a blink was recorded by the eyelink system, and 100 ms before its onset and 100 ms after its offset as missing data. All analyses were repeated with interpolated pupil diameter for blink periods, yielding qualitatively very similar results (data not shown). For the analysis of eye-blinks themselves (Fig. 2D) we used the same criterion, but without the conservative ±100-ms extension. Periods of saccades were also defined as reported by the eye-link system. Blinks and saccades were treated as discrete events, i.e., their traces are set to 1 if there is a blink/saccade and 0 otherwise.
Correlation between pupil diameter and dominance durations.
We denote the times of reported perceptual switching as tn and the preceding and following dominance durations as Tn = tn − tn−1 and Tn+1 = tn+1 − tn, respectively. We define the “relative postswitch (dominance) duration” for the switch at tn as follows:
and refer to 1/(Tn+1 + Tn) as “normalization factor.” We now fix a time t0 relative to the switches, and consider for each tn the pupil diameter d at the time tn + t0. This provides a set of 1,121 pupil diameters for t0: dn(t0) = d(tn + t0). We then compute r(t0) as the correlation between dn(t0) and Dn across all n (Fig. 2B). Furthermore, we compute p(t0) as the P value for the null hypothesis that r(t0) is equal to 0. That is, for each t0, we compute a correlation (and the corresponding P value) over 1,121 values. By applying this analysis for all 6,001 t0 within the ±3 s around the switch, we obtain traces r(t0) and p(t0) (Fig. 2A).
Supplementary Material
ACKNOWLEDGMENTS.
The work was supported by Swiss National Science Foundation Grant PA00A-111447 (to W.E.); the Defense Advanced Research Planning Agency/National Geospatial Intelligence Agency; the National Science Foundation; the National Institute of Mental Health; and the National Health and Medical Research Council (Australia) C.J. Martin Fellowship 368525 (to O.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707727105/DC1.
References
- 1.Blake R, Logothetis N. Visual competition. Nat Rev Neurosci. 2002;3:13–21. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- 2.Leopold D, Logothetis N. Multistable phenomena: Changing views in perception. Trends Cogn Sci. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- 3.Andrews T, Purves D. Similarities in normal and binocular rivalrous viewing. Proc Natl Acad Sci USA. 1997;94:9905–9908. doi: 10.1073/pnas.94.18.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pettigrew J. Searching for the switch: Neural bases for perceptual rivalry alternations. Brain Mind. 2001;2:85–118. [Google Scholar]
- 5.Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10:502–511. doi: 10.1016/j.tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Bárány E, Halidén U. Phasic inhibition of the light reflex of the pupil during retinal rivalry. J Neurophysiol. 1948;11:25–30. doi: 10.1152/jn.1948.11.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Lowe S, Ogle K. Dynamics of the pupil during binocular rivalry. Arch Opthalmol. 1966;75:395–403. doi: 10.1001/archopht.1966.00970050397017. [DOI] [PubMed] [Google Scholar]
- 8.Richards W. Attenuation of the pupil response during binocular rivalry. Vision Res. 1966;6:239–240. doi: 10.1016/0042-6989(66)90044-7. [DOI] [PubMed] [Google Scholar]
- 9.Einhäuser W, Martin K, König P. Are switches in perception of the necker cube related to eye position? Eur J Neurosci. 2004;20:2811–2818. doi: 10.1111/j.1460-9568.2004.03722.x. [DOI] [PubMed] [Google Scholar]
- 10.van Dam L, van Ee R. The role of (micro)saccades and blinks in perceptual bistability from slant rivalry. Vision Res. 2005;45:2417–2435. doi: 10.1016/j.visres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 11.van Dam L, van Ee R. The role of saccades in exerting voluntary control in perceptual and binocular rivalry. Vision Res. 2006;46:787–799. doi: 10.1016/j.visres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Yoshitomi T, Ito Y, Inomata H. Adrenergic excitatory and cholinergic inhibitory innervations in the human iris dilator. Exp Eye Res. 1985;40:453–459. doi: 10.1016/0014-4835(85)90158-7. [DOI] [PubMed] [Google Scholar]
- 13.Loewenfeld I. The Pupil: Anatomy, Physiology, and Clinical Applications. Detroit: Wayne State Univ Press; 1993. [Google Scholar]
- 14.Aston-Jones G, Cohen J. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 15.Koss M. Pupillary dilation as an index of central nervous system α2-adrenoceptor activiation. J Pharmacol Methods. 1986;15:1–19. doi: 10.1016/0160-5402(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Ramage A, Koss M. Pharmacological studies of 8-oh-dpat-induced pupillary dilation in anesthetized rats. Eur J Pharmacol. 2004;489:207–213. doi: 10.1016/j.ejphar.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Usher M, Cohen J, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- 18.Devilbiss D, Page M, Waterhouse B. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurley L, Devilbiss D, Waterhouse B. A matter of focus: Monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Waterhouse B, Moises H, Woodward DJ. Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res. 1998;790:33–44. doi: 10.1016/s0006-8993(98)00117-6. [DOI] [PubMed] [Google Scholar]
- 22.Bouret S, Sara S. Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Brascamp J, van Ee R, Pestman W, van den Berg A. Distrtibutions of alternation rates in various forms of bistable perception. J Vis. 2005;5:287–298. doi: 10.1167/5.4.1. [DOI] [PubMed] [Google Scholar]
- 24.Carter O, Pettigrew J. A common oscillator for perceptual rivalries? Perception. 2003;32:295–305. doi: 10.1068/p3472. [DOI] [PubMed] [Google Scholar]
- 25.Maier A, Wilke M, Logothetis N, Leopold D. Perception of temporally interleaved ambiguous patterns. Curr Biol. 2003;13:1076–1085. doi: 10.1016/s0960-9822(03)00414-7. [DOI] [PubMed] [Google Scholar]
- 26.Beatty J, Lucero-Wagoner B. In: Handbook of Psychophysiology. 2nd Ed. Cacioppo J, Tassinary L, Berntson G, editors. Cambridge, UK: Cambridge Univ Press; 2000. [Google Scholar]
- 27.Mitchell J, Stoner G, Reynolds J. Object-based attention determines dominance in binocular rivalry. Nature. 2004;429:410–413. doi: 10.1038/nature02584. [DOI] [PubMed] [Google Scholar]
- 28.Carter O, et al. Psilocybin links perceptual rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology. 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- 29.Paffen C, Alais D, Verstraten F. Attention speeds binocular rivalry. Psychol Sci. 2006;17:752–756. doi: 10.1111/j.1467-9280.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- 30.Stoner G, Mitchell J, Fallah M, Reynolds J. Interacting competitive selection in attention and binocular rivalry. Prog Brain Res. 2005;149:227–234. doi: 10.1016/S0079-6123(05)49016-0. [DOI] [PubMed] [Google Scholar]
- 31.Aston-Jones G, Foote S, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience. 1985;15:765–777. doi: 10.1016/0306-4522(85)90077-6. [DOI] [PubMed] [Google Scholar]
- 32.Robbins T. Cortical noradrenaline, attention and arousal. Psychol Med. 1984;14:13–21. doi: 10.1017/s0033291700003032. [DOI] [PubMed] [Google Scholar]
- 33.Arnsten A, Goldman-Rakic P. Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res. 1984;306:9–18. doi: 10.1016/0006-8993(84)90351-2. [DOI] [PubMed] [Google Scholar]
- 34.Bouret S, Sara S. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur J Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- 35.Snyder J, Carter O, Lee S, Hannon E, Alain C. Effects of context on auditory stream segregation. J Exp Psychol: Hum Percept Perform. 2008 doi: 10.1037/0096-1523.34.4.1007. in press. [DOI] [PubMed] [Google Scholar]
- 36.Cornelissen F, Peters E, Palmer J. The eyelink toolbox: Eye tracking with matlab and the psychophysics toolbox. Behav Res Methods Instrum Comput. 2002;34:613–617. doi: 10.3758/bf03195489. [DOI] [PubMed] [Google Scholar]
- 37.Genovese C, Lazar N, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.