Abstract
The recently approved drugs 5-azacitidine and 5-aza-2′-deoxyazacytidine are in wide clinical use for the treatment of myelodysplastic syndrome of all types and chronic myelomonocytic leukemia. These agents were developed based upon an understanding of the importance of epigenetic changes in malignancy, and they have been evaluated in randomized clinical trials which demonstrate response rates between 20 and 40% in patients for whom no previous standard of care was available (1,2). As understanding of the epigenetic changes characteristic of the malignant phenotype improves, we are able to target other regulators of chromatin conformation that contribute to aberrant gene transcription and dysregulated cell growth. The histone deacetylase inhibitors belong to one class of therapeutics developed using this paradigm. Although responses using HDAC inhibitors alone in myelodysplastic syndrome have been modest, robust preclinical data drives clinical trials in which they are utilized in combination with DNA methyltransferase inhibitors (3). Combination therapy offers the possibility of hematologic improvemernt and remission to myelodysplastic patients with previously untreatable disease.
Introduction
The chemotherapeutic agents, 5-azacitidine (5AC; Vidaza, Pharmion Corp, Overland KS) and 5-aza-2′-deoxyazacitidine (DAC; Decitabine, Dacogen, MGI Pharma, Bloomington MN), were not initially developed as demethylating or ‘hypomethylating’ agents. In the late 1960s and early 1970s, 5AC was extensively evaluated in phase I and II clinical trials as a classic cytotoxic agent and was found to be effective for the treatment of myeloid malignancy (4). Doses ranging from 100 to 750 mg/m2 were administered for multiple days to patients with relapsed and refractory acute myeloid leukemia (AML). The results of these early phase clinical trials were reasonable response rates, but these encouraging positive data were tempered by profound and prolonged cytopenias and prohibitive gastrointestinal system toxicity (5). Between 1960 and 1990, 5AC was investigated for the treatment of refractory leukemias but was not approved by the Food and Drug Administration (FDA).
In the 1970s, Peter Jones and Shirley Taylor demonstrated that treatment of mouse embryo cells with low concentrations of 5-azanucleotides resulted in differentiation of these cells into myocytes and adipocytes (6). This differentiation was accompanied by a decrease in the frequency of methylcytosine residues within the DNA of the treated cells DNA replication and the incorporation of the 5′-azanucleotides into the daughter strands of DNA were required for these changes (7). Following 5AC or DAC incorporation, even at low levels (<5% substitution), the daughter strands demonstrated a profound decrease in methylation frequency of CG repeat sequences (8). It was hypothesized that this alteration in methylation was mediated by inhibition of a methyltransferase; methylation patterns within the genome might regulate tissue specific gene expression.
Hypermethylation of CpG rich ‘islands’ within the promoter region of certain tumor suppressor genes are hallmarks of malignancy, and that within the normal human genome these CpG rich regions are almost universally free of methylation (9). Hypermethylation of this region within the promoter (in which the concentration of CpGs is greater that 50%) (10), but not other regions within the genome appear to result in a marked decrement in expression of these so called ‘methylated’ genes. Moreover, this hypermethylation is a heritable event that is maintained following cell division (11). Many tumor suppressor genes are specifically methylated in cancer. In myelodysplastic syndromes (MDS), the malignant clone may acquire an increasing number of methylated tumor suppressor genes as the disease progresses; the progressive down regulation of tumor suppressor genes may result in resistance to classic cytotoxic chemotherapy (11).
Although increased methylation of CpG islands within a gene was the presumed primary mechanism leading to gene silencing, it appears that this is only one of a number of ‘epigenetic’ modifications to DNA which contribute to the differential expression of genes within a particular tissue. Epigenetic changes affect the conformation of DNA and indirectly but specifically the expression of genes. DNA exists as euchromatin or heterochromatin; euchromatin has an open structure while heterochromatin is in a more condensed conformation. Heterochromatic DNA is untranscribed and is tightly wrapped around the nucleosome (consisting of eight histones), while DNA in euchromatin is more loosely associated with the nucleosome. Modifications to the lysine tails of histones by a number of proteins, including histone acetyl transferases (HATs), histone deacetylases (HDACs), and histone methyltransferases, mediate the degree of DNA ‘tightness’ and in so doing determine the transcriptional status of genes (12).
Malignant cells depend largely on a specific endogenous DNA methyltransferase (DNMT) known as DNMT1 to induce CpG Island hypermethylation. This enzyme, in addition to the other two endogenous human DNMTs, DNMT3a and DNMT3b, can be irreversibly inhibited by interaction with DNA-incorporated-5′ modified azanucleosides, depleting the intact enzymes and preventing methylation of the daughter strand following DNA replication. Cell lines with a particular methylation signature which have been treated with low doses of 5AC or DAC decrease the methylation density of these genes (13). Both 5AC and DAC induce terminal differentiation in cancer cell lines; this differentiation is associated with upregulation of a number of tumor suppressor gene products (14,15). Both 5AC and DAC have also been studied for the treatment of MDS and chronic myelomonocytic leukemia (CMMoL), and these investigations resulted in FDA approval for both agents.
Hypermethylated CpG islands are associated with hyperacetylated and methylated histone lysine tails, and although it remains unclear whether histone alterations or promoter hypermethylation is the primary signal by which gene expression is determined, the latter mechanism appears to be the more powerful determinant (3). Tumor suppressor genes with hypermethylated promoters recruit transcriptional repression complexes, which include histone deacetylases (HDACs). This molecular interaction prompted investigation of the pharmacodynamic interactions between DNMTs and HDAC inhibitors. Sequential treatment of malignant cell lines, first with 5-azacytosine nucleosides and subsequently with an HDAC inhibitor, results in more robust re-expression of methylated tumor suppressor gene products in vitro than either agent alone (3). This synergy is sequence-dependent, requiring initial exposure to DNMT inhibition followed by HDAC inhibition. The synergistic gene re-expression observed in vitro using this sequence of exposure to agents prompted several clinical trials, which were designed to explore whether more robust clinical responses also could be achieved in patients with MDS and leukemia by sequential treatment with DNMT inhibitors followed by HDAC inhibitors. (Figure 1?)
Figure 1.
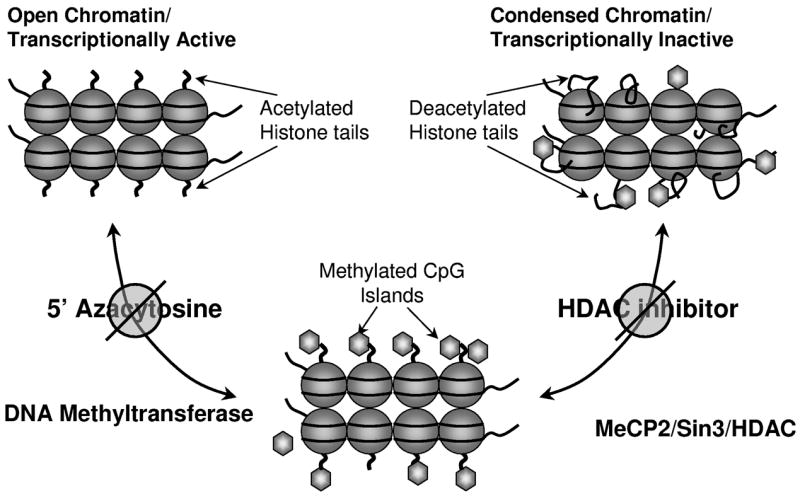
EDITOR PROVIDE LEGEND
DNA Methyltransferase Inhibitors
The DNA methyltransferase inhibitors 5AC and DAC are analogs of the naturally occurring pyrimidine nucleoside cytidine. Their mechanism of action is concentration-dependent, inducing cytotoxicity presumably as a consequence of incorporation into RNA (5AC) and/or DNA (5AC and DAC) at high concentrations, and inducing hypomethylation at lower doses through depletion of cellular DNMTs. The precise mechanism responsible for the responses observed in patients treated with these agents remains controversial, although the rationale underlying the low dose therapy currently approved for MDS stems from the theory that induction of hypomethylation with subsequent re-expression of methylated genes will induce gene expression patterns and beneficially result in differentiation, apoptosis, or senescence of the malignant clone.
Early phase clinical trials of 5AC and DAC demonstrated impressive response rates, including some complete remissions, as summarized in Table 1. The results from these early-phase clinical trials led to large, randomized trials for both 5AC and DAC, which ultimately established these agents as part of the standard armamentarium in the treatment of MDS.
Table 1.
Clinical trials of ‘Epigenetically dosed’ DNMTis in MDS
| Phase | Agent/Dose Schedule | No. Enrolled (disease) | Results | Author |
|---|---|---|---|---|
| I | 5AC 10–35 mg/m2 continuous infusion for 14 days. | 15 (MDS) | ORR 20% (3)
PR 20% (3) |
Chitambar CR et al. Am J Hematol 1991. (39) |
| I | DAC 45 mg/m2 or 50 mg/m2 IV over 4h TID × 3d | 10 (MDS) | CR 40% (4/10) | Zagonel V et al. Leukemia 1993. (40) |
| I | DAC 5,10,15,20 mg/m2 IV 5 days/wk × 2 weeks. | 50 (MDS, AML, CML, ALL) Best MDS responses in 10mg/m2 cohort. | ORR32% (16/50)
CR18% (9) PR 2% (1) HI 8% (4) |
Issa JP et al. Blood 2004. (41) |
| II | 5AC 75mg/m2 SC daily for 7 days every 4 weeks | 43 (MDS) | ORR 49% (21)
CR 12% (5) PR 25% (11) HI 12% (5) |
Silverman LR et al. Leukemia 1993 (42) |
| II | DAC 50–75 mg/m2 by CI for 72h every 6 weeks | 29 (MDS) | ORR 54% (15/29) CR 27%(8) PR 17%(5) HI 7% (2) | Wijermans PW et al Leukemia 1997. (43) |
| Randomized Phase II | DAC: 20 mg/m2 IV daily × 5 days; 20 mg/m2 SQ daily × 5 days; 10 mg/m2 IV daily × 10 days | 95 (77 MDS, 18 CMMoL) | CR 34% (32)
PR 1% (1) HI 14% (13) |
Kantarjian H et al. Blood 2007 (17) |
| III | 5AC 75 mg/m2 SC × 7 days of 28 day cycle; versus supportive care. | 191 (MDS) | ORR 60% (60)
CR 7% (7) PR 16% (16) HI 37% (37) |
Silverman LR et al J Clin Oncol 2002 (1) |
| III | DAC 15 mg/m2 IV every 8 hours × 3 days every 6 weeks versus supportive care. | 170 (MDS) | ORR 17% (15/89)
CR 9% (8), PR 8% (7) HI 13% (12) |
Kantarjian H et al. Cancer 2006(2) |
5AC- 5′-azacytidine
DAC- 5-aza-2′-deoxyazacytidine
ORR- overall response rate
CR- complete response
PR- partial response
HI- hematologic improvement
IV-intravenous
SQ- subcutaneous
ALL- Acute lymphocytic leukemia
CML- Chronic myeloid leukemia
DNMTis- DNA methyltransferase inhibitors
The first randomized open label clinical trial of 5AC was conducted in the Cancer and Leukemia Group B (CALGB) 9221 (1). This trial randomized 191 patients with symptomatic MDS to receive either supportive care or 5AC at 75 mg/m2/day subcutaneously for 7 days every 28 days. Patients received therapy for three cycles after achieving a complete response or ongoing therapy until progressive disease or toxicity if complete response was not achieved. Patients receiving supportive care could cross-over to receive 5AC if they did not improve after four months. A retrospective re-evaluation of the patients treated in this trial has re-classified patients based upon the revised World Health Organization diagnostic criteria for AML and MDS and the International Working Group (IWG) response criteria (16). In this analysis, of the 99 patients with MDS randomized to receive 5AC, 47% had significant responses with 10% of patients achieving a complete response, and 36% of patients demonstrating hematological improvement. The median time to response in this study was three months with responses observed by cycle 4 in 75% of patients, and by cycle 6 in 90% of patients; it is important to note that responses were observed as late as cycle 17. This study did not measure improvements in survival because of its crossover design. However, there was increased time to progression to AML, defined as having 30% blasts in the group of patients who were treated with 5AC, compared to those who received supportive care (including those supportive care patients who crossed over to receive 5AC). Responses were observed in all subtypes of transfusion-dependent MDS; transfusion-ndependence was achieved as frequently for those with low risk disease as for those with high-risk disease. The death rate in the 150 patients who were ultimately treated with 5AC was 1% compared with 2% rate in the observation arm. Median duration of response to 5AC was 15 months, with responses enduring between 2 and 36 months. An international phase III trial designed to specifically investigate the impact of 5AC administration on survival compared to physicians’ choice of care (supportive care, low-dose cytarabine, or cytarabine-based induction chemotherapy, pre-selected for each patient prior to enrollment) has been completed; results are not yet available.
DAC also was evaluated in several early phase clinical trials, summarized in Table 1, followed by a randomized, open label phase III trial involving 170 patients with MDS and an IPSS score ≥ 0.5 (2). In this study, 89 patients were randomized to receive DAC as an intravenous infusion at a dose of 15 mg/m2/dose adminstered over 3 hours, three times daily for 3 days, while 81 patients were randomized to the supportive care arm. Doses were repeated every six weeks but were held depending on the clinical response or toxicity. Intent-to-treat analysis revealed an overall response rate of 17% (15/89) in the treatment arm, with 0% in the supportive care arm, p<0.01. Among the 15 patients who improved, there were 8 complete responses and 7 partial responses. There was no significant difference in the time to AML progression or death in the treatment and supportive care arms, although the Kaplan-Meier curves displayed a trend in favor of DAC, similar to that seen in the CALGB 9221 protocol. Patients received a median of three of a planned 6–8 course of decitabine (range 0–9). Compared to the CALGB 9221 trial, in which the death rate was 1% in the 5AC treated patients, the DAC-treated patients had a 10% mortality, suggesting that the current FDA approved 3 day intravenous infusion schedule of DAC may be more toxic than the previously approved schedule of 5AC. The apparent under-treatment of patients in the DAC trial (presumably because of toxicity) may have decreased the overall response rate and led to failure to find a significant difference in time to AML or death.
A randomized Phase II study investigated DAC at a total dose of 100 mg/m2 in three different schedules: 20 mg/m2/day intravenously for 5 days, 20 mg/m2/day subcutaneously for 5 days and 10 mg/m2/day intravenously for 10 days. Enrolled were 95 patients: 77 with MDS and 18 patients with CMMoL (17). Overall, the authors reported a 34% (32/95) complete response rate, a 1% (1/95) partial responses, and 13% (19/95) hematological improvement. Patients received a median of 6 courses of therapy. This study was designed to investigate whether lower dose intensity would result in a more robust demethylation response. The authors measured methylation-reversal using a LINE1 methylation assay as a surrogate for global changes in methylation, but no statistically significant difference in methylation among the three schedules was found. Additionally, there was no correlation between demethylation of the p15 tumor suppressor gene promoter and response to therapy on any dose schedule. By contrast, with the randomized trial of DAC, there were no deaths in the 95 patients treated with DAC in this study, suggesting that the lower dose, longer duration schedule of DAC may be less toxic than the FDA-approved schedule. A larger, multi-center trial is needed to confirm this important difference.
The randomized trials described above both demonstrated impressive response rates. These two protocols were distinct in terms of study design, with a planned stopping point for therapy incorporated into the DAC trial for patients who did not achieve a CR, while in the CALGB 9221 trial; most patients continued treatment until disease progression. Both trials stopped therapy 2 or 3 cycles past a complete response. The lack of a planned stopping point in CALGB 9221 and superior remission duration in that trial raise the question of whether maintenance therapy for patients who do not achieve a CR has an impact on time to AML disease progression or death, since these outcomes were significantly better in the 5AC-treated patients and was not different in those treated with DAC. At present, there is no consensus regarding maintenance therapy for patients who do not achieve a CR from therapy with a DNA methyltransferase inhibitor.
Both 5AC and DAC require intravenous or subcutaneous administration. In an effort to overcome this limitation, a number of groups are working to develop oral DNA methyltransferase inhibitors. Among the candidates are an oral form for DAC, an oral 5AC, and a novel nucleoside analog called zebularine. Zebularine was attractive because of its superior chemical stability and lowere cytotoxicity compared to prototypical DNA methyltransferase inhibitors, with a similar ability to force re-expression of epigenetically silenced genes in AML cell lines and primary patient samples (18). Unfortunately, early pharmacokinetic analysis in mice, rats, and rhesus monkeys suggest that all of these agents have limited oral bioavailability (6.7%, 61% and <1% respectively for the different animals). (19). By contrast, studies in baboons using oral decitabine and decitabine mesylate achieved similar re-expression of fetal hemoglobin to that observed with subcutaneously administered DAC, suggesting that these agents may be more effectively absorbed (20). As yet there are no published reports for these orally administered drugs in humans.
Histone Deacetylase Inhibitors
A number of different compounds inhibit histone deacetylases, including the short chain fatty acids phenylbutyrate and valproic acid (VPA), the benzamides SNDX-275 (formerly MS-275) and MGCD0103, the cyclic peptide romidepsin, and the hydroxamic acids vorinostat and trichostatin A. HDAC inhibitors modulate gene expression by inhibiting the deacetylation of histone lysine tails, relaxing the chromatin structure by decreasing the interaction between positively charged lysine tails of histones and negatively charged DNA (21). In addition to their impact on gene expression, HDAC inhibitors have additional effects, including induction of reactive oxygen species, direct acetylation of chaperone proteins, alterations to the NFkB pathway and up-regulation of the death receptor pathway (22). Although some of these actions may result from changes in gene expression, others are likely the result of direct acetylation of non-histone proteins, and they may be responsible for the clinical responses seen in patients treated with HDAC inhibitors (23). Some early phase clinical trials have shown evidence HDAC inhibitors’ clinical activity in myeloid malignancy. As a class they are limited by toxicity due to myelosuppression, fatigue and gastrointestinal symptoms (24). The fatty acid HDAC inhibitors characteristically cause somnolence and confusion (24).
Phase I trials
Sodium phenylbutyrate exhibited modest clinical activity in early phase clinical trials in patients with MDS and AML, with responses primarily limited to hematological improvement (25). Further clinical development of this agent was limited by the need for prolonged infusion and by central nervous system toxicity.
VPA has also been investigated as a single agent in a phase I clinical trial, which showed some evidence of clinical activity in MDS (26). Side effects included vertigo and tremor as well as fatigue and thrombocytopenia. These results have prompted further investigation in the phase II setting.
The benzamide HDAC inhibitors have also shown interesting clinical responses. In a phase I dose escalation study of single agent MS-275 administered orally between 4 and 8 mg/m2, the maximum tolerated dose was found to be 8 mg/m2 weekly for 4 weeks of a 6-week cycle. (27). MS275 may be dosed one or twice weekly because of its prolonged half life of 50 hours. This phase I study enrolled 39 patients with relapsed or refractory AML and demonstrated primarily stable disease or hematologic improvement in treated patients. Dose limiting toxicities included infections and neurologic toxicity, manifested by gait instability and somnolence.
Romidepsin, a cyclic tetrapeptide that at very low concentrations in vitro can inhibit HDAC, initially was considered a promising candidate for treatment of myeloid disorders. The first clinical trial of this agent in patients with myeloid malignancy enrolled 10 patients with AML, who were treated at a dose of 13 mg/m2 over 4 hours on days 1, 8, and 15, administered every 28 days (28). Early toxicity studies demonstrated significant gastrointestinal effects and progressive neurological findings, especially dose-limiting fatigue. Clinical trials designed to specifically determine the degree of anticipated cardiac toxicity from this drug did not substantiated this particular concern (29). Despite adequate pharmacokinetics and documented HDAC inhibition, this agent has demonstrated limited single agent activity in myeloid neoplasms.
Vorinostat is a hydroxamic acid derivative that has been approved by the FDA based on phase II data for treatment of cutaneous T-cell lymphoma (30). Vorinostat is an orally bio-available, pan HDAC inhibitor. Although currently in testing in clinical trials in a variety of malignancies including MDS and lymphomas, to date only one phase I study has been reported in patients with MDS, AML and CMMoL. This trial enrolled 41 patients, 3 of whom with MDS. Patients were treated with 100–300 mg of vorinostat 2–3 times per day for 14 days with a week of rest (31). Dose limiting toxicities included diarrhea, nausea, thrombocytopenia, malaise and fatigue. Responses were observed in 7 patients. This agent is not currently in use for the treatment of MDS outside of clinical trials (32).
Phase II Studies
Valproic acid (VPA), a well known anticonvulsive drug, was examined in phase II trails as an antineoplastic agent, after in vitro study demonstrated that it inhibited proliferation and induced differentiation of primitive neuroectodermal tumor cells. In one study of 20 elderly patients with either MDS or AML who were treated with VPA at a dose of 10 mg/kg/day, of 11 who were evaluable for a response, 5 patients showed a hematologic response (33). Another phase II trial evaluated 75 patients with MDS and AML treated continuously with oral VPA in order to obtain a serum concentration between 50 and 100 mcg/ml (34). The response rate in patients with MDS (43/75) was 30%, primarily hematologic improvement. Toxicities with single agent VPA included thrombocytopenia, tremor, and fatigue.
In summary, single agent early phase clinical trials of HDAC inhibitors have demonstrated some evidence of disease response in patients with MDS; however they have not replicated the results observed with single agent DNA methyltransferase inhibitors. As a class, HDAC inhibitors produce side effects which are predominantly gastrointestinal and constitutional, including nausea, vomiting, and, most notably, fatigue. Considering the preclinical data showing marked synergy between DNA methyltransferase inhibitors and HDAC inhibitors, it seems likely that these agents will be mainly employed in combination with other drugs for the treatment of MDS.
Combination Therapy
Optimal re-expression of methylated genes following the serial application of a DNA methyltransferase inhibitor followed by an HDAC inhibitor created significant interest in combination ‘epigenetic therapy’ (3). Phase I and II trials are ongoing to investigate this question, with 12 currently registered on the NCI clinical trials website. A limited number of trials involving combination therapy have been fully reported, and some of the early phase trials of combination therapy have been presented in abstract form.
As the first clinically available HDAC inhibitor, sodium phenylbutyrate, was investigated in combination with 5AC in a phase I trial designed to evaluate the safety and tolerability of this combination (35). 5AC was given subcutaneously at doses ranging from 25–75 mg/m2 for 5–14 days followed by sodium phenylbutyrate at a fixed dose of 375 mg/kg/day for 7 days by continuous infusion to 32 patients with MDS-AML, relapsed AML, MDS or CMMoL. Treatment cycles were repeated every 28 days. No unusual toxicities were seen with this combination of agents. Although responses were observed at all dose levels, the most robust occurred at 5AC of 50 mg/m2 for 10 days and 25mg/m2 for 14 days, with some complete and partial responses observed at these dose levels. Most important, there was a perfect correlation between induction of methylation reversal during the first cycle of therapy and response to therapy in 12 patients who had sequential bone marrow samples available for methylation analysis, suggesting that methylation reversal may be a marker of clinical response, if not mechanistically required. Surprisingly, histone acetylation was markedly increased following administration of 5-azacitidine alone, with additional smaller increment in acetylation following sodium phenylbutyrate. Due to the relative inconvenience of continuous infusion therapy with phenylbutyrate, further investigation has continued with oral HDAC inhibitors.
Concomitant DAC plus VPA was assessed in a phase I/II study of patients with AML (N=48) and MDS (N=6), (44). DAC was administered at 15 mg/m2/day infused intravenously over one hour; VPA was administered at 20, 35, and 50 mg/kg orally, daily for ten days. All dose levels were tolerated, and 50 mg/kg/day was administered in the “Phase II” portion. Eight of 41 patients who were treated at this dose level achieved a complete remission, but with a brief median duration of 5.6 months. Global methylation and p15 promoter methylation diminished transiently in both responders and non-responders; however, baseline p15 methylation appeared less in responding patients. p15 expression did not increase during therapy but p21WAF1/CIP1 did, peaking on day 5. Histone acetylation rose in a minority of patients treated at the highest dose level of VPA, and not at lower doses, confirming that VPA is not a potent HDAC inhibitor.
A similar trial was performed in 25 patients with AML, 6 with antecedent MDS or CMMoL. Eleven patients were treated with decitabine 20 mg/m2/day IV for 10 days with VPA administered at 15, 20, or 25 mg/kg/day orally from days 5 – 21 (the remaining patients received DAC alone). The highest VPA dose was not tolerated due to encephalopathy, occurring within 5 days of beginning VPA. (The differences in central nervous system toxicity between studies remain unexplained.) DAC-induced histone acetylation was observed, with further increment in acetylation following VPA. Expression of the frequently methylated estrogen receptor gene was increased in responding patients compared to patients who did not have a clinical response. No differences in gene expression were observed between patients receiving DAC alone or DAC in combination with VPA. Clinical improvement, including complete responses, were noted in patients treated with and without VPA. No signal of increased clinical activity in the latter group was seen; however, in this phase I study there was neither patient randomization nor stratification between treatment groups (45).
The combination of 5AC, valproic acid, and all trans retinoic acid (ATRA) was investigated in a phase I/II study in which both drugs were given concurrently to 53 patients with relapsed or refractory AML (N=49) or high risk MDS (N=4) (36). 5AC was adminstered at a fixed dose of 75 mg/m2 daily for 7 days and valproic acid was dpsed orally daily over the same period at between 50 and 75mg/kg; ATRA was adminstered at 45 mg/m2 orally daily on days 3–7. In the phase I portion, the maximum tolerated dose of valproic acid was 50 mg/kg, with neurologic system limiting toxicity. In total, 40 patients were treated at this dose level, six in the phase I portion and 34 in the phase II portion, with an overall response rate of 47% (19/40). Ten patients (25%) at the 50 mg/kg dose level showed a complete response and 3 had a complete response but with incomplete platelet recovery. The remaining 6 patients had hematological improvement. The median duration of remission was brief, only 26 weeks with a median time to response of 1 month. The only significant predictor of response to combination therapy was the serum valproic acid level, which was higher in responding patients. There was no correlation with global methylation reversal, and gene specific methylation was not monitored. Expression of p15 increased in a small number of patients, again without association with clinical response.
Results of early phase combination trials with a variety of other HDAC inhibitors have been reported in abstract form and are not presented in detail here. However, responses have been sufficiently interesting to prompt the initiation of a randomized phase II trial comparing the combination of 5AC and SNDX-275 to 5AC alone in the US Intergroup led by the Eastern Cooperative Oncology Group.
Combination therapy with these agents remains an active area of study based upon compelling preclinical data. The superiority of combination therapy over single agent DNA methyltransferase inhibition has yet to be proven, and larger randomized trials comparing the two therapies will be required to ultimately establish whether clinical experience will correlate with preclinical predictions of synergy for these agents.
Mechanism of Action
The putative mechanism underlying responses to therapy with DNMTis and HDAC inhibtors remains reversal of methylation and re-expression of silenced tumor-suppressor gene products. Other hypothetical explanations for response include alterations in differentiation, changes in apoptosis, and induction of a beneficial immune response (37). Unfortunately, data supporting any particular mechanism are limited. There are several authors investigative strategies to evaluate global changes in methylation, including analysis of long interspersed nucleotide (LINE1) repetitive elements as a surrogate for the effective induction of gene hypomethylation (38). Demonstrated decreases in global methylation following treatment with DNMT inhibitors have not correlated with disease response. Others have assayed methylation reversal at particular gene loci, most notably CDKN2B/p15, which is methylated in as many at 70–90% of patients with AML (9,11). As discussed above, only one trial has shown a correlation between reversal of methylation at such a locus and clinical response of the underlying disease (35); in contrast, other attempts at correlation failed to demonstrated clear evidence linking response to gene re-expression or methylation reversal (17). Thus it is impossible to make definitive conclusions concerning the mechanism(s) of action responsible for the clear effects on hematologic diseaese of the DNMT inhibitors. Further investigation into the induction of DNA damage, apoptosis, differentiation and immune modulation are necessary to determine, which if any, of these mechanisms are responsible for clinical efficacy.
Conclusions
At present, patients with symptomatic MDS may be treated with either a DNA methyltransferase inhibitor or lenalidomide (Revlimid, Celgene, Summit NJ) and, for those with a suitable donor, allogeneic bone marrow transplant. Until recently, most patients with high risk MDS and an adequate performance status were referred for transplant. With the advent of epigenetic therapy, an alternative strategy for treatment of myelodysplastic syndromes provides an opportunity for significant improvements in quality of life and possibly prolonged survival. Although it is too early to state conclusively, combination therapy may offer these patients an alternative route to complete remission without the need for intensive chemotherapy. Furthermore, in patients who are suitable transplant recipients, it may be possible to improve transplant outcome by pretreatment with these agents, either alone or in combination, with the goal of remission induction prior to stem cell infusion.
Acknowledgments
This work supported in part by NCI grant K24 CA111717.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth A. Griffiths, Senior Postdoctoral Fellow, Sidney Kimmel Comprehensive Cancer Centre, Johns Hopkins Hospital, CRB 186, 1650 Orleans Street, Baltimore, MD 21231.
Steven D. Gore, Associate Professor, Sidney Kimmel Comprehensive Cancer Center, CRB 288, 1650 Orleans Street, Baltimore, MD 21231, gorest@jhmi.edu.
References
- 1.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 3.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–7. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Slavik M, Muggia FM. 5-Azacytidine. A new anticancer drug with effectiveness in acute myelogenous leukemia. Ann Intern Med. 1976;85:237–245. doi: 10.7326/0003-4819-85-2-237. [DOI] [PubMed] [Google Scholar]
- 5.Saiki JH, McCredie KB, Vietti TJ, Hewlett JS, Morrison FS, Costanzi JJ, et al. 5-azacytidine in acute leukemia. Cancer. 1978;42:2111–2114. doi: 10.1002/1097-0142(197811)42:5<2111::aid-cncr2820420505>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 6.Constantinides PG, Taylor SM, Jones PA. Phenotypic conversion of cultured mouse embryo cells by aza pyrimidine nucleosides. Dev Biol. 1978;66:57–71. doi: 10.1016/0012-1606(78)90273-7. [DOI] [PubMed] [Google Scholar]
- 7.Jones PA, Taylor SM. Hemimethylated duplex DNAs prepared from 5-azacytidine-treated cells. Nucleic Acids Res. 1981;9:2933–2947. doi: 10.1093/nar/9.12.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor SM, Jones PA. Mechanism of action of eukaryotic DNA methyltransferase. Use of 5-azacytosine-containing DNA. J Mol Biol. 1982;163:679–692. doi: 10.1016/0022-2836(82)90395-3. [DOI] [PubMed] [Google Scholar]
- 9.Herman JG, Baylin SB. Promoter-region hypermethylation and gene silencing in human cancer. Curr Top Microbiol Immunol. 2000;249:35–54. doi: 10.1007/978-3-642-59696-4_3. [DOI] [PubMed] [Google Scholar]
- 10.Bird PA. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 11.Galm O, Herman JG, Baylin SB. The fundamental role of epigenetics in hematopoetic malignancies. Blood Rev. 2006;20:1–13. doi: 10.1016/j.blre.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fahrner JA, Eguchi S, German JG, Baylin SB. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62:7213–7218. [PubMed] [Google Scholar]
- 13.Teofili L, Martini M, Di Mario A, Rutella S, Urbano R, Luongo M, Luongo M, et al. Expression of p15(INK4B) gene during megakaryocytic differentiation of normal and myelodysplastic hematopoietic progenitors. Blood. 2001;98:495–497. doi: 10.1182/blood.v98.2.495. [DOI] [PubMed] [Google Scholar]
- 14.Christman JK, Mendelsohn N, Herzog D, Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60) Cancer Res. 1983;43:763–769. [PubMed] [Google Scholar]
- 15.Schmelz K, Wagner M, Dorken B, Tamm I. 5-Aza-2′deoxycydidine induces p21WAF expression by demethylation of p73 leading to apoptosis in myeloid leukemia. Int J Cancer. 2005;114:683–695. doi: 10.1002/ijc.20797. [DOI] [PubMed] [Google Scholar]
- 16.Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further Analysis of Trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the cancer and leukemia group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 18.Scott SA, Lakshimikuttysamma A, Sheridan DP, Sanche SE, Geyer CR, DeCoteau JF. Zebularine inhibits human acute myeloid leukemia cell growth in vitro in association with p15INK4B demethylation and reexpression. Exper Hematol. 2007;35:263–273. doi: 10.1016/j.exphem.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Holleran JL, Parise RA, Joseph E, Eiseman JL, Covey JM, Glaze ER, et al. Plasma pharmacokinetics, oral bioavailability, and interspecies scaling of the DNA methyltransferase inhibitor, zebularine. Clin Cancer Res. 2005;11:3862–3868. doi: 10.1158/1078-0432.CCR-04-2406. [DOI] [PubMed] [Google Scholar]
- 20.Lavelle D, Chin J, Vaitkus K, Redkar S, Phiasivongsa P, Tang C, et al. Oral decitabine reactivates expression of the methylated g-globin gene in Papio anubis. Am J Hematol. 2007 doi: 10.1002/ajh.21020. (in press) [DOI] [PubMed] [Google Scholar]
- 21.Gregory PD, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- 22.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–18. doi: 10.1016/s1535-6108(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 23.Rosato RR, Grant S. Histone deacetylase inhibitors: insights into mechanisms of lethality. Expert Opin Ther Targets. 2005;9:809–824. doi: 10.1517/14728222.9.4.809. [DOI] [PubMed] [Google Scholar]
- 24.Kelly WK, O’Connor OA, Marks PA. Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs. 2002;11:1695–1713. doi: 10.1517/13543784.11.12.1695. [DOI] [PubMed] [Google Scholar]
- 25.Gore SD, Weng LJ, Figg WD, Zhai S, Donehower RC, Dover G, et al. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res. 2002;8:963–970. [PubMed] [Google Scholar]
- 26.Kuendgen A, Strupp C, Aivado M, Bernhardt A, Hildebrant B, Haas R, et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood. 2004;104:1266–1269. doi: 10.1182/blood-2003-12-4333. [DOI] [PubMed] [Google Scholar]
- 27.Gojo I, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor in adults with refractory and relapsed acute leukemias. Blood. 2007;109:2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrd JC, Marcucci G, Parthun MR, Xiao JJ, Klisovic RB, Moran M, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105:959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 29.Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, et al. Cardiac Studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 30.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12:1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, et al. Phase I study of the histone deacetylase inhibitor vorinostat in patients with advance leukemias and myelodysplastic syndromes. Blood. 2007 doi: 10.1182/blood-2007-06-098061. (in press) [DOI] [PubMed] [Google Scholar]
- 32.Marks PA. SAHA as an anticancer agent. Oncogene. 2007;26:1351–1356. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 33.Pilatrino C, Cilloni D, Messa E, Morotti A, Giugliano E, Pautasso M, et al. Increase in platelet count in older, poor-risk patients with acute myeloid leukemia or myelodysplastic syndrome treated with valproic acid and all-trans retinoic acid. Cancer. 2005;104:101–109. doi: 10.1002/cncr.21132. [DOI] [PubMed] [Google Scholar]
- 34.Kuendgen A, Knipp S, Fox F, Strupp C, Hildebrandt B, Steidl C, et al. Ann Hematol. 2005;84:61–66. doi: 10.1007/s00277-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 35.Gore SD, Baylin SB, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 36.Soriano AO, Faderl S, Estrov Z, Giles F, Ravandi F, Cortes J, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 37.Oki Y, Aoki E, Issa JP. Decitabine—Bedside to bench. Crit Rev Oncol. 2007;61:140–152. doi: 10.1016/j.critrevonc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Yang AS, Doshi KD, Choi SW, Mason JB, Manari RK, Gharybian V, et al. DNA methylation changes after 5-aza-2′deoxycytidine therapy in patients with leukemia. Cancer Res. 2006;66:5495–5503. doi: 10.1158/0008-5472.CAN-05-2385. [DOI] [PubMed] [Google Scholar]
- 39.Chitambar CR, Libnoch JA, Matthaeus WG, Ash RC, Ritch PS, Anderson T. Evaluation of continuous infusion low-dose 5-azacytidine in the treatment of myelodysplastic syndromes. Am J Hematol. 1991;37:100–104. doi: 10.1002/ajh.2830370207. [DOI] [PubMed] [Google Scholar]
- 40.Zagonel V, Lo Re G, Marotta G, Babare R, Sardeo G, Gattei V, et al. 5-Aza-2′-deoxycytidine (Decitabine) induces trilineage response in unfavorable myelodysplastic syndromes. Leukemia. 1993;7 (suppl 1):30–35. [PubMed] [Google Scholar]
- 41.Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 42.Silverman LR, Holland JF, Weinberg RS, Alter BP, Davis RB, Ellison RR, et al. Effects of treatment with 5-azacytidine on the in vivo and in vitro hematopoiesis in patients with myelodysplastic syndromes. Leukemia. 1993;7 (Suppl 1):21–29. [PubMed] [Google Scholar]
- 43.Wijermans PW, Krulder JW, Huijgens PC, Neve P. Continuous infusion of low-dose 5-Aza-2′-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia. 1997;11:1–5. doi: 10.1038/sj.leu.2400526. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Ziao L, Faderl S, Estrov Z, Cortes J, O’Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa J-P. Phase ½ study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum W, Klisovic RB, Hackanson B, Liu Z, Liu S, Devine H, Vukosavljovic T, Huynh L, Lozanski G, Kefauver C, Plass C, Devine SM, Heerema NA, Murgo A, Chan KK, Grever MR, Byrd JC, Marcucci M. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. Journal of Clinical Oncology. 2007;25:3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]


