Abstract
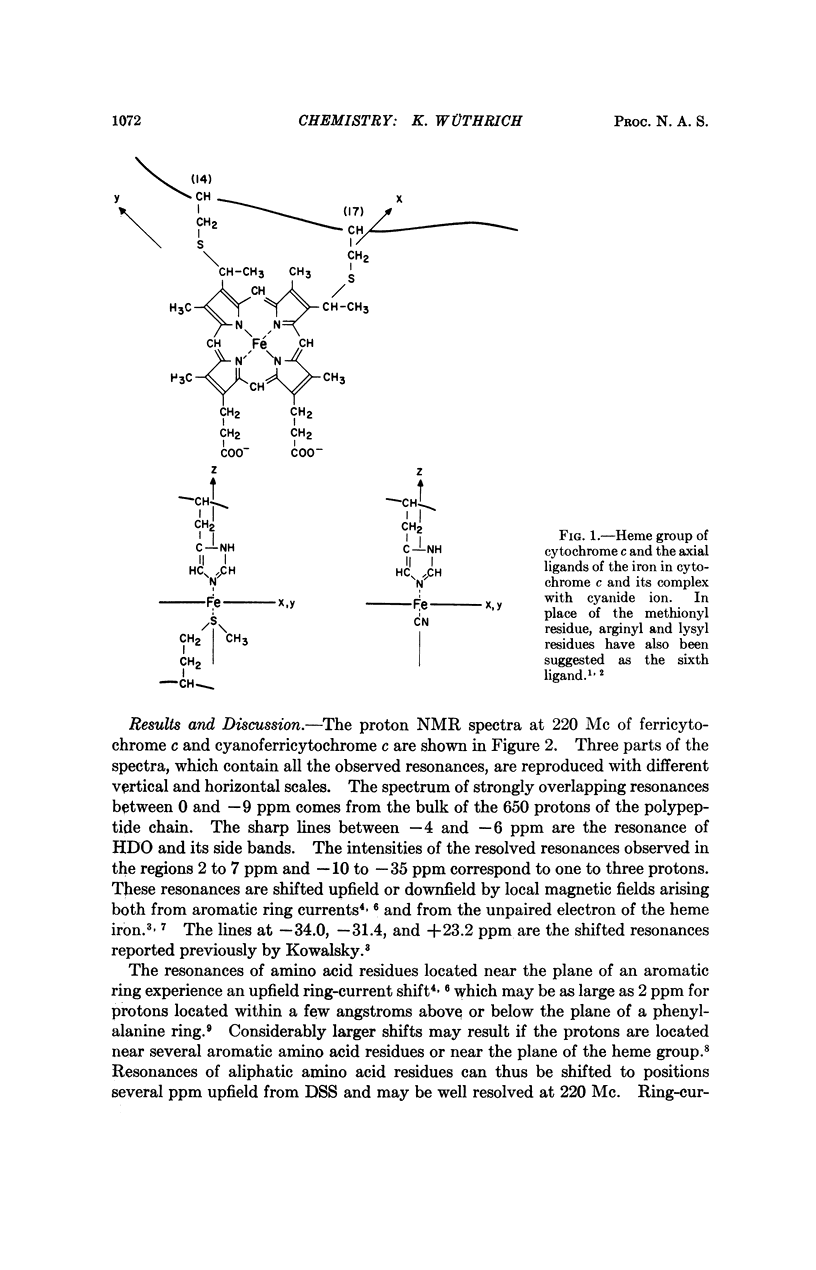
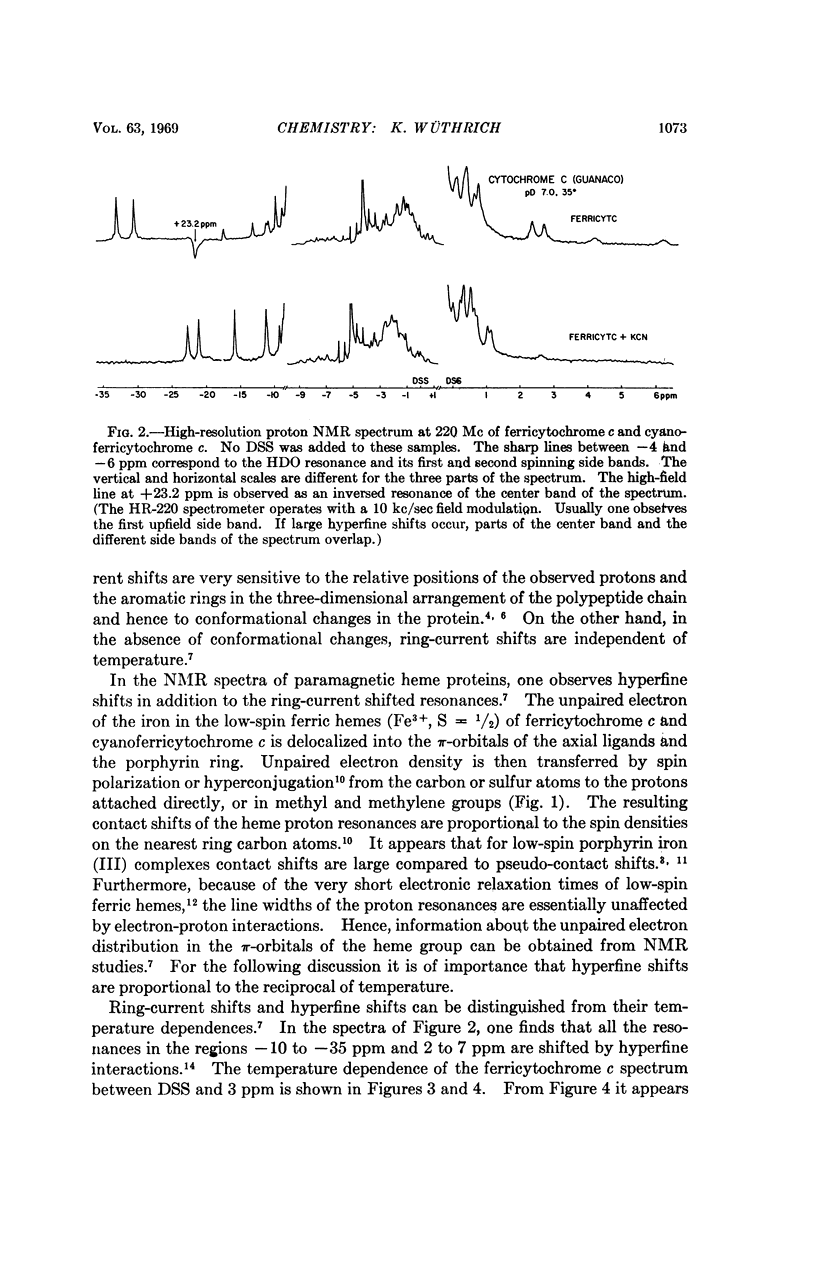
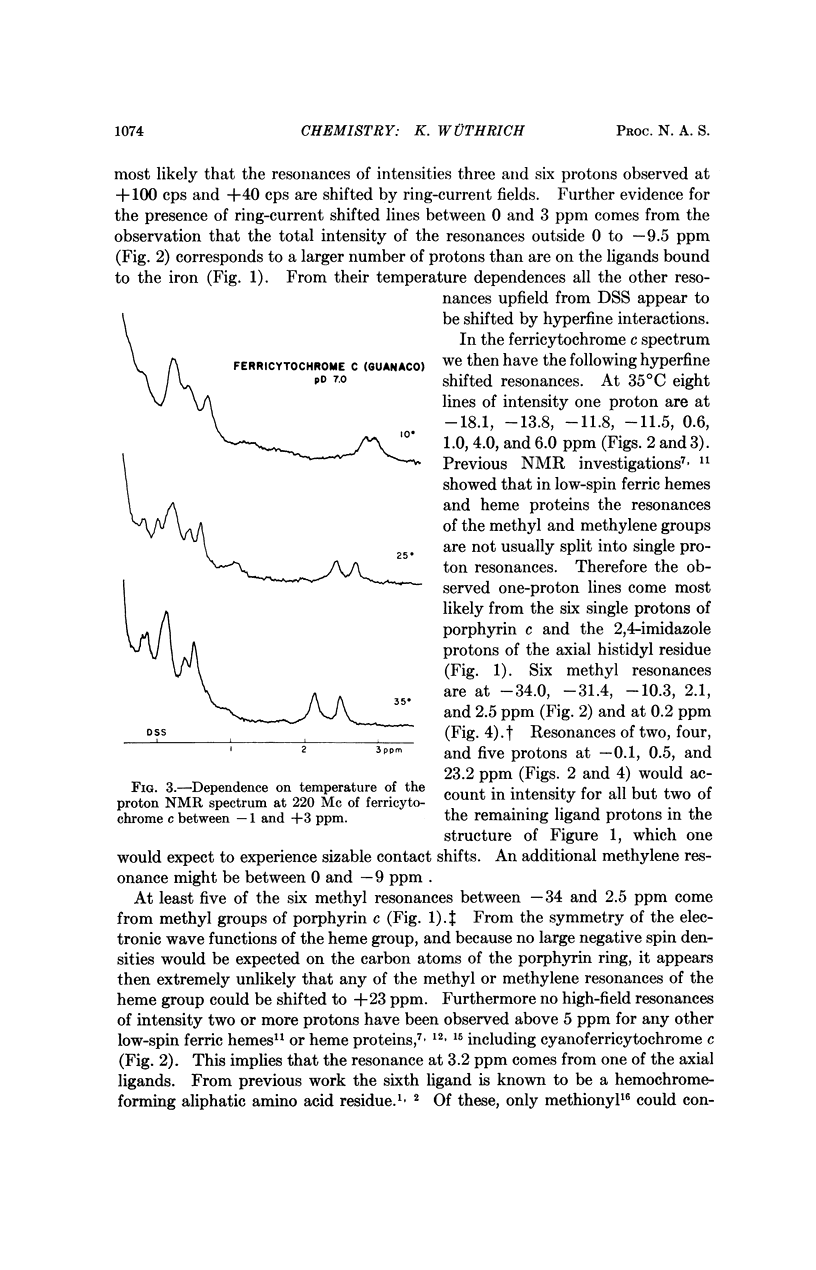
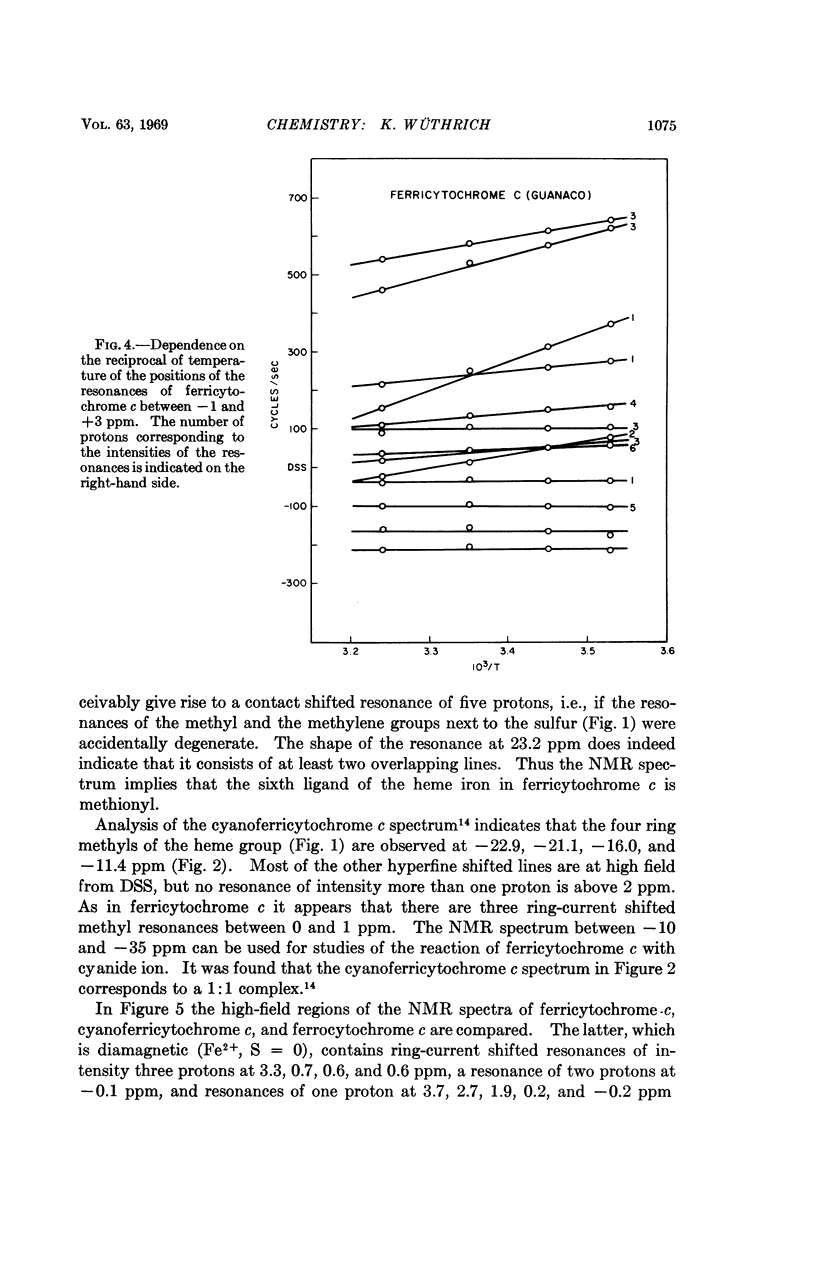
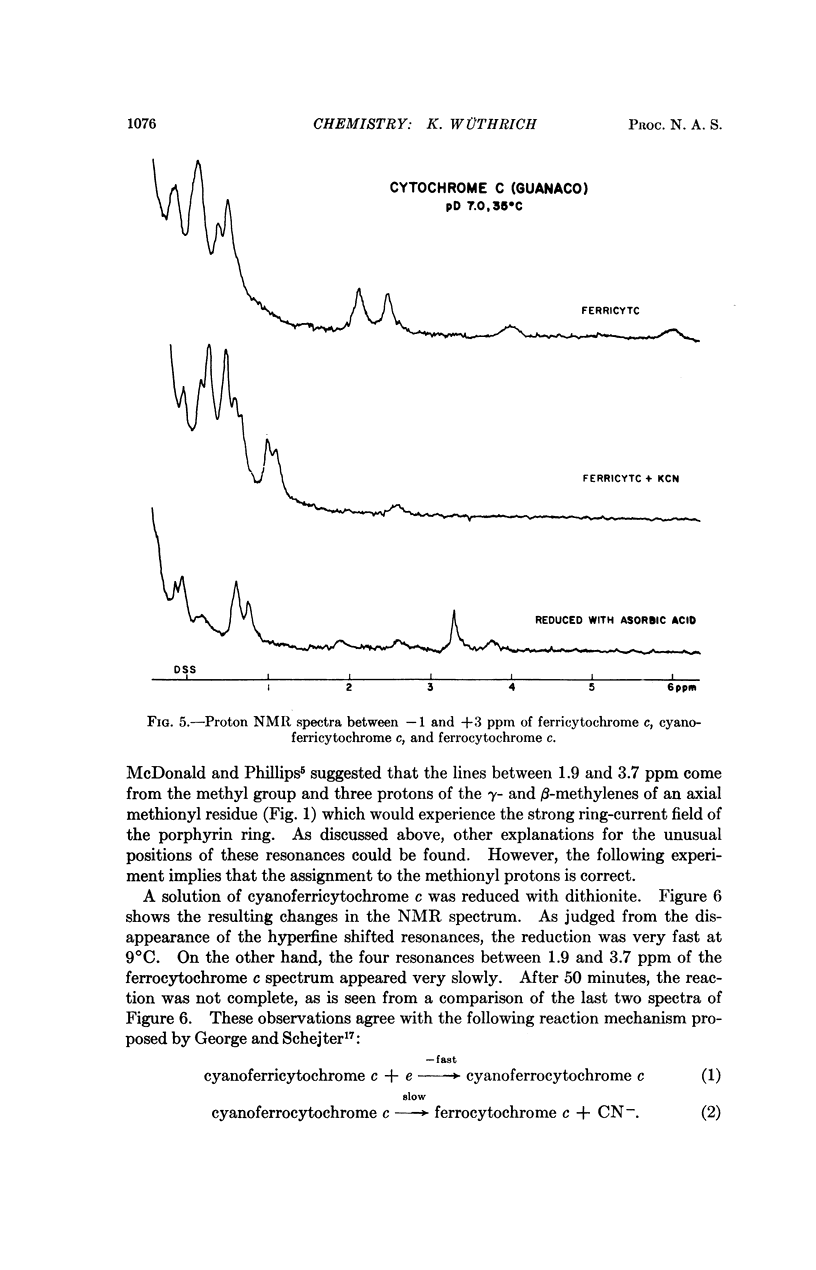
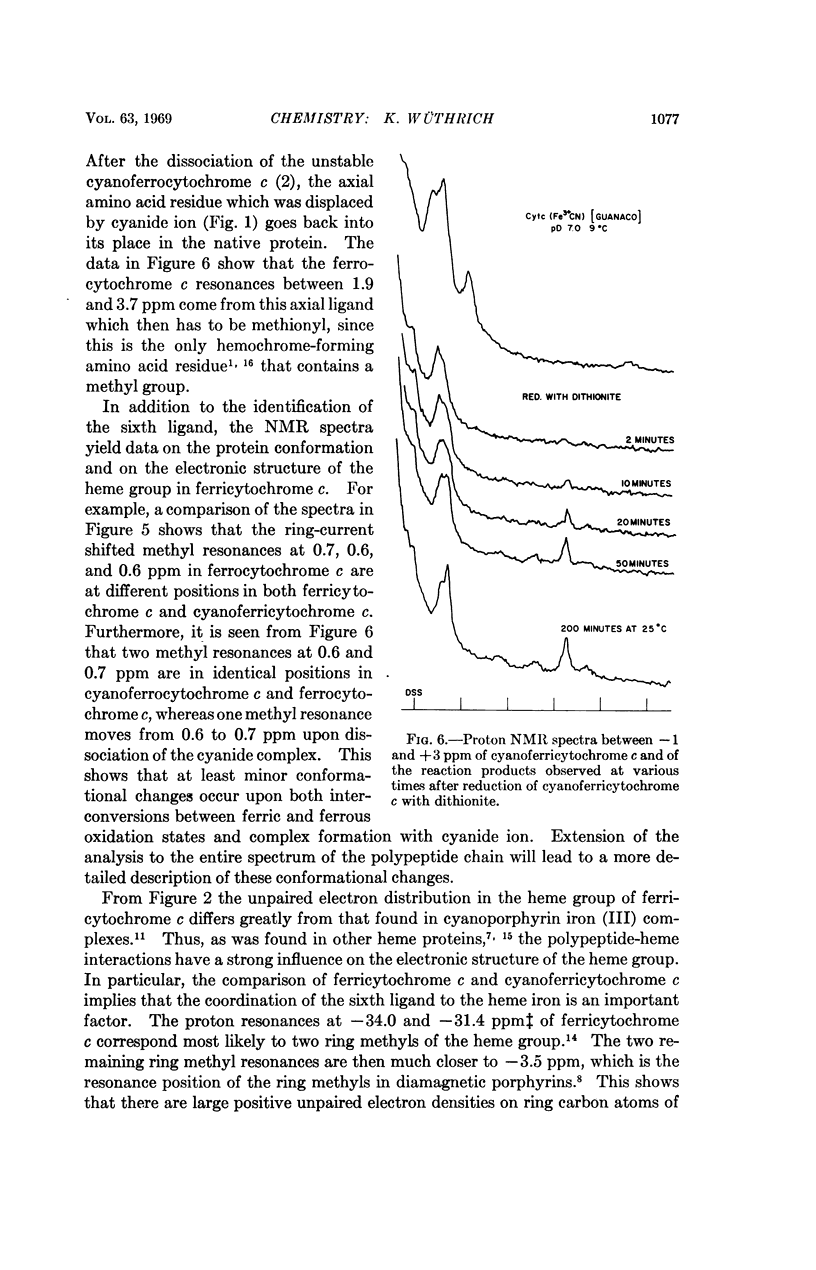
In cytochrome c the axial positions of the heme iron are occupied by two amino acid residues, one of which is known from X-ray studies to be histidyl. Nuclear magnetic resonance spectroscopy provides strong evidence that the sixth ligand is a methionyl residue in both the ferric and ferrous oxidation states. It is further shown that in cyanoferricytochrome c cyanide ion replaces methionyl in the first coordination sphere of the heme iron. Additional data are obtained on the protein conformation and on the electronic structure of the heme group in ferricytochrome c. As in other heme proteins, the interactions with the polypeptide chain greatly affect the unpaired electron distribution in the heme group of cytochrome c. In particular, from a comparison of ferricytochrome c and cyanoferricytochrome c, the importance of the coordination of the sixth ligand is apparent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dickerson R. E., Kopka M. L., Weinzierl J., Varnum J., Eisenberg D., Margoliash E. Location of the heme in horse heart ferricytochrome c by x-ray diffraction. J Biol Chem. 1967 Jun 25;242(12):3015–3018. [PubMed] [Google Scholar]
- GEORGE P., SCHEJTER A. THE REACTIVITY OF FERROCYTOCHROME C WITH IRON-BINDING LIGANDS. J Biol Chem. 1964 May;239:1504–1508. [PubMed] [Google Scholar]
- Harbury H. A., Cronin J. R., Fanger M. W., Hettinger T. P., Murphy A. J., Myer Y. P., Vinogradov S. N. Complex formation between methionine and a heme peptide from cytochrome c. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1658–1664. doi: 10.1073/pnas.54.6.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOWALSKY A. Nuclear magnetic resonance studies of proteins. J Biol Chem. 1962 Jun;237:1807–1819. [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Manifestations of the tertiary structures of proteins in high-frequency nuclear magnetic resonance. J Am Chem Soc. 1967 Nov 22;89(24):6332–6341. doi: 10.1021/ja01000a061. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Peisach J. High-resolution proton magnetic resonance spectra of sperm whale cyanometmyoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):373–380. doi: 10.1073/pnas.60.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Wyluda B. J., Caughey W. S. Proton magnetic resonance studies of porphyrin iron (3) cyanides. Proc Natl Acad Sci U S A. 1969 Mar;62(3):636–643. doi: 10.1073/pnas.62.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K., Shulman R. G., Yamane T. Proton magnetic resonance studies of human cyanomethemoglobin. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1199–1206. doi: 10.1073/pnas.61.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]



