Abstract
Background
Tumor cells have numerous immune surveillance escape mechanisms as well as means of resistance to apoptosis. This study tried to clarify one of these mechanisms in bladder cancer with the hope of being able to develop targeted therapy that will sensitize the tumor cells to immune-mediated apoptosis.
Methods
In this study, electron microscopic examination and expression of TGF-beta-1 protein and TGF-beta-R-1 receptor using immunoelectronmicroscopic and immunocytochemical techniques were investigated in urine and peripheral blood mononuclear cells (PBMNCs). Samples were obtained from 5 healthy controls (Group 1) and 60 study patients who were classified according to the cytopathologic examination of their urine into 2 main subgroups: chronic cystitis (bilharzial and nonbilharzial, Group 2, n = 15) and bladder cancer (transitional cell carcinoma and squamous cell carcinoma, Group 3, n = 45).
Results
Examination of PBMNCs by immunoelectronmicroscopic and immunocytochemical techniques showed a significant increase in the percentage of positive cases expressing both TGF-beta-1 protein and TGF-beta-R-1 receptors in bladder cancer in comparison with the control (P < .01 and P < .05, respectively) and with chronic cystitis (P < .05). By electron microscopic examination, 42 out of 45 bladder cancer cases (93.3%) revealed remarkable apoptotic changes represented by cell shrinkage, surface blebs, nuclear chromatin condensation, and vacuolated cytoplasm. Urine examination by immunoelectronmicroscopic and immunocytochemical techniques revealed a statistically significant decrease in the percentage of positive cases expressing TGF-beta-R1 receptor in bladder cancer in comparison with either chronic cystitis cases or controls (P < .01), while TGF-beta-1 protein was significantly increased (P < .01). By electron microscopic examination, exfoliated necrotic malignant epithelial (urothelial) cells and many inflammatory cells were detected.
Conclusions
This work helps researchers and clinicians to better understand one of the escape mechanisms in bladder cancer that may facilitate the reverse of tumor escape from the immune system. It also draws attention to TGF-beta-1 protein and TGF-beta-R1 receptor; TGF-beta-1 protein can be used as an attractive target for anticancer therapy, and the absence of TGF-beta-R1 can be considered a marker for malignant transformation of urothelial cells in bladder cancer.
Introduction
The incidence of human bladder cancer has increased extensively through the last decades.[1] Despite several attempts to apply immunotherapy to the treatment of this malignant disease, it is still difficult to predict tumor progression, optimal therapy, and finally the clinical outcome.[2] It has long been established that the immune system plays a significant role in preventing tumor development. Experimental tumors develop more frequently in immune suppressed hosts, suggesting a central role of immune surveillance in the pathogenesis of cancer.[3] Likewise, human subjects with congenital or acquired immune suppression have an increased frequency of cancer.[4] The mechanisms of the interplay between tumor-infiltrating lymphocytes and tumor cells were further elucidated by the identification of tumor antigens by T lymphocytes[5] and the molecular mechanisms of the natural killer (NK) cell.[6] In brief, cytotoxic T lymphocytes (CTLs) can recognize tumor-specific antigens restricted by major histocompatibility complex (MHC) molecules and kill tumor cells. In addition, tumor cells that lack the expression of one or more MHC class I alleles become targets for NK-mediation of the cell lysis.[7]
In addition to the regulation of cellular proliferation by apoptosis, cancer cells become refractory to this regulatory signal and thus do not undergo apoptosis under appropriate conditions.[8] Schuster and Krieglstein[9] found that the TGF-beta signaling pathway is proapoptotic in most cases. TGF-beta-induced apoptosis is frequently mediated by the Smad-dependent pathway.[10,11] TGF-beta-induced apoptosis may occur also through both p53-dependent and p53-independent mechanisms[12] and involves caspase activation,[13] upregulation of proapoptotic factors (ie, Bax), and/or downregulation of antiapoptotic factors (ie, Bcl-2 and Bcl-xL).[14] The TGF-beta signaling pathway also interacts with other pathways that regulate apoptosis. For example, TGF-beta is able to enhance Fas-induced apoptosis under conditions in which TGF-beta alone does not induce apoptosis.[15]
Cancer cells are able to produce and secrete TGF-beta,[16] which is able to immunosuppress cancer patients in the absence of cytotoxic treatment.[17] Hersey[18] found elevated levels of TGF-beta in a number of experimental models and in specimens from patients with cancer. Also, increasing expression of TGF-beta in animal models allows immunogenic cell lines to escape immunosurveillance and form tumors.[13] Leach and colleagues[19] found augmented anticancer cell immune responses by blocking the TGF-beta signaling pathway in T cells.
Microscopic examination of urine (urine cytology) has diagnostic and prognostic value in bladder tumors.[20,21] In bladder cancer, it revealed a moderate sensitivity but high specificity; its sensitivity increases with malignancy grade and after the third sample of urine.[22] Many studies reported that urine cytology proved positive in all histologically confirmed cases at follow up. It also can detect cases at times without clinical suspicion of severe abnormality before malignant process is confirmed histologically; the accuracy of urine cytology may differ according to treatment status.[23]
Immunoelectronmicroscopy is an accurate technique that has been of great help in diagnostic and research activities. This is particularly useful in finding difficult-to-locate cells and tissue structures and examining the contents of secretory granules.[24]
Recently, Mansy[25] innovated a technique for processing cells simultaneously for light and electron microscopy called the agarose cell block technique. The application of this technique allowed the processing of cell sediment in a block manner.
In this study, we try to clarify one of the mechanisms of tumor escape and resistance – the decreased sensitivity of tumor cells to immune-mediated apoptosis – with the hope of being able to develop targeted therapy that sensitizes the tumor cells to immune-mediated apoptosis.
Patients and Method
The present study was conducted on 60 patients admitted to the urology department at Theodor Bilharz Research Institute in addition to 5 healthy controls. Patients were subjected to: detailed history taking, complete clinical examination, urine analysis and routine laboratory investigations including urine cytology, abdomino-pelvic ultrasonography, excretory urography, cystoscopic examination, and trans urethral resection biopsies from the apparent lesions.
Samples Collection
Urine samples were collected into clean tubes for cytopathologic diagnosis, immunocytochemical techniques, and electron microscopic examination using agarose cell block technique in addition to the immunoelectronmicroscopic examination.
Blood samples of 10 mL were collected into sterile endotoxin-free vacuum blood collection tubes on potassium EDTA. The PBMNC layer was separated using Ficoll Hypaque (Seromid Biochrom, Berlin, Germany) density gradient centrifugation and washed 3 times with Hank's balanced salt solution (HBSS) without Ca2+ and Mg2+ ions. These cells were prepared for immunocytochemical techniques and electron microscopic examination using agarose cell block technique in addition to the immunoelectronmicroscopic examination.
Cytopathologic Processing of Urine Samples
Part of the collected urine was centrifuged at a rate of 1200–1500 rpm for 15 minutes using Shandon Cytospin (ThermoFisher Scientific, Waltham, Massachusetts). The sediment was smeared on slides that were pretreated with 3-APTES (3-amino-propyl-triethoxy saline, Sigma-Aldrich Ireland Ltd, Dublin, Ireland). Slides were fixed immediately in 95% ethanol for 24 hours and then stained with hematoxylin and eosin for cytopathologic diagnosis and Papanicolaou's stain.
Immunocytochemical Procedures for TGF-beta-1 Protein and TGF-beta-R-1 Receptor on Agarose Cell Block Slides
Part of each urine sediment sample after centrifugation and PBMNCs were fixed in buffered 4% glutaraldehyde for 1 hour. The fixed cells were recentrifuged with melted agarose (molecular biology grade, Promega Corporation, Madison, Wisconsin) for 7 minutes at 1500 rpm according to Mansy.[25] The solidified agarose cell block of each sample was divided into 2 halves. One half was refixed in buffered formalin and embedded in paraffin; 4-mcm-thick sections were prepared on slides pretreated with 3-APTES. A standard avidin-biotin immunoperoxidase technique[26] was used. Paraffin sections were dewaxed in xylene and hydrated in descending grades of ethanol. The sections on the slides were treated with 0.3% hydrogen peroxide to inhibit the activity of endogenous peroxidase. Sections were incubated overnight at 4°C with the primary anti-TGF-beta-1 protein and anti-TGF-beta-R-1 antibodies (Santa Cruz Biotechnology, Santa Cruz, California). Both antibodies were diluted 1:50 in phosphate buffer saline (PBS) and incubated for 24 hours at 4°C. The specificity of these antibodies was demonstrated previously.[27] The following day, the slides were washed 3 times in PBS, then sections were incubated for 15 minutes with biotinylated secondary antibody and then with avidin-biotin complex horseradish peroxidase solution (Vector, Burlingame, California). After incubation for 10 minutes, the peroxidase activity was revealed by the addition of freshly prepared diaminobenzidine (0.03%) for 20 minutes at 37°C in dark then washed 3 times in PBS or tries-HCL buffer pH 7.6. PBMNC slides and urine slides were counter stained with May-Grunwal-Giemsa stain and Meyer's hematoxylin, respectively.
Negative controls were processed in an identical manner by substitution of primary antibody with a normal rabbit IgG.
Assessment of Immunocytochemical Staining
Using a standard light microscope, all slides (specimens) were categorized as either positive or negative in terms of staining for the TGF-beta-I protein and TGF-beta-R-1 receptor. Those specimens with cytoplasmic positive brownish staining were considered positive for TGF-beta-1 protein, and those with brownish positive staining of the cell membrane were considered positive for TGF-beta-R-1 receptor; slides were classified as negative if the staining level was comparable with that of the negative control slides. Cells were counted at 400 magnification. An average of 100 cells within 10 fields was counted/section. All slides were reviewed independently by 2 pathologists.
Electron Microscopic Examination of Agarose Cell Block[28]
The other half of the solidified agarose cell block was refixed in buffered 4% glutaraldehyde for 1 hour then postfixed in 2% osmic acid for 1 hour, dehydrated in ascending alcohol, then infiltrated and embedded in epoxy resin. Ultrathin sections were performed using Leica Ultramicrotome (Leica Microsystems GmbH, Ernst-Leitz-Strasse, Austria). The sections were stained with uranyl acetate and lead citrate and examined using Philips EM 208 S (Eindhoven, The Netherlands).
Immunoelectronmicroscopic Examination of TGF-beta-1 Protein and TGF-beta-R-1 Receptor of Urine and PBMNCs[28]
Another part of each urine sediment and PBMNCs was resuspended in tyrode buffer containing 0.35% bovine serum albumin, fixed for 10 minutes in 0.1 M sodium cacodylate and 1.25% glutaraldehyde, then washed 3 times in a mixture of 0.1 M sodium cacodylate, 0.2 M saccharose, and 0.1 M lysine. Subsequently, immunocytochemistry was carried out on the cells for the revelation of TGF-beta-1 protein and TGF-beta-R-1 receptor as above. Then the cells were post-fixed in 2% osmium tetraoxide and processed as standard preparative procedure for electron microscope examination.
Negative controls were processed in an identical manner by substitution of primary antibody with a normal rabbit IgG.
Statistical Analysis
Version 10 SPSS statistical package (SPSS software, Chicago, Illinois) was used to analyze the data. Descriptive statistics included the mean ± standard deviation. Analytical statistics included comparison between variables among groups using student t-test. The data were considered significant if P values were ≤ .05.
Results
Cytopathologic Diagnosis of Urine Samples
Urine samples were examined for cytopathologic features, including nuclear size, nuclear shape, chromatin texture, chromatin content, nuclear/cytoplasmic ratio, and cytoplasmic features. Cell arrangement (singly, in groups or clusters) and background of the smears were also examined. In addition to the 5 healthy controls who proved cytopathologically free (Group 1), the 60 patients were classified according to Ooms and Veldhuizen[29] into 15 chronic cystitis (bilharzial and nonbilharzial cystitis) cases (Group 2) and 45 malignant (transitional cell carcinoma and squamous cell carcinoma) cases (Group 3).
Immunocytochemical Expression of TGF-beta-1 Protein and TGF-beta-R-1 Receptor on Agarose Cell Block Slides
PBMNCs revealed a significant increase in the percentage of positive cases expressing both TGF-beta-R-1 and TGF-beta-1 protein in bladder cancer cases in comparison with healthy controls (P < .05 and P < .01, respectively) and in the percentage of positive cases expressing TGF-beta-1 protein in bladder cancer in comparison with chronic cystitis cases (P < 0.05) (Table 1, Figure 1, Figure 2).
Table 1.
Expression of TGF-Beta-1 Protein and TGF-Beta-R-1 in PBMNCs Using Agarose Cell Block Technique
| Positive cases with TGF-beta-1 protein in PBMNCs | Positive cases with TGF-beta-R-1 in PBMNCs | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| Control group (n=5) | 2 | 40 | 4 | 80 |
| Chronic cystitis (n=15) | 9 | 60* | 13 | 86.6 |
| Bladder cancer (n=45) | 39 | 86.6†‡ | 41 | 91.1* |
Significant increase in percentage of positive cases in comparison with control group; P < .05
Highly significant increase in number of positive cases in comparison with control group; P < .01
Significant increase in percentage of positive cases in comparison with chronic cystitis; P < .05
Figure 1.
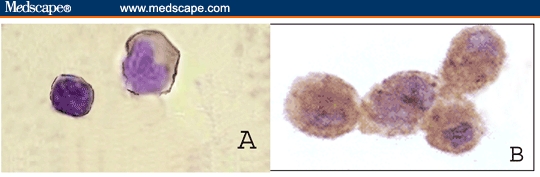
PBMNCs from healthy control showing positive immunoperoxidase reaction either tracing the cell membrane for TGF-beta-R-1 (A) or intracellular for TGF-beta-1 protein (B) (counterstained with May-Grunwal-Giemsa stain; × 400).
Figure 2.
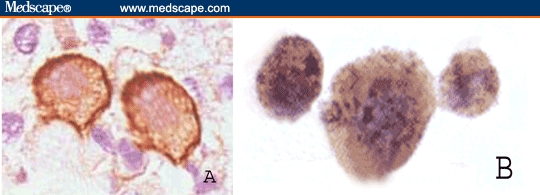
PBMNCs from a patient with bladder cancer showing positive immunoperoxidase reaction either tracing the cell membrane for TGF-beta-R-1 (A) or intracellular for TGF-beta-1 protein (B) (contrasted section with May-Grunwal-Giemsa stain; × 400).
With regard to urine examination, there was a statistically significant decrease in the percentage of positive cases expressing TGF-beta-R-1 in bladder cancer cases compared with either chronic cystitis cases or controls (P < .01). The percentage of positive cases expressing TGF-beta-1 protein significantly increased (P < .05) (Table 2, Figure 3, Figure 4).
Table 2.
Expression of TGF-Beta-1 Protein and TGF-Beta-R-1 in Urine Using Agarose Cell Block Technique
| Positive cases with TGF-beta-1 protein in urine | Positive cases with TGF-beta-R-1 in urine | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| Control group (n=5) | – | – | 2 | 40 |
| Chronic cystitis (n=15) | 7 | 46.6 | 10 | 66.6 |
| Bladder cancer (n=45) | 37 | 82.2* | 12 | 26.6† |
Significant increase in percentage of positive cases in comparison with either chronic cystitis or control; P < .05
Highly significant decrease in number of positive cases in comparison with either chronic cystitis or control; P < .01
Figure 3.
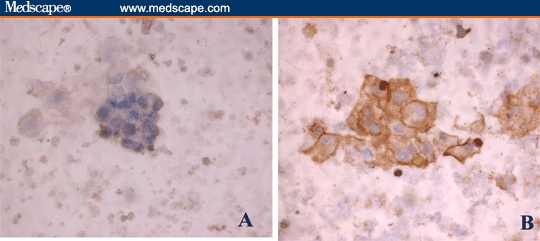
Urine sediment of a patient with transitional cell carcinoma showing positive immunoperoxidase reaction either tracing the cell membrane for TGF-beta-R-1 (A) or intracellular for TGF-beta-1 protein (B) (Immunostain; DAB × 400).
Figure 4.
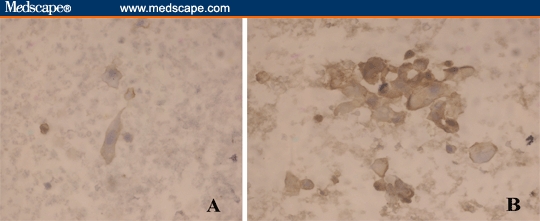
Urine sediment of a patient with squamous cell carcinoma showing negative immunoperoxidase reaction for TGF-beta-R-1 (A) and positive for TGF-beta-1 protein (Immunostain; DAB × 400).
Immunoelectronmicroscopic Examination of TGF-beta-1 Protein and TGF-beta-R-1 Receptor
PBMNC examination revealed a significant increase in the percentage of positive cases expressing both TGF-beta-1 protein and TGF-beta-R-1 in bladder cancer in comparison with the control group (P < .01 and P < .05, respectively) and in the percentage of positive cases expressing TGF-beta-1 protein in comparison with chronic cystitis (P < .05) (Table 3, Figure 5, Figure 6).
Table 3.
Immunoelectronmicroscopic Examination of TGF-Beta-1 Protein and TGF-beta-R-1 Receptors of PBMNCs
| Positive cases with TGF-beta-1 protein in PBMNCs | Positive cases with TGF-beta-R-1 in PBMNCs | |||
|---|---|---|---|---|
| No. | % | No. | % | |
| Control group (n=5) | 3 | 60 | 4 | 80 |
| Chronic cystitis (n=15) | 12 | 80* | 14 | 93.3* |
| Bladder cancer (n=45) | 42 | 93.3†‡ | 44 | 97.7* |
Significant increase in percentage of positive cases in comparison with control group; P < .05
Highly significant increase in number of positive cases in comparison with control group; P < .01
Significant increase in percentage of positive cases in comparison with chronic cystitis; P < .05
Figure 5.
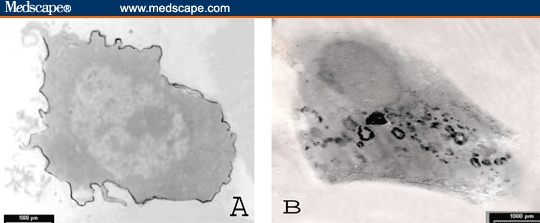
TEM photomicrograph of immunoperoxidase-labeled normal PBMNC showing patchy distribution of TGF-beta-R-1 alongside the cell membrane (A) and intracellular electron dense deposits for TGF-beta-1 protein (uncontrasted section; × 1800).
Figure 6.
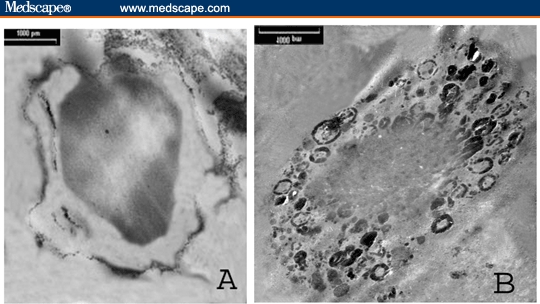
TEM photomicrograph of immunoperoxidase-labeled PBMNC of patient with bladder cancer showing patchy distribution of TGF-beta-R-1 alongside the cell membrane (A) and intracellular electron dense deposits for TGF-beta-1 protein (uncontrasted section; × 3100).
Urine examination yielded a highly significant decrease in percentage of positive cases expressing TGF-beta-R-1 in bladder cancer when compared with either chronic cystitis cases or controls (P < .01), while the percentage of positive cases expressing TGF-beta-1 protein highly significantly increased (P < .01) (Table 4, Figure 7, Figure 8).
Table 4.
Immunoelectronmicroscopic Examination of TGF-Beta-1 Protein and TGF-beta-R-1 Receptors of Exfoliated Malignant Epithelial (Urothelial) Cells in Urine
| Positive cases with TGF-beta-1 protein in urine | Positive cases with TGF-beta-R-1 in urine | |||
|---|---|---|---|---|
| No | % | No | % | |
| Control group (n=5) | – | – | 3 | 60 |
| Chronic cystitis (n=15) | 10 | 66.6 | 13 | 86.6 |
| Bladder cancer (n=45) | 42 | 93.3* | 17 | 37.7† |
Highly significant increase in percentage of positive cases in comparison with either chronic cystitis or control; P < .01
Highly significant decrease in number of positive cases in comparison with either chronic cystitis or control; P < .01
Figure 7.
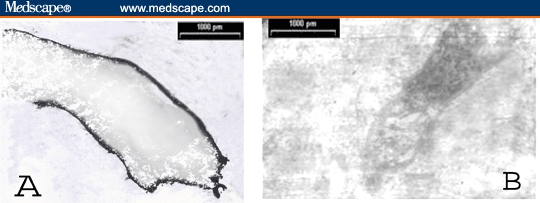
TEM photomicrograph of exfoliated epithelial (urothelial) cell in urine of patient with cystitis showing even distribution of the +ve immunoelectron labeling for TGF-beta-R-1 circumscribing the cell membrane (A) and −ve immunoperoxidase reaction for TGF-beta-1 protein (B). (uncontrasted section; × 3000)
Figure 8.
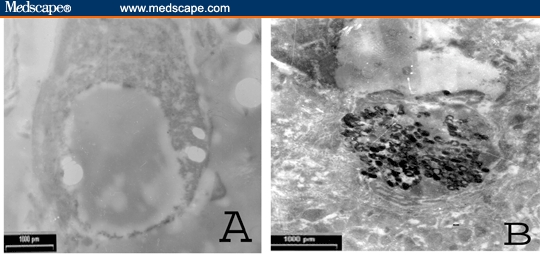
TEM photomicrograph of immunoperoxidase labeled exfoliated malignant epithelial (urothelial) cells in urine of patient with SQCC showing negative reaction for TGF-beta-R-1 (A) and positive reaction for TGF-beta-1 protein (B) (uncontrasted section; × 3000)
Three PBMNC samples and 5 urine samples showed positivity by this technique while they were negative by the immunocytochemical technique using the light microscope.
Electron Microscopic Examination of Agarose Cell Block
Electron microscopic examination of PBMNCs revealed remarkable apoptotic changes in the form of cell shrinkage, surface blebbing, nuclear chromatin condensation, and vacuolated cytoplasm in 42 of 45 bladder cancer cases (93.3%) (Figure 9A).
Figure 9.
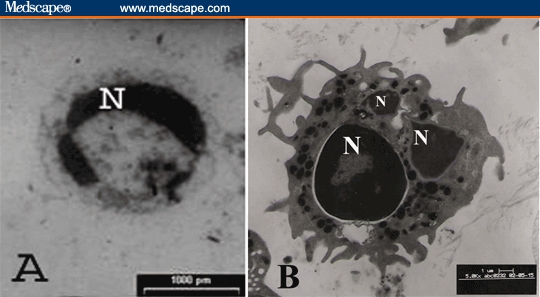
A – PBMNCs in patient with bladder cancer, showing clumped nuclear chromatin (N) and vacuolated cytoplasm (× 2300). B – Exfoliated inflammatory cell (mature neutrophil) in the urine of patient with bladder carcinoma (× 6500).
Electron microscopic examination of the urine samples revealed exfoliated necrotic malignant epithelial (urothelial) cells and many inflammatory cells in all bladder cancer cases (Figure 9B).
Discussion
Although we have many complex mechanisms for tumor surveillance by the immune systems, tumor cells continue to grow, invade, and metastasize.[2] The lack of clinical response in bladder cancer cases has generated more focus on analyzing tumor resistance to the immune system.
For diagnosis of urinary abnormalities, urine cytology, using Cytospin, has some limitations in clarifying accurate changes occurring in urothelial cells, so a more sophisticated technique is recommended.[29] In this study, the agarose cell block technique was applied for the immunocytochemical and electron microscopic examination of the urine and, for the first time, for PBMNC examination .We found that, by this technique, plenty of cells were well formed and collected due to the prohibition of their loss during manipulation. Moreover, the same block was simultaneously processed for light and electron microscopic examination, which may offer a new prospect for cytopathology in addition to its effectiveness in increasing the sensitivity of cytology.[25]
In PBMNCs, the significant increase in percentage of positive cases expressing TGF-beta-R1 in our bladder cancer cases compared with healthy controls might be explained by assuming that they are expressed under normal conditions on T cells[30] while being in a latent form and ready for rapid local activation when stressed.[31]
The significant increase in percentage of positive cases expressing TGF-beta-1 protein in bladder cancer is in agreement with Kehrl and colleagues,[32] who found that TGF-beta is produced mainly by T cells that can block production of interleukin 2 (IL-2) to inhibit IL-2-dependent proliferation of T cells. Brandes and colleagues[33] demonstrated the immunosuppressive effects of TGF-beta in malignant cases on both T cells that are mediated predominantly through CD4+CD25+ regulatory T cells that secrete TGF-beta-1 and express cell surface-bound TGF-beta-1,[34] and antigen-presenting cells. TGF-beta also inhibits the differentiation of T cells and prevents naive T cells from acquiring effector (cytotoxic or helper) functions.[35]
Recent reports have shown that phagocytosis of apoptotic cells by macrophages leads to release of TGF-beta. Monocytes may therefore increase their secretion of TGF-beta in the peripheral blood as a result of ingestion of increased numbers of apoptotic cells[36]; this potentially explains our findings of increased intracellular production of TGF-beta by monocytes in bladder cancer.
Ultrastructural studies of PBMNCs imply evident features of apoptosis in 93.3% of bladder cancer cases. These findings are supported by the proapoptotic role of the TGF-beta signaling pathway,[9] whose expression was significantly increased in our bladder cancer cases.
In our study, urine examination by immunoelectronmicroscopic and immunocytochemical techniques revealed a statistically significant increase in the percentage of bladder cancer cases expressing TGF-beta protein. Similar results were found in studies by De Visser and Kast[37] and Wojtowicz-Praga,[38] who found that TGF-beta protein is overexpressed in bladder cancer. De Visser and Kast[37] also found that high levels of TGF-beta are produced by cancer cells and have a negative impact on surrounding cells, including the host immune cells. Chen and colleagues[39] studied the role of TGF-beta as a potent immunosuppressor that helps tumor cells evade the immune system; they found that it can interact with T cells to efficiently block their IL-2 production and proliferation. In addition, TGF-beta interferes with the generation of CTLs and inactivates both NK cell and lymphokine-activated killer cell cytotoxicity, most likely by the inhibition of tumor necrosis factor alpha and beta secretion.[40,41] Dendritic cell (DC) maturation and antigen presentation is also impaired by TGF-beta[42] that is produced by tumor cells and significantly reduced the potency of DC/tumor fusion.[43]
The statistically significant decrease in the percentage of bladder cancer cases expressing TGF-beta-R1 compared with either chronic cystitis cases or the control group could be attributed to the loss of tumor cell receptors, mutation in the receptors, or dysregulation in TGF-beta signaling pathways, whereas immune cells remain sensitive.[44] Villanueva and colleagues[45] and Grady and colleagues[46] reported that all epithelial-derived tumors (> 85% of all human cancers) become resistant to the growth-inhibitory effects of TGF-beta with defects in the Smad proteins (predominately Smad4)and TGF-beta receptors (predominately TGF-beta RII). Proposed alternative mechanisms for resistance to TGF-beta include decreased expression of TGF-beta-R1,[47] TGF-beta-RII,[48] or TGF-beta-RIII[49] on the tumor cell surface, increased expression of the inhibitory Smad,[50] repression of TGF-beta signaling by a variety of oncoproteins,[51] and reduced expression or inactivation of tumor suppressors that directly regulate the TGF-beta signaling pathway.[52]
Our results revealed the accuracy of immunoelectronmicroscopic examination as 3 PBMNC samples and 5 urine samples showed positivity by this technique while they were negative by the immunocytochemical technique using the light microscope. This result agrees with Karen and colleagues,[24] who found the significantly increased yield of this technique due to its ability to reveal the secretory granules' contents and in locating cell receptors.
In this study, urine cytology, immunocytochemistry, electron microscopic examination using agarose cell block technique, and immunoelectronmicroscopic technique help us to better understand one of the escape mechanisms in bladder cancer. This mechanism is represented by the significant increase in the expression of TGF-beta protein in malignant cases that resist the apoptotic effect of this protein by the negative expression of its receptors on their surfaces. The increased understanding of this mechanism may facilitate the reverse of tumor escape from the immune system. It also draws the attention to TGF-beta-1 protein and TGF-beta-R1 receptor, where TGF-beta-1 protein can be used as an attractive target for anticancer therapy and the absence of TGF-beta-R1 can be considered a marker for malignant transformation of urothelial cells in bladder cancer.
Footnotes
Reader Comments on: The Role of TGF-beta-1 Protein and TGF-beta-R-1 Receptor in Immune Escape Mechanism in Bladder Cancer See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at amira7121956@yahoo.com or to Paul Blumenthal, MD, Deputy Editor of MedGenMed, for the editor's eyes only or for possible publication as an actual Letter in MedGenMed via email: pblumen@stanford.edu
Contributor Information
Amira Helmy, Theodor Bilharz Research Institute, Electron Microscopy Department, Cairo, Egypt Amira Helmy, MD's email: amira7121956@yahoo.com.
Olfat Ali Hammam, Theodor Bilharz Research Institute, Pathology Department, Cairo, Egypt.
Tarek Ramzy El Lithy, Theodor Bilharz Research Institute, Urology Department, Cairo, Egypt.
Mohamed Mohi El Deen Wishahi, Theodor Bilharz Research Institute, Urology Department, Cairo, Egypt.
References
- 1.Stein JP, Grossfeld GD, Ginsberg DA, et al. Prognostic markers in bladder cancer: a contemporary review of the literature. J Urol. 1998;160:645–659. doi: 10.1016/S0022-5347(01)62747-2. [DOI] [PubMed] [Google Scholar]
- 2.Burchardt M, Burchardt T, Shabsigh A, de la Taille A, Benson MC, Sawczuk I. Current concepts in biomarker technology for bladder cancers. Clin Chem. 2000;46:595–605. [PubMed] [Google Scholar]
- 3.Shankaran V, Ikeda H, Bruce AT, et al. IFNg and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 5.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Botet M, Moretta L, Strominger J. NK-cell receptors and recognition of MHC class I molecules. Immunol Today. 1996;17:212–214. doi: 10.1016/0167-5699(96)30009-1. [DOI] [PubMed] [Google Scholar]
- 7.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 8.Elliott RL, Blobe GC. Role of transforming growth factor beta in human cancer. J Clin Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Schuster N, Krieglstein K. Mechanisms of TGF-beta-mediated apoptosis. Cell Tissue Res. 2002;307:1–14. doi: 10.1007/s00441-001-0479-6. [DOI] [PubMed] [Google Scholar]
- 10.Atfi A, Buisine M, Mazars A. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-beta through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997;272:24731–24734. doi: 10.1074/jbc.272.40.24731. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura Y, Hua X, Bergelson S. Critical role of smads and AP-1 complex in TGF-beta-dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- 12.Motyl T, Grzelkowska K, Zimowska W. Expression of bcl-2 and bax in TGF-beta 1-induced apoptosis of L1210 leukemic cells. Eur J Cell Biol. 1998;75:367–374. doi: 10.1016/s0171-9335(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 13.Torre-Amione G, Beauchamp RD, Koeppen H, et al. A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci U S A. 1990;87:1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inman GJ, Allday MJ. Apoptosis induced by TGF-beta 1 in Burkitt's lymphoma cells is caspase 8 dependent but is death receptor independent. J Immunol. 2000;165:2500–2510. doi: 10.4049/jimmunol.165.5.2500. [DOI] [PubMed] [Google Scholar]
- 15.Hagimoto N, Kuwano K, Inoshima I. TGF-beta 1 as an enhancer of Fas-mediated apoptosis of lung epithelial cells. J Immunol. 2002;168:6470–6478. doi: 10.4049/jimmunol.168.12.6470. [DOI] [PubMed] [Google Scholar]
- 16.Bodmer S, Strommer K, Frei K. Immunosuppression and transforming growth factor-beta in glioblastoma: preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–3229. [PubMed] [Google Scholar]
- 17.Von Bernstorff W, Voss M, Freichel S. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7:925s–932s. [PubMed] [Google Scholar]
- 18.Hersey P. Impediments to successful immunotherapy. Pharmacol Ther. 1999;81:111–119. doi: 10.1016/s0163-7258(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 19.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 20.Caron F. Bacteriologic diagnosis and antibiotic therapy of urinary tract infections. Rev Prat. 2003;53:1790–1799. [PubMed] [Google Scholar]
- 21.Koss LG, Deitch D, Ramanathan R, Sherman AB. Diagnostic value of cytology of voided urine. Acta Cytol. 1985;29:810–816. [PubMed] [Google Scholar]
- 22.Wiener HG, Vooijs GP, Van't Hof-Grootenboer B. Accuracy of urinary cytology in the diagnosis of primary and recurrent bladder cancer. Acta Cytol. 1993;37:163–169. [PubMed] [Google Scholar]
- 23.Planz B, Jochims E, Deix T, Caspers HP, Jakse G, Boecking A. The role of urinary cytology for detection of bladder cancer. Eur J Surg Oncol. 2005;31:304–308. doi: 10.1016/j.ejso.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.De Mesy-Jensen KL, di Sant'Agnese PA. Large block embedding and “pop-off” technique for immunoelectron microscopy. Ultrastructural Pathology. 1992;16:51–59. doi: 10.3109/01913129209074550. [DOI] [PubMed] [Google Scholar]
- 25.Mansy S. Agarose cell block: innovated technique for the processing of urine cytology for electron microscopic examination. Ultrastructural Pathology. 2004;28:15–21. [PubMed] [Google Scholar]
- 26.Hsu SM, Raiue L. Protein A avidin and biotin in immunohistochemistry. J Histochem Cytochem. 1981;29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- 27.Kim WS, Park C, Jung YS. Reduced transforming growth factor-beta type II receptor (TGF-beta RII) expression in adenocarcinoma of the lung. Anticancer Res. 1999;19:301–306. [PubMed] [Google Scholar]
- 28.Grimaud JA, Druget M, Peyrol S, Chevalier D. Collagen immunotyping in human liver: light and electron microscope study. J Histochem Cytol. 1980;28:1145–1151. doi: 10.1177/28.11.7000887. [DOI] [PubMed] [Google Scholar]
- 29.Ooms ECM, Veldhuizen RW. Cytological criteria and diagnostic terminology in urinary cytology. Cyto Pathology. 1993;4:51–54. doi: 10.1111/j.1365-2303.1993.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 30.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Ann Rev Immunol. 1998;13:51–69. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 31.Nicolas OF, Hatzfeld A, Jacques AH. Transforming growth factor-beta pleiotropic role in the regulation of hematopoiesis. Blood. 96:2022–2036. 200. [PubMed] [Google Scholar]
- 32.Kehrl JH, Wakefield LM, Roberts AB, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandes ME, Wakefield LM, Wahl SM. Modulation of monocyte type I transforming growth factor-beta receptors by inflammatory stimuli. J Biol Chem. 1991;266:19697–19703. [PubMed] [Google Scholar]
- 34.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 36.Hodge SJ, Hodge GL, Reynolds PN, Scicchitano R, Holmes M. Increased production of TGF-beta and apoptosis of T lymphocytes. Am J Physiol Lung Cell Mol Physiol. 2003;285:L492–L499. doi: 10.1152/ajplung.00428.2002. [DOI] [PubMed] [Google Scholar]
- 37.De Visser KE, Kast MW. Effects of TGF-beta on the immune system: implications for cancer immunotherapy. Leukemia. 1999;13:1188–1199. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 38.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression by TGF-b inhibitors. Invest New Drugs. 2003;21:21–32. doi: 10.1023/a:1022951824806. [DOI] [PubMed] [Google Scholar]
- 39.Chen CH, Seguin-Devaux C, Burke NA, et al. Transforming growth factor b blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197:1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray JD, Hirokawa M, Ohtsuka K, Horwitz DA. Generation of an inhibitory circuit involving CD8+ T cells, IL-2, and NK cell-derived TGF-b : contrasting effects of anti-CD2 and anti-CD3. J Immunol. 1988;160:2248–2254. [PubMed] [Google Scholar]
- 41.Ebert O, Ropke G, Marten A, et al. TNF-alpha secretion and apoptosis of lymphocytes mediated by gene transfer. Cytokines Cell Mol Ther. 1999;5:165–173. [PubMed] [Google Scholar]
- 42.Geissmann F, Revy P, Regnault A, et al. TGF-beta1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- 43.Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta1 reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol. 2003;170:3806–3811. doi: 10.4049/jimmunol.170.7.3806. [DOI] [PubMed] [Google Scholar]
- 44.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-b in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 45.Villanueva A, Garcia C, Paules AB. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–1978. doi: 10.1038/sj.onc.1202118. [DOI] [PubMed] [Google Scholar]
- 46.Grady WM, Myeroff LL, Swinler SE. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 47.Nicolas FJ, Hill CS. Attenuation of the TGF-beta-Smad signaling pathway in pancreatic tumor cells confers resistance to TGF-beta-induced growth arrest. Oncogene. 2003;22:3698–3711. doi: 10.1038/sj.onc.1206420. [DOI] [PubMed] [Google Scholar]
- 48.Chen C, Wang XF, Sun L. Expression of transforming growth factor beta (TGFbeta) type III receptor restores autocrine TGFbeta1 activity in human breast cancer MCF-7 cells. J Biol Chem. 1997;272:12862–12867. doi: 10.1074/jbc.272.19.12862. [DOI] [PubMed] [Google Scholar]
- 49.Kleeff J, Ishiwata T, Maruyama H. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–5372. doi: 10.1038/sj.onc.1202909. [DOI] [PubMed] [Google Scholar]
- 50.Ewen ME, Oliver CJ, Sluss HK. p53-dependent repression of CDK4 translation in TGF-beta-induced G1 cell-cycle arrest. Genes Dev. 1995;9:204–217. doi: 10.1101/gad.9.2.204. [DOI] [PubMed] [Google Scholar]
- 51.Kaji H, Canaff L, Lebrun JJ. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–3842. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumacher YL, Ribas A. Overcoming tumor resistance to immunotherapy. Cancer Ther. 2006;4:13–26. [Google Scholar]


