Abstract
The adaptor protein AP-1 is the major coat protein involved in the formation of clathrin-coated vesicles at the trans-Golgi network. The prevailing view is that AP-1 recruitment involves coincident binding to multiple low-affinity sites comprising adenosine diphosphate ribosylation factor 1 (Arf-1)–guanosine triphosphate (GTP), cargo sorting signals, and phosphoinositides. We now show that binding of cargo signal peptides to AP-1 induces a conformational change in its core domain that greatly enhances its interaction with Arf-1–GTP. In addition, we provide evidence for cross talk between the dileucine and tyrosine binding sites within the AP-1 core domain such that binding of a cargo signal to one site facilitates binding to the other site. The stable association of AP-1 with Arf-1–GTP, which is induced by cargo signals, would serve to provide sufficient time for adaptor polymerization and clathrin recruitment while ensuring the packaging of cargo molecules into the forming transport vesicles.
Introduction
The adaptor protein complex AP-1 (a heterotetramer composed of γ, β1, μ1, and σ1 subunits) plays a major role in the assembly of clathrin-coated vesicles (CCVs) at the TGN, serving to select and link cargo molecules with the growing clathrin lattice. AP-1 binds cargo molecules, mainly via two types of sorting determinants, a tyrosine-based YXXφ motif (where φ is a bulky hydrophobic residue), which binds to the μ1 subunit, and the dileucine-based [D/E]XXXL[L/I/M] sequence, which binds to the γ/σ1 hemicomplex (Traub, 2005). The prevailing model of AP-1 targeting to the TGN ascribes a major role to the small GTPase ADP ribosylation factor 1 (Arf-1) as the primary docking site in the initial recruitment step. Activation of Arf-1 involves GTP-for-GDP exchange, which is catalyzed by guanine nucleotide exchange factors, which in turn exposes the covalently linked myristoyl moiety on the Arf-1 allowing it to insert into membranes. It is thought that the ensuing conformational change in the membrane-associated GTP-bound form of Arf-1 is what allows it to weakly associate with and initially recruit AP-1 (Edeling et al., 2006). Additional components of the TGN, such as phosphoinositides and the cytosolic domains of sorting signal–bearing cargo proteins, are believed to function together with Arf-1 in providing a combinatorial targeting mechanism for the Golgi localization of AP-1. This concept of coincidence detection suggests that the association of AP-1 with the various TGN components, though insufficient in themselves, is significantly enhanced through multiple simultaneous interactions that together result in the proper membrane targeting of AP-1 (Carlton and Cullen, 2005). In support of this hypothesis, it has been demonstrated that a membrane-anchored tyrosine-based sorting signal, together with myristoylated Arf-1–GTP, constitutes a minimal machinery for the recruitment of AP-1 to chemically defined liposomes and that the process can be further stimulated by specific phosphoinositides (Crottet et al., 2002; Baust et al., 2006). This finding that myristoylated Arf-1 alone cannot recruit AP-1 to liposomes is indicative of the fact that whatever conformational switch occurs in the membrane-associated Arf-1 is insufficient for binding AP-1.
An alternate mechanism, which is not mutually exclusive to the coincident detection hypothesis, is one where sorting signal binding to AP-1 influences its association with Arf-1–GTP. To distinguish between these two models, we synthesized soluble peptides corresponding to known dileucine- or tyrosine-based sorting signals and determined their effects on the interaction of AP-1 with activated Arf-1. Our experiments show that both types of sorting determinants are able to strongly enhance the AP-1–Arf-1–GTP interaction. We further demonstrate that the peptide-induced stimulation of this interaction is accompanied by a conformational change in the adaptor core domain, which we propose serves to stabilize the association of AP-1 with Golgi membranes.
Results and discussion
AP-1 binding to Arf-1–GTP is stimulated by dileucine- and tyrosine-based sorting signals
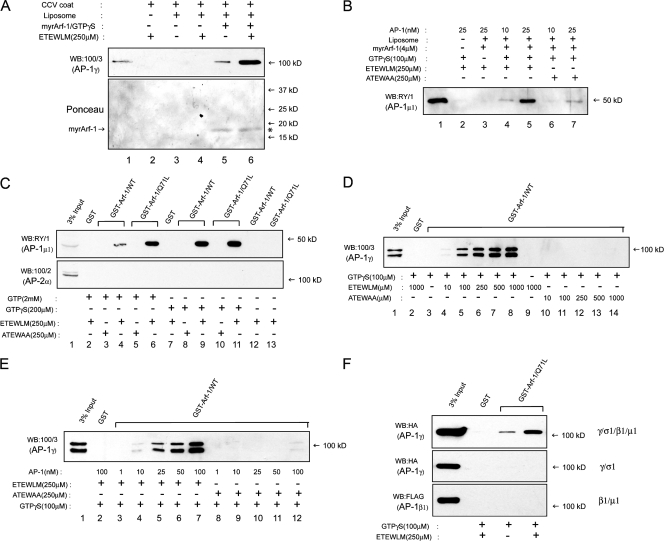
In considering a possible role for sorting signals in the modulation of AP-1 function at the TGN, we first tested whether a soluble 14-aa peptide molecule corresponding to the cation-independent mannose 6-phosphate receptor (CI-MPR) internal dileucine-type sequence (ETEWLM) could affect AP-1 binding to activated Arf-1 in a liposome recruitment assay. As shown in Fig. 1 A, recruitment of AP-1 from a CCV coat fraction was greatly increased in the presence of the wild-type (WT) ETEWLM peptide (compare lanes 5 and 6). This recruitment required activated Arf-1, as no AP-1 association occurred in the absence of myristoylated Arf-1–GTPγS (Fig. 1 A, lane 4). To rule out the idea that some other component of the CCV coat fraction mediated the peptide-stimulated association of AP-1 with liposome, recruitment assays were performed with purified AP-1. As in Fig. 1 A, the WT ETEWLM (Fig. 1 B, lanes 4 and 5), but not the mutant ATEWAA, peptide (Fig. 1 B, lanes 6 and 7) stimulated recruitment of purified AP-1 in a GTPγS-dependent manner. The ETEWLM peptide also stimulated binding of cytosolic AP-1 to Arf-1, immobilized as a GST fusion protein. Fig. 1 C shows that AP-1 from bovine brain cytosol (BBC) failed to bind either WT GST–Arf-1 or the constitutively active Q71L mutant (which is unable to hydrolyze GTP) in the presence of GTPγS, which is in agreement with previous observations that AP-1 and Arf-1 do not interact in solution (Austin et al., 2000). In contrast, the ETEWLM, but not the ATEWAA, peptide greatly stimulated binding of cytosolic AP-1 to both forms of Arf-1 in a GTPγS-dependent manner (Fig. 1 C, lanes 8–13) or to Arf-1 Q71L in a GTP-dependent manner (Fig. 1 C, lanes 3–6). There was no binding to AP-2 under any condition, which highlights the specificity of the AP-1 interaction with Arf-1. This effect was dependent on the concentration of both free peptide (Fig. 1 D) and purified adaptor (Fig. 1 E). Furthermore, the cargo signal–dependent binding of AP-1 to Arf-1 requires the tetrameric form of the adaptor complex, as neither the γ/σ1 nor β1/μ1 hemicomplex bound to GST–Arf-1 Q71L in the presence of the ETEWLM peptide (Fig. 1 F). This result is consistent with the finding that Arf-1 is cross-linked to both the γ and β1 subunits of AP-1 on immature secretory granule membranes (Austin et al., 2000), which suggests that productive binding of AP-1 to activated Arf-1 requires an interaction of Arf-1 with both large subunits of the adaptor.
Figure 1.
Binding of the CI-MPR ETEWLM peptide to AP-1 stimulates its association with activated Arf-1. (A) Recruitment of CCV-derived AP-1 to soybean liposomes is greatly enhanced only in the presence of 250 μM ETEWLM peptide (top, compare lanes 5 and 6) even though similar amounts of activated Arf-1 are found on the liposomes, as seen in the Ponceau stain of the blot (bottom, *). Lane 1 represents 5% of the CCV coat fraction input. (B) Recruitment of 25 nM AP-1 purified from BAC to liposomes is stimulated by the ETEWLM peptide (lanes 5 vs. 7) in a GTPγS-dependent manner (lanes 3 vs. 5). The reactions contained 4 μM of myristoylated Arf-1, 100 μM GTPγS, and the indicated concentrations of AP-1. Lane 1 represents 20% of the input from the reaction containing 25 nM AP-1. (C) Pulldown of AP-1 from BBC with both WT GST–Arf-1 and the Q71L mutant preloaded with 2mM GTP or 100 μM GTPγS is dependent on the presence of the ETEWLM peptide. No binding to AP-2 was detected under these conditions. (D and E) Binding of AP-1, purified from BAC, to GST–Arf-1 is a function of the concentration of the ETEWLM peptide (D) and purified adaptor (E). The AP-1 concentration was kept constant at 25 nM in D, whereas the peptide concentrations were kept constant at 250 μM in E. The doublet observed on the blots in D and E, and in subsequent experiments where BAC served as the source of cytosolic AP-1, is the consequence of partial proteolysis of the appendage domain of the γ subunit of AP-1 in our adrenal cytosol preparations. The γ subunit of AP-1 in our bovine brain CCV coat preparations did not undergo such proteolysis, as shown in A. (F) The dileucine peptide–dependent binding of AP-1 to activated GST–Arf-1 requires the tetramer because neither hemicomplex bound.
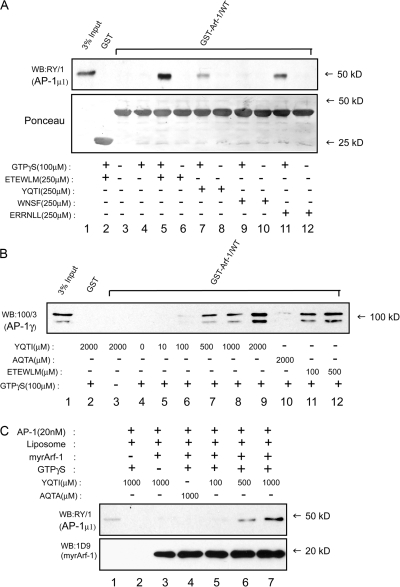
To ascertain if other cargo sorting signals are also functional in our assay, we tested two other soluble peptides, YQTI and ERRNLL, corresponding to known sorting signals in Lamp1 and Vamp4, respectively (Guarnieri et al., 1993; Peden et al., 2001). Both the YQTI and ERRNLL peptides, but not the γ appendage–binding WNSF peptide (Bai et al., 2004; Yamada et al., 2005), stimulated binding of purified AP-1 to GST–Arf-1 (Fig. 2 A). In our assays, a four to fivefold molar excess of the YQTI peptide was necessary to achieve the same effect in stimulating Arf-1 binding as the ETEWLM peptide (Fig. 2 B, lanes 7–12). It has previously been demonstrated that the YQTI peptide, when immobilized as a peptidoliposome, is able to recruit purified AP-1 in the presence of Arf-1–GTP (Crottet et al., 2002). Fig. 2 C shows that a soluble YQTI peptide is also functional in the liposome recruitment assay in an Arf-1– and GTPγS-dependent manner (lanes 2–7).
Figure 2.
A tyrosine-based sorting peptide stimulates binding of AP-1 to Arf-1–GTP. (A) Binding of 25 nM of purified AP-1 to GST–Arf-1, either preloaded with GTPγS or in the absence of nucleotide, was tested in pulldown assays in the presence of either of the indicated peptides. Cargo sorting signals (ETEWLM, YQTI, and ERRNLL) are able to stimulate AP-1 binding to activated Arf-1 (top, lanes 5, 7, and 11) but not the γ appendage binding WNSF peptide (lane 9). A Ponceau stain of the blot (bottom) shows equal loading of GST–Arf-1 in all lanes. (B) Binding of purified AP-1 to activated GST–Arf-1 required a higher concentration of YQTI peptide relative to the ETEWLM peptide to achieve the same degree of stimulation (lanes 7–12). (C) Liposome recruitment of purified AP-1 is stimulated by the YQTI, but not the AQTA, peptide in a GTPγS-dependent fashion (top). Even though myristoylated Arf-1 pelleted with the liposomes in the absence of nucleotide (bottom, lane 3), it is not able to associate with AP-1, underscoring the importance of activated Arf-1 for the interaction. Lane 1 represents 20% of the AP-1 input.
Dileucine peptide induces conformational change in the AP-1 core domain
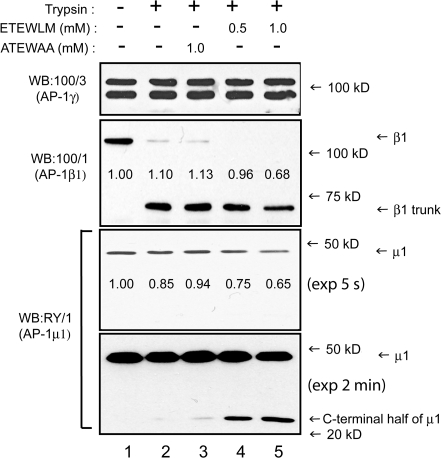
These findings suggest that binding of sorting signals to the AP-1 core (AP-1 tetramer minus the hinge and appendage domains of γ and β1) may induce a structural change in AP-1 that increases its affinity for Arf-1–GTP. To test this possibility, limited trypsin proteolysis of AP-1 immunoisolated from cytosol was performed under conditions where the majority of the appendage and hinge domains of β1 adaptin were cleaved but the β1 trunk and the γ and μ1 subunits remained intact (Fig. 3, compare lanes 1 and 2; Traub et al., 1995). In the presence of the ETEWLM peptide, a significant fraction (35%) of the μ1 subunit was sensitive to proteolysis, as shown by the release of the C-terminal fragment (Fig. 3, lanes 4 and 5). Furthermore, the β1 trunk fragment underwent additional proteolysis (Fig. 3, lane 5). The increased tryptic sensitivity of μ1 and the β1 trunk are consistent with a peptide-induced structural change within the AP-1 core that could facilitate and stabilize its association with activated Arf-1.
Figure 3.
The ETEWLM peptide induces a conformational change in cytosolic AP-1, as assessed by trypsin sensitivity. Trypsin treatment (5 μg/ml trypsin for 15 min at 37°C) of AP-1 immunoisolated from BBC cleaved the majority of β1 into the trunk domain (β1 trunk), whereas the γ1 and μ1 subunits were not affected under these conditions (lanes 1 vs. 2; Traub et al., 1995). The presence of the ETEWLM peptide (lanes 4 and 5) but not the ATEWAA peptide (lane 3) increased the trypsin sensitivity of μ1, as shown by the release of the C-terminal fragment (detected by the RY1 polyclonal antibody). The β1 trunk (detected by the 100/1 mAb) was slightly more susceptible to trypsin in the presence of the ETEWLM peptide. When quantitated by densitometry (numbers in blots), ∼35% of μ1 was cleaved in the presence of the ETEWLM peptide, but not the mutant peptide, under the conditions used.
Cross talk occurs between the dileucine- and tyrosine-based binding sites of AP-1
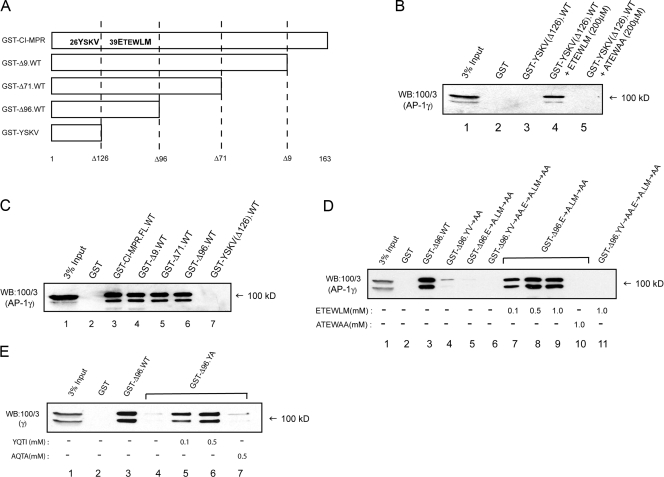
The crystal structure of the AP-1 core indicates that the YXXφ binding pocket in the μ1 subunit is occupied by a β1 chain hydrophobic residue, suggesting that a conformational change in the molecule is required to expose the C-terminal half of μ1 to permit unhindered access of a tyrosine-based sorting signal (Heldwein et al., 2004). We next asked if the conformational change induced by the ETEWLM peptide is sufficient to allow binding of AP-1 to an immobilized tyrosine motif, namely the CI-MPR YSKV motif fused to GST (Fig. 4 A). As expected, binding of cytosolic AP-1 to GST-YSKV was either extremely poor or undetectable under our assay conditions (Fig. 4 B, lane 3), reflecting the functionally closed state of the adaptor in solution. The presence of the WT, but not the mutant, peptide in the assay promoted AP-1 binding (Fig. 4 B, lanes 4 and 5), indicating that the ETEWLM peptide induces the open conformation of AP-1, allowing its simultaneous association with both activated Arf-1 and cargo molecules.
Figure 4.
Binding of the ETEWLM peptide to the core domain of cytosolic AP-1 exposes the tyrosine binding site on μ1. (A) The bovine CI-MPR cytosolic tail contains the tyrosine (YSKV) and the dileucine (ETEWLM)-based motifs arranged in tandem as shown. 163, number of amino acids in the CI-MPR cytoplasmic tail; Δ values, amino acids where truncations were made. (B) The lack of AP-1 binding to GST-YSKV (Δ126) is indicative of the closed structure of the adaptor in solution (lane 3) (Heldwein et al., 2004). The ETEWLM, but not the ATEWAA, peptide stimulated binding of AP-1 (lanes 4 and 5), indicating that the dileucine peptide–induced change within the core is sufficient to permit the YSKV motif access to the cargo binding site on C-μ1. (C) The minimal construct (GST-Δ96) that displayed AP-1 binding retained both motifs. (D) Mutation of the dileucine motif (GST-Δ96E→A.LM→AA) abolished AP-1 binding (lane 5), which is restored by the addition of the ETEWLM but not the ATEWAA peptide (lanes 7 to 10). (E) Bidirectional cross talk occurs between the dileucine- and tyrosine-based binding sites because the YQTI, but not the AQTA, peptide stimulated binding of AP-1 to GST-Δ96.YV→AA (lanes 5–7).
The internal dileucine signal in the bovine CI-MPR 163-aa cytoplasmic tail occurs in tandem with the tyrosine-based YSKV motif (Fig. 4 A). To determine if this ETEWLM sequence indeed plays a role in facilitating the interaction of the YSKV motif with cytosolic AP-1 when it is part of the same molecule, we initially constructed several truncations within the CI-MPR tail in the context of a GST fusion protein (Fig. 4 A). Binding of cytosolic AP-1 to the various GST tail fusions was unaffected if both the dileucine- and tyrosine-based motifs remained intact, as in the GST-Δ96 construct (Fig. 4, A and C [lane 6]). When the YSKV motif was mutated to ASKA (GST-Δ96.YV→AA), a low level of binding was observed, corresponding to that mediated by the ETEWLM sequence (Fig. 4 D, lane 4). Mutation of ETEWLM to ATEWAA (Fig. 4 D, lane 5, GST-Δ96.E→A.LM→AA) almost completely abrogated AP-1 binding, but this binding was completely restored by the addition of the WT but not the mutant peptide (Fig. 4 D, lanes 7–10). As expected, the ETEWLM peptide was without effect if both the YSKV and the ETEWLM motifs were mutated (Fig. 4 D, lane 11). These findings indicate that the binding of cytosolic AP-1 to the GST-Δ96 protein is not simply the consequence of increased avidity when the adaptor molecule engages the two individual motifs simultaneously. If this were the case, the soluble ETEWLM peptide would not restore binding of AP-1 to the GST-Δ96.E→A.LM→AA fusion protein. Instead, our results demonstrate that cytosolic AP-1 is indeed in a closed conformation and that binding of the dileucine sequence to the γ/σ1 hemicomplex serves to reconfigure the β1/μ1 hemicomplex within the tetramer into a state competent to engage tyrosine-based sorting signals.
Finally, we investigated whether bidirectional cross talk occurs between the dileucine-based and tyrosine-based binding sites within the AP-1 core. As shown in Fig. 4 E, the soluble YQTI peptide does indeed stimulate binding of AP-1 to GST-Δ96.YV→AA, suggesting that binding of the tyrosine-based peptide to μ1 optimizes the γ/σ1 hemicomplex for engaging the CI-MPR dileucine-based signal.
In summary, we present evidence that cargo sorting signal peptide binding to AP-1 in solution impacts the conformation of the core domain of the adaptor such that its interaction with Arf-1 is strongly stimulated. Although both the dileucine- and tyrosine-based sorting signals within the CI-MPR cytoplasmic tail are able to perform this function, the latter required a markedly higher peptide concentration to achieve the same effect. This is likely because the equilibrium of cytosolic AP-1 strongly favors the conformation in which the tyrosine binding site within μ1 is not accessible. Our data further indicate that binding of the dileucine motif does not require AP-1 to be in the open conformation but, instead, drives the equilibrium toward this state to facilitate binding of the tyrosine signal to μ1with a concomitant increase in affinity for activated Arf-1. This mode of AP-1 activation to promote coupling of cargo protein selection and coat nucleation goes well beyond the simple coincidence-detector role currently ascribed to AP-1. In the latter model, AP-1 seeks out a combination of low-affinity membrane components, including Arf-1, phosphatidylinositol lipids, and sorting signals in cargo proteins, that together create a high-affinity binding site for AP-1 on the membrane (Wang et al., 2003; Baust et al., 2006). Instead, our results indicate that cargo sorting signals play an active role in promoting their own sorting into transport vesicles by ensuring the stable association of AP-1 with activated Arf-1 in a temporally controlled manner to permit nucleation of coat protein assembly. A similar mechanism has been proposed for AP-2 and COPII coat vesicle formation (Springer and Schekman, 1998; Haucke and De Camilli, 1999), suggesting that the mode of coated vesicle formation along the secretory pathway is more universally conserved than was previously thought.
Materials and methods
DNA constructs, antibodies, reagents, and peptides
GST–CI-MPRΔ96 was constructed from the plasmid encoding the 163-aa bovine CI-MPR tail fused to GST (Zhu et al., 2001) by inserting a stop codon at amino acid K2403, downstream of the internal dileucine-based sequence (ETEWLM). GST-Δ96.YA and GST-Δ96.E→A.LM→AA were subsequently made by mutating the YSKV and ETEWLM sequences to ASKA and ATEWAA, respectively. GST-YSKV was constructed by mutating amino acid E2373, upstream of the ETEWLM sequence, to a stop codon. The GST-Δ9 and -Δ71 constructs have been previously described (Ghosh and Kornfeld, 2004). GST–Arf-1 was made by PCR from a cDNA clone (provided by D. Haslam, Washington University, St. Louis, MO) and inserted into the BamHI and XhoI sites of the vector pGEX6P1 (GE Healthcare). All mutant constructs were made, using primers incorporating the desired mutations, with the QuikChange system (Stratagene). All constructs and mutations were confirmed to be correct by dideoxynucleotide sequencing.
The anti-μ1 subunit polyclonal antibody RY/1 was provided by L. Traub (University of Pittsburgh School of Medicine, Pittsburgh, PA). The anti-HA mAb was purchased from Covance, whereas the anti–FLAG-tag mAb was purchased from Stratagene. The mAbs 100/3 and 100/2 against the clathrin adaptors AP-1 and AP-2, respectively, were obtained from Sigma-Aldrich. Anti-ARF mAb 1D9 was purchased from Affinity BioReagents. Trypsin and l-α-phosphatidylcholine from soybeans containing 20% phosphatidylcholine were purchased from Sigma-Aldrich. Glutathione Sepharose 4B was obtained from GE Healthcare, whereas GTPγS was purchased from Roche. Frozen bovine brain and adrenal glands were purchased from Pel-Freez Biologicals. All peptides were synthesized by Biomolecules Midwest. The amino acid sequences of the peptides used in this study are as follows (bold, WT and mutated residues): ETEWLM → CEADENETEWLMEEI (WT CI-MPR peptide); ATEWAA→CEADENATEWAAEEI (mutant CI-MPR peptide); YQTI→CRKRSHAGYQTI (WT Lamp1 peptide); AQTA→CRKRSHAAQTA (mutant Lamp1 peptide); ERRNLL→SVKSERRNLLEDD (WT Vamp4 peptide); and WNSF→SLDGTGWNSFQSSDAT (WT GGA1 hinge peptide).
Protein expression and purification
All GST fusion proteins were expressed in the Escherichia coli strain BL-21 (RIL; Stratagene) and purified essentially as described previously (Doray and Kornfeld, 2001). Myristoylated Arf-1 was made by coexpression of bovine Arf-1 and human N-myristoyltransferase in E. coli strain BL21 (DE3) as previously described (Liang and Kornfeld, 1997). The expression of the γ1/σ1A and β1/μ1A hemicomplexes in Sf9 cells has been previously described (Doray et al., 2007). BBC, bovine adrenal cytosol (BAC), and bovine brain CCVs were prepared from frozen tissue as previously described (Zhu et al., 1998). AP-1 was purified from BAC by coupling the anti-γ subunit mAb 100/3 to cyanogen bromide–activated glutathione Sepharose 4B as previously described (Zhu et al., 1998).
Liposome recruitment and binding assays
AP-1 recruitment assays were performed with soybean liposomes essentially as previously described (Zhu et al., 1999). GST pulldown assays with BBC and BAC in assay buffer were performed as previously described (Doray and Kornfeld, 2001). For insect cell–expressed proteins, typically 100–150 μl of total cell lysates (5–10 mg/ml) was used for each GST pulldown assay. Typically, 40% of pellet fractions and 3% of unbound fractions were analyzed by SDS-PAGE and Western blotting. Nitrocellulose membranes were routinely stained with Ponceau solution to ascertain equal loadings of fusion protein.
Controlled tryptic digestion
Controlled tryptic digestion of AP-1 was performed using the previously described procedure (Traub et al., 1995) with some modifications. AP-1 from BAC was first immunoisolated using the 100/3 mAb and protein G–Sepharose. After several wash steps, protein bound to beads was diluted into 50 μl of assay buffer containing 5 μg/ml trypsin with either 0.5 or 1 mM of the ETEWLM peptide or 1 mM of the ATEWAA peptide. Samples were incubated at 37°C for 15 min, after which SDS sample buffer was added and the samples were boiled before being subjected to SDS-PAGE and Western blotting.
Acknowledgments
We thank Daniel Ory, Douglas Tollefsen, and David Haslam for critical reading of the manuscript and valuable comments.
This work was supported by National Institutes of Health grant RO1 CA-08759 (S. Kornfeld).
I. Lee and B. Doray contributed equally to this paper.
Abbreviations used in this paper: Arf-1, ADP ribosylation factor 1; BAC, bovine adrenal cytosol; BBC, bovine brain cytosol; CCV, clathrin-coated vesicle; CI-MPR, cation-independent mannose 6-phosphate receptor; WT, wild-type.
References
- Austin, C., I. Hinners, and S.A. Tooze. 2000. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 275:21862–21869. [DOI] [PubMed] [Google Scholar]
- Bai, H., B. Doray, and S. Kornfeld. 2004. GGA1 interacts with the adaptor protein AP-1 through a WNSF sequence in its hinge region. J. Biol. Chem. 279:17411–17417. [DOI] [PubMed] [Google Scholar]
- Baust, T., C. Czupalla, E. Krause, L. Bourel-Bonnet, and B. Hoflack. 2006. Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc. Natl. Acad. Sci. USA. 103:3159–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton, J.G., and P.J. Cullen. 2005. Coincidence detection in phosphoinositide signaling. Trends Cell Biol. 15:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crottet, P., D.M. Meyer, J. Rohrer, and M. Spiess. 2002. Arf-1.GTP, tyrosine-based signals, and phosphatidylinositol 4,5-bisphosphate constitute a minimal machinery to recruit the AP-1 clathrin adaptor to membranes. Mol. Biol. Cell. 13:3672–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray, B., and S. Kornfeld. 2001. γ subunit of the AP-1 adaptor complex binds clathrin: implications for cooperative binding in clathrin vesicle assembly. Mol. Biol. Cell. 12:1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray, B., I. Lee, J. Knisely, G. Bu, and S. Kornfeld. 2007. The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell. 18:1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling, M.A., C. Smith, and D. Owen. 2006. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 7:32–44. [DOI] [PubMed] [Google Scholar]
- Ghosh, P., and S. Kornfeld. 2004. The cytoplasmic tail of the cation-independent mannose 6-phosphate receptor contains four binding sites for AP-1. Arch. Biochem. Biophys. 426:225–230. [DOI] [PubMed] [Google Scholar]
- Guarnieri, F.G., L.M. Arterburn, M.B. Penno, Y. Cha, and J.T. August. 1993. The motif tyr-X-X-hydrophobic residue mediates lysosomal membrane targeting of lysosome-associated membrane protein 1. J. Biol. Chem. 268:1941–1946. [PubMed] [Google Scholar]
- Haucke, V., and P. De Camilli. 1999. AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science. 285:1268–1271. [DOI] [PubMed] [Google Scholar]
- Heldwein, E.E., E. Macia, J. Wang, H.L. Yin, T. Kirchhausen, and S.C. Harrison. 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA. 101:14108–14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, J.O., and S. Kornfeld. 1997. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J. Biol. Chem. 272:4141–4148. [DOI] [PubMed] [Google Scholar]
- Peden, A.A., G.Y. Park, and R.H. Scheller. 2001. The Di-leucine motif of vesicle-associated membrane protein 4 is required for its localization and AP-1 binding. J. Biol. Chem. 276:49183–49187. [DOI] [PubMed] [Google Scholar]
- Springer, S., and R. Schekman. 1998. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 281:698–700. [DOI] [PubMed] [Google Scholar]
- Traub, L.M., S. Kornfeld, and E. Ungewickell. 1995. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 270:4933–4942. [DOI] [PubMed] [Google Scholar]
- Traub, L.M. 2005. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim. Biophys. Acta. 1744:415–437. [DOI] [PubMed] [Google Scholar]
- Wang, Y.J., J. Wang, H.Q. Sun, M. Martinez, Y.X. Sun, E. Macia, T. Kirchhausen, J.P. Albanesi, M.G. Roth, and H.L. Yin. 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 114:299–310. [DOI] [PubMed] [Google Scholar]
- Yamada, Y., M. Inoue, T. Shiba, M. Kawasaki, R. Kato, K. Nakayama, and S. Wakatsuki. 2005. Structure determination of GGA-GAE and γ1-ear in complex with peptides: crystallization of low-affinity complexes in membrane traffic. Acta Crystallogr. D Biol. Crystallogr. 61:731–736. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., L.M. Traub, and S. Kornfeld. 1998. ADP-ribosylation factor 1 transiently activates high affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol. Biol. Cell. 9:1323–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., M.T. Drake, and S. Kornfeld. 1999. ADP-ribosylation factor 1 dependent clathrin-coat assembly on synthetic liposomes. Proc. Natl. Acad. Sci. USA. 96:5013–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., B. Doray, A. Poussu, V.P. Lehto, and S. Kornfeld. 2001. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science. 292:1716–1718. [DOI] [PubMed] [Google Scholar]