Abstract
Eukaryotic cells are able to discriminate between native and non-native polypeptides, selectively transporting the former to their final destinations. Secretory proteins are scrutinized at the endoplasmic reticulum (ER)–Golgi interface. Recent findings reveal novel features of the underlying molecular mechanisms, with several chaperone networks cooperating in assisting the maturation of complex proteins and being selectively induced to match changing synthetic demands. ‘Public' and ‘private' chaperones, some of which enriched in specializes subregions, operate for most or selected substrates, respectively. Moreover, sequential checkpoints are distributed along the early secretory pathway, allowing efficiency and fidelity in protein secretion.
Keywords: endoplasmic reticulum, ER signalling, folding, protein degradation, protein secretion
Introduction
As the per-capita income increases in Western societies, the quality of the products that appear in the market is becoming more important than their quantity. Depending on high quality and innovative design, industries employ abundant personnel and devices to ensure a stringent control of their products, the quality of which must fulfill strict pre-determined standards. This key activity is usually referred to as ‘quality control' (QC). At the same time, the need for innovation makes it very hard to dictate a fixed set of standards and rules. Moreover, selling more (high quality) products remains a common goal of any commercial activity. Italian parents often say to their hasty offspring ‘Presto e bene raro avviene' (Fast and good is a rare combination). How can our factories contradict this rather wise saying? And, of more interest for The EMBO Journal readership, how do cells cope with somewhat similar problems? In this essay, we analyze the mechanisms that cells employ to couple abundant synthesis and high quality for secretory proteins.
After synthesis, proteins must rapidly fold to perform their biological activities. Folding takes place in three main sub-cellular compartments, cytosol, endoplasmic reticulum (ER) and mitochondria. Each organelle is equipped with a specific set of chaperones and folding enzymes optimized to work in the local conditions. In all cases, the final outcome must be a native molecule devoid of errors. Moreover, structural maturation must be completed within a rather short time frame. In the crowded environment of the cell, unfolded proteins are a danger as they may aggregate and become toxic. In viable cells, extensive aggregation is prevented by several proteolytic systems that rapidly dispose of aberrant or damaged polypeptides (see Goldberg, Liberek, Haas, this issue).
A considerable fraction of the proteome consists of molecules that are destined to the extracellular space (Chen et al, 2005): these are either secreted by the cell or inserted in membranes, to act as ligands and receptors, respectively. Proteins destined to the extracellular space are synthesized on ER-bound ribosomes, and are cotranslationally translocated into the ER lumen where they attain their native conformation, before being transported to the Golgi and downstream compartments (Figure 1). Secreted and membrane proteins are the main devices of intercellular communications. The fidelity of ligand–receptor interactions requires that both molecules attain the very conformations that allow signals to be properly transmitted and understood. Protein folding in the secretory pathway must therefore be controlled in the tightest way.
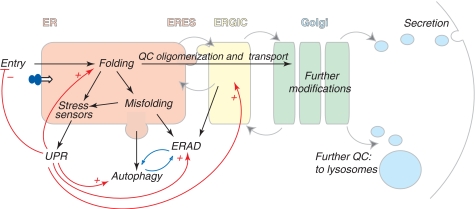
Figure 1.
The early secretory pathway. Proteins destined to the extracellular space or to organelles of the secretory route are synthesized by ER-bound ribosomes and cotranslationally translocated (entry) into the ER. Here they attain their native structure (folding), under strict QC scrutiny. Only properly folded and assembled proteins can reach the Golgi, where they are further modified, to be transported to the extracellular space or to lysosomes. Gray arrows indicate the direction of vesicles moving among different compartments; dark arrows indicate the pathways followed by cargoes in the early secretory pathway; red lines show homeostatic control pathways (+ stimulatory, − inhibitory). Misfolded proteins are recognized, retained and eventually routed to degradation by ERAD or autophagy (which are likely reciprocally regulated, as indicated by the blue arrows). Some misfolded soluble ERAD substrates are transported to ERGIC or cis-Golgi before retrotranslocation and degradation. Too high load for the folding machinery or the accumulation of misfolded proteins activate resident ER stress sensors, which elicit the UPR. ER stress can selectively inhibit protein entry into the ER, and increase the capacity of folding and degradation (via ERAD and autophagy). The UPR induces also molecules acting downstream of the ER.
Protein QC in the secretory compartment
In the late 1980s, work on oligomeric viral proteins (Kreis and Lodish, 1986; Boulay et al, 1988; Gething and Sambrook, 1989), the T-cell receptor (Bonifacino et al, 1989; Sancho et al, 1989) and immunoglobulins (Bole et al, 1986; Sitia et al, 1987; Hendershot and Kearney, 1988) revealed that assembly is a requisite for transport to the Golgi apparatus and onwards along the secretory route. Klausner (1989) referred to this phenomenon as ‘Architectural editing'; the term ‘ER quality control' (Hurtley and Helenius, 1989; Hurtley et al, 1989) eventually stuck to indicating the processes of conformation-dependent molecular sorting of secretory proteins. Until then, the lysosome was considered the site where secretory molecules are degraded. Since proteins retained in the ER cannot reach downstream lysosomes, the question arose as to how aberrant proteins are degraded (Klausner and Sitia, 1990).
In the mid 1990s, studies on CFTR and MHC class I (Michalek et al, 1993; Ward et al, 1995; Wiertz et al, 1996) revealed that proteins that fail to fold or assemble are eventually retrotranslocated (or dislocated) across the ER membrane for degradation by cytosolic proteasomes. The players, mechanisms and physiopathologic implications of this process (ER-associated degradation, ERAD) remain a hot topic in molecular cell biology (Yoshida, 2007). During their lifetime, cells must integrate all the different reactions schematized in Figure 1, and adapt them to face possible changes in the quality and quantity of secretory proteins they produce during differentiation. As colocalized signals can dictate assembly, retention and degradation of membrane and soluble cargo proteins (Bonifacino et al, 1990; Fra et al, 1993), a competition between ER export and degradation can explain homeostatic control.
An interesting mathematical model has been recently introduced, which considers protein folding in the ER (ERAF, ER-assisted folding), ERAD and ER export as single biochemical parameters (see Wiseman et al, 2007 and references therein). Despite the limits imposed by the simplification, this approach leads to some interesting and testable predictions: export efficiency of a particular cargo protein depends on the activity of the ERAF, ERAD and export systems, which in turn are influenced by the proteome expressed by the cell. This partially simplified model could be further expanded and tested to integrate new emerging evidence. Recent data highlight a spatial subdivision of the early secretory compartment that seems particularly suited for the biogenesis of complex, multimeric proteins. Both parallel and sequential QC pathways coexist in cells, some common to all polypeptides, others specific for particularly demanding proteins. This diversity likely evolved to cope with the myriads of polypeptides that our cells produce, often in exuberant amounts.
Protein QC (Box 1) is intimately linked to the processes of folding (Ellgaard and Helenius, 2003; Sitia and Braakman, 2003). Both rely on chaperones and devoted resident enzymes. QC serves different roles: (i) it prevents the deployment of aberrant protein conformers, ensuring that only native proteins proceed along the secretory pathway; (ii) it retains precursors in an environment suitable for their maturation; (iii) it increases their local concentration to favor assembly and polymerization; (iv) it reduces the risks of proteotoxicity by inhibiting aggregation and degrading terminally misfolded proteins; (v) it maintains homeostasis in the early secretory pathway; (vi) it is involved in the developmental regulation of protein secretion (IgM, adiponectin, see below) and (vii) it is important for storing proteins for regulated secretion. In certain plants, in fact, ER retention/accumulation is utilized to store abundant proteins during seed formation (Larkins et al, 1993; Jolliffe et al, 2005; Vicente-Carbajosa and Carbonero, 2005).
Table 1.
Box 1 The logics of QC
| 1. Preventing the deployment of aberrant protein conformers |
| 2. Retaining precursor proteins in an environment suitable for their maturation |
| 3. Favoring correct assembly by increasing subunit concentration |
| 4. Reducing the risks of proteotoxicity by inhibiting aggregation and degrading terminally misfolded proteins |
| 5. Maintaining homeostasis in the early secretory pathway |
| 6. Developmental regulation of protein secretion (IgM, adiponectin) |
| 7. Storing proteins for regulated secretion (plants, adipocytes) |
| QC, quality control. |
Protein folding in the ER
Upon cotranslational translocation, nascent secretory proteins enter the crowded environment of the ER lumen and soon begin folding into more stable, lower energy, conformation(s) (Dobson, 2004). While the basic principles governing folding are common to other cellular compartments (Anfinsen and Scheraga, 1975; Dobson, 2004), the ER is unique in sustaining a set of covalent modifications, which include removal of the signal sequences, disulfide bond formation, N-glycosylation and GPI addition. A plethora of enzymes and assistants are found in the early secretory pathway, which catalyze each step (Box 2). How is their synthesis regulated so as to have the right balance in different cell types? How are they functionally interconnected? How are the different steps executed in the right order? How are unfolded proteins recognized (Box 3)?
Table 2.
Box 2 Workers in the secretory protein factory (an incomplete list)
| (A) ‘Public' chaperones and enzymes | |||
|---|---|---|---|
| Class | Name | Localization | Function |
| Chaperones | BiP/GRP78 | ER | Folding assistant/unfolding Regulation of IRE1, PERK and ATF6 in ER signaling Translocon gating and regulation |
| GRP94 | ER | Folding assistant | |
| ORP150 | ER | Folding assistant, hypoxia | |
| HERP | ER membrane | ERAD | |
| SEL1L | ER membrane | ERAD | |
| Co-chaperones | Sil1/BAP ERdjs | ER ER | ATP exchange factor BiP cofactors |
| Lectins | CNX | ER membrane | Folding |
| CRT | ER soluble | Folding | |
| ERGIC-53 | ERGIC | Transport F5, F8, CatZ, CatC, IgM polymers | |
| VIPL | ER | Transport | |
| VIP-36 | Cis-Golgi | Transport | |
| EDEM1, 2, 3 | ER subregion | ERAD | |
| OS9 | ER membrane | ERAD | |
| Erlectin/XTP3-B | ER membrane | ERAD | |
| Enzymes redox | Ero1α | ER+ERGIC | Oxidase |
| Ero1β | ER | Oxidase | |
| PDI | ER | Oxidase, isomerase, reductase Subunit of prolyl 4-hydroxylase Subunit of microsomal triacylglycerol transfer protein | |
| ERp57 | ER | Isomerase, oxidase? | |
| ERp72 | ER | Unclear | |
| ERp44 | ERGIC-cis-Golgi | Thiol-mediated retention/IP3R1 regulation | |
| Proline isomerases | PPIases Cyclophilins | ER ER, mitochondria, nucleus, cytosol | Proline isomerization |
| Proline isomerization | |||
| Sugar processing | Glucosidase I | ER | |
| Glucosidase II | ER | ||
| ER Man I | ER | ||
| ER Man II | ER | ||
| UGGT | ER | Folding sensor | |
| Man IA, IB, IC | Golgi | ||
| (B) ‘Private' tissue- or substrate-specific factors | |||
| Name |
Tissue expression |
Substrates/function |
|
| Hsp47 | Fibroblasts | Collagen biosynthesis/chaperone | |
| PDIp | Exocrine pancreas | Zymogens/oxidoreductase | |
| PDILT | Sertoli cells in testis | Calmegin, Δ-somatostatin/ chaperone | |
| Egasyn | Ubiquitous | β-Glucuronidase/chaperone | |
| Invariant chain | APC | MHC class II assembly and transport | |
| Tapasin | Ubiquitous | MHC class I assembly | |
| SCAP-RAP Boca/Mesd | Ubiquitous | LDL receptor assembly and transport | |
| APC, antigen-presenting cell; CNX, calnexin; CRT, calreticulin; ER Man, ER α1,2-mannosidase; ER, endoplasmic reticulum; ERAD, ER-associated degradation; ERGIC, ER–Golgi intermediate compartment; LDL, low-density lipoprotein; MHC, major histocompatibility complex; UGGT, UDP-glucose glycoprotein glucosyltransferase. | |||
Table 3.
Box 3 Monitoring non-native structure
| 1. Exposure of hydrophobic patches |
| 2. Presence of immature glycans |
| 3. Exposure of reactive thiols |
Owing to the fact that N-glycosylation is unique to secretory proteins, the folding and QC of glycoproteins have been analyzed in great detail and can be used as a prototypic example of labor organization in the ER protein factory. As in all assembly lines, transport must follow the execution of a given step. The time allocated to the latter, however, must be precisely controlled in order to allow efficiency and prevent jams along the line. The sequential modifications of the oligosaccharides provide an elegant solution to dictate and time the manufacture of cargo glycoproteins.
N-glycosylation involves binding of a preformed oligosaccharide (Glc3Man9GlcNAc2) to asparagine side chains in the sequence NXS/T, where X is any amino acid other than proline (Khalkhall and Marshall, 1975). The sugar moieties are then progressively trimmed by resident enzymes of the secretory pathway. Soon after synthesis, glucosidases I and II sequentially remove the three glucose moieties from the A branch of the oligosaccharide moieties (Figure 2). UDP-glucose glycoprotein glucosyltransferase (UGGT) adds back a glucose residue to N-glycans positioned near regions of disorders (Taylor et al, 2004). Therefore, UGGT acts as a folding sensor and produces monoglucosylated proteins (Glc1Man9GlcNAc2) that can interact with calnexin (CNX) or calreticulin (CRT), two ER chaperones with lectin activity (Waisman et al, 1985; Ahluwalia et al, 1992; reviewed in Williams, 2006). CNX and CRT retain misfolded substrates in the ER, prevent their aggregation and promote oxidative folding via interactions with ERp57 (Ellgaard et al, 2001; Schrag et al, 2001; Frickel et al, 2002; Russell et al, 2004). By removing the terminal glucose, glucosidase II dissociates the substrate from CNX/CRT for a novel round of inspection by UGGT.
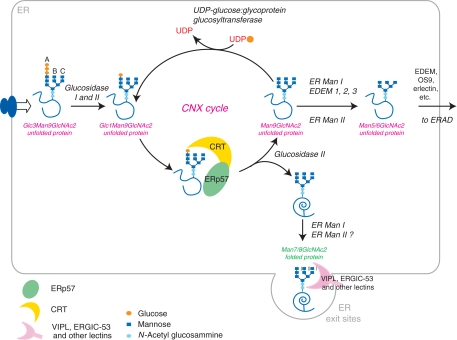
Figure 2.
The CNX/CRT cycle. After transfer of the preformed core oligosaccharide (Glc3Man9GlcNAc2) onto nascent proteins, glucosidase I and II sequentially remove the two terminal glucoses from the A branch. The monoglucosylated Glc1Man9GlcNAc2 unfolded protein can now interact with the lectin chaperones CNX and CRT. In association with the oxidoreductase ERp57, CNX and CRT prevent aggregation and facilitate glycoprotein folding. Removal of the glucose by glucosidase II (Man9GlcNAc2) interrupts the interaction of the protein with CNX/CRT. If the protein has attained its native structure, it can now proceed along the secretory pathway by bulk flow or by interaction with specific lectin transporters such as ERGIC-53 or VIPL. If unfolding persists, the glycoprotein is recognized by UGGT1, which places a single glucose back onto the A branch, causing the protein to enter the CNX/CRT cycle again. Mannose trimming causes exit from the CNX/CRT cycle. Misfolded proteins can be recognized by specific lectins (EDEMs, OS9, etc) and targeted to degradation.
How do terminally misfolded proteins escape the cycle? Glycan processing again comes into action, because removing the terminal mannose moieties inhibits glucose re-addition. Mannose trimming hence acts as a timer, discriminating between junior proteins (which should be given the time to fold) and senior ones, which should be either secreted or sent to degradation. Many proteins with mannosidase activity reside in the early secretory apparatus (e.g., ER α1,2-mannosidase I (ER Man I), EDEM1 and 3, Golgi Man IA, IB, IC, ER Man II). Man I inhibitors (e.g., kifunensine), which prevent removal of the terminal B-branch mannose, stabilize ERAD glycoprotein substrates, but do not prevent secretion of native species. Overexpression of ER Man I and EDEMs accelerates degradation (Hosokawa et al, 2006 and references therein). ER–Golgi intermediate compartment-53 (ERGIC-53) (a protein transporter with lectin activity, cycling between the ER and the ERGIC, see below) and possibly other L-type lectins (e.g., VIPL, VIP36; Kamiya et al, 2007) bind high-mannose cargoes, facilitating their forward transport. Further mannose trimming in the ER may favor degradation, possibly also because reducing the hydrodynamic volume of substrate glycoproteins could facilitate their retrotranslocation. It will be of great interest to determine the precise binding specificities and fate of the various intermediates in glycan processing (Helenius and Aebi, 2001).
Another well-characterized folding pathway is based on Binding Protein (BiP, also called GRP78), an abundant chaperone of the hsp70 family, which serves also a key regulatory role in ER signalling (Bertolotti et al, 2000). BiP was first isolated as a protein associating with unassembled Ig-H chains (Haas and Wabl, 1983). It consists of an N-terminal ATPase domain and a C-terminal domain with affinity for hydrophobic patches (Flynn et al, 1991; Blond-Elguindi et al, 1993). The affinity for substrates depends on ATP binding at the N-terminal domain. When ATP is hydrolyzed to ADP, a conformational change occurs, which determines substrate release. Thus, substrates can undergo cycles of BiP binding and release, depending on ATP hydrolysis (Gething, 1999). Owing to the weak BiP ATPase activity, hsp40-like co-chaperones containing J domains (ERdj) play a key regulatory role. Five ERdj proteins have been isolated so far (Shen et al, 2002; Cunnea et al, 2003; Kroczynska et al, 2004; Shen and Hendershot, 2005). One of them, ERdj5, also displays oxidoreductase acitivity, possibly linking BiP-dependent folding/unfolding and disulfide bond formation, isomerization or reduction (Nagata et al, personal communication).
Very rarely glycoproteins are found to bind simultaneously to BiP and CNX or CRT. Therefore, it seems that a given glycoprotein enters first either the BiP or the CNX/CRT pathway (Figure 3). The initial choice is dictated by the localization of the N-glycans: the closer these are to the N-terminus of the nascent protein, the higher the tendency to use CNX as a chaperone system (Molinari and Helenius, 2000). If the first attempts to fold fail, the protein can shift to the alternative pathway. Altogether, these data imply that sites of conjunction exist in which the substrate can jump from one pathway to another. In principle, however, it should be possible for large multi-domain proteins to engage with both. It would be of interest to determine whether ER sub-regions exist that are enriched in either pathway.
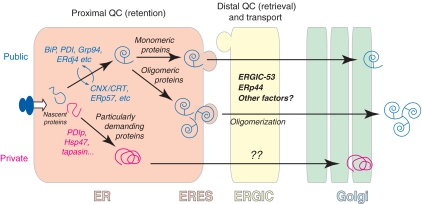
Figure 3.
QC in the early secretory pathway. Chaperones and folding assistants can be grouped in different classes according to their specificity and subcellular localization. The majority of secretory proteins utilize public chaperones: some initially go with BiP, PDI and their partners, others enter the CNX/CRT cycle, the choice depending on the location of the N-glycans. Certain proteins that are produced in large amounts, or are intrinsically difficult to fold, are assisted by specific (private) chaperones and enzymes (see also Box 2). In addition, QC can occur in sequential steps. After a proximal QC, certain substrates (generally multimeric proteins) seem to undergo also distal QC checkpoints in ERGIC and cis-Golgi. This model could mediate cargo concentration and selective export of oligomerized species, thus coupling fidelity and efficiency in the secretory protein factory. While proximal QC can rely on simple retention, the distal checkpoints likely imply substrate retrieval to the ER, either for further attempts to fold, or for retrotranslocation and degradation.
Supplementary Table 1S shows the phenotypes of cells, mice and patients in which individual chaperones, enzymes or sensor molecules are insufficient or absent altogether. BiP−/−, ERp57−/−, CNX−/− and CRT−/− mice show embryonic or perinatal lethality, but their phenotypes vary considerably: CRT−/− mice have severe problems in cardiac development, while large myelinated fibers in peripheral nerves are the main targets in CNX−/− animals (Mesaeli et al, 1999; Denzel et al, 2002; Garbi et al, 2006). BiP is essential also for survival of cells in culture, in agreement with its role in regulating translocation and ER signalling. In contrast, CRT−/− and CNX−/− cells are viable, and their phenotypes surprisingly mild, suggesting redundancy in substrate recognition by the two chaperones.
Oxidative folding
In terms of ionic composition and redox potential, the ER is similar to the extracellular space, providing an ideal folding place/test bench for proteins destined to the external world. Although certainly important, the higher ratio between oxidized and reduced glutathione (GSSG/GSH) in the ER, compared with the cytosol (Hwang et al, 1992), is not enough to guarantee efficient oxidative folding. Indeed, for many proteins to fold correctly, disulfide bond isomerization, and sometimes also reduction (Jansens et al, 2002), is needed. A hyper-oxidizing environment in the ER lumen may hence inhibit folding of proteins with multiple disulfides, and promote aggregation (Molteni et al, 2004). Therefore, oxidative folding relies primarily on protein–protein interchange relays. The main pathway involves disulfide transfer from PDI or PDI-like proteins to nascent cargoes. PDI consists of four thioredoxin (trx) domains: the two lateral domains (a and a′) are endowed with oxidoreductase activity, while the two central ones, b and b′, provide a hydrophobic surface suited to bind and present nascent proteins to the active sites in a and a′. This overall structure is likely important for the redox-dependent chaperone function of PDI (Wilkinson and Gilbert, 2004; Forster et al, 2006a), particularly with terminally misfolded proteins, which must be reduced before dislocation to the cytosol for proteasomal degradation (Gillece et al, 1999; Fagioli et al, 2001; Tsai et al, 2001, 2002; Molinari et al, 2002).
After transferring a disulfide bond to nascent proteins, PDI is re-oxidized by members of the Ero1 flavoprotein family (Frand and Kaiser, 1998; Pollard et al, 1998; Cabibbo et al, 2000; Pagani et al, 2000; Mezghrani et al, 2001). In vitro, yeast Ero1p can use molecular oxygen as terminal electron acceptor, in a reaction that produces hydrogen peroxide in stoichiometric amounts to the disulfides formed (Tu and Weissman, 2002; Gross et al, 2006). Studies are ongoing in several laboratories to determine whether H2O2 is generated in living cells as a byproduct of oxidative folding, because this could serve signalling purposes. However, at least in yeast, disulfide bond formation can proceed in anaerobic conditions (Gross et al, 2006), suggesting that alternative electron acceptors exist.
Over the last years, many other ER-resident PDI-like oxidoreductases have been characterized in mammalian cells. The precise role(s) of these molecules, as well as the mechanisms controlling their redox state and activity, remain to be clarified (Ellgaard and Ruddock, 2005). Some of them, for example, PDIp (Desilva et al, 1996) and PDILT (van Lith et al, 2005; van Lith et al, 2007), are selectively expressed in pancreas and testis, respectively, and hence belong to the growing group of substrate- or tissue-specific (‘private') chaperones, including Hsp47, etc. (Box 2B).
Disulfide bond formation is crucial in the folding and QC of secretory proteins. Since they increase the stability of the native conformation, their absence, or even worse, their mispairing, generally produces severely misfolded species. Furthermore, an exposed cysteine in the proper amino acid context is sufficient to cause retention and degradation of otherwise transport competent intermediates (Fra et al, 1993; Guenzi et al, 1994), likely because the reactivity of thiol groups favors the formation of mixed disulfides with PDI, ERp44 and other resident proteins (Reddy et al, 1996; Anelli et al, 2003, 2007). The thiol-dependent retention mechanisms, originally described in the developmental control of IgM (Sitia et al, 1990), have recently been shown to control also adiponectin secretion (Wang et al, 2007).
Bulk flow, retention, retrieval and selective export
Since the discovery of the KDEL motif as a means to localize soluble proteins in the ER (Munro and Pelham, 1987), the problem arose as to how these extremely abundant residents rarely saturate the KDEL receptors. A possible answer lies in the discovery of supra-molecular complexes comprising different chaperones (BiP, GRP94, ERdj3 and UGGT, but no CRT; Meunier et al, 2002; Gilchrist et al, 2006). Because of their different diffusibility, these complexes could be excluded from forward moving vesicles, and form a matrix to retain folding intermediates in a suitable environment for their maturation. The presence of UGGT in these complexes may be important for shifting misfolded substrates to the CNX–CRT pathway.
Unless retained by interactions with resident proteins, a protein could exit from the ER by bulk flow (Wieland et al, 1987). However, many proteins are actively transported out of the ER by interaction with specialized export machineries (see Gurkan et al, 2006 for a review). Export from the ER occurs at ER exit sites (ERES; Mezzacasa and Helenius, 2002), where budding of COPII-coated vesicle takes place. It is now evident that transportable cargoes contribute to the formation of ERES- and COPII-coated vesicles (Forster et al, 2006b). Moreover, in exocrine pancreatic cells, the ER–Golgi interface is where different secretory proteins reach their highest intracellular concentration (Martinez-Menarguez et al, 1999; Oprins et al, 2001), which could have important consequences for the biogenesis of oligomeric proteins (see below).
Specific transporter molecules mediate the exit from the ER of certain glycoproteins, concentrating them into forward transport vesicles (see Hauri et al, 2002; Lee et al, 2004 for reviews). In mammalian cells, one of the best characterized is ERGIC-53, a hexameric transmembrane lectin (Schindler et al, 1993) that derives its name from being particularly abundant in the ERGIC. ERGIC-53 is described to capture high-mannose glycoproteins in the ER, and release them in the ERGIC in a Ca2+- and pH-dependent manner (Appenzeller-Herzog et al, 2004). Mutations in ERGIC-53 (also known as LMAN1) or in MCFD2, a gene encoding a small soluble protein that associates with ERGIC-53 (Zhang et al, 2003, 2005; Baines and Zhang, 2007), are responsible for most cases of combined deficiency of coagulation factors V and VIII (F5F8D), a recessive bleeding disorder characterized by decreased serum levels of both clotting factors (Nichols et al, 1998; Neerman-Arbez et al, 1999). The rather limited phenotype of patients who lack functional ERGIC-53 suggests that other lectins serve redundant functions in controlling glycoprotein traffic (e.g., VIP36 (Fiedler et al, 1994), VIPL (Neve et al, 2003), ERGL (Yerushalmi et al, 2001; see Hauri et al, 2002 for a review)). The specificity of these lectins has recently been analyzed (Kamiya et al, 2007): VIPL and VIP36 interact preferentially with glycans carrying, on their A branch, three mannoses but no terminal glucoses, (see also Fullekrug et al, 1999). Unexpectedly, ERGIC-53 displays low-affinity and broad-specificity interactions with high-mannose oligosaccharides also when monoglucosylated at the A branch. Moreover, while VIPL and ERGIC-53 bind better at pH 7, (as found in the ER), VIP36 has an optimum at pH 6.5 (as in the Golgi). From these data, it has been suggested that VIPL binds de-glucosylated cargoes exiting the CNX/CRT cycle, protecting them from de-mannosylation and hence degradation. The cargo is then passed to ERGIC-53, perhaps owing to its hexameric structure, and exported toward the Golgi. In the cis-Golgi, VIP36 may play additional roles in glycoprotein QC and transport.
Sequential QC checkpoints along the early secretory pathway
For many ER-synthesized proteins, attaining the native structure is a long endeavor, requiring several hours or more. This generally reflects the sequential execution of multiple steps, often including oligomerization. In a factory, engineers would most likely design a sequential assembly line, in which each step is carefully executed and monitored. Evidence in favor of such a physical compartmentalization within the early secretory pathway of mammalian cells is accumulating.
ERGIC-53, ERp44 and IgM polymerization
IgM polymers are planar, multimeric proteins with an overall hexameric shape, secreted as either [(μ2L2)5−J] or (μ2L2)6. Individual subunits are linked by disulfide bonds involving Cys575 in the C-terminal tailpiece of secretory μ(μs) chains. Cys575 acts also as a retention and degradation signal for unpolymerized subunits (Fra et al, 1993). The first assembly step (μ2L2 formation) is fast and efficient in both B and plasma cells: its fidelity is mainly checked by BiP (see Hendershot and Sitia, 2005 and references therein). In contrast, polymerization is slow and occurs only in plasma cells. Recent studies suggest that a post-ER QC mechanism plays a key role in the sequential assembly of IgM polymers. An unexpected finding was that ERp44, a soluble member of the trx family equipped with an RDEL localization motif (Anelli et al, 2002), accumulates in the ERGIC and cis-Golgi (Gilchrist et al, 2006; Anelli et al, 2007; Wang et al, 2007). ERp44 mediates the thiol-dependent retention of μ2L2, μL and other unpolymerized IgM subunits that have already passed the BiP-dependent checkpoints (Anelli et al, 2003, 2007). ERp44 localization partly depends on interactions with ERGIC-53, which also binds IgM subunits (Mattioli et al, 2006). As a hexameric membrane-embedded lectin, ERGIC-53 may provide a platform for IgM polymerization (Anelli et al, 2007). In the cis-Golgi, ERp44 could capture unpolymerized IgM subunits and retrieve them via RDEL-dependent mechanisms. In view of its ability of binding Ero1 (Otsu et al, 2006), ERp44 could also provide oxidative power to the polymerization machinery. In this scenario, the compartmentalization of assembly and polymerization in the early secretory pathway of professional antibody secreting cells may couple QC and transport, thus achieving high production capacity.
ERp44, Ero1α and adiponectin oligomerization
Another example of sequential QC comes from adiponectin, a plasma protein secreted by adipocytes (Scherer et al, 1995; Chandran et al, 2003), low levels of which associate with diabetes and cardiovascular diseases (Hotta et al, 2000; Phillips et al, 2003; Shetty et al, 2004). Plasma adiponectin is composed of trimers, hexamers and higher molecular weight oligomers, the latter being more active in preventing diseases (Tonelli et al, 2004; Bobbert et al, 2005; Lara-Castro et al, 2006). ERp44 was shown to retain folded adiponectin trimers, forming mixed disulfides with Cys39 in one subunit (Qiang et al, 2007; Wang et al, 2007). Ero1α, whose synthesis is regulated by hypoxia, SIRT1 and PPARγ, sequesters ERp44 and favors secretion of adiponectin oligomers (Wang et al, 2007). As in the case of IgM, therefore, folded adiponectin intermediates are retained by thiol-dependent mechanisms. ERp44-bound adiponectin may provide a reservoir of molecules easily mobilized for rapid release. The wide distribution of ERp44 in secretory cells (see www.hpr.se) and its high evolutionary conservation in worms and insects imply a wider role for ERp44 in the early secretory compartment, including the coupled control of Ca2+ and redox homeostasis and signalling (Higo et al, 2005).
Oligomeric membrane proteins
Different mechanisms exist for ER retrieval of membrane proteins (reviewed in Lee et al, 2004). Yeast Rer1p, a Golgi transmembrane protein, binds and mediates the retrieval of the ER-localized proteins Sec12p, Sec63p, Sec71p via their transmembrane domains (Sato et al, 1995, 1996, 1997, 2001). Interestingly, yeast Rer1p has also a role as quality controller for the iron transporter subunit Fet3p, retrieving it back in the ER by interactions with the TMD, unless the subunit is correctly assembled with its partner Ftr1p (Sato et al, 2004). The mammalian homologue of Rer1p localizes in the Golgi (Fullekrug et al, 1997). RER1 binds unassembled nicastrin and regulates transport of the γ-secretase complex (Spasic et al, 2007). On the contrary, the thermosensitive mutant tsO45 VSV-G protein can no longer be retained once it reaches the ERGIC or Golgi (Mezzacasa and Helenius, 2002), impying that the machineries recognizing mutant tsO45 VSV-G protein are not present downstream of the ER.
Therefore, QC occurs in multiple stations of the early secretory compartments, each station likely recognizing particular features in cargo molecules.
Multiple ways for ERADicating proteins from the early secretory pathway
Because of mutations and/or lacking of cofactors, partner proteins or prosthetic groups, some proteins cannot reach their native conformation and hence must be degraded. Substrates vary widely in size, structure and topology, and multiple pathways are active in cells to handle them. However, some common features exist. Aberrant proteins must be first recognized. As anticipated above, mannose trimming allows discrimination between unfolded and terminally misfolded glycoproteins (Helenius and Aebi, 2001); the latter are recognized by the concerted action of α1/α2 mannosidase-like proteins like Htm1p/Mnl1p in yeast (EDEM1, 2, 3 in mammalian cells) (Molinari et al, 2003; Oda et al, 2003; Mast et al, 2005; Olivari et al, 2005; Hirao et al, 2006), yeast Yos9p (Buschhorn et al, 2004) and mammalian OS-9 and erlectin/XTP3-B. The latter two mammalian lectins interact with SEL1L (J Christianson and R Kopito, personal communication), a membrane-bound molecule involved in ERAD (Mueller et al, 2006) and capable of binding the E3 ligase HRD1 (Neuber et al, 2005), providing a possible link between substrate recognition, translocation and ubiquitination. Much less is known on how terminally misfolded proteins that lack N-glycans are targeted to destruction.
Once recognized, aberrant proteins likely undergo partial unfolding and reduction (Tortorella et al, 1998; Fagioli et al, 2001) before retrotranslocation. Both BiP and PDI have been implicated in this process (Nishikawa et al, 2001; Tsai et al, 2001; Molinari et al, 2002). Certain exogenous toxins utilize ERAD pathways to reach the cytosol. Since their folding occurs in the producer cell (generally a bacterium or a plant), toxins provide powerful tools to analyze the interactions occurring during retrotranslocation independently from the ones occurring during folding. Using these models, reduced PDI was shown to bind and unfold cholera toxin before dislocation. Upon Ero1-dependent oxidation, PDI releases dislocation-competent A subunits, thus acting as a redox-dependent chaperone (Tsai et al, 2001, 2002; Tsai and Rapoport, 2002).
ERdj5 might play an important role in the dislocation of certain substrates that form interchain disulfide bonds. Both its oxidoreductase and DnaJ activities are essential, suggesting that ERdj5 could couple reduction and BiP-mediated unfolding (K Nagata, personal communication).
Dislocation is the least understood step in ERAD. Besides Sec61 (Gillece et al, 2000; Jarosch et al, 2002; Clemons et al, 2004; Ng et al, 2007), additional transmembrane proteins (yeast Der1p and its homologues Derlin1, 2 and 3 in mammalian cells) have been implicated. It remains to be seen whether derlins directly mediate dislocation, or act by recruiting additional essential components such as the AAA-ATPase p97, which provides part of the energy necessary for this step (Ye et al, 2001, 2004; Rabinovich et al, 2002; Oda et al, 2006). It has been recently suggested that also lipid-based mechanisms mediate the transport of large substrates across the ER membrane, as observed for the entry in the cell of certain non enveloped viruses.
Once they emerge into the cytosol, ERAD substrates are ubiquitinated by E2–E3 complexes generally associated with the ER membrane (yeast E3 ligase Doa10p or Hrd1p and E2 ubiquitin conjugating enzyme Ubc7p-Cue1p (Carvalho et al, 2006; Denic et al, 2006). Then, an N-glycanase removes the oligosaccharide moieties from glycoproteins (Hirsch et al, 2003, 2004). Proteasome inhibitors generally impair dislocation (Chillaron and Haas, 2000; Mancini et al, 2000), implying that the above steps (dislocation, ubiquitination, proteolysis) are tightly coupled. The association of proteasomes with the ER membranes (Kalies et al, 2005) may be important in coupling substrate extraction and degradation.
Experiments in yeast and mammalian cells have recently shown that molecules involved in ERAD form multiprotein complexes specialized in the handling of topological classes of proteins (Vashist and Ng, 2004; Lilley and Ploegh, 2005; Carvalho et al, 2006). In yeast, the ERAD-L pathway recognizes substrates with misfolded ER luminal domains (on both soluble and membrane proteins). It needs the cytosolic factor Cdc48p (p97), the E3 ubiquitin ligase Hrd1p (HRD1 in mammalian cells) and its transmembrane cofactor Hrd3p (SEL1L), the putative channel component Der1p (Derlin1), the luminal lectins Yos9p (OS9 and XTP3-B) and Htm1p (EDEMs). SEL1L binds substrate proteins via its luminal domain, possibly favoring their inspection by OS9, EDEM and other molecules capable of discriminating between native and non-native species. Proteins with defects in their transmembrane regions utilize instead the ERAD-M pathway. In yeast, ERAD-M substrates require Cdc48p, Hrd1p and Der1p, but not Yos9 and Hrd3p. Finally, ERAD-C takes care of transmembrane proteins with misfolded cytosolic domains. These substrates appear to be handled in a way similar to ERAD-M substrates, but in yeast they use the E3 ubiquitin ligase Doa10p instead of Hrd1p.
In both yeast and mammalian cells, certain ERAD substrates are stabilized when ER–Golgi transport is blocked, while others are disposed of efficiently. An Hrd1p-independent pathway (HIP) has been described in yeast that implies substrate transport through the Golgi (Haynes et al, 2002). In mammalian cells, Golgi α1,2-mannosidases were shown to accelerate the degradation of a terminally misfolded α1-antitrypsin variant primarily localized in the ER, suggesting that mannose trimming in distal compartments contributes to ERAD (Hosokawa et al, 2007). Altogether, these findings underscore the existence of sequential checkpoints along the early secretory compartments, finalized to optimize protein folding and QC.
Autophagy
In addition to ERAD, cells can dispose of aberrant proteins accumulating in the ER by autophagy. In yeast, autophagy was shown to remodel the ER after its expansion during the unfolded protein response (UPR) (ER-phagy; Bernales et al, 2007). Additional links are emerging between the two pathways. On the one hand, blocking ERAD stimulates autophagy via signalling pathways that involve elements of the UPR- and Ire1-dependent JNK phosphorylation (Yorimitsu et al, 2006; Hosokawa et al, 2007; Kouroku et al, 2007; Yorimitsu and Klionsky, 2007). On the other, since EDEM is constitutively degraded by autophagic pathways (M Molinari, personal communication), inhibition of the latter stimulates glycoprotein dislocation and degradation. An intriguing possibility is that EDEM, which concentrates in defined ER subregions (Zuber et al, 2007), targets some glycoproteins to autophagic degradation. Also ER Man I is rapidly degraded by non-proteasomal pathways in hepatoma cells (Wu et al, 2007b). It will be of interest to identify additional short-lived ER-resident proteins, as they could include molecules that integrate distinct proteolytic pathways and hence, ER homeostasis.
Adapting the factory to new demands: ER stress and signalling
The accumulation of misfolded proteins in the ER lumen elicits a multidimensional signalling cascade finalized to relieve ER stress. The UPR activates several mechanisms to handle the increase of unfolded proteins (Ma and Hendershot, 2004; Bernales et al, 2006; Ron and Walter, 2007): (i) decreased protein translation, (ii) increased transcription of genes enhancing protein folding (ER-resident chaperones and folding enzymes) and ERAD, (iii) decreased entry of proteins into the ER (Kang et al, 2006; Orsi et al, 2006) and (iv) selective degradation of certain mRNAs encoding secretory proteins (Hollien and Weissman, 2006). If these measures are not sufficient for eliminating misfolded proteins from the ER, apoptotic pathways are activated (reviewed in Szegezdi et al, 2006). The UPR serves a key role also during the development of professional secretory cells. A challenging problem, with important medical implications, is what turns an adaptive response (finalized to increase the ER folding capacity, as in the case in professional secretory cells) into a mal-adaptive response that causes cell death (Lin et al, 2007; Rutkowski and Kaufman, 2007; Wu et al, 2007a). On the one hand, the latter can be viewed as an organismal defence mechanism against cells producing potentially harmful polypeptides. On the other, it is increasingly clear that apoptosis caused by chronic ER stress underlies many diseases, such as diabetes, and that preventing apoptotic pathways retards disease progression (Ozcan et al, 2004, 2006).
QC and disease
Disturbances in the QC mechanisms are the cause of many diseases (Supplementary Table 1S; Otsu and Sitia, 2007). Diseases can arise because of mutations in cargo proteins as well as in folding, transport or signalling molecules. Clearly, different therapeutic strategies have to be envisaged in each of the above classes (Wiseman et al, 2007).
The most frequent mutation in cystic fibrosis mutations does not preclude chloride transport by CFTR: it is its absence from the plasma membrane that causes a loss of function. In such cases, therapy should aim at weakening QC. Instead, gain of function often arises from defective degradation. Serpinopathies are ER storage disorders in which transport-incompetent mutants form large polymers in the ER (Lomas, 2005). Not only the intrinsic tendency of a protein to polymerize, but also the interactions it establishes in the secretory pathway determine the extent and site of condensation (Mattioli et al, 2006). Accumulation in post-ER compartments has been described for mutant pro-insulin in the Akita diabetes model (Zuber et al, 2004; Fan et al, 2007) and vasopressin V2 receptor in nephrogenic diabetes insipidus (Oueslati et al, 2007), in line with the existence of sequential checkpoints. It remains to be seen whether these unusual localizations reflect accumulation in a specialized early secretory pathway subregion, the so-called ‘quality control compartment' originally described for ASGPR H2a mutants (Kamhi-Nesher et al, 2001; Kondratyev et al, 2007).
The phenotypes of ERQC disorders generally involve tissues specialized in secretion, such as exocrine and endocrine glands, osteoblasts, plasma cells. Pancreatic β-cells are particularly sensitive, likely because the circadian oscillations in insulin biosynthesis require stringent translational control. Accordingly, the absence or insufficiency of ER stress sensors, particularly PERK and downstream elements, are responsible for many cases of diabetes (see Supplementary Table 1S). The absence of CHOP (a UPR factor involved in apoptosis) delays β-cell destruction and hyperglycemia. Type II diabetes can also ensue from environmental factors (e.g., a high-fat diet). The JNK-dependent phosphorylation of IRS1 inhibits insulin signalling, thus creating an exaggerated demand on β-cells, which in turn leads to stress and apoptosis (Ozcan et al, 2004). Confirming the role of ER stress, systemic administration of tauro-urso desoxy cholic acid (an analog of which is particularly abundant in the bile of bears, a common prescription in traditional Chinese medicine) leads to normalization of hyperglycemia, restoration of systemic insulin sensitivity and enhancement of insulin action in a murine model of type II diabetes, the ob/ob mice (Ozcan et al, 2006).
Concluding remarks
The subdivision of the early secretory pathway into distinct functional regions provides an efficient and dynamic factory capable of handling myriads of polypeptides, while maintaining stringent homeostatic control. The various elements that regulate folding and QC of the secretory proteome need to work in tight synergy and change their relative abundance (and qualitative composition) when facing a particular synthetic task. Signalling pathways are being identified that regulate the efficiency of ER folding, export and degradation so as to adapt to the changing demands of the ER protein factory during differentiation. Clearly, a better understanding of these pathways is bound to improve dramatically our capability to deal with many severe chronic diseases.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Ari Helenius, Mauri Molinari and Larry Wrabetz, and the members of our laboratory for helpful suggestions and criticism. We are grateful to AIRC, Cariplo (NoBEL project) and Telethon (grant no. GGp06155) for generous financial support.
References
- Ahluwalia N, Bergeron JJ, Wada I, Degen E, Williams DB (1992) The p88 molecular chaperone is identical to the endoplasmic reticulum membrane protein, calnexin. J Biol Chem 267: 10914–10918 [PubMed] [Google Scholar]
- Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R (2003) Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J 22: 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Alessio M, Mezghrani A, Simmen T, Talamo F, Bachi A, Sitia R (2002) ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family. EMBO J 21: 835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Ceppi S, Bergamelli L, Cortini M, Masciarelli S, Valetti C, Sitia R (2007) Sequential steps and checkpoints in the early exocytic compartment during secretory IgM biogenesis. EMBO J 26: 4177–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anfinsen CB, Scheraga HA (1975) Experimental and theoretical aspects of protein folding. Adv Protein Chem 29: 205–300 [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Roche AC, Nufer O, Hauri HP (2004) pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. J Biol Chem 279: 12943–12950 [DOI] [PubMed] [Google Scholar]
- Baines AC, Zhang B (2007) Receptor-mediated protein transport in the early secretory pathway. Trends Biochem Sci 32: 381–388 [DOI] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P (2006) Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol 22: 487–508 [DOI] [PubMed] [Google Scholar]
- Bernales S, Schuck S, Walter P (2007) ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy 3: 285–287 [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332 [DOI] [PubMed] [Google Scholar]
- Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething MJ (1993) Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75: 717–728 [DOI] [PubMed] [Google Scholar]
- Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, Mohlig M, Pfeiffer AF, Spranger J (2005) Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes 54: 2712–2719 [DOI] [PubMed] [Google Scholar]
- Bole DG, Hendershot LM, Kearney JF (1986) Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol 102: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Cosson P, Klausner RD (1990) Colocalized transmembrane determinants for ER degradation and subunit assembly explain the intracellular fate of TCR chains. Cell 63: 503–513 [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Suzuki CK, Lippincott-Schwartz J, Weissman AM, Klausner RD (1989) Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol 109: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay F, Doms RW, Webster RG, Helenius A (1988) Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol 106: 629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschhorn BA, Kostova Z, Medicherla B, Wolf DH (2004) A genome-wide screen identifies Yos9p as essential for ER-associated degradation of glycoproteins. FEBS Lett 577: 422–426 [DOI] [PubMed] [Google Scholar]
- Cabibbo A, Pagani M, Fabbri M, Rocchi M, Farmery MR, Bulleid NJ, Sitia R (2000) ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J Biol Chem 275: 4827–4833 [DOI] [PubMed] [Google Scholar]
- Carvalho P, Goder V, Rapoport TA (2006) Distinct ubiquitin–ligase complexes define convergent pathways for the degradation of ER proteins. Cell 126: 361–373 [DOI] [PubMed] [Google Scholar]
- Chandran M, Phillips SA, Ciaraldi T, Henry RR (2003) Adiponectin: more than just another fat cell hormone? Diabetes Care 26: 2442–2450 [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Yin Y, Gao G, Li S, Jiang Y, Gu X, Luo J (2005) SPD—a web-based secreted protein database. Nucleic Acids Res 33: D169–D173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillaron J, Haas IG (2000) Dissociation from BiP and retrotranslocation of unassembled immunoglobulin light chains are tightly coupled to proteasome activity. Mol Biol Cell 11: 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons WM Jr, Menetret JF, Akey CW, Rapoport TA (2004) Structural insight into the protein translocation channel. Curr Opin Struct Biol 14: 390–396 [DOI] [PubMed] [Google Scholar]
- Cunnea PM, Miranda-Vizuete A, Bertoli G, Simmen T, Damdimopoulos AE, Hermann S, Leinonen S, Huikko MP, Gustafsson JA, Sitia R, Spyrou G (2003) ERdj5, an endoplasmic reticulum (ER)-resident protein containing DnaJ and thioredoxin domains, is expressed in secretory cells or following ER stress. J Biol Chem 278: 1059–1066 [DOI] [PubMed] [Google Scholar]
- Denic V, Quan EM, Weissman JS (2006) A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126: 349–359 [DOI] [PubMed] [Google Scholar]
- Denzel A, Molinari M, Trigueros C, Martin JE, Velmurgan S, Brown S, Stamp G, Owen MJ (2002) Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol Cell Biol 22: 7398–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desilva MG, Lu J, Donadel G, Modi WS, Xie H, Notkins AL, Lan MS (1996) Characterization and chromosomal localization of a new protein disulfide isomerase, PDIp, highly expressed in human pancreas. DNA Cell Biol 15: 9–16 [DOI] [PubMed] [Google Scholar]
- Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15: 3–16 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Riek R, Herrmann T, Guntert P, Braun D, Helenius A, Wuthrich K (2001) NMR structure of the calreticulin P-domain. Proc Natl Acad Sci USA 98: 3133–3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Ruddock LW (2005) The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 6: 28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagioli C, Mezghrani A, Sitia R (2001) Reduction of interchain disulfide bonds precedes the dislocation of Ig-mu chains from the endoplasmic reticulum to the cytosol for proteasomal degradation. J Biol Chem 276: 40962–40967 [DOI] [PubMed] [Google Scholar]
- Fan JY, Roth J, Zuber C (2007) Expression of mutant Ins2(C96Y) results in enhanced tubule formation causing enlargement of pre-Golgi intermediates of CHO cells. Histochem Cell Biol 128: 161–173 [DOI] [PubMed] [Google Scholar]
- Fiedler K, Parton RG, Kellner R, Etzold T, Simons K (1994) VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J 13: 1729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn GC, Pohl J, Flocco MT, Rothman JE (1991) Peptide-binding specificity of the molecular chaperone BiP. Nature 353: 726–730 [DOI] [PubMed] [Google Scholar]
- Forster ML, Sivick K, Park YN, Arvan P, Lencer WI, Tsai B (2006a) Protein disulfide isomerase-like proteins play opposing roles during retrotranslocation. J Cell Biol 173: 853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, Pepperkok R (2006b) Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol 16: 173–179 [DOI] [PubMed] [Google Scholar]
- Fra AM, Fagioli C, Finazzi D, Sitia R, Alberini CM (1993) Quality control of ER synthesized proteins: an exposed thiol group as a three-way switch mediating assembly, retention and degradation. EMBO J 12: 4755–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frand AR, Kaiser CA (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1: 161–170 [DOI] [PubMed] [Google Scholar]
- Frickel EM, Riek R, Jelesarov I, Helenius A, Wuthrich K, Ellgaard L (2002) TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci USA 99: 1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullekrug J, Boehm J, Rottger S, Nilsson T, Mieskes G, Schmitt HD (1997) Human Rer1 is localized to the Golgi apparatus and complements the deletion of the homologous Rer1 protein of Saccharomyces cerevisiae. Eur J Cell Biol 74: 31–40 [PubMed] [Google Scholar]
- Fullekrug J, Scheiffele P, Simons K (1999) VIP36 localisation to the early secretory pathway. J Cell Sci 112 (Part 17): 2813–2821 [DOI] [PubMed] [Google Scholar]
- Garbi N, Tanaka S, Momburg F, Hammerling GJ (2006) Impaired assembly of the major histocompatibility complex class I peptide–loading complex in mice deficient in the oxidoreductase ERp57. Nat Immunol 7: 93–102 [DOI] [PubMed] [Google Scholar]
- Gething MJ (1999) Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 10: 465–472 [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J (1989) Protein folding and intracellular transport: studies on influenza virus haemagglutinin. Biochem Soc Symp 55: 155–166 [PubMed] [Google Scholar]
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ (2006) Quantitative proteomics analysis of the secretory pathway. Cell 127: 1265–1281 [DOI] [PubMed] [Google Scholar]
- Gillece P, Luz JM, Lennarz WJ, de La Cruz FJ, Romisch K (1999) Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol 147: 1443–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece P, Pilon M, Romisch K (2000) The protein translocation channel mediates glycopeptide export across the endoplasmic reticulum membrane. Proc Natl Acad Sci USA 97: 4609–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E, Sevier CS, Heldman N, Vitu E, Bentzur M, Kaiser CA, Thorpe C, Fass D (2006) Generating disulfides enzymatically: reaction products and electron acceptors of the endoplasmic reticulum thiol oxidase Ero1p. Proc Natl Acad Sci USA 103: 299–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi S, Fra AM, Sparvoli A, Bet P, Rocco M, Sitia R (1994) The efficiency of cysteine-mediated intracellular retention determines the differential fate of secretory IgA and IgM in B and plasma cells. Eur J Immunol 24: 2477–2482 [DOI] [PubMed] [Google Scholar]
- Gurkan C, Stagg SM, Lapointe P, Balch WE (2006) The COPII cage: unifying principles of vesicle coat assembly. Nat Rev 7: 727–738 [DOI] [PubMed] [Google Scholar]
- Haas IG, Wabl M (1983) Immunoglobulin heavy chain binding protein. Nature 306: 387–389 [DOI] [PubMed] [Google Scholar]
- Hauri HP, Nufer O, Breuza L, Tekaya HB, Liang L (2002) Lectins and protein traffic early in the secretory pathway. Biochem Soc Symp 69: 73–82 [DOI] [PubMed] [Google Scholar]
- Haynes CM, Caldwell S, Cooper AA (2002) An HRD/DER-independent ER quality control mechanism involves Rsp5p-dependent ubiquitination and ER–Golgi transport. J Cell Biol 158: 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science (New York, NY) 291: 2364–2369 [DOI] [PubMed] [Google Scholar]
- Hendershot L, Sitia R (2005) Immunoglobulin assembly and secretion. In Molecular Biology of B Cells, Honjo T, Alt FW, Neuberger MS (eds), pp 261–273. Amsterdam, NL: Elsevier Acad Press [Google Scholar]
- Hendershot LM, Kearney JF (1988) A role for human heavy chain binding protein in the developmental regulation of immunoglobin transport. Mol Immunol 25: 585–595 [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98 [DOI] [PubMed] [Google Scholar]
- Hirao K, Natsuka Y, Tamura T, Wada I, Morito D, Natsuka S, Romero P, Sleno B, Tremblay LO, Herscovics A, Nagata K, Hosokawa N (2006) EDEM3, a soluble EDEM homolog, enhances glycoprotein endoplasmic reticulum-associated degradation and mannose trimming. J Biol Chem 281: 9650–9658 [DOI] [PubMed] [Google Scholar]
- Hirsch C, Blom D, Ploegh HL (2003) A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J 22: 1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C, Misaghi S, Blom D, Pacold ME, Ploegh HL (2004) Yeast N-glycanase distinguishes between native and non-native glycoproteins. EMBO Rep 5: 201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science (New York, NY) 313: 104–107 [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Wada I, Natsuka Y, Nagata K (2006) EDEM accelerates ERAD by preventing aberrant dimer formation of misfolded alpha1-antitrypsin. Genes Cells 11: 465–476 [DOI] [PubMed] [Google Scholar]
- Hosokawa N, You Z, Tremblay LO, Nagata K, Herscovics A (2007) Stimulation of ERAD of misfolded null Hong Kong alpha1-antitrypsin by Golgi alpha1,2-mannosidases. Biochem Biophys Res Commun 362: 626–632 [DOI] [PubMed] [Google Scholar]
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T et al. (2000) Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599 [DOI] [PubMed] [Google Scholar]
- Hurtley SM, Bole DG, Hoover-Litty H, Helenius A, Copeland CS (1989) Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol 108: 2117–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A (1989) Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol 5: 277–307 [DOI] [PubMed] [Google Scholar]
- Hwang C, Sinskey AJ, Lodish HF (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science (New York, NY) 257: 1496–1502 [DOI] [PubMed] [Google Scholar]
- Jansens A, van Duijn E, Braakman I (2002) Coordinated nonvectorial folding in a newly synthesized multidomain protein. Science (New York, NY) 298: 2401–2403 [DOI] [PubMed] [Google Scholar]
- Jarosch E, Geiss-Friedlander R, Meusser B, Walter J, Sommer T (2002) Protein dislocation from the endoplasmic reticulum—pulling out the suspect. Traffic (Copenhagen, Denmark) 3: 530–536 [DOI] [PubMed] [Google Scholar]
- Jolliffe NA, Craddock CP, Frigerio L (2005) Pathways for protein transport to seed storage vacuoles. Biochem Soc Trans 33: 1016–1018 [DOI] [PubMed] [Google Scholar]
- Kalies KU, Allan S, Sergeyenko T, Kroger H, Romisch K (2005) The protein translocation channel binds proteasomes to the endoplasmic reticulum membrane. EMBO J 24: 2284–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ (2001) A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell 12: 1711–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Kamiya D, Yamamoto K, Nyfeler B, Hauri HP, Kato K (2007) Molecular basis of sugar recognition by the human L-type lectins ERGIC-53, VIPL and VIP36. J Biol Chem (in press) [DOI] [PubMed] [Google Scholar]
- Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS (2006) Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127: 999–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalkhall Z, Marshall RD (1975) Glycosylation of ribonuclease A catalysed by rabbit liver extracts. Biochem J 146: 299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD (1989) Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol 1: 3–8 [PubMed] [Google Scholar]
- Klausner RD, Sitia R (1990) Protein degradation in the endoplasmic reticulum. Cell 62: 611–614 [DOI] [PubMed] [Google Scholar]
- Kondratyev M, Avezov E, Shenkman M, Groisman B, Lederkremer GZ (2007) PERK-dependent compartmentalization of ERAD and unfolded protein response machineries during ER stress. Exp Cell Res 313: 3395–3407 [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14: 230–239 [DOI] [PubMed] [Google Scholar]
- Kreis TE, Lodish HF (1986) Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell 46: 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczynska B, Evangelista CM, Samant SS, Elguindi EC, Blond SY (2004) The SANT2 domain of the murine tumor cell DnaJ-like protein 1 human homologue interacts with alpha1-antichymotrypsin and kinetically interferes with its serpin inhibitory activity. J Biol Chem 279: 11432–11443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT (2006) Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55: 249–259 [PubMed] [Google Scholar]
- Larkins BA, Lending CR, Wallace JC (1993) Modification of maize-seed-protein quality. Am J Clin Nutr 58: 264S–269S [DOI] [PubMed] [Google Scholar]
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bidirectional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20: 87–123 [DOI] [PubMed] [Google Scholar]
- Lilley BN, Ploegh HL (2005) Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci USA 102: 14296–14301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science (New York, NY) 318: 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomas DA (2005) Molecular mousetraps, alpha1-antitrypsin deficiency and the serpinopathies. Clin Med 5: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM (2004) ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28: 51–65 [DOI] [PubMed] [Google Scholar]
- Mancini R, Fagioli C, Fra AM, Maggioni C, Sitia R (2000) Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J 14: 769–778 [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez JA, Geuze HJ, Slot JW, Klumperman J (1999) Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell 98: 81–90 [DOI] [PubMed] [Google Scholar]
- Mast SW, Diekman K, Karaveg K, Davis A, Sifers RN, Moremen KW (2005) Human EDEM2, a novel homolog of family 47 glycosidases, is involved in ER-associated degradation of glycoproteins. Glycobiology 15: 421–436 [DOI] [PubMed] [Google Scholar]
- Mattioli L, Anelli T, Fagioli C, Tacchetti C, Sitia R, Valetti C (2006) ER storage diseases: a role for ERGIC-53 in controlling the formation and shape of Russell bodies. J Cell Sci 119: 2532–2541 [DOI] [PubMed] [Google Scholar]
- Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause KH, Opas M, MacLennan DH, Michalak M (1999) Calreticulin is essential for cardiac development. J Cell Biol 144: 857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Usherwood YK, Chung KT, Hendershot LM (2002) A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol Biol Cell 13: 4456–4469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani A, Fassio A, Benham A, Simmen T, Braakman I, Sitia R (2001) Manipulation of oxidative protein folding and PDI redox state in mammalian cells. EMBO J 20: 6288–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacasa A, Helenius A (2002) The transitional ER defines a boundary for quality control in the secretion of tsO45 VSV glycoprotein. Traffic (Copenhagen, Denmark) 3: 833–849 [DOI] [PubMed] [Google Scholar]
- Michalek MT, Grant EP, Gramm C, Goldberg AL, Rock KL (1993) A role for the ubiquitin-dependent proteolytic pathway in MHC class I-restricted antigen presentation. Nature 363: 552–554 [DOI] [PubMed] [Google Scholar]
- Molinari M, Calanca V, Galli C, Lucca P, Paganetti P (2003) Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science (New York, NY) 299: 1397–1400 [DOI] [PubMed] [Google Scholar]
- Molinari M, Galli C, Piccaluga V, Pieren M, Paganetti P (2002) Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol 158: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M, Helenius A (2000) Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science (New York, NY) 288: 331–333 [DOI] [PubMed] [Google Scholar]
- Molteni SN, Fassio A, Ciriolo MR, Filomeni G, Pasqualetto E, Fagioli C, Sitia R (2004) Glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum. J Biol Chem 279: 32667–32673 [DOI] [PubMed] [Google Scholar]
- Mueller B, Lilley BN, Ploegh HL (2006) SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER. J Cell Biol 175: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HR (1987) A C-terminal signal prevents secretion of luminal ER proteins. Cell 48: 899–907 [DOI] [PubMed] [Google Scholar]
- Neerman-Arbez M, Johnson KM, Morris MA, McVey JH, Peyvandi F, Nichols WC, Ginsburg D, Rossier C, Antonarakis SE, Tuddenham EG (1999) Molecular analysis of the ERGIC-53 gene in 35 families with combined factor V-factor VIII deficiency. Blood 93: 2253–2260 [PubMed] [Google Scholar]
- Neuber O, Jarosch E, Volkwein C, Walter J, Sommer T (2005) Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat Cell Biol 7: 993–998 [DOI] [PubMed] [Google Scholar]
- Neve EP, Svensson K, Fuxe J, Pettersson RF (2003) VIPL, a VIP36-like membrane protein with a putative function in the export of glycoproteins from the endoplasmic reticulum. Exp Cell Res 288: 70–83 [DOI] [PubMed] [Google Scholar]
- Ng W, Sergeyenko T, Zeng N, Brown JD, Romisch K (2007) Characterization of the proteasome interaction with the Sec61 channel in the endoplasmic reticulum. J Cell Sci 120: 682–691 [DOI] [PubMed] [Google Scholar]
- Nichols WC, Seligsohn U, Zivelin A, Terry VH, Hertel CE, Wheatley MA, Moussalli MJ, Hauri HP, Ciavarella N, Kaufman RJ, Ginsburg D (1998) Mutations in the ER–Golgi intermediate compartment protein ERGIC-53 cause combined deficiency of coagulation factors V and VIII. Cell 93: 61–70 [DOI] [PubMed] [Google Scholar]
- Nishikawa SI, Fewell SW, Kato Y, Brodsky JL, Endo T (2001) Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J Cell Biol 153: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Hosokawa N, Wada I, Nagata K (2003) EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science (New York, NY) 299: 1394–1397 [DOI] [PubMed] [Google Scholar]
- Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K (2006) Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 172: 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari S, Galli C, Alanen H, Ruddock L, Molinari M (2005) A novel stress-induced EDEM variant regulating endoplasmic reticulum-associated glycoprotein degradation. J Biol Chem 280: 2424–2428 [DOI] [PubMed] [Google Scholar]
- Oprins A, Rabouille C, Posthuma G, Klumperman J, Geuze HJ, Slot JW (2001) The ER to Golgi interface is the major concentration site of secretory proteins in the exocrine pancreatic cell. Traffic (Copenhagen, Denmark) 2: 831–838 [DOI] [PubMed] [Google Scholar]
- Orsi A, Fioriti L, Chiesa R, Sitia R (2006) Conditions of endoplasmic reticulum stress favor the accumulation of cytosolic prion protein. J Biol Chem 281: 30431–30438 [DOI] [PubMed] [Google Scholar]
- Otsu M, Bertoli G, Fagioli C, Guerini-Rocco E, Nerini-Molteni S, Ruffato E, Sitia R (2006) Dynamic retention of Ero1alpha and Ero1beta in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid Redox Signal 8: 274–282 [DOI] [PubMed] [Google Scholar]
- Otsu M, Sitia R (2007) Diseases originating from altered protein quality control in the endoplasmic reticulum. Curr Med Chem 14: 1639–1652 [DOI] [PubMed] [Google Scholar]
- Oueslati M, Hermosilla R, Schonenberger E, Oorschot V, Beyermann M, Wiesner B, Schmidt A, Klumperman J, Rosenthal W, Schulein R (2007) Rescue of a nephrogenic diabetes insipidus-causing vasopressin V2 receptor mutant by cell-penetrating peptides. J Biol Chem 282: 20676–20685 [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science (New York, NY) 306: 457–461 [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Fabbri M, Benedetti C, Fassio A, Pilati S, Bulleid NJ, Cabibbo A, Sitia R (2000) Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J Biol Chem 275: 23685–23692 [DOI] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, Baxi S, Mudaliar SR, Henry RR (2003) Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52: 667–674 [DOI] [PubMed] [Google Scholar]
- Pollard MG, Travers KJ, Weissman JS (1998) Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol Cell 1: 171–182 [DOI] [PubMed] [Google Scholar]
- Qiang L, Wang H, Farmer SR (2007) Adiponectin secretion is regulated by SIRT1 and the ER oxidoreductase Ero1-L{alpha}. Mol Cell Biol 27: 4698–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S (2002) AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol 22: 626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Sparvoli A, Fagioli C, Fassina G, Sitia R (1996) Formation of reversible disulfide bonds with the protein matrix of the endoplasmic reticulum correlates with the retention of unassembled Ig light chains. EMBO J 15: 2077–2085 [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Russell SJ, Ruddock LW, Salo KE, Oliver JD, Roebuck QP, Llewellyn DH, Roderick HL, Koivunen P, Myllyharju J, High S (2004) The primary substrate binding site in the b′ domain of ERp57 is adapted for endoplasmic reticulum lectin association. J Biol Chem 279: 18861–18869 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ (2007) That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci 32: 469–476 [DOI] [PubMed] [Google Scholar]
- Sancho J, Chatila T, Wong RC, Hall C, Blumberg R, Alarcon B, Geha RS, Terhorst C (1989) T-cell antigen receptor (TCR)-alpha/beta heterodimer formation is a prerequisite for association of CD3-zeta 2 into functionally competent TCR.CD3 complexes. J Biol Chem 264: 20760–20769 [PubMed] [Google Scholar]
- Sato K, Nishikawa S, Nakano A (1995) Membrane protein retrieval from the Golgi apparatus to the endoplasmic reticulum (ER): characterization of the RER1 gene product as a component involved in ER localization of Sec12p. Mol Biol Cell 6: 1459–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A (1997) Rer1p as common machinery for the endoplasmic reticulum localization of membrane proteins. Proc Natl Acad Sci USA 94: 9693–9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Sato M, Nakano A (2001) Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J Cell Biol 152: 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A (1996) Endoplasmic reticulum localization of Sec12p is achieved by two mechanisms: Rer1p-dependent retrieval that requires the transmembrane domain and Rer1p-independent retention that involves the cytoplasmic domain. J Cell Biol 134: 279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A (2004) Endoplasmic reticulum quality control of unassembled iron transporter depends on Rer1p-mediated retrieval from the Golgi. Mol Biol Cell 15: 1417–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270: 26746–26749 [DOI] [PubMed] [Google Scholar]
- Schindler R, Itin C, Zerial M, Lottspeich F, Hauri HP (1993) ERGIC-53, a membrane protein of the ER–Golgi intermediate compartment, carries an ER retention motif. Eur J Cell Biol 61: 1–9 [PubMed] [Google Scholar]
- Schrag JD, Bergeron JJ, Li Y, Borisova S, Hahn M, Thomas DY, Cygler M (2001) The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell 8: 633–644 [DOI] [PubMed] [Google Scholar]
- Shen Y, Hendershot LM (2005) ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP's interactions with unfolded substrates. Mol Biol Cell 16: 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Meunier L, Hendershot LM (2002) Identification and characterization of a novel endoplasmic reticulum (ER) DnaJ homologue, which stimulates ATPase activity of BiP in vitro and is induced by ER stress. J Biol Chem 277: 15947–15956 [DOI] [PubMed] [Google Scholar]
- Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A (2004) Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 27: 2450–2457 [DOI] [PubMed] [Google Scholar]
- Sitia R, Braakman I (2003) Quality control in the endoplasmic reticulum protein factory. Nature 426: 891–894 [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger M, Alberini C, Bet P, Fra A, Valetti C, Williams G, Milstein C (1990) Developmental regulation of IgM secretion: the role of the carboxy-terminal cysteine. Cell 60: 781–790 [DOI] [PubMed] [Google Scholar]
- Sitia R, Neuberger MS, Milstein C (1987) Regulation of membrane IgM expression in secretory B cells: translational and post-translational events. EMBO J 6: 3969–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic D, Raemaekers T, Dillen K, Declerck I, Baert V, Serneels L, Fullekrug J, Annaert W (2007) Rer1p competes with APH-1 for binding to nicastrin and regulates gamma-secretase complex assembly in the early secretory pathway. J Cell Biol 176: 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Ferguson AD, Bergeron JJ, Thomas DY (2004) The ER protein folding sensor UDP-glucose glycoprotein-glucosyltransferase modifies substrates distant to local changes in glycoprotein conformation. Nat Struct Mol Biol 11: 128–134 [DOI] [PubMed] [Google Scholar]
- Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M (2004) Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes 53: 1621–1629 [DOI] [PubMed] [Google Scholar]
- Tortorella D, Story CM, Huppa JB, Wiertz EJ, Jones TR, Bacik I, Bennink JR, Yewdell JW, Ploegh HL (1998) Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol 142: 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Rapoport TA (2002) Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J Cell Biol 159: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B, Rodighiero C, Lencer WI, Rapoport TA (2001) Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104: 937–948 [DOI] [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA (2002) Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev 3: 246–255 [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS (2002) The FAD- and O(2)-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol Cell 10: 983–994 [DOI] [PubMed] [Google Scholar]
- van Lith M, Hartigan N, Hatch J, Benham AM (2005) PDILT, a divergent testis-specific protein disulfide isomerase with a non-classical SXXC motif that engages in disulfide-dependent interactions in the endoplasmic reticulum. J Biol Chem 280: 1376–1383 [DOI] [PubMed] [Google Scholar]
- van Lith M, Karala AR, Bown D, Gatehouse JA, Ruddock LW, Saunders PT, Benham AM (2007) A developmentally regulated chaperone complex for the endoplasmic reticulum of male haploid germ cells. Mol Biol Cell 18: 2795–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist S, Ng DT (2004) Misfolded proteins are sorted by a sequential checkpoint mechanism of ER quality control. J Cell Biol 165: 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Carbajosa J, Carbonero P (2005) Seed maturation: developing an intrusive phase to accomplish a quiescent state. Int J Dev Biol 49: 645–651 [DOI] [PubMed] [Google Scholar]
- Waisman DM, Salimath BP, Anderson MJ (1985) Isolation and characterization of CAB-63, a novel calcium-binding protein. J Biol Chem 260: 1652–1660 [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE (2007) Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol 27: 3716–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CL, Omura S, Kopito RR (1995) Degradation of CFTR by the ubiquitin–proteasome pathway. Cell 83: 121–127 [DOI] [PubMed] [Google Scholar]
- Wieland FT, Gleason ML, Serafini TA, Rothman JE (1987) The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50: 289–300 [DOI] [PubMed] [Google Scholar]
- Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL (1996) Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384: 432–438 [DOI] [PubMed] [Google Scholar]
- Wilkinson B, Gilbert HF (2004) Protein disulfide isomerase. Biochim Biophys Acta 1699: 35–44 [DOI] [PubMed] [Google Scholar]
- Williams DB (2006) Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci 119: 615–623 [DOI] [PubMed] [Google Scholar]
- Wiseman RL, Powers ET, Buxbaum JN, Kelly JW, Balch WE (2007) An adaptable standard for protein export from the endoplasmic reticulum. Cell 131: 809–821 [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ (2007a) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13: 351–364 [DOI] [PubMed] [Google Scholar]
- Wu Y, Termine DJ, Swulius MT, Moremen KW, Sifers RN (2007b) Human endoplasmic reticulum mannosidase I is subject to regulated proteolysis. J Biol Chem 282: 4841–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA (2001) The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414: 652–656 [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA (2004) A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429: 841–847 [DOI] [PubMed] [Google Scholar]
- Yerushalmi N, Keppler-Hafkemeyer A, Vasmatzis G, Liu XF, Olsson P, Bera TK, Duray P, Lee B, Pastan I (2001) ERGL, a novel gene related to ERGIC-53 that is highly expressed in normal and neoplastic prostate and several other tissues. Gene 265: 55–60 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ (2007) Endoplasmic reticulum stress: a new pathway to induce autophagy. Autophagy 3: 160–162 [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281: 30299–30304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H (2007) ER stress and diseases. FEBS J 274: 630–658 [DOI] [PubMed] [Google Scholar]
- Zhang B, Cunningham MA, Nichols WC, Bernat JA, Seligsohn U, Pipe SW, McVey JH, Schulte-Overberg U, de Bosch NB, Ruiz-Saez A, White GC, Tuddenham EG, Kaufman RJ, Ginsburg D (2003) Bleeding due to disruption of a cargo-specific ER-to-Golgi transport complex. Nat Genet 34: 220–225 [DOI] [PubMed] [Google Scholar]
- Zhang B, Kaufman RJ, Ginsburg D (2005) LMAN1 and MCFD2 form a cargo receptor complex and interact with coagulation factor VIII in the early secretory pathway. J Biol Chem 280: 25881–25886 [DOI] [PubMed] [Google Scholar]
- Zuber C, Cormier JH, Guhl B, Santimaria R, Hebert DN, Roth J (2007) EDEM1 reveals a quality control vesicular transport pathway out of the endoplasmic reticulum not involving the COPII exit sites. Proc Natl Acad Sci USA 104: 4407–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber C, Fan JY, Guhl B, Roth J (2004) Misfolded proinsulin accumulates in expanded pre-Golgi intermediates and endoplasmic reticulum subdomains in pancreatic beta cells of Akita mice. FASEB J 18: 917–919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information