Abstract
The etiologies of neurodegenerative diseases may be diverse; however, a common pathological denominator is the formation of aberrant protein conformers and the occurrence of pathognomonic proteinaceous deposits. Different approaches coming from neuropathology, genetics, animal modeling and biophysics have established a crucial role of protein misfolding in the pathogenic process. However, there is an ongoing debate about the nature of the harmful proteinaceous species and how toxic conformers selectively damage neuronal populations. Increasing evidence indicates that soluble oligomers are associated with early pathological alterations, and strikingly, oligomeric assemblies of different disease-associated proteins may share common structural features. A major step towards the understanding of mechanisms implicated in neuronal degeneration is the identification of genes, which are responsible for familial variants of neurodegenerative diseases. Studies based on these disease-associated genes illuminated the two faces of protein misfolding in neurodegeneration: a gain of toxic function and a loss of physiological function, which can even occur in combination. Here, we summarize how these two faces of protein misfolding contribute to the pathomechanisms of Alzheimer's disease, frontotemporal lobar degeneration, Parkinson's disease and prion diseases.
Keywords: Alzheimer, FTLD, misfolding, Parkinson, prion
Oligomeric protein assemblies in a deadly cascade
The most frequent neurodegenerative disorder is Alzheimer's disease (AD). Major progress has been made in this field due to the identification of the deposited amyloidogenic proteins and genetically linked mutations, which accelerate amyloid formation and disease onset (Haass and Selkoe, 2007). Two major proteins are found within the pathological hallmarks of AD. Amyloid-β peptide (Aβ) is deposited in amyloid plaques within the parenchyma and tau in neurofibrillary tangles within neurons. It is now becoming more and more clear that both proteins are required to confer neurotoxicity in a process called the amyloid cascade (Hardy and Selkoe, 2002; Figure 1A). Aβ is derived by proteolytic processing from the β-amyloid precursor protein (βAPP, see below) and exists as several species of distinct lengths (Haass, 2004). The most abundant 40 amino-acid species (Aβ40) is rather benign, whereas the less abundant 42 amino-acid variant (Aβ42) aggregates much faster and is therefore directly related to disease pathology (for a review, see Haass and Selkoe, 2007). However, it is currently unclear how the addition of the two amino acids at the C-terminus of Aβ changes the biophysical properties of the peptide in a way that it aggregates faster. The amyloid cascade is probably initiated by subtle changes in the Aβ42/Aβ40 ratio, a total increase of Aβ generation, or reduced clearance of Aβ (Figure 1A). There is strong genetic evidence that specifically the Aβ42/Aβ40 ratio is affected by mutations in one of the Aβ-generating enzymes (see below) and the βAPP substrate itself (Haass, 2004). In addition, environmental factors and specifically genetic predisposition such as the ApoE status can influence disease onset (Figure 1A; Martins et al, 2006). All Aβ species are secreted from healthy neurons throughout life, but specifically the longer Aβ42 species tends to form soluble oligomers (Haass and Selkoe, 2007). These in vivo generated oligomeric assemblies can be as small as dimers or trimers (Podlisny et al, 1995) or as large as dodecamers (Lesne et al, 2006). Probably, long before these oligomers are deposited in disease-characterizing pathological plaques, they inhibit the maintenance of long-term potentiation (Walsh et al, 2002). This by itself may lead to mild cognitive impairment (MCI) at early stages of AD (Haass and Selkoe, 2007). One should keep in mind that brains of MCI patients may already contain some deposits, so it may be difficult to conclude that exclusively soluble Aβ oligomers are involved in early memory loss. However, the effects of Aβ oligomers on long-term potentiation were observed in rats lacking any amyloid deposits, making it likely that such soluble oligomers could indeed be responsible for the very first symptoms of memory loss in human patients. Inflammatory responses involving microglia and astrocytes follow the deposition of Aβ and may enhance progressive synaptic and neuronal injury. As a result, ion homeostasis may be affected and oxidative stress occurs. Strikingly, these events then directly can affect tau metabolism (Blurton-Jones and Laferla, 2006). Tau, a microtubule-binding protein, is required for microtubule stabilization (Mandelkow et al, 2007). Misphosphorylation of tau, which is a pathological signature of all AD cases, reduces binding of tau to microtubules, which then detaches. Unbound tau is apparently misfolded and begins to aggregate due to its enhanced free concentration in the cytoplasm. Finally, hyperphosphorylated tau forms the paired helical filaments found within tangles (Mandelkow and Mandelkow, 1998; Ballatore et al, 2007). A strong support of a direct induction of tau pathology by Aβ came from the observation that pathological phosphorylation of tau is induced by the intracerebral injection of Aβ42 fibrils (Gotz et al, 2001). Moreover, double transgenic mice expressing a tau mutation, together with mutant APP, showed enhanced tau pathology (Lewis et al, 2001). Strikingly, Oddo et al (2003) demonstrated that Aβ accumulation precedes tau pathology by several months in transgenic mouse models of AD. Consistent with these findings, a reduction of Aβ by an anti-Aβ vaccination strategy (Haass and Selkoe, 2007) in brains of transgenic mice reduces tau pathology. Importantly, under these conditions, early tau pathology is selectively reduced, whereas late tau pathology is apparently not affected (Oddo et al, 2004). Recent evidence further supported the connection between Aβ and tau within the amyloid cascade by demonstrating that memory deficits can be prevented in a transgenic model for AD pathology upon removal of the tau gene (Roberson et al, 2007). Thus, there is culminating evidence that Aβ is at the beginning of the amyloid cascade and initiates tau mislocalization, misfolding and toxicity. Plaques may serve as reservoirs for a continuing supply of soluble oligomers of Aβ, which can diffuse and cause neuronal dysfunction and cell death even far away from amyloid deposits (Haass and Selkoe, 2007). Thus, a direct correlation of the amyloid plaque load with memory loss is not necessarily to be expected. The same may be true for tau, as oligomeric species composed of 8–14 tau molecules have been implicated in neurotoxicity (Wille et al, 1992).
Figure 1.
Pathomechanisms in AD (A), FTLD linked to chromosome 17 (B), PD (C) and prion diseases (D). (A) In AD, environmental factors, genetic predisposition and mutations in βAPP and PS can affect the metabolism of Aβ. Initially, small and soluble oligomeric assemblies of Aβ42 are produced, which then cause synaptic dysfunction as well as an induction of the amyloid cascade. Note the ‘shortcut' to tau pathology and FTLD via chromosome 17-linked tau mutations. (B) The major variants of chromosome 17-linked FTLD. On the left panel, FTLD cases with tau-positive inclusions (tauopathies) are described. On the right panel, the tau-negative, ubiquitin-positive cases are shown. (C) In sporadic PD and familial PD there are common pathophysiological alterations, such as oxidative stress, mitochondrial dysfunction and protein misfolding, which ultimately result in the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta. (D) In the classical form of prion diseases, conversion of PrPC to PrPSc leads to a neurodegenerative and infectious disorder. The conformational transition can occur spontaneously (sporadic), or can be induced by invading PrPSc (acquired) or mutations (inherited). Transgenic mouse models indicated that expression of mutant PrPs can trigger neurodegeneration in the absence of infectious prion propagation; whether such disease entities exist in animals or humans is unknown. PrPSc: self-propagating isoform, essential component of infectious prions; CtmPrP: a transmembrane form of PrP with the C-terminus facing the cytosol; cytoPrP: cytosolically localized PrP; PG14PrP: mutant PrP containing a nine octarepeat insertion; PrPΔHD: mutant PrP lacking the internal HD.
Interestingly, in the absence of amyloid toxicity aggregation of tau can result in a different neurodegenerative disorder, named frontotemporal lobar degeneration (FTLD) (Figure 1B). In other words, abnormal tau by itself can induce a ‘shortcut' within the above-described amyloid cascade (Figure 1A and B). The chromosome 17-linked FTLD cases, which are characterized by tau-positive inclusions (tangles), are caused by mutations within the tau gene (Ballatore et al, 2007; Mandelkow et al, 2007). These mutations affect tau function by several distinct mechanisms. Some mutations change the splicing pattern of tau and lead to the accumulation of the four repeat variant (Hutton et al, 1998). The change in the ratio from four-repeat to three-repeat tau is apparently directly related to its neurotoxic propensity and its ability to cause neurodegeneration, but it is currently not known how it affects neuronal viability. Other mutations within tau do not affect splicing but are missense mutations causing single-amino-acid exchanges. These seem to affect the microtubule-binding capacity of tau and/or increase its aggregation (Mandelkow et al, 2007).
Gain of neurotoxic and loss of protective function in combination?
Deposition of aggregated α-synuclein in Lewy bodies and Lewy neurites is a pathological hallmark of Parkinson's disease (PD) (Figure 1C) and some other neurodegenerative entities, collectively termed α-synucleinopathies. The physiological function of α-synuclein, which is abundantly expressed in the central nervous system, is not fully understood. Its enrichment in presynaptic terminals and its association with vesicles suggests a role of α-synuclein in synaptic dynamics. In a manner similar to tau, α-synuclein is a natively unfolded or intrinsically disordered protein with considerable conformational plasticity (for reviews, see Volles and Lansbury, 2003; Beyer, 2007; Uversky, 2007). In vitro, different α-synuclein conformers can be populated: monomers, which adopt a N-terminal α-helical structure upon membrane binding, morphologically diverse β-sheet-rich oligomers, called protofibrils, amorphous aggregates and amyloid fibrils with a characteristic cross-β structure. Three α-synuclein missense mutations as well as genomic multiplications promote the propensity of α-synuclein to aggregate and are associated with autosomal dominant PD (Polymeropoulos et al, 1997; Kruger et al, 1998; Singleton et al, 2003; Chartier-Harlin et al, 2004; Farrer et al, 2004; Ibanez et al, 2004; Zarranz et al, 2004). As in the case of Aβ, it is currently discussed that not the final aggregates, but rather oligomeric intermediates might be the toxic species (for review, see Lansbury and Lashuel, 2006; Haass and Selkoe, 2007; Uversky, 2007). Remarkably, dopamine can modify the aggregation pathway of α-synuclein, facilitating the formation of oligomeric intermediates (Conway et al, 2001; Li et al, 2004; Cappai et al, 2005; Norris et al, 2005; Mazzulli et al, 2006). In support of a role of dopamine in enhancing the toxic potential of α-synuclein, inhibition of dopamine synthesis blocked cell death induced by α-synuclein overexpression in a cell culture model (Xu et al, 2002). Various mechanisms have been proposed to explain the toxic effects of α-synuclein based on observations in different model systems (for a review, see Cookson and van der Brug, 2007), including impairment of proteasomal or lysosomal protein degradation (Stefanis et al, 2001; Tanaka et al, 2001; Petrucelli et al, 2002; Snyder et al, 2003; Cuervo et al, 2004; Lindersson et al, 2004), induction of endoplasmic reticulum (ER) stress (Smith et al, 2005; Cooper et al, 2006), Golgi fragmentation (Gosavi et al, 2002), sequestration of antiapoptotic proteins into aggregates (Xu et al, 2002) and the formation of pores on cellular membranes (Volles et al, 2001; Lashuel et al, 2002). Basic insight into early events of α-synuclein toxicity came from a recent study in Saccharomyces cerevisiae. Overexpression of wild-type or mutant α-synuclein resulted in defective ER-to-Golgi vesicular transport, and Rab1, a small GTPase identified in a screen for modifiers of α-synuclein toxicity, protected against neuronal loss in some animal models (Drosophila melanogaster, Caenorhabditis elegans) and in primary cultures of rat midbrain neurons (Cooper et al, 2006). Furthermore, Sept4, a presynaptic scaffold protein, has recently been shown to suppress α-synuclein toxicity in a transgenic mouse model (Ihara et al, 2007). Clearly, model systems to study the toxicity of α-synuclein have intrinsic limitations, and to discriminate causal events from secondary effects is a difficult task. However, it seems plausible that more than one single mechanism contributes to the complex pathogenesis of α-synucleinopathies, which proceeds over decades in patients. To add another layer of complexity, α-synuclein may also play a neuroprotective role (for a review, see Lee et al, 2006). Transgenic expression of α-synuclein has been shown to prevent neurodegeneration caused by the deletion of cysteine-string protein-α, a molecular chaperone that is crucial for folding and refolding of synaptic SNARE proteins (Chandra et al, 2005). If and how the physiological activity of α-synuclein is coupled to its pathological potential and which conformers constitute the functional or toxic species is still a challenge for future research.
A deadly and infectious variant of protein misfolding
Protein misfolding can also lead to neurotoxicity and infectivity. The concept that a conformational transition of the cellular prion protein (PrPC) to the pathological prion protein (PrPSc for scrapie PrP) implicates the formation of a neurotoxic conformer that in addition is infectious, is unprecedented (Prusiner, 1982; Figure 1D). After it had been proposed that heritable infectious proteins are responsible for mammalian prion diseases, studies in fungi indicated that self-propagating protein conformers might be of broader biological significance (Wickner, 1994). In prion disease research, numerous studies have been focused on the enigmatic composition of the infectious agent. A conclusive answer is still missing partly due to the fact that the purest infectious preparation (prion rods) still contains components in addition to PrPSc. Nucleic acids longer than 25 nucleotides can definitely be excluded as essential components for infectivity (Safar et al, 2005), but a possible role of the polysaccharide scaffold, which accounts for 5–15% of prion rods, remains to be established (Dumpitak et al, 2005). The first successful attempts to generate infectivity in vitro with recombinantly expressed PrP were reported, but with extremely low infectious titer, and PrPC-overexpressing mice were used for the bioassays (Legname et al, 2004, 2005). Even if infectivity is entirely deciphered in the conformation of PrPSc, it seems plausible that auxiliary components can significantly modulate various aspects in the pathogenesis of prion diseases (for a review, see Caughey and Baron, 2006).
At present, there is only little understanding of how PrPSc or neurotoxic PrP mutants cause neurodegeneration (for review, see Hunter, 2006). Interestingly, expression of PrPC in neuronal cells seems to be required to mediate neurotoxic effects of PrPSc (Brandner et al, 1996; Mallucci et al, 2003; Chesebro et al, 2005). Neurotoxicity of PrPSc could be linked to its propagation in neuronal cells, or PrPSc might elicit a deadly signal through a PrPC-dependent signaling pathway. Indeed, these observations might provide a link to the physiologcial function of PrPC. A stress-protective activity of PrPC was first observed in cell culture experiments with primary neurons (Kuwahara et al, 1999). It was then shown that PrPC-knockout mice display enlarged infarct volumes after ischemic brain injury and an increased sensitivity to kainate-induced seizures (Walz et al, 1999; McLennan et al, 2004; Shyu et al, 2005; Spudich et al, 2005; Weise et al, 2006; Mitteregger et al, 2007; Rangel et al, 2007). In one study, the neuroprotective activity of PrPC was linked to the octarepeat region, located within the unstructured N-terminal domain (Mitteregger et al, 2007). Based on these and other studies with established cell lines, it is plausible to propose that PrPC can modulate signaling cascades, in particular stress-protective pathways (for review, see Flechsig and Weissmann, 2004; Roucou and LeBlanc, 2005; Westergard et al, 2007). Moreover, a loss of PrPC function could be implicated in the pathogenesis of prion diseases and PrPC-dependent pathways might be involved in neurotoxic signaling. For example, in vivo crosslinking of PrPC by antibodies triggered neuronal apoptosis (Solforosi et al, 2004) and PrPC-dependent receptors were postulated to explain neurotoxic effects of a PrP mutant lacking the hydrophobic domain (HD) (see below).
Transgenic mouse models revealed that several aberrant conformers of PrP distinct from PrPSc can induce neuronal cell death in the absence of infectious prion propagation (Muramoto et al, 1997; Chiesa et al, 1998; Hegde et al, 1998; Shmerling et al, 1998; Ma et al, 2002; Flechsig et al, 2003; Baumann et al, 2007; Li et al, 2007; Figure 1D). From one class of PrP mutants it emerged that PrPC can acquire a neurotoxic potential by deleting the internal HD (Shmerling et al, 1998; Baumann et al, 2007; Li et al, 2007). PrPΔHD is complex glycosylated and linked to the plasma membrane via a GPI anchor, suggesting a similar cellular location to PrPC (Winklhofer et al, 2003b). Two different models were proposed to explain the neurotoxic activity of PrPΔHD. In one model, it was suggested that PrPΔHD blocks neurotrophic signaling via binding to a yet unidentified cell-surface receptor (Shmerling et al, 1998). In another model, PrPΔHD competes with PrPC for binding to a hypothetical signal transducing protein. In this scenario, PrPC induces neuroprotective signaling, while binding of PrPΔHD triggers a neurotoxic cascade (Li et al, 2007).
Acquisition of a neurotoxic activity is not restricted to PrP conformers present in the secretory pathway. Spontaneous neurodegeneration of transgenic mice expressing a PrP mutant without the N-terminal ER-targeting sequence indicated a toxic potential of PrP when located in the cytosolic compartment (cytoPrP) (Ma et al, 2002). Toxicity of cytoPrP seems to be dependent on its association with intracellular membranes (Wang et al, 2006) and its binding to Bcl-2, an antiapoptotic protein present at the cytosolic side of ER and mitochondrial membranes (Rambold et al, 2006). Might the toxic potential of misfolded PrP in the cytosol be relevant to the pathogenesis of prion diseases? First, some pathogenic mutants linked to inherited prion diseases in humans are partially mistargeted to the cytosol (Zanusso et al, 1999; Heske et al, 2004). Second, access to the cytosol is possible via retrograde translocation of PrP out of the ER (Ma and Lindquist, 2001; Yedidia et al, 2001). Third, most recent data revealed an impairment of the ubiquitin-proteasome system (UPS) in prion-infected mice. In conjunction with in vitro and cell culture approaches, it was proposed that prion neurotoxicity is linked to PrPSc oligomers, which translocate to the cytosol and inhibit the UPS (Kristiansen et al, 2007).
Loss of a protective function
So far we have discussed how aggregation-prone proteins, such as Aβ, tau, PrP and α-synuclein can cause neurodegeneration by a gain of toxic function. In the following, we will concentrate on proteins associated with autosomal recessive diseases, which can lose their function due to misfolding. Mutations in parkin, a gene encoding an E3 ubiquitin ligase (Kitada et al, 1998), are responsible for the majority of autosomal recessive PD (Figure 1C). E3 ubiquitin ligases catalyze the covalent attachment of ubiquitin to lysine residues of substrate proteins. Ubiquitin is best known for its role in targeting proteins for proteasomal degradation, therefore, it has been proposed that a loss of parkin function due to pathogenic mutations causes the accumulation of parkin substrates, which ultimately damage dopaminergic neurons. Various putative parkin substrates have been described; however, an accumulation was observed for only two putative substrates in one parkin knockout model, and the pathophysiological relevance of this observation is still unclear (Ko et al, 2005, 2006). Notably, degradation-independent functions of ubiquitylation have been implicated in various cellular functions, such as signal transduction, transcriptional regulation, DNA repair, endocytosis and cellular trafficking (for review, see Pickart and Fushman, 2004; Haglund and Dikic, 2005). Recent research from different laboratories revealed that parkin can indeed promote non-degradative ubiquitylation (Doss-Pepe et al, 2005; Lim et al, 2005; Hampe et al, 2006; Matsuda et al, 2006; Henn et al, 2007) and that a neuroprotective activity of parkin is linked to this mode of ubiquitylation (Fallon et al, 2006; Henn et al, 2007).
Since its discovery in 1998 (Kitada et al, 1998), a large number and a wide spectrum of pathogenic mutations have been identified, including exon deletions and rearrangements, missense, nonsense and frameshift mutations (for a review, see Mata et al, 2004). Accumulating evidence indicates that misfolding of parkin is a major mechanism of parkin inactivation. All pathogenic C-terminal deletion mutants spontaneously adopt a misfolded conformation and form aggregates in cell culture models (Winklhofer et al, 2003a; Henn et al, 2005). Aberrant parkin folding not necessarily induces the accumulation of misfolded conformers, but can also lead to destabilization and rapid proteasomal degradation, exemplified by some missense mutations within the N-terminal ubiquitin-like domain of parkin (Henn et al, 2005). Alterations in the detergent solubility or cellular localization of parkin have also been described for various missense mutants (Ardley et al, 2003; Cookson et al, 2003; Gu et al, 2003; Muqit et al, 2004; Sriram et al, 2005; Wang et al, 2005b; Hampe et al, 2006). Remarkably, recent publications provide a scientific rationale for the hypothesis that inactivation of parkin by misfolding may also play a role in sporadic PD. Based on the observation that parkin is prone to misfolding and aggregation in the presence of high-level oxidative stress (Winklhofer et al, 2003a), LaVoie et al (2005) could demonstrate that in the substantia nigra of patients suffering from sporadic PD, detergent-insoluble parkin is present, which is covalently modified by an oxidation product of dopamine. In support of this concept, nitrosative stress has been reported to impair the E3 ligase activity of parkin, and S-nitrosylated parkin was indeed detected in the brains of PD patients (Chung et al, 2004; Yao et al, 2004). How could the inactivation of parkin promote the demise of dopaminergic neurons? Parkin has the capacity to maintain neuronal integrity under various moderate stress conditions, including mitochondrial stress, excitotoxicity and ER stress. Mechanistic insight into this activity emerged from recent work, showing that parkin can stimulate pro-survival pathways (Fallon et al, 2006; Henn et al, 2007). Dopaminergic neurons are characterized by a high oxidative burden and thus require an effective stress-response management, yet parkin is inactivated under severe and dopamine induced stress (Winklhofer et al, 2003a; LaVoie et al, 2005, 2007; Wang et al, 2005a; Wong et al, 2007). This inherent imbalance might explain why dopaminergic neurons are particularly vulnerable to a loss of parkin function.
Strikingly, studies in Drosophila indicated a genetic link between parkin and another PD-associated gene, namely PINK1 (PTEN-induced kinase 1). Mutations in the PINK1 gene encoding a mitochondrial kinase are the second most common cause of autosomal recessive PD (Valente et al, 2004). In Drosophila, PINK1 and parkin loss-of-function mutants show a similar phenotype, including mitochondrial defects. Remarkably, parkin can compensate for the loss of PINK1 function, but not vice versa, suggesting that parkin acts downstream of PINK1 (Clark et al, 2006; Park et al, 2006; Yang et al, 2006). Now rescue activity of parkin has also been observed in PINK1-deficient mammalian cells (Exner et al, 2007). It will now be an important endeavor to elucidate the underlying mechanism.
In addition to parkin and PINK1, DJ-1 has been associated with autosomal recessive PD (Bonifati et al, 2003). While PINK1 and parkin genetically interact as described above, DJ-1 is not part of this signaling cascade, as recent evidence demonstrated that DJ-1 fails to rescue the pathological phenotype caused by PINK1 reduction (Exner et al, 2007; Yang et al, 2006). Mutations in the DJ-1 gene are extremely rare, accounting for about 1% of early onset PD. Diverse cellular functions have been attributed to DJ-1; the most relevant function linked to the pathogenesis of PD might be a role of DJ-1 in the response to oxidative stress. DJ-1 has been shown to protect cells against oxidative stress-induced cell death in various cell culture and animal models. Different mechanisms have been proposed to explain the protective activity of DJ-1. It could serve as a sensor of oxidative stress via modification of a cysteine residue to sulfinic acid, and/or it might have an intrinsic antioxidative or chaperone activity (for a review, see Moore et al, 2005). A recent study has provided evidence for an atypical peroxiredoxin-like peroxidase activity of DJ-1 implicated in the scavenging of mitochondrial H2O2 (Andres-Mateos et al, 2007). Furthermore, DJ-1 has been shown to influence signaling pathways implicated in the regulation of cell death; it stimulates the pro-survival PI3K/Akt pathway and inhibits the pro-apoptotic ASK1 pathway (Junn et al, 2005; Yang et al, 2005; Gorner et al, 2007). There is reason to assume that the generation of a non-native conformation also plays a role in the inactivation of DJ-1. The L166P mutant impairs the formation of functional DJ-1 dimers, resulting in a highly unstable protein (Macedo et al, 2003; Miller et al, 2003; Moore et al, 2003; Gorner et al, 2004, 2007; Olzmann et al, 2004). Whether misfolding of other PD-associated gene products, such as PINK1 or LRRK2 (leucine-rich repeat kinase 2, dardarin), might contribute to the pathogenesis of PD, has not been reported so far. Mutations in the LRRK2 gene are regarded as the most common cause of genetic PD; they are responsible for the majority of autosomal dominant PD typically associated with late-onset and are also found in some cases which would have been classified as sporadic PD (Paisan-Ruiz et al, 2004; Zimprich et al, 2004). The LRRK2 gene encodes a large multidomain protein, including a kinase domain related to the mixed lineage kinase family, a Rho/Ras-like GTPase domain, a WD40-repeat domain and leucine-rich repeats. Some pathogenic mutations seem to increase the kinase activity of LRRK2 in vitro, assessed by autophosphorylation or phosphorylation of generic substrates, which may suggest a toxic gain-of-function mechanism (West et al, 2005; Gloeckner et al, 2006; Greggio et al, 2006; Smith et al, 2006). However, we still do not know the physiological and pathological function of LRRK2 in vivo.
Lack of a neuroprotective factor may also play a role in a different neurodegenerative disorder. In addition to the tau-positive FTLD cases, a significant number of FTLD patients carried tau- and α-synuclein-negative but ubiquitin-positive neuronal inclusions (Rademakers et al, 2002; Figure 1B). These inclusions define a novel type of FTLD, called fronotemporal lobar degeneration with ubiquitin-positive inclusions, FTLD-U (Pickering-Brown, 2007). The 43 kDa TAR DNA-binding protein (TDP-43) has been shown to be a major component of these inclusions (Neumann et al, 2006). Strikingly, TDP-43-positive inclusions were also found in sporadic and familial non-SOD1 amyotrophic lateral sclerosis (Neumann et al, 2006; Dickson et al, 2007; Mackenzie et al, 2007; Tan et al, 2007). TDP-43 is a nuclear protein, which may be involved in RNA binding (Buratti et al, 2001; Zuccato et al, 2004; Ayala et al, 2006) or DNA binding (Ou et al, 1995), and accumulates frequently in cytoplasmic and sometimes also in nuclear deposits in FTLD-U cases (Neumann et al, 2006, 2007; Cairns et al, 2007; Davidson et al, 2007; Seelaar et al, 2007). These neuronal deposits contain insoluble hyperphosphorylated, proteolytically generated C-terminal fragments of the full-length protein (Neumann et al, 2006). Currently, it is unclear if these deposits are toxic entities of the disease and/or if they result in a loss-of-function of TDP-43 due to its reduced concentrations within the nucleus, where it is normally located and expected to be functional, for example as an mRNA-stabilizing factor (Strong et al, 2007). While familial cases of FTLD with tau pathology often carry mutations in the tau gene on chromosome 17, three genes and an uncharacterized locus on chromosome 9p have been linked to familial FTLD-U: the valosin-containing protein (VCP) gene on chromosome 9 (Watts et al, 2004), the charged multivesicular body protein 2B gene (CHMP2B) on chromosome 3 (Skibinski et al, 2005) and the progranulin gene (PGRN) on chromosome 17 in close vicinity of the tau locus (Baker et al, 2006; Cruts et al, 2006). VCP/p97 is a multifunctional AAA (ATPases associated with a variety of activities) ATPase, which has been implicated in the ubiquitin-proteasome pathway, ER-associated protein degradation (ERAD), membrane fusion and cell-cycle control (for review, see Wang et al, 2004; Halawani and Latterich, 2006). It has been reported that some pathogenic VCP mutants impair the ubiquitin-proteasome and ERAD pathway (Weihl et al, 2006). CHMP2B constitutes a subunit of the endosomal sorting complex required for transport III (ESCRTIII), involved in the trafficking of ubiquitylated proteins along the endosomal pathway via multivesicular bodies to lysosomes (for a review, see Williams and Urbe, 2007). Expression of mutant CHMP2B has recently been associated with ESCORTIII dysfunction, resulting in the accumulation of autophagosomes (Lee et al, 2007). PGRN is a secreted protein, which has properties of a growth factor or wound-healing factor (Zanocco-Marani et al, 1999; He et al, 2003). Numerous nonsense, frameshift and splice-site mutations have now been identified, which all lead to haploinsufficiency (for review, see Kumar-Singh and Van Broeckhoven, 2007; Mackenzie and Rademakers, 2007; van der Zee et al, 2007a). Apparently, all these mutations result in the degradation of the mutant mRNA by nonsense-mediated mRNA decay (Baker et al, 2006; Cruts et al, 2006). Thus, these mutations cause a loss-of-function by neutralizing the mRNA derived from one mutant allele. Together with the fact that PGRN has many properties of wound healing and growth factors, this suggests that the loss-of-function of PGRN probably results in reduced neuroprotection. This is supported by the observation that PGRN is upregulated in activated microglia surrounding amyloid plaques of AD patients (Baker et al, 2006). If that indicates a general function of PRGN in neuroprotection is currently unknown. Interestingly, three exceptional PRGN mutations have been observed, which all lead to the exchange of only one amino acid within the mature protein (PGRN P248L and PGRN R432C; Schymick et al, 2007; van der Zee et al, 2007b) or the signal sequence (PGRN A9D; Mukherjee et al, 2006). The two mutations occurring C-terminal to the signal sequence allow the synthesis of the immature protein and its translocation into the ER (Shankaran et al, 2007). However, possibly due to misfolding (van der Zee et al, 2007b) these proteins fail to be efficiently transported through the secretory pathway, which leads to a significant reduction in PGRN secretion. In contrast, the mutation within the signal sequence is hardly expressed at all. This protein is mislocated to the cytoplasm, where it is rapidly degraded by the proteasome (Shankaran et al, 2007). Thus, three independent mechanisms, nonsense-mediated mRNA decay, reduced secretion and degradation upon mislocalisation all lead to the same result, that is a loss-of-function of a putative neuroprotective factor (Figure 1B). It is currently unclear if and how the loss-of-function of PGRN is associated with the cytoplasmic deposition of TDP-43. However, very recently it has been suggested that a reduction of PGRN leads to caspase-mediated generation and subsequent accumulation of insoluble TDP-43 fragments, similar to those observed in human patients (Zhang et al, 2007).
A loss-of-function, which results in a gain-of-function?
We have now described a number of pathological consequences, which occur upon a loss or a gain-of-function; however, there is still one rather surprising and very challenging variant to be described, namely a loss-of-function that may result in a gain of (toxic) function. This occurs in at least some autosomal dominant AD-associated presenilin (PS1 and PS2) mutations. PSs are directly involved in the proteolytic generation of Aβ from its precursor, the βAPP. Aβ is produced from APP by proteolytic processing mediated by secretases. First, β-secretase (BACE1) has to cleave at the N-terminus of the Aβ domain to generate a membrane-retained C-terminal stub. This is the immediate substrate for the subsequent γ-secretase cleavage, which liberates Aβ (Haass, 2004). The intramembrane cleavage mediated by γ-secretase is heterogenous and can take place at several positions within the transmembrane domain. Familial AD (FAD)-associated mutations shift the cleavage from position 40 to position 42 of the Aβ domain to produce a more aggregation prone Aβ species (Aβ42) or at least increase the ratio of Aβ42/Aβ40 (Haass and Selkoe, 2007). This is clearly a toxic gain-of-function, which is directly related to the disease, as disease onset and the amount of Aβ42 generated roughly correlate (Duering et al, 2005; Page et al, 2007).
PSs harbor the catalytically active center of the γ-secretase complex. More than 150 missense mutations are spread throughout the entire amino-acid sequence with no obvious hot spots observed (Haass, 2004). However, all of the mutations investigated so far specifically affect the precision of the γ-secretase cut. This can only be explained by slight structural changes, which all affect the catalytically active center in a similar manner. In fact, studies using fluoresecence resonance energy transfer suggested that PS mutations can cause alterations in its conformation (Berezovska et al, 2005). Moreover, certain drugs, such as the nonsteroidal anti-inflammatory drugs (NSAIDs) modulate the γ-secretase cleavage by shifting the cut from amino acid 42 to amino acid 38, thereby reducing the generation of the neurotoxic Aβ42 (Weggen et al, 2001). Strikingly, this shift in cleavage precision is accompanied by a structural change of the domains containing the catalytic center within PS (Lleo et al, 2004). These findings suggest that FAD-associated PS mutations result in a gain of (toxic) function due to structural changes affecting the catalytic center of the protease. However, there is also strong evidence that at least some PS mutations are associated with a loss-of-function (Bentahir et al, 2006; De Strooper, 2007; Wolfe, 2007). Apparently, these PS mutants, if expressed in cells lacking endogenous PS1 and PS2, show reduced Aβ production. As Aβ40 is more severely reduced than Aβ42, these mutations still affect the Aβ42/Aβ40 ratio (Bentahir et al, 2006). How can such an apparent paradox be explained? The γ-secretase complex is physiologically required for Notch signaling, a process which requires the liberation of the cytoplasmic domain and its subsequent nuclear translocation (Haass, 2004). Already very early evidence suggested that FAD-associated PS mutations do not efficiently rescue a Notch-related loss-of-function of the C. elegans PS homolog sel-12 (Levitan et al, 1996; Baumeister et al, 1997; De Strooper, 2007; Wolfe, 2007). Moreover, some FAD-associated mutations reduce intramembrane proteolysis of Notch and thus its nuclear signaling capacity (Moehlmann et al, 2002; Bentahir et al, 2006). However, in mice the knock-in of certain FAD-associated mutations rescued the PS1-knockout phenotype (Guo et al, 1999; Nakano et al, 1999; Siman et al, 2000). But which model is true? We suggest that a somewhat reduced function of at least selected PS mutants is associated with a favored production of Aβ42. This may be explained by a packman-like processing model starting from the cytoplasmic end of the transmembrane domain. If in line with the data in C. elegans, PS mutations reduce the catalytic activity of the γ-secretase, one may assume that the enzyme simply cleaves slower and slower until it reaches position 42. If the substrate is then released from the complex before further cleavage at the subsequent sites (positions 40 and 38) occurs, the Aβ42/Aβ40 ratio may be changed by an apparent loss-of-function. However, one should keep in mind that this may not be a general mechanism of all FAD-associated mutations, but rather of individual mutations, which in most cases are extremely aggressive and cause an unusually strong increase of Aβ42. Although mutations may generally affect the structure of the active site of PS within the γ-secretase complex (Berezovska et al, 2005), individual mutations may affect the PS structure more severely and thus cause a measurable loss-of-function.
Perspective
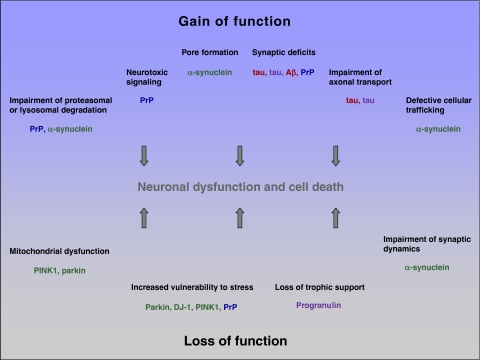
Aberrations in protein folding, processing and/or degradation are common features of neurodegenerative diseases, resulting in the accumulation of misfolded conformers. The identification of genes, which are responsible for rare familial variants of neurodegenerative diseases, provided important insights into common as well as specific features of different disease entities. Clearly, amyloidogenic proteins, such as Aβ, tau, PrP and α-synuclein, which accumulate in sporadic and autosomal dominant forms of the respective diseases, can acquire a toxic gain-of-function. Considerable evidence from recent research indicates that soluble oligomeric assemblies and not amyloid fibers or the final aggregates mediate the toxic effects. However, a disease-relevant impact of large deposits cannot be excluded: on one hand they can sequester and thereby inactivate toxic oligomers, on the other hand they may serve as a dynamic reservoir for the liberation of soluble oligomers. Different mechanisms have been proposed to explain the toxic potential of misfolded protein conformers as summarized in Figure 2. Although some aspects of toxicity may be specific for a distinct entity, certain common mechanisms have emerged. Strikingly, prion diseases illustrated that misfolding can induce not only a toxic but also a self-propagating protein species. The transmissible nature of prion diseases brings up the question how this feature can be explained and how it can be demarcated from seeding effects observed after the experimental inoculation of misfolded conformers. Whether transmissibility is associated with a specific pathological conformation and/or with structural dynamics of the physiological isoform remains to be seen. In some neurodegenerative diseases, protein misfolding is implicated in the pathogenesis via a loss-of-function mechanism, exemplified by autosomal recessive PD (parkin, DJ-1) and FTLD-U with PGRN mutations. In these diseases, the deficiency of a neuroprotective factor may be associated with an increased neuronal vulnerability. In addition, there is experimental evidence suggesting that PrPC as well as α-synuclein may have a neuroprotective capacity, which is lost upon the formation of misfolded conformers. Finally, a rather puzzling case occurs with the FAD-associated PS mutations, where a loss-of-function can surprisingly result in a gain-of-function, in at least some cases.
Figure 2.
Examples for gain- and loss-of-function mechanisms leading to neuronal dysfunction and cell death. red: AD; blue: prion diseases; green: PD; purple: FTLD.
How can our current understanding of neurodegenerative diseases be translated into the development of therapeutic strategies? Remarkably, different oligomeric assemblies, formed by either Aβ, α-synuclein or PrPSc, share structural features, suggesting a common harmful potential of these species (Kayed et al, 2003). Defining the toxic signature of these oligomers might pave the way for immunization approaches. Strikingly, anti-Aβ vaccination has been shown to prevent cognitive deficits and disease progression in mouse models of AD (Roberson and Mucke, 2006; Haass and Selkoe, 2007). Another therapeutic target may be the modulation of the protein quality control machinery, above all molecular chaperones, which are the first line of defence to encounter misfolded conformers.
Acknowledgments
We thank Dr H Steiner, Dr S Lichtenthaler and Dr R Page for critically reading the manuscript. We apologize to those whose work has not been cited due to space limitations. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 596 and the Center for Integrated Protein Science Munich (CiPSM), and the Bundesministerium für Bildung and Forschung (Kompetenznetz Degenerative Demengen). CH is supported by a ‘Forschungsprofessur' provided by the LMU Excellence Program.
References
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, Dawson TM, Dawson VL (2007) DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA 104: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardley HC, Scott GB, Rose SA, Tan NG, Markham AF, Robinson PA (2003) Inhibition of proteasomal activity causes inclusion formation in neuronal and non-neuronal cells overexpressing Parkin. Mol Biol Cell 14: 4541–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, Pagani F, Baralle FE (2006) TDP43 depletion rescues aberrant CFTR exon 9 skipping. FEBS Lett 580: 1339–1344 [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C et al. (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442: 916–919 [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8: 663–672 [DOI] [PubMed] [Google Scholar]
- Baumann F, Tolnay M, Brabeck C, Pahnke J, Kloz U, Niemann HH, Heikenwalder M, Rulicke T, Burkle A, Aguzzi A (2007) Lethal recessive myelin toxicity of prion protein lacking its central domain. EMBO J 26: 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C (1997) Human presenilin-1, but not familial Alzheimer's disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct 1: 149–159 [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B (2006) Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem 96: 732–742 [DOI] [PubMed] [Google Scholar]
- Berezovska O, Lleo A, Herl LD, Frosch MP, Stern EA, Bacskai BJ, Hyman BT (2005) Familial Alzheimer's disease presenilin 1 mutations cause alterations in the conformation of presenilin and interactions with amyloid precursor protein. J Neurosci 25: 3009–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer K (2007) Mechanistic aspects of Parkinson's disease: alpha-synuclein and the biomembrane. Cell Biochem Biophys 47: 285–299 [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Laferla FM (2006) Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res 3: 437–448 [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259 [DOI] [PubMed] [Google Scholar]
- Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A (1996) Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379: 339–343 [DOI] [PubMed] [Google Scholar]
- Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE (2001) Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J 20: 1774–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A et al. (2007) TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 171: 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai R, Leck SL, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF (2005) Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J 19: 1377–1379 [DOI] [PubMed] [Google Scholar]
- Caughey B, Baron GS (2006) Prions and their partners in crime. Nature 443: 803–810 [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123: 383–396 [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364: 1167–1169 [DOI] [PubMed] [Google Scholar]
- Chesebro B, Trifilo M, Race R, Meade-White K, Teng C, LaCasse R, Raymond L, Favara C, Baron G, Priola S, Caughey B, Masliah E, Oldstone M (2005) Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308: 1435–1439 [DOI] [PubMed] [Google Scholar]
- Chiesa R, Piccardo P, Ghetti B, Harris DA (1998) Neurological illness in transgenic mice expressing a prion protein with an insertional mutation. Neuron 21: 1339–1351 [DOI] [PubMed] [Google Scholar]
- Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM (2004) S-nitrosylation of parkin regulates ubiquitination and compromises Parkin's protective function. Science 304: 1328–1331 [DOI] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441: 1162–1166 [DOI] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294: 1346–1349 [DOI] [PubMed] [Google Scholar]
- Cookson MR, Lockhart PJ, McLendon C, O'Farrell C, Schlossmacher M, Farrer MJ (2003) RING finger 1 mutations in Parkin produce altered localization of the protein. Hum Mol Genet 12: 2957–2965 [DOI] [PubMed] [Google Scholar]
- Cookson MR, van der Brug M (2007) Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp Neurol; September 29 [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP et al. (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442: 920–924 [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305: 1292–1295 [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM (2007) Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol (Berlin) 113: 521–533 [DOI] [PubMed] [Google Scholar]
- De Strooper B (2007) Loss-of-function presenilin mutations in Alzheimer disease. Talking point on the role of presenilin mutations in Alzheimer disease. EMBO Rep 8: 141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Josephs KA, Amador-Ortiz C (2007) TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol (Berlin) 114: 71–79 [DOI] [PubMed] [Google Scholar]
- Doss-Pepe EW, Chen L, Madura K (2005) Alpha-synuclein and parkin contribute to the assembly of ubiquitin lysine 63-linked multiubiquitin chains. J Biol Chem 280: 16619–16624 [DOI] [PubMed] [Google Scholar]
- Duering M, Grimm MO, Grimm HS, Schroder J, Hartmann T (2005) Mean age of onset in familial Alzheimer's disease is determined by amyloid beta 42. Neurobiol Aging 26: 785–788 [DOI] [PubMed] [Google Scholar]
- Dumpitak C, Beekes M, Weinmann N, Metzger S, Winklhofer KF, Tatzelt J, Riesner D (2005) The polysaccharide scaffold of PrP 27-30 is a common compound of natural prions and consists of alpha-linked polyglucose. Biol Chem 386: 1149–1155 [DOI] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmström K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken H-H, Gasser T, Krüger R, Winklhofer KF, Vogel F, Reichert A, Auburger G, Kahle PJ, Schmid B, Haass C (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27: 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon L, Belanger CM, Corera AT, Kontogiannea M, Regan-Klapisz E, Moreau F, Voortman J, Haber M, Rouleau G, Thorarinsdottir T, Brice A, van Bergen En Henegouwen PM, Fon EA (2006) A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K-Akt signalling. Nat Cell Biol 8: 834–842 [DOI] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW (2004) Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol 55: 174–179 [DOI] [PubMed] [Google Scholar]
- Flechsig E, Hegyi I, Leimeroth R, Zuniga A, Rossi D, Cozzio A, Schwarz P, Rulicke T, Gotz J, Aguzzi A, Weissmann C (2003) Expression of truncated PrP targeted to Purkinje cells of PrP knockout mice causes Purkinje cell death and ataxia. EMBO J 22: 3095–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig E, Weissmann C (2004) The role of PrP in health and disease. Curr Mol Med 4: 337–353 [DOI] [PubMed] [Google Scholar]
- Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M (2006) The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet 15: 223–232 [DOI] [PubMed] [Google Scholar]
- Gorner K, Holtorf E, Odoy S, Nuscher B, Yamamoto A, Regula JT, Beyer K, Haass C, Kahle PJ (2004) Differential effects of Parkinson's disease-associated mutations on stability and folding of DJ-1. J Biol Chem 279: 6943–6951 [DOI] [PubMed] [Google Scholar]
- Gorner K, Holtorf E, Waak J, Pham TT, Vogt-Weisenhorn DM, Wurst W, Haass C, Kahle PJ (2007) Structural determinants of the C-terminal helix–kink–helix motif essential for protein stability and survival promoting activity of DJ-1. J Biol Chem 282: 13680–13691 [DOI] [PubMed] [Google Scholar]
- Gosavi N, Lee HJ, Lee JS, Patel S, Lee SJ (2002) Golgi fragmentation occurs in the cells with prefibrillar alpha-synuclein aggregates and precedes the formation of fibrillar inclusion. J Biol Chem 277: 48984–48992 [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293: 1491–1495 [DOI] [PubMed] [Google Scholar]
- Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23: 329–341 [DOI] [PubMed] [Google Scholar]
- Gu WJ, Corti O, Araujo F, Hampe C, Jacquier S, Lucking CB, Abbas N, Duyckaerts C, Rooney T, Pradier L, Ruberg M, Brice A (2003) The C289G and C418R missense mutations cause rapid sequestration of human Parkin into insoluble aggregates. Neurobiol Dis 14: 357–364 [DOI] [PubMed] [Google Scholar]
- Guo Q, Fu W, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP (1999) Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med 5: 101–106 [DOI] [PubMed] [Google Scholar]
- Haass C (2004) Take five-BACE and the g-secretase quartet conduct Alzheimer's amyloid b-peptide generation. EMBO J 23: 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101–112 [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I (2005) Ubiquitylation and cell signaling. EMBO J 24: 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halawani D, Latterich M (2006) p97: the cell's molecular purgatory? Mol Cell 22: 713–717 [DOI] [PubMed] [Google Scholar]
- Hampe C, Ardila-Osorio H, Fournier M, Brice A, Corti O (2006) Biochemical analysis of Parkinson's disease-causing variants of Parkin, an E3 ubiquitin-protein ligase with monoubiquitylation capacity. Hum Mol Genet 15: 2059–2075 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- He Z, Ong CH, Halper J, Bateman A (2003) Progranulin is a mediator of the wound response. Nat Med 9: 225–229 [DOI] [PubMed] [Google Scholar]
- Hegde RS, Mastrianni JA, Scott MR, DeFea KA, Tremblay P, Torchia M, DeArmond SJ, Prusiner SB, Lingappa VR (1998) A transmembrane form of the prion protein in neurodegenerative disease. Science 279: 827–834 [DOI] [PubMed] [Google Scholar]
- Henn IH, Bouman L, Schlehe JS, Schlierf A, Schramm JE, Wegener E, Nakaso K, Culmsee C, Berninger B, Krappmann D, Tatzelt J, Winklhofer KF (2007) Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J Neurosci 27: 1868–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn IH, Gostner JM, Tatzelt J, Winklhofer KF (2005) Pathogenic mutations inactivate parkin by distinct mechanisms. J Neurochem 92: 114–122 [DOI] [PubMed] [Google Scholar]
- Heske J, Heller U, Winklhofer KF, Tatzelt J (2004) The C-terminal domain of the prion protein is necessary and sufficient for import into the endoplasmic reticulum. J Biol Chem 279: 5435–5443 [DOI] [PubMed] [Google Scholar]
- Hunter P (2006) Shedding a negative image. Research into their mechanism of infectivity reveals that prions might have important biological roles. EMBO Rep 7: 1196–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J et al. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393: 702–705 [DOI] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A (2004) Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet 364: 1169–1171 [DOI] [PubMed] [Google Scholar]
- Ihara M, Yamasaki N, Hagiwara A, Tanigaki A, Kitano A, Hikawa R, Tomimoto H, Noda M, Takanashi M, Mori H, Hattori N, Miyakawa T, Kinoshita M (2007) Sept4, a component of presynaptic scaffold and lewy bodies, is required for the suppression of alpha-synuclein neurotoxicity. Neuron 53: 519–533 [DOI] [PubMed] [Google Scholar]
- Junn E, Taniguchi H, Jeong BS, Zhao X, Ichijo H, Mouradian MM (2005) Interaction of DJ-1 with Daxx inhibits apoptosis signal-regulating kinase 1 activity and cell death. Proc Natl Acad Sci USA 102: 9691–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489 [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N (1998) Mutations in the parkin gene cause autosomal parkinsonism. Nature 392: 605–608 [DOI] [PubMed] [Google Scholar]
- Ko HS, Kim SW, Sriram SR, Dawson VL, Dawson TM (2006) Identification of far upstream element-binding protein-1 as an authentic Parkin substrate. J Biol Chem 281: 16193–16196 [DOI] [PubMed] [Google Scholar]
- Ko HS, von Coelln R, Sriram SR, Kim SW, Chung KK, Pletnikova O, Troncoso J, Johnson B, Saffary R, Goh EL, Song H, Park BJ, Kim MJ, Kim S, Dawson VL, Dawson TM (2005) Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci 25: 7968–7978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen M, Deriziotis P, Dimcheff DE, Jackson GS, Ovaa H, Naumann H, Clarke AR, van Leeuwen FW, Menendez-Benito V, Dantuma NP, Portis JL, Collinge J, Tabrizi SJ (2007) Disease-associated prion protein oligomers inhibit the 26S proteasome. Mol Cell 26: 175–188 [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet 18: 106–108 [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Van Broeckhoven C (2007) Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol 17: 104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, Kubosaki A, Matsumoto Y, Saeki K, Matsumoto Y, Yokoyama T, Itohara S, Onodera T (1999) Prions prevent neuronal cell-line death. Nature 400: 225–226 [DOI] [PubMed] [Google Scholar]
- Lansbury PT, Lashuel HA (2006) A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nature 443: 774–779 [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418: 291. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Cortese GP, Ostaszewski BL, Schlossmacher MG (2007) The effects of oxidative stress on parkin and other E3 ligases. J Neurochem; September 19 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ (2005) Dopamine covalently modifies and functionally inactivates parkin. Nat Med 11: 1214–1221 [DOI] [PubMed] [Google Scholar]
- Lee HG, Zhu X, Takeda A, Perry G, Smith MA (2006) Emerging evidence for the neuroprotective role of alpha-synuclein. Exp Neurol 200: 1–7 [DOI] [PubMed] [Google Scholar]
- Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB (2007) ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol 17: 1561–1567 [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB (2004) Synthetic mammalian prions. Science 305: 673–676 [DOI] [PubMed] [Google Scholar]
- Legname G, Nguyen HO, Baskakov IV, Cohen FE, Dearmond SJ, Prusiner SB (2005) Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci USA 102: 2168–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440: 352–357 [DOI] [PubMed] [Google Scholar]
- Levitan D, Doyle TG, Brousseau D, Lee MK, Thinakaran G, Slunt HH, Sisodia SS, Greenwald I (1996) Assessment of normal and mutant human presenilin function in Caenorhabditis elegans. Proc Natl Acad Sci USA 93: 14940–14944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293: 1487–1491 [DOI] [PubMed] [Google Scholar]
- Li A, Christensen HM, Stewart LR, Roth KA, Chiesa R, Harris DA (2007) Neonatal lethality in transgenic mice expressing prion protein with a deletion of residues 105–125. EMBO J 26: 548–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL (2004) Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson's and Alzheimer's disease. FASEB J 18: 962–964 [DOI] [PubMed] [Google Scholar]
- Lim KL, Chew KC, Tan JM, Wang C, Chung KK, Zhang Y, Tanaka Y, Smith W, Engelender S, Ross CA, Dawson VL, Dawson TM (2005) Parkin mediates nonclassical, proteasomal-independent ubiquitination of synphilin-1: implications for Lewy body formation. J Neurosci 25: 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH (2004) Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem 279: 12924–12934 [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Herl L, Raju S, Deng A, Bacskai BJ, Frosch MP, Irizarry M, Hyman BT (2004) Nonsteroidal anti-inflammatory drugs lower Abeta42 and change presenilin 1 conformation. Nat Med 10: 1065–1066 [DOI] [PubMed] [Google Scholar]
- Ma J, Lindquist S (2001) Wild-type PrP and a mutant associated with prion disease are subject to retrograde transport and proteasome degradation. Proc Natl Acad Sci USA 98: 14955–14960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wollmann R, Lindquist S (2002) Neurotoxicity and neurodegeneration when PrP accumulates in the cytosol. Science 298: 1781–1785 [DOI] [PubMed] [Google Scholar]
- Macedo MG, Anar B, Bronner IF, Cannella M, Squitieri F, Bonifati V, Hoogeveen A, Heutink P, Rizzu P (2003) The DJ-1L166P mutant protein associated with early onset Parkinson's disease is unstable and forms higher-order protein complexes. Hum Mol Genet 12: 2807–2816 [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61: 427–434 [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Rademakers R (2007) The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics 8: 857–868 [DOI] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klohn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302: 871–874 [DOI] [PubMed] [Google Scholar]
- Mandelkow E, von Bergen M, Biernat J, Mandelkow EM (2007) Structural principles of tau and the paired helical filaments of Alzheimer's disease. Brain Pathol 17: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E (1998) Tau in Alzheimer's disease. Trends Cell Biol 8: 425–427 [DOI] [PubMed] [Google Scholar]
- Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN (2006) Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Mol Psychiatry 11: 721–736 [DOI] [PubMed] [Google Scholar]
- Mata IF, Lockhart PJ, Farrer MJ (2004) Parkin genetics: one model for Parkinson's disease. Hum Mol Genet 13: R127–R133 [DOI] [PubMed] [Google Scholar]
- Matsuda N, Kitami T, Suzuki T, Mizuno Y, Hattori N, Tanaka K (2006) Diverse effects of pathogenic mutations of Parkin that catalyze multiple monoubiquitylation in vitro. J Biol Chem 281: 3204–3209 [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Mishizen AJ, Giasson BI, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H (2006) Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci 26: 10068–10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan NF, Brennan PM, McNeill A, Davies I, Fotheringham A, Rennison KA, Ritchie D, Brannan F, Head MW, Ironside JW, Williams A, Bell JE (2004) Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol 165: 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DW, Ahmad R, Hague S, Baptista MJ, Canet-Aviles R, McLendon C, Carter DM, Zhu PP, Stadler J, Chandran J, Klinefelter GR, Blackstone C, Cookson MR (2003) L166P mutant DJ-1, causative for recessive Parkinson's disease, is degraded through the ubiquitin-proteasome system. J Biol Chem 278: 36588–36595 [DOI] [PubMed] [Google Scholar]
- Mitteregger G, Vosko M, Krebs B, Xiang W, Kohlmannsperger V, Nolting S, Hamann GF, Kretzschmar HA (2007) The role of the octarepeat region in neuroprotective function of the cellular prion protein. Brain Pathol 17: 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H (2002) Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci USA 99: 8025–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci 28: 57–87 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Dawson TM, Dawson VL (2003) A missense mutation (L166P) in DJ-1, linked to familial Parkinson's disease, confers reduced protein stability and impairs homo-oligomerization. J Neurochem 87: 1558–1567 [DOI] [PubMed] [Google Scholar]
- Mukherjee O, Pastor P, Cairns NJ, Chakraverty S, Kauwe JS, Shears S, Behrens MI, Budde J, Hinrichs AL, Norton J, Levitch D, Taylor-Reinwald L, Gitcho M, Tu PH, Tenenholz Grinberg L, Liscic RM, Armendariz J, Morris JC, Goate AM (2006) HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 60: 314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqit MM, Davidson SM, Payne Smith MD, MacCormac LP, Kahns S, Jensen PH, Wood NW, Latchman DS (2004) Parkin is recruited into aggresomes in a stress-specific manner: over-expression of parkin reduces aggresome formation but can be dissociated from parkin's effect on neuronal survival. Hum Mol Genet 13: 117–135 [DOI] [PubMed] [Google Scholar]
- Muramoto T, DeArmond SJ, Scott M, Telling GC, Cohen FE, Prusiner SB (1997) Heritable disorder resembling neuronal storage disease in mice expressing prion protein with deletion of an alpha-helix. Nat Med 3: 750–755 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Kondoh G, Kudo T, Imaizumi K, Kato M, Miyazaki JI, Tohyama M, Takeda J, Takeda M (1999) Accumulation of murine amyloidbeta42 in a gene-dosage-dependent manner in PS1 ‘knock-in' mice. Eur J Neurosci 11: 2577–2581 [DOI] [PubMed] [Google Scholar]
- Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS (2007) TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 66: 152–157 [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133 [DOI] [PubMed] [Google Scholar]
- Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM (2005) Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem 280: 21212–21219 [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM (2004) Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43: 321–332 [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39: 409–421 [DOI] [PubMed] [Google Scholar]
- Olzmann JA, Brown K, Wilkinson KD, Rees HD, Huai Q, Ke H, Levey AI, Li L, Chin LS (2004) Familial Parkinson's disease-associated L166P mutation disrupts DJ-1 protein folding and function. J Biol Chem 279: 8506–8515 [DOI] [PubMed] [Google Scholar]
- Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB (1995) Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol 69: 3584–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RM, Baumann K, Tomioka M, Perez-Revuelta BI, Fukumori A, Jacobsen H, Flohr A, Luebbers T, Ozmen L, Steiner H, Haass C (2007) Generation of Abeta 38 and Abeta 42 is independently and differentially affected by FAD-associated presenilin 1 mutations and gamma-secretase modulation. J Biol Chem; October 24 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44: 595–600 [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161 [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O'Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR (2002) Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36: 1007–1019 [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D (2004) Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol 8: 610–616 [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM (2007) Progranulin and frontotemporal lobar degeneration. Acta Neuropathol (Berlin) 114: 39–47 [DOI] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem 270: 9564–9570 [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045–2047 [DOI] [PubMed] [Google Scholar]
- Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216: 136–144 [DOI] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van den Broeck M, Backhovens H, van Swieten J, van Duijn CM, Van Broeckhoven C (2002) Tau negative frontal lobe dementia at 17q21: significant finemapping of the candidate region to a 4.8 cM interval. Mol Psychiatry 7: 1064–1074 [DOI] [PubMed] [Google Scholar]
- Rambold AS, Miesbauer M, Rapaport D, Bartke T, Baier M, Winklhofer KF, Tatzelt J (2006) Association of Bcl-2 with misfolded prion protein is linked to the toxic potential of cytosolic PrP. Mol Biol Cell 17: 3356–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Burgaya F, Gavin R, Soriano E, Aguzzi A, Del Rio JA (2007) Enhanced susceptibility of Prnp-deficient mice to kainate-induced seizures, neuronal apoptosis, and death: role of AMPA/kainate receptors. J Neurosci Res 85: 2741–2755 [DOI] [PubMed] [Google Scholar]
- Roberson ED, Mucke L (2006) 100 years and counting: prospects for defeating Alzheimer's disease. Science 314: 781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 316: 750–754 [DOI] [PubMed] [Google Scholar]
- Roucou X, LeBlanc AC (2005) Cellular prion protein neuroprotective function: implications in prion diseases. J Mol Med 83: 3–11 [DOI] [PubMed] [Google Scholar]
- Safar JG, Kellings K, Serban A, Groth D, Cleaver JE, Prusiner SB, Riesner D (2005) Search for a prion-specific nucleic acid. J Virol 79: 10796–10806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymick J, Yang Y, Andersen P, Vonsattel J, Greenway M, Momeni P, Elder J, Chio A, Restagno G, Robberecht W, Dahlberg C, Mukherjee O, Goate A, Graff-Radford N, Caselli R, Hutton M, Gass J, Cannon A, Rademakers R, Singleton A et al. (2007) Progranulin mutations and ALS or ALS-FTD phenotypes. J Neurol Neurosurg Psychiatry 78: 754–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H, Jurgen Schelhaas H, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, de Rijik MC, Rizzu P, Brummelhuis MT, van Doorn PA, Kamphorst W, Willemsen R, van Swieten JC (2007) TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain 130: 1375–1385 [DOI] [PubMed] [Google Scholar]
- Shankaran SS, Capell A, Hruscha AT, Fellerer K, Neumann M, Schmid B, Haass C (2007) TTLD-U linked progranulin missense mutations affect their secretion but transient knockdown is not sufficient to induce cytoplasmic TDP-43 depositon. J Biol Chem; November 5 [E-pub ahead of print] [Google Scholar]
- Shmerling D, Hegyi I, Fischer M, Blättler T, Brandner S, Götz J, Rülicke T, Flechsig E, Cozzio A, von Mehring C, Hangartner C, Aguzzi A, Weissmann C (1998) Expression of animo-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93: 203–214 [DOI] [PubMed] [Google Scholar]
- Shyu A-B, Wilkinson MF, van Hoof A (2008) Messenger RNA regulation: to translate or to degrade. EMBO J (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Ding DC, Li KW, Chen SF, Yang HI, Li H (2005) Overexpression of PrPC by adenovirus-mediated gene targeting reduces ischemic injury in a stroke rat model. J Neurosci 25: 8967–8977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R, Reaume AG, Savage MJ, Trusko S, Lin YG, Scott RW, Flood DG (2000) Presenilin-1 P264L knock-in mutation: differential effects on abeta production, amyloid deposition, and neuronal vulnerability. J Neurosci 20: 8717–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J et al. (2003) alpha-Synuclein locus triplication causes Parkinson's disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J (2005) Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 37: 806–808 [DOI] [PubMed] [Google Scholar]
- Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14: 3801–3811 [DOI] [PubMed] [Google Scholar]
- Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA (2006) Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci 9: 1231–1233 [DOI] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B (2003) Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem 278: 11753–11759 [DOI] [PubMed] [Google Scholar]
- Solforosi L, Criado JR, McGavern DB, Wirz S, Sanchez-Alavez M, Sugama S, DeGiorgio LA, Volpe BT, Wiseman E, Abalos G, Masliah E, Gilden D, Oldstone MB, Conti B, Williamson RA (2004) Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303: 1514–1516 [DOI] [PubMed] [Google Scholar]
- Spudich A, Frigg R, Kilic E, Kilic U, Oesch B, Raeber A, Bassetti CL, Hermann DM (2005) Aggravation of ischemic brain injury by prion protein deficiency: role of ERK-1/-2 and STAT-1. Neurobiol Dis 20: 442–449 [DOI] [PubMed] [Google Scholar]
- Sriram SR, Li X, Ko HS, Chung KK, Wong E, Lim KL, Dawson VL, Dawson TM (2005) Familial-associated mutations differentially disrupt the solubility, localization, binding and ubiquitination properties of parkin. Hum Mol Genet 14: 2571–2586 [DOI] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA (2001) Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci 21: 9549–9560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, Shoesmith C (2007) TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci 35: 320–327 [DOI] [PubMed] [Google Scholar]
- Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H (2007) TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol (Berlin) 113: 535–542 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, Dawson VL, Dawson TM, Ross CA (2001) Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum Mol Genet 10: 919–926 [DOI] [PubMed] [Google Scholar]
- Uversky VN (2007) Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem 103: 17–37 [DOI] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B et al. (2004) Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304: 1158–1160 [DOI] [PubMed] [Google Scholar]
- van der Zee J, Gijselinck I, Pirici D, Kumar-Singh S, Cruts M, Van Broeckhoven C (2007a) Frontotemporal lobar degeneration with ubiquitin-positive inclusions: a molecular genetic update. Neurodegener Dis 4: 227–235 [DOI] [PubMed] [Google Scholar]
- van der Zee J, Le Ber I, Maurer-Stroh S, Engelborghs S, Gijselinck I, Camuzat A, Brouwers N, Vandenberghe R, Sleegers K, Hannequin D, Dermaut B, Schymkowitz J, Campion D, Santens P, Martin JJ, Lacomblez L, De Pooter T, Peeters K, Mattheijssens M, Vercelletto M et al. (2007b) Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat 28: 416. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lansbury PT Jr (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson's disease. Biochemistry 42: 7871–7878 [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT Jr (2001) Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry 40: 7812–7819 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid b protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539 [DOI] [PubMed] [Google Scholar]
- Walz R, Amaral OB, Rockenbach IC, Roesler R, Izquierdo I, Cavalheiro EA, Martins VR, Brentani RR (1999) Increased sensitivity to seizures in mice lacking cellular prion protein. Epilepsia 40: 1679–1682 [DOI] [PubMed] [Google Scholar]
- Wang C, Ko HS, Thomas B, Tsang F, Chew KC, Tay SP, Ho MW, Lim TM, Soong TW, Pletnikova O, Troncoso J, Dawson VL, Dawson TM, Lim KL (2005a) Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet 14: 3885–3897 [DOI] [PubMed] [Google Scholar]
- Wang C, Tan JM, Ho MW, Zaiden N, Wong SH, Chew CL, Eng PW, Lim TM, Dawson TM, Lim KL (2005b) Alterations in the solubility and intracellular localization of parkin by several familial Parkinson's disease-linked point mutations. J Neurochem 93: 422–431 [DOI] [PubMed] [Google Scholar]
- Wang Q, Song C, Li CC (2004) Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J Struct Biol 146: 44–57 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang F, Arterburn L, Wollmann R, Ma J (2006) The interaction between cytoplasmic prion protein and the hydrophobic lipid core of membrane correlates with neurotoxicity. J Biol Chem 281: 13559–13565 [DOI] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36: 377–381 [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH (2001) A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414: 212–216 [DOI] [PubMed] [Google Scholar]
- Weihl CC, Dalal S, Pestronk A, Hanson PI (2006) Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet 15: 189–199 [DOI] [PubMed] [Google Scholar]
- Weise J, Sandau R, Schwarting S, Crome O, Wrede A, Schulz-Schaeffer W, Zerr I, Bahr M (2006) Deletion of cellular prion protein results in reduced Akt activation, enhanced postischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke 37: 1296–1300 [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM (2005) Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 102: 16842–16847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergard L, Christensen HM, Harris DA (2007) The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim Biophys Acta 1772: 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB (1994) (URE3) as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae (see comments). Science 264: 566–569 [DOI] [PubMed] [Google Scholar]
- Wille H, Drewes G, Biernat J, Mandelkow EM, Mandelkow E (1992) Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol 118: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RL, Urbe S (2007) The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8: 355–368 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Henn IH, Kay-Jackson P, Heller U, Tatzelt J (2003a) Inactivation of parkin by oxidative stress and C-terminal truncations; a protective role of molecular chaperones. J Biol Chem 278: 47199–47208 [DOI] [PubMed] [Google Scholar]
- Winklhofer KF, Heske J, Heller U, Reintjes A, Muranji W, Moarefi I, Tatzelt J (2003b) Determinants of the in vivo-folding of the prion protein: a bipartite function of helix 1 in folding and aggregation. J Biol Chem 278: 14961–14970 [DOI] [PubMed] [Google Scholar]
- Wolfe MS (2007) When loss is gain: reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep 8: 136–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ES, Tan JM, Wang C, Zhang Z, Tay SP, Zaiden N, Ko HS, Dawson VL, Dawson TM, Lim KL (2007) Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem 282: 12310–12318 [DOI] [PubMed] [Google Scholar]
- Xu J, Kao SY, Lee FJ, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of alpha-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat Med 8: 600–606 [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, Takahashi R, Lu B (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA 102: 13670–13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 103: 10793–10798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D, Gu Z, Nakamura T, Shi ZQ, Ma Y, Gaston B, Palmer LA, Rockenstein EM, Zhang Z, Masliah E, Uehara T, Lipton SA (2004) Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA 101: 10810–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedidia Y, Horonchik L, Tzaban S, Yanai A, Taraboulos A (2001) Proteasomes and ubiquitin are involved in the turnover of the wild-type prion protein. EMBO J 20: 5383–5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R (1999) Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res 59: 5331–5340 [PubMed] [Google Scholar]
- Zanusso G, Petersen RB, Jin T, Jing Y, Kanoush R, Ferrari S, Gambetti P, Singh N (1999) Proteasomal degradation and N-terminal protease resistance of the codon 145 mutant prion protein. J Biol Chem 274: 23396–23404 [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173 [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L (2007) Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci 27: 10530–10534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44: 601–607 [DOI] [PubMed] [Google Scholar]
- Zuccato E, Buratti E, Stuani C, Baralle FE, Pagani F (2004) An intronic polypyrimidine-rich element downstream of the donor site modulates cystic fibrosis transmembrane conductance regulator exon 9 alternative splicing. J Biol Chem 279: 16980–16988 [DOI] [PubMed] [Google Scholar]