Abstract
During pregnancy crucial anatomic, physiologic, and metabolic changes challenge the mother and the fetus. The placenta is a remarkable organ that allows the mother and the fetus to adapt to the new metabolic, immunologic, and angiogenic environment imposed by gestation. One of the physiologic systems that appears to have evolved to sustain this metabolic regulation is mediated by peroxisome proliferator-activated receptors (PPARs). In clinical pregnancy-specific disorders, including preeclampsia, gestational diabetes, and intrauterine growth restriction, aberrant regulation of components of the PPAR system parallels dysregulation of metabolism, inflammation and angiogenesis. This review summarizes current knowledge on the role of PPARs in regulating human trophoblast invasion, early placental development, and also in the physiology of clinical pregnancy and its complications. As increasingly indicated in the literature, pregnancy disorders, such as preeclampsia and gestational diabetes, represent potential targets for treatment with PPAR ligands. With the advent of more specific PPAR agonists that exhibit efficacy in ameliorating metabolic, inflammatory, and angiogenic disturbances, further studies of their application in pregnancy-related diseases are warranted.
1. INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs) are major regulators of lipid and glucose metabolism, inflammation, and angiogenesis [1–6] that allow adaptation of the mother to the nutritional and perfusion requirements of the fetus [3, 7, 8]. PPARs, members of the nuclear hormone receptor superfamily, are ligand-activated transcription factors. The PPAR amino acid sequence can be divided into five modular domains: A/B, C, D, E, and F. Domain E is the ligand binding domain (LBD) and contains a ligand-dependent transcriptional activation function (AF-2). Domain C is the DNA binding domain, formed of two typical zinc fingers. PPARs activate DNA direct repeat response elements by binding as heterodimers with retinoic acid receptor (RXR) partners [9]. There are three PPAR isotypes, PPARα, PPARγ, and PPARβ/δ, that are highly conserved across species, with mouse, rat, and human sequences sharing >80% amino acid homology [6, 10]. The conserved expression of different PPAR and RXR isotypes in both rat and human placentas [11] suggests that these receptors play functional roles in placental lipid transfer and homeostasis. PPARα has a wide distribution and is prominent in tissues with high metabolic rates such as liver, heart, skeletal muscle, and kidney and in steroidogenic organs such as the adrenals [12]. PPARγ has three isoforms (PPARγ1, γ2, and γ3) and is expressed in brown and white adipose tissue, large intestine, to a lesser extent in immune cells (monocytes, macrophages, Peyer’s patches of the digestive tract), the mucosa of colon and cecum, and placental trophoblasts [13– 16]. PPARβ/δ is distributed in all tissues tested with particularly high expression in placenta and large intestine [8, 17, 18]. PPARα and PPARγ are involved in adipocyte differentiation, lipid metabolism, insulin action, and in the regulation of inflammatory responses [1, 5, 16], particularly involving the macrophage [19]. PPARβ/δ is known to be involved in lipid metabolism and inflammation, as well as keratinocyte differentiation and wound healing [5, 20, 21].
The PPAR system is intimately involved in cardiovascular disease, obesity, as well as pregnancy-specific diseases [6, 22]. Over the past decade studies have shown that all three PPAR isotypes are expressed in human placental trophoblast cells [11] and that they are involved in the regulation of pregnancy physiology and its clinical complications. Physiological and pathophysiological conditions that modulate the PPAR system [22– 35] influence the risk and course of preeclampsia (PE), gestational diabetes mellitus (GDM), or intrauterine growth restriction (IUGR) [36– 53]. Some of these diseases and factors involving the PPAR system are summarized in Tables 1 and 2.
Table 1.
Effects of physiological and pathophysiological conditions on PPAR.
| Influence on PPAR action | |||
|---|---|---|---|
| Conditions | PPAR-action | Model | Reference |
| Diabetes | Increases PPARγ in skeletal muscle | Murine | Park et al. [22] |
|
| |||
| Age | Increases PPARγ in subcutaneous fat in older man | Human | Imbeault et al. [23] |
| Decreases PPARα in heart | Murine | Iemitsu et al. 2002 [24] | |
|
| |||
| Hypertension | Increases PPARα and γ in aorta and mesenteric arteries | Murine | Diep and Schiffrin [25] |
|
| |||
| Diet | Soy extract increases PPARα and γ in macrophages | In vitro | Mezei et al. [28] |
| High-fat diet increases adipose tissue expression of PPARγ and induces PPARγ2 mRNA expression in liver (obese mice) | Murine | Vidal-Puig et al. [26] | |
| Hyperlipid diet reduces PPARγ in colonic epithelium | Murine | Delage et al. [29] | |
| Low-calorie diet decreases PPARγ in subcutaneous fat | Human | Bastard et al. [27] | |
|
| |||
| Exercise | Increases PPARγ DNA binding activity in fat depots | Murine | Petridou et al. [30] |
| Increases PPARα in heart | Murine | Iemitsu et al. [24] | |
| Increases PPARβ/δ in skeletal muscle | Human | Fritz et al. [34] | |
|
| |||
| Obesity | Increases of PPARγ2 and PPARγ2/PPARγ1 ratio in adipose tissue | Human | Vidal-Puig et al. [31] |
|
| |||
| Metabolic syndrome | Dominant-negative mutation in PPARγ induces metabolic syndrome | Human | Savage et al. [35] |
|
| |||
| Insulin resistance (IR) | Pioglitazone ameliorates IR | Murine | Ding et al. [33] |
| PPARγ Ala allele protects against hyperinsulinemia | Human | Jaziri et al. [32] | |
|
| |||
| Vitamin A | Increases PPARγ in colonic mucosa | Murine | Delage et al. [29] |
Table 2.
Effects of metabolic conditions on pregnancy-specific diseases (GDM: gestational diabetes mellitus; PE: preeclampsia; IUGR: Intrauterine growth restriction; −: reduced risk; +: increased risk).
| Influence on pregnancy-specific diseases | ||||
|---|---|---|---|---|
| Conditions | GDM | PE | IUGR | Reference |
| Diabetes | — | + | — | Ostlund et al. [36] |
| Advanced maternal age | + | + | + | Delbaere et al. [53] Odibo et al. [37] |
| Hypertension | — | + | + | Sibai et al. [38] |
| Optimal nutrition | − | − | − | Artal et al. [41] Saftlas et al. [43] Scholl et al. [39] |
|
| ||||
| Optimal exercise | − | − | — | Artal et al. [41] Zhang et al. [44] Sorensen et al. [42] Saftlas et al. [43] |
|
| ||||
| Obesity | + | + | + | Cedergren [48] Saftlas et al. [43] O’Brien et al. [47] Ros et al. [45] Sebire et al. [46] Bodnar et al. [49] |
|
| ||||
| Metabolic syndrome | + | + | + | Ray et al. [50] |
| Insulin resistance | — | + | — | Wolf et al. [51] |
| Periconceptional multivitamins | — | − | — | Bodnar et al. [52] |
In early pregnancy, immediately after embryonic implantation, major maternal physiologic changes occur in the cardiovascular, hepatic, and endocrine systems with resultant anatomical and metabolic modifications that serve to promote maternal immune tolerance of the conceptus and to provide the fetus with its increased nutritional needs [54, 55]. Metabolic changes (including increased availability of glucose, low density lipoprotein, and fatty acids) increased insulin resistance and altered amino acid metabolism, immunologic, and hematologic changes (including an increase in plasma volume). Establishment of a thrombophilic state and extensive placental and decidual angiogenesis are observed in pregnancy, and these changes require a complex activation of regulating mediators [56–58].
Pregnancy complications result when the mother and/or fetus fail to adapt to these new metabolic, angiogenic, and thrombogenic challenges. Women with preexisting compromise to their vascular homeostasis, such as underlying hypertension, diabetes mellitus, or metabolic syndrome, have a significantly increased risk of developing pregnancy complications (see Table 2). Placenta-associated complications also can lead to impaired growth or fetal demise [59, 60]. These placental conditions share vasculopathological mechanisms in common with atherosclerosis and represent early markers for maternal risk of cardiovascular disease [61, 62] and hypertension [61,63, 64]. Curiously, a prior history of preeclampsia appears to confer protection against the future development of endometriosis and some cancers [65, 66].
PPARs can be activated by natural ligands, like prostaglandins (PGs), fatty acids, and their derivatives, as well as by synthetic ligands. PPAR medications have been developedand discovered to be relatively safe drugs with benefits in multiple disease states including diabetes and cardiovascular disease [67]. Fibrate drugs used to treat hyperlipidemia, and thiazolidinedione drugs used to treat type 2 diabetes are potent and relatively specific ligand activators of PPARα and γ, respectively, and are widely used clinically [68, 69]. A number of naturally-occurring PPAR ligands have been identified, including long-chain fatty acids (C16 and greater), eicosanoids such as 8(S)-HETE (PPARα) and 9-and13-HODE (PPARγ), and PGs such as PGA1,which binds to PPARα, PPARβ/δ, and 15-deoxy-delta12,14-prostaglandin J2(15dPGJ2), which in turn binds to PPARγ [70– 72]. Both the expression of PPAR and the production of their potential ligands are altered during pregnancy and its related diseases. We postulate that pathologic diversion of fatty-acid metabolism away from the production of eicosanoid ligands in preeclampsia and gestational diabetes might be corrected using synthetic ligands.
2. PPARs IN TROPHOBLAST INVASION AND PLACENTAL DEVELOPMENT
In first trimester, human placental bed biopsies, PPAR-γ is expressed predominantly in invasive trophoblasts, whereas in the second-trimester PPARγ is expressed in the columns of anchoring villi and cytotrophoblasts [73, 74]. In the third trimester, PPARγ principally localizes to extravillous cytotrophoblasts (EVCT) and villous syncytiotrophoblasts [75], where it appears to regulate placental hormone production and secretion. Although the focus of this review is to summarize findings on PPAR/RXR heterodimers in human placentation, much of the direct evidence for a role of these receptors in trophoblast invasion and placental development has emerged from studies in knockout mouse models. This topic is reviewed comprehensively in Schaiff et al. [3], and is summarized briefly here and in Table 3 [76–81].
Table 3.
PPAR knock out models and placental pathology (PRIP: peroxisome proliferator-activated receptor-(PPAR) interacting protein; RAP 250: nuclear receptor-activating protein 250).
| PPAR knockout model | Placental pathology | Lethality | Reference |
|---|---|---|---|
| PPARα | No significant effect on placentation | 20% | Yessoufou et al. [76] |
| PPARβ/δ | Poor placentation | >90% | Barak et al. [77] |
| PPARγ | Poorly developed labyrinth | 100% | Barak et al. [15] Kubota et al. [82] |
| PPARγ coactivator PRIP | Reduced spongiotrophoblast layer | 100% | Zhu et al. [79] |
| PPARγ coactivator RAP250 | Reduced spongiotrophoblast layer | 100% | Antonson et al. [80] |
| RXRα or β | Lack of labyrinth zone | 100% | Sapin et al. [81] |
PPARγ/RXRα heterodimers play a key regulatory role in murine placental development. PPARγ deficiency was shown to interfere with terminal trophoblast differentiation and placental vascularization [78]; embryos without this gene show massive placental defects that can be rescued by restoration of the trophoblast PPARγ gene via tetraploid chimeras [15]. Deletion of RXRα and RXRβ also leads to embryo lethality [15, 81, 83]. Both PPAR-interacting protein (PRIP) and nuclear receptor-activating protein 250 (RAP250) encode nuclear receptor coactivators that associate with PPARs, RXRs, and other nuclear receptor proteins. Genetic disruption of PRIP or RAP250 in mouse models results in embryonic lethality at postconception days 11.5 and 13.5, respectively [79, 80]. Placentas of PRIP (−/−) and RAP250 (−/−) embryos exhibited dramatically reduced spongiotrophoblast and labyrinth layers as well as failure of blood vessel maturation in the region bordering the spongiotrophoblast [79, 80].
In addition to placentation per se, PPARγ appears to play an important role in the uterine preparation for embryonic implantation. Peeters et al. demonstrated that PPARγ ligands reduced the production of the endometrial angiogenic factor VEGF, and postulated that this pathway might influence early embryonic vascularization [84]. By contrast, PPARγ agonists induce angiogenesis in cardiac myofibroblasts, smooth muscle cells, and macrophages [85– 87]. Recent preliminary data by our lab and others suggest that the PPARγ system also stimulates VEGF expression in trophoblast (JEG-3) cells (Depoix et al., unpublished).
The functional role of PPARγ activity is well studied in trophoblast physiology (Table 4). PPARγ agonists inhibit invasion of cultured EVCT isolated from human first-trimester placenta, whereas PPARγ antagonists promoted EVCT invasion and repressed the PPARγ agonist-mediated effects [78]. PPARγ controls mucin (MUC)-1 transcription and regulates maternal-fetal transport in mouse models [88]. Moreover, PPARγ and RXRα play a role in human chorionic gonadotropin (hCG) expression, trophoblast differentiation, and regulation of fatty acid transport and storage in human placental trophoblasts [89, 90]. PPARγ diminishes leptin-induced inflammatory responses in the human placenta [91] and inhibits PAPP-A expression [92].
Table 4.
PPAR action in trophoblast development and placental function (MUC-1: mucin-1; EVCT: extravillous cytotrophoblast; hCG: human chorionic gonadotropin; Th2 T-helper 2 cell).
| PPAR action in trophoblast development and placentation | |||
|---|---|---|---|
| PPAR | PPAR action | Model | Reference |
| PPARγ | Inhibits EVCT invasion | In vitro | Fournier et al. [78] |
| Promotes trophoblast differentiation hCG secretion | In vitro | Tarrade et al. [89] | |
| Induces hCG production | In vitro | Schild et al. [93] | |
| Antiinflammatory | In vitro | Lappas et al. [91] | |
| Regulates fatty acid transport | In vitro | Schaiff et al. [90] | |
| Increases VEGF expression | In vitro | Depoix, unpublished | |
| Terminal differentiation, placental vascularization | Murine | Barak et al. [15] | |
| Controls MUC-1 expression | Murine | Shalom-Barak et al. [88] | |
| Stimulates trophoblast maturation | Murine | Asami-Miyagishi et al. [94] | |
| Modulates placental lipid metabolism | Murine | Capobianco et al. [95] | |
|
| |||
| PPARβ/δ | Promotes placental development | Murine | Nadra et al. [8] |
|
| |||
| PPARα | Regulates placental lipid transfer | Murine/Human | Wang et al. [74] |
|
| |||
| PPAR action in pregnancy | |||
|
| |||
| PPARγ | Antiinflammatory | In vitro | Lappas et al. [96] |
| Involved in inflammatory control and remodeling in the placenta | In vitro | Marvin et al. [97] | |
| Increased circulating PPARγ activators in normal pregnancy | In vitro/human | Waite et al. [73] | |
| Decreases in fetal membrane with labor | Human | Dunn-Albanese et al. [98] | |
|
| |||
| PPARβ/δ | Increases in amnion with labor | Human | Berry et al. [99] |
|
| |||
| PPARα | Stimulates Th2 cytokine pattern during pregnancy | Murine | Yessoufou et al. [76] |
| Declines in choriodecidua with labor | Human | Berry et al. [99] | |
Regulation of PPARγ in human placental tissues is thought to occur through natural ligands (e.g., 15dPGJ2, 9-HODE, 13-HODE, and 15-HETE) through direct binding to the receptor’s ligand binding pocket [11, 100]. These ligands are likely to be synthesized locally within the placenta. Furthermore, crosstalk between the mitogen-activated protein kinase (MAPK) p38 and PPARγ occurs within cultured trophoblast cells [101]. PPARγ decreases IGFII secretion and is thought to inhibit trophoblast invasion via the PAPP-A cascade [92].
In young PPARα knock out mice, no major phenotypic differences of gross pathology of internal organs were described [76, 102]. However, disturbance of the Th1/Th2 T-lymphocyte ratio, rather than placental malformation, is thought to be responsible for an increased abortion rate (20%) in PPARα null mice. During normal pregnancy Th1 cytokines are downregulated and Th2 cytokines are upregulated [103].
The third distinct PPAR, PPARβ/δ also is essential for placentation as demonstrated in PPARβ/δ knockout mice (Table 3) [77], and is involved in the regulation of implantation in other animal models [17, 104, 105]. The implantation of cultured embryos is enhanced by PPARβ/δ activation and this receptor even has been postulated as a novel therapeutic target to improve clinical IVF outcomes [104]. PPARβ/δ is induced during decidualization of the implantation site and requires close contact with the blastocyst. PPARβ/δ null mice die between 9.5 to 10.5 embryonic days due to abnormal cell-cell communication at the placental-decidual interface [8].
Together these data suggest that PPARs are required not only for trophoblast invasion and differentiation but also for establishment of the placental maternal-fetal transport.
3. PPARs AND PREGNANCY
Based on its regulatory functions and known eicosanoid ligands, PPARγ has emerged as an excellent candidate to play a role in the regulation of maternal metabolism, maintenance of uterine quiescence, and onset of labor by regulating proinflammatory cytokines and prostaglandins (Table 4). Normal pregnancy is accompanied by changes in lipid and glucose metabolism, but further dysregulation of these pathways can lead to pregnancy complications such as PE or GDM. Hence, PPAR regulators of these metabolic pathways might be expected to be important in human pregnancy.
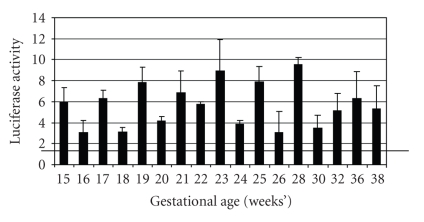
Some of our initial studies in this field were designed to screen for potential activators of PPARγ in the circulation of pregnant women. Human choriocarcinoma JEG-3 cells were transfected with peroxisome-proliferator responsive reporter plasmids; and pooled sera from pregnant and nonpregnant women were added to the cell culture medium [73]. Peroxisome proliferator responsive element (PPRE) luciferase reporter activation was dramatically increased by sera from pregnant women compared to nonpregnant women (Figures 1 and 2). We showed that PPARγ (and to some extent PPARα) activity is increased from the earliest stages of pregnancy (Figure 2). The findings suggested that circulating PPARγ-activating factors, presumably eicosanoids, were present throughout the course of gestation. We hypothesized that activation of PPARγ by sera of pregnant women is a regulatory adaptation of the maternal organism to increased lipid and glucose loading in pregnancy [73].
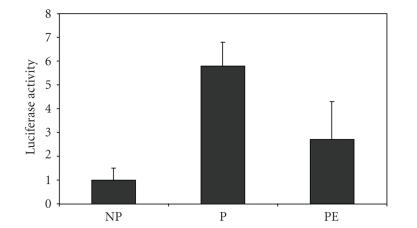
Figure 1.
JEG-3 cells were transfected with PPRE-luciferase reporter vectors and treated with pooled sera (10%) from non-pregnant (NP), pregnant (P) and preeclamptic (PE) women. Luciferase acitivity, relative to cells treated with 10% dextran charcoal-shipped fetal calf serum (DCSS), is reported on the ordinate.
Figure 2.
PPARγ activation is present throughout the course of normal pregnancy. All serum samples were collected from the same subject and PPRE-luciferase reporter experiments were performed using 10% serum as described in Figure 1. Luciferase activity was normalized to DCSS to determine relative activation. Black horizontal bar represents the level of signaling seen with 10% serum from the same woman six weeks after delivery.
It also has been hypothesized that PPARγ activation regulates uterine quiescence by influencing Nuclear Factor-Kappa B (NFκB) and cyclooxygenase (COX-2) expression [96, 97, 106]. Reciprocal expression of PPARγ and (COX)-2 in human term placenta suggests a role of the PPAR system in the initiation of labor [98]. Under conditions of high PPARγ expression, antiinflammatory actions dominate; however, with onset of labor PPARγ levels drop and COX-2 concomitantly increases in the fetal membranes [98]. Elevated COX-2 activity in the human amnion is observed in the settings of term and idiopathic preterm labor, contributing to the generation of uterotonic prostaglandins (PGs), which are known to participate in parturition [107]. PPARγ ligands have been shown to antagonize NF-κB activation and reduce inflammatory cytokine gene expression (IL-1β, IL-6, IL-10 and TNF-α) and COX-2 [108]. Both natural (e.g., 15dPGJ2) and synthetic ligands (e.g., troglitazone) were shown to have anti-inflammatory effects in human gestational tissues, significantly decreasing basal and LPS-stimulated PGE2 and PGF2α release from placenta and amnion [108]. PGF2α , also a marker of oxidative stress, is increased in women with preeclampsia [109]. Given the inflammatory changes observed in pregnancy-specific diseases, a potential role of PPAR agonist treatment has been entertained for the treatment of PE, GDM, and other pregnancy-specific diseases such as the prevention of preterm labor [96].
PPARα and β/δ also play a role in maintaining pregnancy and parturition. PPARα and β/δ are expressed in the amnion, choriodecidua, and villous placental tissues. Data from PPARα knockout mice suggest that PPARα maintains pregnancy by stimulating a Th2 cytokine response [76]. In normal pregnancy, expression of PPARα declines in the choriodecidua with the onset of labor [99]. By contrast, PPARβ/δ expression, which is temporally upregulated between the first and third trimester of pregnancy [99], increases further in the amnion coincidental with the onset of labor [99].
Few studies have elucidated substantial risk of PPAR agonists during pregnancy in animal models, but these drugs carry a “C” classification from the FDA. For example, rosiglitazone did not damage blastocyst development in vitro or harm mouse fetuses when given during murine pregnancy [110]. While the use of rosiglitazone during pregnancy is generally considered to be safe [110]; more data need to be acquired before these drugs can be recommended.
4. PPARs and PREGNANCY-SPECIFIC DISEASES
Failure of metabolic adaptation to pregnancy can result in pregnancy-specific complications such as PE and GDM. We and others have postulated that angiogenic factors and cytokines that lead to pathological gestational changes are likely to be regulated by the PPAR system (Table 5).
Table 5.
PPAR in pregnancy-specific diseases.
| PPAR | PPAR-action | Disease | Model | Reference |
|---|---|---|---|---|
| PPARγ | Reduced circulating PPARγ activators in serum from women with PE | PE | In vitro | Waite et al. [111] |
| Placental 15dPGJ2level are decreased in diabetes | GDM | Murine | Capobianco et al. [95] | |
| Association of PPAR-γ2 Pro12Ala with weight gain | GDM | Human | Tok et al. [112] | |
| Placental 15dPGJ2levels are decreased | GDM | Human | Javerbaum et al. [113] | |
| Decreased | Hydatidiform mole | Human | Capparuccia et al. [114] | |
| Decreased | Choriocarcinoma | Human | Capparuccia et al. [114] | |
| Placental PPAR expression is not involved | IUGR | Human | Rodie et al. [115] | |
| Association of PPAR-γ2 Pro12Ala polymorphism | Preterm birth | Human | Meirhaeghe et al. [116] | |
|
| ||||
| PPARα | Lack of PPAR-α upregulates Th1 cytokines | Abortion/neonatal mortality | Murine | Yessoufou et al. [76] |
4.1. PPARs and preeclampsia
PE is a multifactorial, pregnancy-related disorder that is defined by new-onset hypertension and proteinuria after 20 weeks of gestation [117]. PE is a common cause of maternal and infant morbidity and mortality worldwide, and is responsible for about 20% of pregnancy-related maternal deaths in the US [118]. Women with PE have increased insulin resistance as well as hypertriglyceridemia relative to normal pregnant women [119]. To date, no effective treatment has been found that either prevents or reverses the development of the disease. Modern concepts of PE pathophysiology invoke a two-stage process. The first stage is believed to be initiated by impaired trophoblast invasion and abnormal uterine vessel remodeling. The second stage is postulated to result from circulating factors claimed to be derived from the ischemic placenta that stimulate an inflammatory activation of maternal vascular endothelial cells. PE presents clinically in the second or third trimester, however, fundamental inflammatory and angiogenic biomarkers in the serum are detectable as early as the first trimester in women with PE. Elevated concentrations of IL-2, TNFα, and sVEGFR-1 and reduced concentrations of PlGF, IGFBP-1, and HLA-G in the maternal serum precede the clinical manifestations of PE [119–123].
While the cause of PE remains unknown, several environmental and genetic risk factors have been identified (Table 2). Relevant to this review are hypertension, diabetes, and high (>29) body mass index (BMI) [47, 124,125]. Black race also appears to be a risk factor for PE [126] although this may be confounded by increased rates of the above risk factors. Key inflammatory and angiogenic pathways involved in the pathogenesis of PE are regulated by the PPAR system, which itself is influenced by environmental and genetic factors. We believe that exogenous and endogenous lipid regulators of PPAR play a role in maternal metabolism and immune functionin normal and pathological pregnancies. For example, dietary factors and physical activity that modulate the PPAR system have been shown to reduce the risk and course of PE (Table 2).
Similarly, genetic variations in the PPARγ gene have been proposed to modify the risk of PE. For example, the Pro467Leu mutation of PPARγ [127–129] is a dominant negative mutant resulting from a C-to-T transition in exon 6. A report of two individuals (one woman, one man) with this mutation showed that they developed type 2 diabetes at young ages (26 and 27 years at diagnosis), as well as early hypertension (37 and 27 years at diagnosis). Intriguingly, the woman had two pregnancies, both of which were complicated by severe PE. The Pro12Ala polymorphism occurs in PPARγ2 [130], a second isoform of PPARγ that is expressed mainly in adipose tissue. This mutation is the result of a C-to-G transversion in exon B. This is by far the most studied allelic variation in any PPAR, and occurs at a rate of about 12% in the Caucasian US population. While the resulting phenotype is highly diverse and even apparently contradictory, it appears that the penetrance of this mutation is influenced by other genetic, environmental, ethnic, and gender differences. The studies generally agree that the presence of the Ala allele is associated with increased BMI, an independent risk factor for PE. Thus, this polymorphism is a candidate affecting pregnancy outcome. Preliminary data of a study on the PPAR gene variations (in PPAR gene) showed no association with PE or severity of PE in a Finnish population [131]. Further studies on the association of PPAR α, β, and γ gene variations of mothers and offspring and pregnancy-specific diseases need to be performed in different ethnic populations.
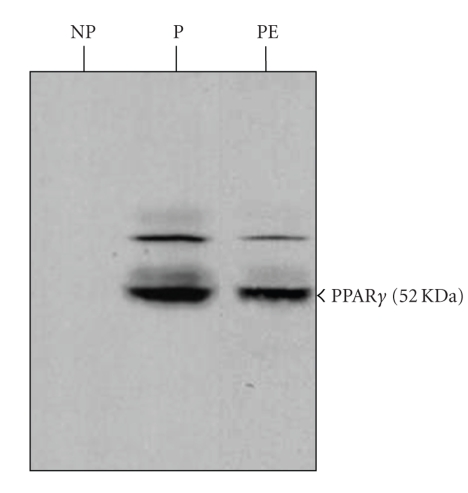
PE is marked by hyperlipidemia, and is characterized by a state of oxidative stress. Circulating lipids in PE women are more highly oxidized, and oxidized low-density lipoproteins (oxLDLs), in particular, are highly elevated [132]. Given the circulating plasma lipid disturbances in PE, our group performed experiments comparing sera from normal and PE patients. We found that serum from women with severe PE had reduced levels of PPAR activating lipids compared with serum of parity and gestational age-matched women and also diminished the expression of PPARγ in trophoblast cells (Figures 1 and 3) [111]. The reduction of transcriptional activity observed in preeclamptic women’s sera was shown for PPARγ and PPARα, however not for PPARβ/δ or RXR. The reduction in potential circulating PPAR activatorswas observed weeks and sometimes months before the onset of maternal symptoms and clinicaldiagnosis of PE [133]. Our results are consistent with other clinical evidence that antiinflammatory regulation is challenged and further compromised in the maternal syndrome of PE. Normal pregnancy manifests as a physiologic inflammatory state postulated to be tolerated to serve the nutritional needs of the fetus, whereas, in PE regulatory inflammatory mechanisms are excessively amplified, leading to vascular damage in the mother [133]. In this “hyperinflammatory” state of PE [134], the cytokines TNFα and IL-1β which are typically controlled by the NF-κB pathway in a negative-feedback loop with PPAR, are elevated [26, 60, 119]. Elevated inflammatory parameters in PE accompany altered levels of PG metabolites and circulating fatty acids. As noted, PG metabolites as well as fatty acids are important ligands of the PPAR system [135]. PG metabolism is altered during normal pregnancy with levels of vasorelaxants suchas prostacyclin increasing, whereas vasoconstrictive prostaglandin levelstend to be suppressed [136]. Failure of these alterations have been suggested to lead to pregnancy complications (e.g., PE) [137]. For example, PGF2α, which itself is stimulated by factorsin the plasma of women with PE [138], can inhibit PPARγ effects [135]. Levels of circulating free fatty acids are in the normal range duringmost of pregnancy, but rise dramatically during the final weeks of pregnancy and drop precipitously at term [136]. In PE these levels are increased from 20 weeks’ gestation [133, 139]. We postulate that altered PG metabolism in this setting [138] results in decreased PPARγ ligation and subsequent cytokine activation. If this proposal is supported by more data, the use of PPAR ligands might be proposed to ameliorate symptoms such as hypertension and inflammation. Unfortunately, at present, the mechanism and site of this salutary of PPAR ligand effect remain unknown in pregnancy, confounded by PPAR expression in many cell types, including endothelial cells.
Figure 3.
Immunoblot of JEG-3 cells treated with pooled sera (10%) from nonpregnant (NP), pregnant (P), and preeclamptic (PE) women. Cell lysates were analyzed using a specific mouse anti-human PPARγ monoclonal antibody. Equal amounts of protein (50 μg) were loaded into each lane. Factors in pregnant serum up-regulate JEG-3 PPARγ expression. A decrease in PPARγ protein was observed in cells exposed to PE sera (PE) compared to sera from normal pregnant women (P).
4.2. PPARs and gestational diabetes
During normal pregnancy, maternal lipid, and glucose metabolism is profoundly altered [140]. The developing fetus uses glucose as its predominant energy source, which puts a continuous demand on the mother to provide this substrate [141]. This constant need for glucose results in frequent hypoglycemia and postprandial hyperglycemia during normal pregnancy [141]. Problems with energy metabolism such as GDM are not uncommon and are often observed in susceptible women at this time. GDM is defined as any degreeof glucose intolerance with onset or first recognition during pregnancy. In women with GDM, defective β-cells function cannot adequately compensate for free fatty acid-mediated insulin resistance [142]. As elsewhere in our society, the incidence of obesity, diabetes, and gestational diabetes mellitus are increasing in the pregnant population [143]. In the United States, the incidence of obesity among pregnant women ranges from 18.5% to 38.3% [144]; obesity comprises a major risk factor for GDM [145]. Morphological changes have been identified in the syncytiotrophoblast, cytotrophoblast, trophoblastic basement membrane, and fetal vessels within the placentae of these cases [146]. GDM is associated with several severe neonatal complications (such as macrosomia, brachial plexus palsy, premature delivery, IUGR, and intrauterine death) and maternal birth injuries also are common [125, 147]. Furthermore, GDM has emerged as a risk factor for the development of diabetes mellitus type 2 (DM2) and cardiovascular disease in later life and shares a number of epidemiologic, pathophysiologic, and genetic characteristics with DM2 [148]. GDM also has detrimental effects on the postnatal infants [149].
The PPAR system regulates the metabolic and pathways involved in the establishment of GDM. PPAR-agonists have antidiabetogenic, antiinflammatory, and antioxidant effects, which are all potentially beneficial in the treatment of GDM [5].
Environmental factors, such as diet and exercise and genetic factors influence PPARα, γ activity [130, 150] as well as the risk for insulin resistance and GDM (Table 2). Exercise activity initiated prepregnancy was shown to reduce the risk of GDM and its complications [40, 41, 44, 151,152]. Nutritional counseling, moderate physical exercise, weight loss, and diet are successful therapies in some women with GDM, improving glycemic control, reducing the incidence of LGA infants, and decreasing the need for cesarean deliveries for cephalopelvic disproportion [41, 153].
Candidate genes for GDM risk include TNFα, β3 adrenoreceptor (ADRB3), and PPARα and γ. The PPARγ Pro12Ala polymorphism was not associated with increased insulin resistance in Turkish women with GDM, however it was associated with weight gain [112]. The PPARγ coactivator-1alpha (PGC-1) polymorphism also failed to be associated with the development of GDM [154]. More studies on the association of various genetic PPARα and γ variants and GDM in different ethnic populations will be of interest.
15dPGJ2 is a potent antiinflammatory agent that represses the expression of a number of inflammatory genes and regulating factors including the transcription factor NF-κB [33, 108]. The concentration of 15dPGJ2 was reduced in placentae from diabetic rats (Table 5) [95]. Placental 15dPGJ2 was noted to be diminished in women with gestational and pregestational diabetes when compared to controls, whereas levels of nitric oxide (a stimulator of placental invasiveness, differentiation, and proliferation) were higher in term placental explants from diabetic patients when compared to controls [113]. As PPARγ can prevent nitric oxide overproduction in placenta from pregestational diabetic women [113], it may have the potential to improve fetal outcome in this condition.
Sulfonylurea agents including gliumepiride and glibenclamide exhibit PPARγ activity [155]. A randomized controlled trial to test the effectiveness and safety of the sulfonylurea agent glyburide in the management of women with GDM showed similar efficacy to insulin treatment [156]. Both the insulin- and glyburide-treated women were able to achieve satisfactory glucose control and had similar perinatal outcome [156].
4.3. PPARs and other pregnancy-specific diseases
Trophoblast research has emphasized the similarities between the proliferative, migratory, andinvasive properties of placental cells and those of cancer cells [157]. PPARγ, PPARβ/δ, and RXR appear to be linked to gestational trophoblastic neoplasms, conditions associated with malignant trophoblast behavior [114]. PPARγ agonists inhibit invasion of normal extravillous cytotrophoblast isolated from human first-trimester placenta, and PPAR activity has been shown to be downregulated in trophoblastic diseases including hydatidiform mole and choriocarcinoma [114].
PPARγ has an effect on fetal and placental size influencing intrauterine growth. In an intrauterine growth restriction (IUGR) model, glucocorticoids inhibited fetal and placental growth partly by suppression of PPARγ in the labyrinth zone of the placenta [158]. Activation of PPARγ in the labyrinth trophoblasts is hypothesized to induce angiogenic factors and stimulate the growth of fetal blood vessels, thereby promoting placental growth. However, treatment of pregnant mice with rosiglitazone led to reduced thickness of the spongiotrophoblast layer and the surface area of labyrinthine vasculature, and it altered expression of proteins implicated in placental development [159].
In vitro and in vivo experiments as well as animal models studies suggest a link between the PPAR system and gestational duration, preterm labor, and birth weight [116]. Variations in the PPAR genes influence other pregnancy-related mechanisms including birth weight and gestational duration. In an Irish population, the PPARγ Ala12 allele was associated with shorter gestational duration [116].
PPAR ligands regulate apoptotic mechanisms involved in rupture of the fetal membranes and may play a role in preterm delivery, a condition associated with increased risk of neonatal sepsis and newborn trauma [160]. 15d-PGJ2induced morphological characteristics of apoptosis within 2 hours in an amniotic cell line [160]. In addition, ciglitizone also induced apoptosis, whereas rosiglitazone had no effect on cell viability [160]. Prevention of apoptosis may have therapeutic potential in preterm labor and premature rupture of the membranes and necessitates further investigations.
Interestingly, PPARα deficiency is associated with miscarriage, neonatal mortality, and a shift from Th2 to a Th1 cytokine phenotype [76]. Th1 predominant immunity is closely associated with inflammation, endothelial dysfunction, and pregnancy complications. For example, interferonγ is significantly reduced in the spleens of PPARα null mice [76]. Twenty percent of PPARα knockout mice aborted, and offspring of PPAR-α null mice exhibited increased neonatal mortality (13.3%). However the mechanism whereby PPARα induces a Th2 phenotype shift remains to be determined. PPARγ ligands also were shown to decrease production of inflammatory ligands in activated macrophages and T cells and to induce a shift from Th1 to Th2 cytokine phenotype [161, 162].
5. CONCLUSIONS
PPARs are involved in trophoblast invasion, placental development, parturition, and pregnancy-specific diseases, particularly PE and GDM. The role of the PPAR system in pregnancy under physiologic and pathologic conditions has remained partly unclear due to lack of knowledge about endogenous PPAR ligands. Pharmacological ligand research is ahead of the identification of physiologic ligands. Partially characterized inflammatory, angiogenic, and metabolic disturbances in pregnancy-related diseases suggest that these synthetic PPAR agonists may be of potential use in these conditions. Ongoing basic studies have elucidated the metabolic, antiinflammatory, and angiogenic benefits of PPARα/β/δ and PPARγ/β/δ dual agonists and PPAR pan agonists for treatment purposes. However, some experimental and clinical data have uncovered unfortunate side effects of PPAR ligands, including cancer progression and increased cardiac event rates. New generations of PPAR modulators are under development and these promise to be more receptor-specific, and hopefully will activate only a specific subset of target genes and metabolic pathways to reduce untoward side effects. The potential role of PPARs in regulation of inflammation and angiogenesis is intriguing and warrants further studies. We submit that PPAR agonists may become beneficial drugs for pregnancy-specific diseases, once their risks have been fully evaluated.
ACKNOWLEDGMENT
The authors’ studies described in this article were supported by NIH Grants P01-HD30367 and R01-HL73469.
References
- 1.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Current Opinion in Pharmacology. 2006;6(4):421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Margeli A, Kouraklis G, Theocharis S. Peroxisome proliferator activated receptor- (PPAR-) ligands and angiogenesis. Angiogenesis. 2003;6(3):165–169. doi: 10.1023/B:AGEN.0000021377.13669.c0. [DOI] [PubMed] [Google Scholar]
- 3.Schaiff WT, Barak Y, Sadovsky Y. The pleiotropic function of PPAR in the placenta. Molecular and Cellular Endocrinology. 2006;249(1-2):10–15. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. Journal of Endocrinology. 2006;189(2):199–209. doi: 10.1677/joe.1.06667. [DOI] [PubMed] [Google Scholar]
- 5.Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 6.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 7.Holness MJ, Greenwood GK, Smith ND, Sugden MC. Peroxisome proliferator-activated receptor- and glucocorticoids interactively regulate insulin secretion during pregnancy. Diabetes. 2006;55(12):3501–3508. doi: 10.2337/db06-0666. [DOI] [PubMed] [Google Scholar]
- 8.Nadra K, Anghel SI, Joye E, et al. Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor . Molecular and Cellular Biology. 2006;26(8):3266–3281. doi: 10.1128/MCB.26.8.3266-3281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krey G, Mahfoudi A, Wahli W. Functional interactions of peroxisome proliferator-activated receptor, retinoid-X receptor, and Sp1 in the transcriptional regulation of the acyl- coenzyme-A oxidase promoter. Molecular Endocrinology. 1995;9(2):219–231. doi: 10.1210/mend.9.2.7776972. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. American Journal of Physiology - Renal Physiology. 1997;273(6):F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 11.Fournier T, Tsatsaris V, Handschuh K, Evain-Brion D. PPARs and the placenta. Placenta. 2007;28(2-3):65–76. doi: 10.1016/j.placenta.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-, -, and - in the adult rat. Endocrinology. 1996;137(1):354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 13.Fajas L, Fruchart J-C, Auwerx J. PPAR3 mRNA: a distinct PPAR mRNA subtype transcribed from an independent promoter. FEBS Letters. 1998;438(1-2):55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 14.Peraldi P, Xu M, Spiegelman BM. Thiazolidinediones block tumor necrosis factor--induced inhibition of insulin signaling. Journal of Clinical Investigation. 1997;100(7):1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barak Y, Nelson MC, Ong ES, et al. PPAR is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Young HA. PPAR and immune system—what do we know? International Immunopharmacology. 2002;2(8):1029–1044. doi: 10.1016/s1567-5769(02)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord E, Murphy BD, Desmarais JA, Ledoux S, Beaudry D, Palin M-F. Modulation of peroxisome proliferator-activated receptor and transcripts in swine endometrial tissue during early gestation. Reproduction. 2006;131(5):929–942. doi: 10.1530/rep.1.00657. [DOI] [PubMed] [Google Scholar]
- 18.Higashiyama H, Billin AN, Okamoto Y, Kinoshita M, Asano S. Expression profiling of Peroxisome proliferator-activated receptor- (PPAR-) in mouse tissues using tissue microarray. Histochemistry and Cell Biology. 2007;127(5):485–494. doi: 10.1007/s00418-007-0279-5. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR- dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nature Medicine. 2001;7(1):48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 20.Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A. Role of AMP kinase and PPAR in the regulation of lipid and glucose metabolism in human skeletal muscle. Journal of Biological Chemistry. 2007;282(27):19313–19320. doi: 10.1074/jbc.M702329200. [DOI] [PubMed] [Google Scholar]
- 21.Adjuik. M, Babiker A, Garner P, Olliaro P, Taylor W, White N. Artesunate combinations for treatment of malaria: meta-analysis. The Lancet. 2004;363(9402):9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- 22.Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. PPAR- gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes. 1997;46(7):1230–1234. doi: 10.2337/diab.46.7.1230. [DOI] [PubMed] [Google Scholar]
- 23.Imbeault P, Vidal H, Tremblay A, et al. Age-related differences in messenger ribonucleic acid expression of key proteins involved in adipose cell differentiation and metabolism. Journal of Clinical Endocrinology & Metabolism. 2001;86(2):828–833. doi: 10.1210/jcem.86.2.7203. [DOI] [PubMed] [Google Scholar]
- 24.Iemitsu M, Miyauchi T, Maeda S, et al. Aging-induced decrease in the PPAR- level in hearts is improved by exercise training. American Journal of Physiology - Heart and Circulatory Physiology. 2002;283(5):H1750–H1760. doi: 10.1152/ajpheart.01051.2001. [DOI] [PubMed] [Google Scholar]
- 25.Diep QN, Schiffrin EL. Increased expression of peroxisome proliferator-activated receptor- and - in blood vessels of spontaneously hypertensive rats. Hypertension. 2001;38(2):249–254. doi: 10.1161/01.hyp.38.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, et al. Regulation of PPAR gene expression by nutrition and obesity in rodents. Journal of Clinical Investigation. 1996;97(11):2553–2561. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bastard JP, Hainque B, Dusserre E, et al. Peroxisome proliferator activated receptor-, leptin and tumor necrosis factor- mRNA expression during very low calorie diet in subcutaneous adipose tissue in obese women. Diabetes/Metabolism Research and Reviews. 1999;15(2):92–98. doi: 10.1002/(sici)1520-7560(199903/04)15:2<92::aid-dmrr21>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese zucker rats and murine RAW 264.7 cells. Journal of Nutrition. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 29.Delage B, Bairras C, Buaud B, Pallet V, Cassand P. A high-fat diet generates alterations in nuclear receptor expression: prevention by vitamin A and links with cyclooxygenase-2 and -catenin. International Journal of Cancer. 2005;116(6):839–846. doi: 10.1002/ijc.21108. [DOI] [PubMed] [Google Scholar]
- 30.Petridou A, Tsalouhidou S, Tsalis G, Schulz T, Michna H, Mougios V. Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor in rat adipose tissue. Metabolism. 2007;56(8):1029–1036. doi: 10.1016/j.metabol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, et al. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. Journal of Clinical Investigation. 1997;99(10):2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaziri R, Lobbens S, Aubert R, et al. The PPARG Pro12Ala polymorphism is associated with a decreased risk of developing hyperglycemia over 6 years and combines with the effect of the APM1 G-11391A single nucleotide polymorphism. Diabetes. 2006;55(4):1157–1162. doi: 10.2337/diabetes.55.04.06.db05-0676. [DOI] [PubMed] [Google Scholar]
- 33.Ding SY, Shen ZF, Chen YT, Sun SJ, Liu Q, Xie MZ. Pioglitazone can ameliorate insulin resistance in low-dose streptozotocin and high sucrose-fat diet induced obese rats. Acta Pharmacologica Sinica. 2005;26(5):575–580. doi: 10.1111/j.1745-7254.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 34.Fritz T, Krämer DK, Karlsson HK, et al. Low-intensity exercise increases skeletal muscle protein expression of PPAR and UCP3 in type 2 diabetic patients. Diabetes/Metabolism Research and Reviews. 2006;22(6):492–498. doi: 10.1002/dmrr.656. [DOI] [PubMed] [Google Scholar]
- 35.Savage DB, Tan GD, Acerini CL, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor- . Diabetes. 2003;52(4):910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 36.Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;113(1):12–16. doi: 10.1016/j.ejogrb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Odibo AO, Nelson D, Stamilio DM, Sehdev HM, Macones GA. Advanced maternal age is an independent risk factor for intrauterine growth restriction. American Journal of Perinatology. 2006;23(5):325–328. doi: 10.1055/s-2006-947164. [DOI] [PubMed] [Google Scholar]
- 38.Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The calcium for preeclampsia prevention (CPEP) study group. American Journal of Obstetrics & Gynecology. 1997;177(5):1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 39.Scholl TO, Leskiw M, Chen X, Sims M, Stein TP. Oxidative stress, diet, and the etiology of preeclampsia. American Journal of Clinical Nutrition. 2005;81(6):1390–1396. doi: 10.1093/ajcn/81.6.1390. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia. 2006;49(11):2604–1613. doi: 10.1007/s00125-006-0422-1. [DOI] [PubMed] [Google Scholar]
- 41.Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Applied Physiology, Nutrition, and Metabolism. 2007;32(3):596–601. doi: 10.1139/H07-024. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41(6):1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 43.Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. American Journal of Epidemiology. 2004;160(8):758–765. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C, Solomon CG, Manson JE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Archives of Internal Medicine. 2006;166(5):543–548. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]
- 45.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. American Journal of Epidemiology. 1998;147(11):1062–1070. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 46.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287 213 pregnancies in London. International Journal of Obesity and Related Metabolic Disorders. 2001;25(8):1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368–374. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- 48.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics & Gynecology. 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 49.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2):234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 50.Ray JG, Vermeulen MJ, Schull MJ, McDonald S, Redelmeier DA. Metabolic syndrome and the risk of placental dysfunction. Journal of Obstetrics and Gynaecology Canada. 2005;27(12):1095–1101. doi: 10.1016/s1701-2163(16)30391-7. [DOI] [PubMed] [Google Scholar]
- 51.Wolf M, Sandler L, Muñoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: a prospective study. Journal of Clinical Endocrinology & Metabolism. 2002;87(4):1563–1568. doi: 10.1210/jcem.87.4.8405. [DOI] [PubMed] [Google Scholar]
- 52.Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. American Journal of Epidemiology. 2006;164(5):470–477. doi: 10.1093/aje/kwj218. [DOI] [PubMed] [Google Scholar]
- 53.Delbaere I, Verstraelen H, Goetgeluk S, Martens G, De Backer G, Temmerman M. Pregnancy outcome in primiparae of advanced maternal age. European Journal of Obstetrics Gynecology & Reproductive Biology. 2007;135(1):41–46. doi: 10.1016/j.ejogrb.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Kalhan SC. Protein metabolism in pregnancy. American Journal of Clinical Nutrition. 2000;71(5, supplement):1249S–1255S. doi: 10.1093/ajcn/71.5.1249s. [DOI] [PubMed] [Google Scholar]
- 55.Sivan E, Boden G. Free fatty acids, insulin resistance, and pregnancy. Current Diabetes Reports. 2003;3(4):319–322. doi: 10.1007/s11892-003-0024-y. [DOI] [PubMed] [Google Scholar]
- 56.Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. American Journal of Obstetrics & Gynecology. 1979;133(2):165–170. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 57.Bonnar J, Daly L, Sheppard BL. Changes in the fibrinolytic system during pregnancy. Seminars in Thrombosis and Hemostasis. 1990;16(3):221–229. doi: 10.1055/s-2007-1002673. [DOI] [PubMed] [Google Scholar]
- 58.Sherer DM, Abulafia O. Angiogenesis during implantation, and placental and early embryonic development. Placenta. 2001;22(1):1–13. doi: 10.1053/plac.2000.0588. [DOI] [PubMed] [Google Scholar]
- 59.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. The Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 60.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. The Lancet. 1993;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 61.Manten GTR, Sikkema MJ, Voorbij HAM, Visser GHA, Bruinse HW, Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertension in Pregnancy. 2007;26(1):39–50. doi: 10.1080/10641950601146574. [DOI] [PubMed] [Google Scholar]
- 62.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175(2):189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 63.Newstead J, von Dadelszen P, Magee LA. Preeclampsia and future cardiovascular risk. Expert Review of Cardiovascular Therapy. 2007;5(2):283–294. doi: 10.1586/14779072.5.2.283. [DOI] [PubMed] [Google Scholar]
- 64.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. Journal of the American Medical Association. 2005;294(21):2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 65.Brosens IA, De Sutter P, Hamerlynck T, et al. Endometriosis is associated with a decreased risk of pre-eclampsia. Human Reproduction. 2007;22(6):1725–1729. doi: 10.1093/humrep/dem072. [DOI] [PubMed] [Google Scholar]
- 66.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncology. 2007;8(12):1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 67.Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. The Lancet. 1999;354(9173):141–148. doi: 10.1016/S0140-6736(98)10364-1. [DOI] [PubMed] [Google Scholar]
- 68.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor (PPAR) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 69.Vosper H, Khoudoli GA, Graham TL, Palmer CN. Peroxisome proliferator-activated receptor agonists, hyperlipidaemia, and atherosclerosis. Pharmacology & Therapeutics. 2002;95(1):47–62. doi: 10.1016/s0163-7258(02)00232-2. [DOI] [PubMed] [Google Scholar]
- 70.Chambrier C, Bastard J-P, Rieusset J, et al. Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor . Obesity Research. 2002;10(6):518–525. doi: 10.1038/oby.2002.70. [DOI] [PubMed] [Google Scholar]
- 71.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin metabolite binds peroxisome proliferator-activated receptor and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 72.Chinetti G, Fruchart J-C, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Research. 2000;49(10):497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 73.Waite L, Person EC, Zhou Y, Lim K-H, Scanlan TS, Taylor RN. Placental peroxisome proliferator-activated receptor- is up-regulated by pregnancy serum. Journal of Clinical Endocrinology & Metabolism. 2000;85(10):3808–3814. doi: 10.1210/jcem.85.10.6847. [DOI] [PubMed] [Google Scholar]
- 74.Wang Q, Fujii H, Knipp GT. Expression of PPAR and RXR isoforms in the developing rat and human term placentas. Placenta. 2002;23(8-9):661–671. doi: 10.1053/plac.2002.0855. [DOI] [PubMed] [Google Scholar]
- 75.Tarrade A, Lai Kuen R, Malassiné A, et al. Characterization of human villous and extravillous trophoblasts isolated from first trimester placenta. Laboratory Investigation. 2001;81(9):1199–1211. doi: 10.1038/labinvest.3780334. [DOI] [PubMed] [Google Scholar]
- 76.Yessoufou A, Hichami A, Besnard P, Moutairou K, Khan NA. Peroxisome proliferator-activated receptor deficiency increases the risk of maternal abortion and neonatal mortality in murine pregnancy with or without diabetes mellitus: modulation of T cell differentiation. Endocrinology. 2006;147(9):4410–4418. doi: 10.1210/en.2006-0067. [DOI] [PubMed] [Google Scholar]
- 77.Barak Y, Liao D, He W, et al. Effects of peroxisome proliferator-activated receptor on placentation, adiposity, and colorectal cancer. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fournier T, Pavan L, Tarrade A, et al. The role of PPAR-/RXR- heterodimers in the regulation of human trophoblast invasion. Annals of the New York Academy of Sciences. 2002;973:26–30. doi: 10.1111/j.1749-6632.2002.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhu Y-J, Crawford SE, Stellmach V, et al. Coactivator PRIP, the peroxisome proliferator-activated receptor-interacting protein, is a modulator of placental, cardiac, hepatic, and embryonic development. Journal of Biological Chemistry. 2003;278(3):1986–1990. doi: 10.1074/jbc.C200634200. [DOI] [PubMed] [Google Scholar]
- 80.Antonson P, Schuster GU, Wang L, et al. Inactivation of the nuclear receptor coactivator RAP250 in mice results in placental vascular dysfunction. Molecular and Cellular Biology. 2003;23(4):1260–1268. doi: 10.1128/MCB.23.4.1260-1268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sapin V, Dollé P, Hindelang C, Kastner P, Chambon P. Defects of the chorioallantoic placenta in mouse RXR null fetuses. Developmental Biology. 1997;191(1):29–41. doi: 10.1006/dbio.1997.8687. [DOI] [PubMed] [Google Scholar]
- 82.Kubota N, Terauchi Y, Miki H, et al. PPAR mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Molecular Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 83.Wendling O, Chambon P, Mark M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(2):547–551. doi: 10.1073/pnas.96.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peeters LLH, Vigne J-L, Tee MK, Zhao D, Waite L, Taylor RN. PPAR represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2006;8(4):373–379. doi: 10.1007/s10456-005-9027-4. [DOI] [PubMed] [Google Scholar]
- 85.Bamba H, Ota S, Kato A, Kawamoto C, Fujiwara K. Prostaglandins up-regulate vascular endothelial growth factor production through distinct pathways in differentiated U937 cells. Biochemical and Biophysical Research Communications. 2000;273(2):485–491. doi: 10.1006/bbrc.2000.2969. [DOI] [PubMed] [Google Scholar]
- 86.Chintalgattu V, Harris GS, Akula SM, Katwa LC. PPAR- agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovascular Research. 2007;74(1):140–150. doi: 10.1016/j.cardiores.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 87.Yamakawa K, Hosoi M, Koyama H, et al. Peroxisome proliferator-activated receptor- agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2000;271(3):571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 88.Shalom-Barak T, Nicholas JM, Wang Y, et al. Peroxisome proliferator-activated receptor controls Muc1 transcription in trophoblasts. Molecular and Cellular Biology. 2004;24(24):10661–10669. doi: 10.1128/MCB.24.24.10661-10669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarrade A, Schoonjans K, Guibourdenche J, et al. PPAR/RXR heterodimers are involved in human CG synthesis and human trophoblast differentiation. Endocrinology. 2001;142(10):4504–4514. doi: 10.1210/endo.142.10.8448. [DOI] [PubMed] [Google Scholar]
- 90.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor- and retinoid X receptor signaling regulate fatty acid uptake by primary human placental Trophoblasts. Journal of Clinical Endocrinology & Metabolism. 2005;90(7):4267–4275. doi: 10.1210/jc.2004-2265. [DOI] [PubMed] [Google Scholar]
- 91.Lappas M, Permezel M, Rice GE. Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-B, peroxisomal proliferator-activated receptor- and extracellularly regulated kinase 1/2. Endocrinology. 2005;146(8):3334–3342. doi: 10.1210/en.2005-0406. [DOI] [PubMed] [Google Scholar]
- 92.Handschuh K, Guibourdenche J, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Modulation of PAPP-A expression by PPAR in human first trimester trophoblast. Placenta. 2006;27(1):127–134. doi: 10.1016/j.placenta.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 93.Schild RL, Schaiff WT, Carlson MG, Cronbach EJ, Nelson DM, Sadovsky Y. The activity of PPAR in primary human trophoblasts is enhanced by oxidized lipids. Journal of Clinical Endocrinology & Metabolism. 2002;87(3):1105–1110. doi: 10.1210/jcem.87.3.8284. [DOI] [PubMed] [Google Scholar]
- 94.Asami-Miyagishi R, Iseki S, Usui M, Uchida K, Kubo H, Morita I. Expression and function of PPAR in rat placental development. Biochemical and Biophysical Research Communications. 2004;315(2):497–501. doi: 10.1016/j.bbrc.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 95.Capobianco E, Jawerbaum A, Romanini MC, et al. 15-deoxy--prostaglandin and peroxisome proliferator-activated receptor (PPAR) levels in term placental tissues from control and diabetic rats: modulatory effects of a PPAR agonist on nitridergic and lipid placental metabolism. Reproduction, Fertility and Development. 2005;17(4):423–433. doi: 10.1071/rd04067. [DOI] [PubMed] [Google Scholar]
- 96.Lappas M, Permezel M, Georgiou HM, Rice GE. Regulation of proinflammatory cytokines in human gestational tissues by peroxisome proliferator-activated receptor-: effect of 15-deoxy-- and troglitazone. Journal of Clinical Endocrinology & Metabolism. 2002;87(10):4667–4672. doi: 10.1210/jc.2002-020613. [DOI] [PubMed] [Google Scholar]
- 97.Marvin KW, Eykholt RL, Keelan JA, Sato TA, Mitchell MD. The 15-deoxy--prostaglandin receptor, peroxisome proliferator activated receptor- (PPAR) is expressed in human gestational tissues and is functionally active in JEG3 choriocarcinoma cells. Placenta. 2000;21(4):436–440. doi: 10.1053/plac.1999.0485. [DOI] [PubMed] [Google Scholar]
- 98.Dunn-Albanese LR, Ackerman IV WE, Xie Y, Iams JD, Kniss DA. Reciprocal expression of peroxisome proliferator-activated receptor- and cyclooxygenase-2 in human term parturition. American Journal of Obstetrics & Gynecology. 2004;190(3):809–816. doi: 10.1016/j.ajog.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 99.Berry EBE, Eykholt R, Helliwell RJA, Gilmour RS, Mitchell MD, Marvin KW. Peroxisome proliferator-activated receptor isoform expression changes in human gestational tissues with labor at term. Molecular Pharmacology. 2003;64(6):1586–1590. doi: 10.1124/mol.64.6.1586. [DOI] [PubMed] [Google Scholar]
- 100.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy--prostaglandin is a ligand for the adipocyte determination factor PPAR . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 101.Schild RL, Sonnenberg-Hirche CM, Schaiff WT, Bildirici I, Nelson DM, Sadovsky Y. The kinase p38 regulates peroxisome proliferator activated receptor-γ in human trophoblasts. Placenta. 2006;27(2-3):191–199. doi: 10.1016/j.placenta.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 102.Lee SS-T, Gonzalez FJ. Targeted disruption of the peroxisome proliferator-activated receptor gene, PPAR . Annals of the New York Academy of Sciences. 1996;804(1):524–529. doi: 10.1111/j.1749-6632.1996.tb18642.x. [DOI] [PubMed] [Google Scholar]
- 103.Dealtry GB, O'Farrell MK, Fernandez N. The Th2 cytokine environment of the placenta. International Archives of Allergy and Immunology. 2000;123(2):107–119. doi: 10.1159/000024441. [DOI] [PubMed] [Google Scholar]
- 104.Huang JC, Wun WS, Goldsby JS, Wun IC, Noorhasan D, Wu KK. Stimulation of embryo hatching and implantation by prostacyclin and peroxisome proliferator-activated receptor activation: implication in IVF. Human Reproduction. 2007;22(3):807–814. doi: 10.1093/humrep/del429. [DOI] [PubMed] [Google Scholar]
- 105.Lim H, Dey SK. PPAR functions as a prostacyclin receptor in blastocyst implantation. Trends in Endocrinology & Metabolism. 2000;11(4):137–142. doi: 10.1016/s1043-2760(00)00243-5. [DOI] [PubMed] [Google Scholar]
- 106.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biology of Reproduction. 2002;67(2):668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 107.Törnblom SA, Patel FA, Byström B, et al. 15-Hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2909–2915. doi: 10.1210/jc.2003-031149. [DOI] [PubMed] [Google Scholar]
- 108.Lappas M, Permezel M, Rice GE. 15-deoxy--rostaglandin and troglitazone regulation of the release of phospholipid metabolites, inflammatory cytokines and proteases from guman gestational tissues. Placenta. 2006;27(11-12):1060–1072. doi: 10.1016/j.placenta.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 109.McKinney ET, Shouri R, Hunt RS, Ahokas RA, Sibai BM. Plasma, urinary, and salivary 8-epi-prostaglandin levels in normotensive and preeclamptic pregnancies. American Journal of Obstetrics & Gynecology. 2000;183(4):874–877. doi: 10.1067/mob.2000.108877. [DOI] [PubMed] [Google Scholar]
- 110.Klinkner DB, Lim HJ, Strawn EY, Jr, Oldham KT, Sander TL. An in vivo murine model of rosiglitazone use in pregnancy. Fertility and Sterility. 2006;86(4, supplement 1):1074–1079. doi: 10.1016/j.fertnstert.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 111.Waite L, Louie RE, Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. Journal of Clinical Endocrinology & Metabolism. 2005;90(2):620–626. doi: 10.1210/jc.2004-0849. [DOI] [PubMed] [Google Scholar]
- 112.Tok EC, Ertunc D, Bilgin O, Erdal EM, Kaplanoglu M, Dilek S. PPAR-2 Pro12Ala polymorphism is associated with weight gain in women with gestational diabetes mellitus. European Journal of Obstetrics Gynecology & Reproductive Biology. 2006;129(1):25–30. doi: 10.1016/j.ejogrb.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 113.Jawerbaum A, Capobianco E, Pustovrh C, et al. Influence of peroxisome proliferator-activated receptor activation by its endogenous ligand 15-deoxy prostaglandin on nitric oxide production in term placental tissues from diabetic women. Molecular Human Reproduction. 2004;10(9):671–676. doi: 10.1093/molehr/gah090. [DOI] [PubMed] [Google Scholar]
- 114.Capparuccia L, Marzioni D, Giordano A, et al. PPAR expression in normal human placenta, hydatidiform mole and choriocarcinoma. Molecular Human Reproduction. 2002;8(6):574–579. doi: 10.1093/molehr/8.6.574. [DOI] [PubMed] [Google Scholar]
- 115.Rodie VA, Young A, Jordan F, Sattar N, Greer IA, Freeman DJ. Human placental peroxisome proliferator-activated receptor and expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. Journal of the Society for Gynecologic Investigation. 2005;12(5):320–329. doi: 10.1016/j.jsgi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Meirhaeghe A, Boreham CAG, Murray LJ, et al. A possible role for the PPARG Pro12Ala polymorphism in preterm birth. Diabetes. 2007;56(2):494–498. doi: 10.2337/db06-0915. [DOI] [PubMed] [Google Scholar]
- 117.Higgins JR, de Swiet M. Blood-pressure measurement and classification in pregnancy. The Lancet. 2001;357(9250):131–135. doi: 10.1016/S0140-6736(00)03552-2. [DOI] [PubMed] [Google Scholar]
- 118.Mackay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics & Gynecology. 2001;97(4):533–538. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 119.Taylor RN, de Groot CJM, Cho YK, Lim K-H. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Seminars in Reproductive Endocrinology. 1998;16(1):17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 120.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor- levels before the clinical manifestations of preeclampsia. American Journal of Reproductive Immunology. 1997;38(2):89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 121.Wathén K-A, Tuutti E, Stenman U-H, et al. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. Journal of Clinical Endocrinology & Metabolism. 2006;91(1):180–184. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 122.Yie S-M, Li L-H, Li Y-M, Librach C. HLA-G protein concentrations in maternal serum and placental tissue are decreased in preeclampsia. American Journal of Obstetrics & Gynecology. 2004;191(2):525–529. doi: 10.1016/j.ajog.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 123.Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble FMS-like tyrosine kinase 1 and risk for preeclampsia. Journal of Clinical Endocrinology & Metabolism. 2004;89(2):770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 124.Coonrod DV, Hickok DE, Zu K, Easterling TR, Daling JR. Risk factors for preeclampsia in twin pregnancies: a population-based cohort study. Obstetrics & Gynecology. 1995;85(5, part 1):645–650. doi: 10.1016/0029-7844(95)00049-w. [DOI] [PubMed] [Google Scholar]
- 125.McMahon MJ, Ananth CV, Liston RM. Gestational diabetes mellitus: risk factors, obstetric complications and infant outcomes. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 1998;43(4):372–378. [PubMed] [Google Scholar]
- 126.Tanaka M, Jaamaa G, Kaiser M, et al. Racial disparity in hypertensive disorders of pregnancy in New York state: a 10-year longitudinal population-based study. American Journal of Public Health. 2007;97(1):163–170. doi: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beamer BA, Yen C-J, Andersen RE, et al. Association of the Pro12Ala variant in the peroxisome proliferator- activated receptor-2 gene with obesity in two Caucasian populations. Diabetes. 1998;47(11):1806–1808. doi: 10.2337/diabetes.47.11.1806. [DOI] [PubMed] [Google Scholar]
- 128.Hasstedt SJ, Ren Q-F, Teng K, Elbein SC. Effect of the peroxisome proliferator-activated receptor-2 Pro12Ala variant on obesity, glucose homeostasis, and blood pressure in members of familial type 2 diabetic kindreds. Journal of Clinical Endocrinology & Metabolism. 2001;86(2):536–541. doi: 10.1210/jcem.86.2.7205. [DOI] [PubMed] [Google Scholar]
- 129.Li W-D, Lee JH, Price RA. The peroxisome proliferator-activated receptor 2 Pro12Ala mutation is associated with early onset extreme obesity and reduced fasting glucose. Molecular Genetics and Metabolism. 2000;70(2):159–161. doi: 10.1006/mgme.2000.2999. [DOI] [PubMed] [Google Scholar]
- 130.Deeb SS, Fajas L, Nemoto M, et al. A Pro12Ala substitution in PPAR2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nature Genetics. 1998;20(3):284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- 131.Laasanen J, Heinonen S, Hiltunen M, Mannermaa A, Laakso M. Polymorphism in the peroxisome proliferator-activated receptor- gene in women with preeclampsia. Early Human Development. 2002;69(1-2):77–82. doi: 10.1016/s0378-3782(02)00069-5. [DOI] [PubMed] [Google Scholar]
- 132.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proceedings of the Society for Experimental Biology and Medicine. 1999;222(3):222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 133.Arbogast BW, Leeper SC, Merrick RD, Olive KE, Taylor RN. Which plasma factors bring about disturbance of endothelial function in pre-eclampsia? The Lancet. 1994;343(8893):340–341. doi: 10.1016/s0140-6736(94)91169-x. [DOI] [PubMed] [Google Scholar]
- 134.Redman CWG, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. 2003;24(1):S21–S27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 135.Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor . Journal of Biological Chemistry. 1998;273(4):1855–1858. doi: 10.1074/jbc.273.4.1855. [DOI] [PubMed] [Google Scholar]
- 136.Ogburn PL, Jr, Johnson SB, Williams PP, Holman RT. Levels of free fatty acids and arachidonic acid in pregnancy and labor. Journal of Laboratory and Clinical Medicine. 1980;95(6):943–949. [PubMed] [Google Scholar]
- 137.Ogburn PL, Jr, Williams PP, Johnson SB, Holman RT. Serum arachidonic acid levels in normal and preeclamptic pregnancies. American Journal of Obstetrics & Gynecology. 1984;148(1):5–9. doi: 10.1016/s0002-9378(84)80023-x. [DOI] [PubMed] [Google Scholar]
- 138.de Groot CJM, Murai JT, Vigne J-L, Taylor RN. Eicosanoid secretion by human endothelial cells exposed to normal pregnancy and preeclampsia plasma in vitro. Prostaglandins Leukotrienes and Essential Fatty Acids. 1998;58(2):91–97. doi: 10.1016/s0952-3278(98)90146-6. [DOI] [PubMed] [Google Scholar]
- 139.Hornung D, Ryan IP, Chao VA, Vigne J-L, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. Journal of Clinical Endocrinology & Metabolism. 1997;82(5):1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 140.Blackburn S, Loper D. Maternal Fetal and Neonatal Physiology: A Clinical Perspective. Philadelphia, Pa, USA: Harcourt Brace Jovanovic; 1992. [Google Scholar]
- 141.Hay WW., Jr Placental transport of nutrients to the fetus. Hormone Research. 1994;42(4-5):215–222. doi: 10.1159/000184196. [DOI] [PubMed] [Google Scholar]
- 142.Jovanovic L, Pettitt DJ. Gestational diabetes mellitus. Journal of the American Medical Association. 2001;286(20):2516–2518. doi: 10.1001/jama.286.20.2516. [DOI] [PubMed] [Google Scholar]
- 143.Rudra CB, Sorensen TK, Leisenring WM, Dashow E, Williams MA. Weight characteristics and height in relation to risk of gestational diabetes mellitus. American Journal of Epidemiology. 2007;165(3):302–308. doi: 10.1093/aje/kwk007. [DOI] [PubMed] [Google Scholar]
- 144.Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. American Journal of Clinical Nutrition. 2000;71(5, supplement):1242S–1248S. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- 145.Catalano PM. Increasing maternal obesity and weight gain during pregnancy: the obstetric problems of plentitude. Obstetrics & Gynecology. 2007;110(4):743–744. doi: 10.1097/01.AOG.0000284990.84982.ba. [DOI] [PubMed] [Google Scholar]
- 146.Jones CJP, Fox H. Placental changes in gestational diabetes. An ultrastructural study. Obstetrics & Gynecology. 1976;48(3):274–280. [PubMed] [Google Scholar]
- 147.Åberg A, Rydhstroem H, Frid A. Impaired glucose tolerance associated with adverse pregnancy outcome: a population-based study in southern Sweden. American Journal of Obstetrics & Gynecology. 2001;184(2):77–83. doi: 10.1067/mob.2001.108085. [DOI] [PubMed] [Google Scholar]
- 148.Carr DB, Utzschneider KM, Hull RL, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–2083. doi: 10.2337/dc05-2482. [DOI] [PubMed] [Google Scholar]
- 149.Lee H, Jang HC, Park HK, Cho NH. Early manifestation of cardiovascular disease risk factors in offspring of mothers with previous history of gestational diabetes mellitus. Diabetes Research and Clinical Practice. 2007;78(2):238–245. doi: 10.1016/j.diabres.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 150.Morifuji M, Sanbongi C, Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. British Journal of Nutrition. 2006;96(3):469–475. [PubMed] [Google Scholar]
- 151.Zhang C, Liu S, Solomon CG, Hu FB. Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care. 2006;29(10):2223–2230. doi: 10.2337/dc06-0266. [DOI] [PubMed] [Google Scholar]
- 152.Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstetrics & Gynecology. 2006;108(5):1200–1207. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weissgerber TL, Wolfe LA, Davies GAL, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Applied Physiology, Nutrition and Metabolism. 2006;31(6):661–674. doi: 10.1139/h06-060. [DOI] [PubMed] [Google Scholar]
- 154.Leipold H, Knoefler M, Gruber C, Huber A, Haslinger P, Worda C. Peroxisome proliferator-activated receptor coactivator-1 gene variations are not associated with gestational diabetes mellitus. Journal of the Society for Gynecologic Investigation. 2006;13(2):104–107. doi: 10.1016/j.jsgi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 155.Fukuen S, Iwaki M, Yasui A, Makishima M, Matsuda M, Shimomura I. Sulfonylurea agents exhibit peroxisome proliferator-activated receptor agonistic activity. Journal of Biological Chemistry. 2005;280(25):23653–23659. doi: 10.1074/jbc.M412113200. [DOI] [PubMed] [Google Scholar]
- 156.Langer O, Conway DL, Berkus MD, Xenakis EM-J, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. New England Journal of Medicine. 2000;343(16):1134–1138. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 157.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Human Reproduction Update. 2007;13(2):121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 158.Hewitt DP, Mark PJ, Waddell BJ. Placental expression of peroxisome proliferator-activated receptors in rat pregnancy and the effect of increased glucocorticoid exposure. Biology of Reproduction. 2006;74(1):23–28. doi: 10.1095/biolreprod.105.045914. [DOI] [PubMed] [Google Scholar]
- 159.Schaiff WT, Knapp FF, Jr, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor alters placental morphology and placental fatty acid uptake in mice. Endocrinology. 2007;148(8):3625–3634. doi: 10.1210/en.2007-0211. [DOI] [PubMed] [Google Scholar]
- 160.Keelan JA, Helliwell RJA, Nijmeijer BE, et al. 15-deoxy--prostaglandin -induced apoptosis in amnion-like WISH cells. Prostaglandins & Other Lipid Mediators. 2001;66(4):265–282. doi: 10.1016/s0090-6980(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 161.Hasegawa H, Takano H, Zou Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor activator, ameliorates experimental autoimmune myocarditis by modulating Th1/Th2 balance. Journal of Molecular and Cellular Cardiology. 2005;38(2):257–265. doi: 10.1016/j.yjmcc.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 162.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferator-activated receptor- . Molecular Pharmacology. 2007;72(4):934–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]