Abstract
Freshly isolated mouse prostate epithelial cells regenerate fully differentiated prostate tissue when combined with embryonic urogenital sinus mesenchyme and grafted in vivo. We show here that this regenerative capacity, which has been attributed to a small population of pleuripotential progenitor epithelial cells, is rapidly lost when the cells are placed in monolayer culture but can be maintained by culture in anchorage-independent conditions. Epithelial cells placed in anchorage-independent culture formed proliferating spheres that could be serially passaged and exhibited increased expression of putative stem cell markers as compared to cells grown in monolayer culture. Epithelial cells isolated from the fetal urogenital sinus, the newborn and adult prostate formed spheres with similar efficiency, while cells isolated from the post-castration prostate exhibited significantly higher sphere-forming abilities. When passaged spheres were recombined with E17 rat urogenital sinus mesenchyme and grafted in vivo, they generated fully differentiated mouse prostate glandular epithelium containing both p63+ basal cells and p63- luminal cells and expressing a variety of prostate-specific and terminal differentiation markers.
Keywords: prostate stem cell, prostasphere, anchorage-independent culture, prostate regeneration, stem cell markers, castration
Introduction
The adult mouse prostate is a male accessory sex organ located near the base of the urinary bladder. It is composed of distinct anterior, dorsal and ventral lobes that surround the male urethra and contribute androgen-dependent secretory proteins to the ejaculate. The origin of the secretory ducts is the prostatic anlagen of the urogenital sinus (UGS). The UGS is comprised of two layers: an outer layer of mesenchyme and an inner layer of stratified epithelium (Price, 1963; Wang et al., 2001). In mouse, epithelial buds invade the surrounding mesenchyme and give rise to the main ducts of the prostatic ductal system at E17.5. This is an androgen-induced event, and under the continued influence of testosterone, the main prostatic ducts undergo extensive branching morphogenesis to generate a complex ductal system surrounded by a complex periductal stroma. The ducts of the mature prostate are lined by a differentiated luminal epithelium with at least three major cell types. Basal cells express cytokeratins 5 and 14, and transformation-related protein 63 (p63) (Hayward et al., 1996; Yang et al., 1998; Wang et al., 2001; Kurita et al., 2004). Luminal epithelial cells express cytokeratin 8 and 18 and secrete prostate-specific proteins such as probasin (Cussenot et al., 1994; Johnson et al., 2000). Epithelial cells co-expressing cytokeratins 5, 14, 8 and 18 are hypothesized to represent either intermediates of the basal to luminal cell lineage (Cussenot et al., 1994; Peehl et al., 1994; Verhagen et al., 1988) or a pleuripotent progenitor cell population (Wang et al., 2001). The adult prostate is an androgen dependent organ. In response to castration, there is widespread epithelial apoptosis and involution preferentially affecting the distal duct segments. Testosterone supplementation induces complete regeneration of the prostate ductal system by stimulating outgrowth from the spared proximal duct segments (Sugimura et al., 1986).
The regenerative potential of the adult prostate fueled early efforts to identify prostate specific stem cells. Early studies by Kinbara and colleagues (1996) demonstrated that epithelial cells isolated from different lobes of the adult rodent prostate exhibited features expected of stem cells, including enormous proliferative potential and the potential for re-programmed epithelial differentiation. These characteristics recapitulated the progenitor phenotype of epithelial cells isolated from the UGS prior to the induction of prostate development and suggested a functional equivalence between them. A number of studies of tissue-specific stem cells in the adult prostate followed (Robinson, et al., 1998; Hudson et al., 2000; Collins et al., 2001; Foster et al., 2002) and Tjusimura et al. (2002) performed in vivo labeling during postnatal prostate development followed by multiple rounds of castration-regeneration to localize label-retaining cells within the adult prostate. These efforts demonstrated that label-retaining cells localized preferentially to the proximal segments of the prostate ducts, and subsequent studies using in vivo recombination techniques demonstrated that the regenerative capacity of the adult prostate epithelium was predominantly located in the proximal duct segments (Burger et al., 2005; Xin et al., 2005). More recent efforts have focused on identifying specific stem cell markers. Richardson et al. (2004) identified CD133 as a putative stem cell marker. Stem cell antigen-1 (Sca-1) was also identified as a putative stem cell marker in prostate, and the proximal duct segments were shown to be enriched for cells co-expressing Sca-1 and α6-integrin (Burger et al., 2005). More recent efforts used FACS to isolate cells expressing combinations of these stem cell markers to enrich for epithelial cells with increased regenerative potential (Lawson et al., 2007). The regenerative potential of epithelial cells from the proximal duct segments can be maintained through serial passages in vivo and correlates with formation of viable spheres in a collagen gel assay (Goto et el., 2006; Lawson et al., 2007), however, successful culture and passage of bona fide progenitor cells in vitro has not been previously reported.
In the studies reported here, we show that epithelial cells isolated from the E16 UGS, the P1 prostate and the adult prostate all generate viable and proliferating spheres of cells when plated in anchorage-independent culture. The “prostaspheres” were morphologically indistinguishable, could be serially passaged in vitro, and generated glandular tissue with characteristic prostatic histology and expression of terminal differentiation markers when recombined with embryonic rat UGS mesenchyme (rUGM) in vivo. We show that epithelial cells isolated from the adult prostate and cultured in anchorage independent conditions recapitulate the differentiation potential of UGS epithelial cells and show, furthermore, that in utero BrdU labeling of the urogenital epithelium yields a fraction of label-retaining basal epithelial cells in duct segments of the adult prostate. Taken together, these findings suggest that slow cycling progenitor cells of the adult prostate epithelium may derive, at least in part, from a subpopulation of cells that actively proliferate during early prostate development.
Materials and Methods
Animals
Animal procedures were performed in accordance with the guidelines of the University of Wisconsin-Madison’s Animal Care and Use Committee. CD-1 wild-type and nude mice were purchased from Charles River Laboratories (Wilmington, MA) unless otherwise stated. The actin-EGFP (C57 BL/6 TG (ACTB-EGFP/1Osb/j, stock # 003291) was ordered from The Jackson Laboratory (Bar Harbor, ME). Mice were sedated with isoflurane, euthanized by cervical dislocation, and UGS and prostates were harvested by dissection in Dulbecco’s PBS (Invitrogen, Carlsbad, CA). Tissue samples were either snap frozen in liquid nitrogen and stored at −80°C for RNA isolation or fixed in 4% paraformaldehyde.
BrdU labeling
The developing prostate was labeled in utero by IP injection of 0.5 ml BrdU (10 mM) per mouse as instructed (Roche Diagnostic GmbH, Mannheim, Germany).
Prostasphere Generation
E16 UGS tissues were treated with 1% trypsin (GIBCO, Cat. # 27250-018, Invitrogen, Carlsbad, CA) for 60 minutes at 4°C, and the epithelium and mesenchyme was mechanically separated as previously described (Lamm et al., 2001). The isolated epithelium was treated with 0.5% collagenase Type I (GIBCO, Cat. # 17100-017, Invitrogen, Calsbad, CA), dissociated using a fire-polished glass Pasteur pipette and passed through a 40 μm cell strainer to produce a single cell suspension. Cells were plated on an standard cell culture plate for 60 to 120 minutes at 37°C to remove contaminating mesenchymal cells detected by RT-PCR for the expression of the mesenchyme-specific marker BMP4 (Ravindranath and Dym, 1999; Lamm et al., 2001).
The isolated anterior (AP), ventral (VP) and, dorsal-lateral prostate (DLP) lobes of the adult prostate were minced to 1 mm pieces, digested with 1% collagenase in DMEM media with 10% FBS 37°C for 30 minutes; the prostate tissues were digested in 1% collagenase and 0.5% hydrouronidase for another 30 minutes and incubated in PBS containing 1% trypsin for 30 to 60 minute. The separated cells were collected and passed through a 40 μm cell strainer to produce a single cell suspension and counted using a hemocytometer. Plating on monolayer tissue culture dishes with DMEM/F12 media supplemented with 10% FBS without DHT for 12 to 14 hours was used to remove contaminating stromal cells. The epithelial cells remaining in the supernatant were collected and grown on ultralow attachment plates (Corning Inc., Acton, MA) to generate prostaspheres. The isolated cells were cultured at the density of 500 cells/ml (unless stated otherwise) in 100 mm diameter ultralow attachment plates with 20 ml sphere culture media. The sphere culture media is serum free DMEM/F12 50/50 (Mediatech, Inc., Harndon, VA, Cat. 10-090-CV) supplemented with 20 ng/ml EGF (BD Biosciences, MA), 10 ng/ml bFGF (BD Biosciences, MA), 5 μg/ml heparin (Sigma, MO), 2 nM Glutamate (Sigma, MO), other components of the media include 1% penicillin-streptomycin (50 IU penicillin and 50 μg/ml streptomycin, Mediatech, Inc., Harndon, VA Cat. 30-001-CI), 0.2% bovine serum albumin (BSA, Fisher, Cat. 9048-46-8), 1X B27 without Vitamine A (GIBCO, 12578-010), 1X Insulin-Transferrin-Selenium-A (Gibco/BRL 51300-044). The sphere culture media was changed every 72 hours until the majority of prostaspheres reached 200 - 300 μm in diameter (approximately 15 days). For monolayer culture, the same number of cells were plated in either serum free prostate epithelial cell culture media supplemented with defined growth factors (PrEBM, from Cambrex, Walkersville, MD) or DMEM/F12 with 1% FBS supplemented with 20 ng/ml of EGF.
Passage of Prostaspheres
Prostaspheres were collected by gentle centrifugation (400Xg for 5 minutes) dissociated with 0.25% trypsin–EDTA and mechanically disrupted with a fire-polished Pasteur pipette. The cell suspension was sieved through a 40 μm cell strainer, plated on a monolayer culture plates for 60 minutes to remove contaminating stromal cells and then re-plated.
Cell count
Sphere diameter and cell number was determined for 20 prostaspheres on day 3, 6, 9, 12 and 15 of culture. Prostasphere diameter was determined by digital imaging through a dissecting microscope and measured using an NIH image analysis program (http://rsb.info.nih.gov/nih-image/). Cell number was determined for triplicate samples of 20 prostaspheres by trypsination and counting on a hemocytometer.
Renal grafts
Thirty to forty second generation (G2) prostaspheres or an equivalent number of cells (~100,000 cells) generated from E16 UGS or adult prostate epithelial cells were mixed with freshly isolated E17 rat UGM (rUGM) and 40 μl of neutralized rat-tail collagen, incubated at 37°C for 2 hours and grafted under the renal capsule of an adult male nude mouse. The details for recombinant generation and grafting are described (Shaw et al., 2006). 40 μl of neutralized rat-tail collagen was mixed with prostasphere-only or rUGM-only and grafted as controls. Grafted tissues were harvested 4, 8 or 12 weeks, and half were fixed for sectioning and half snap frozen.
Histology
Dissected tissues were fixed in 4% paraformaldehyde overnight at 4°C, and 5 μm sections were obtained, dewaxed and stained with Mayer’s haematoxylin and eosin (H&E) for histological evaluation. Examination was performed on at least 10 fields from each of 5 sections per graft to determine the presence or absence of glandular structures.
Hoechst 33258 Staining
Dewaxed tissue sections were stained with Hoechst 33258 according to methods described previously (Cunha and Vanderslice, 1984).
Indirect immunofluorescent staining
Dewaxed sections were blocked with 3% BSA plus 10% goat or monkey normal serum (secondary antibody species). The primary antibody was added in blocking buffer (3% BSA in PBS) at concentration and incubation time listed below. The primary antibodies were: Mouse anti-E-cadherin (BD Biosciences, 1:200, Cat. #612130, room temperature 1 hour); Rabbit anti-Ki67 (Vector, 1:100, Cat. #VP-K451, 4°C overnight); Mouse anti-SMA (Sigma. 1:200, Cat.#A5228; room temperature 1 hour); Mouse anti-CK14 (Biogenix, 1:200, Cat.#AM146; 4°C overnight); Mouse anti-p63 (Santa Cruz, 1:50, Cat. #SC8431; 4°C overnight); Goat anti-Probasin (Santa Cruz, 1:200, Cat.#SC17124; room temperature 2 hours); Rabbit anti-Androgen Receptor (Santa Cruz, 1:250, Cat. #SC-816; 4°C overnight); Rabbit anti-GFP (Chemicon, 1:100; Cat. #B3080; 4°C overnight); Mouse anti-BrdU (Roche, 1:10, Cat. #1299964; 37°C 30 minutes); Rabbit anti-chromogranin A (Zymed, 1:100, Cat# 18-0094; 4°C overnight). After washing 3 times with PBS, the secondary antibody was added and incubated at room temperature or 1 hour. All secondary antibodies were diluted 1:200 in blocking buffer. The secondary antibodies were: Goat anti mouse Alexa 488 (Molecular Probes, Cat. #A11029); Goat anti rabbit Alexa 546 (1:200, Molecular Probes, Cat. #A 11035); Donkey anti-rabbit Alexa 594 (Molecular Probes, Cat. #A21207); Donkey anti goat Alexa 546 (Molecular Probes, Cat. #A11056). Fluorescent images were captured using an Olympus-BX51 fluorescent microscope (Olympus, Center Valley, PA).
RT-PCR
RNA was prepared using QIAshredder (QIAGEN, Valencia, CA) and RNeasy mini kit (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. One microgram of RNA was subjected to reverse transcription using SuperScript III first-strand cDNA synthesis system (Invitrogen, Carlsbad, CA) in a volume of 25 μl. Real-time PCR was carried out in an iCycler Thermal Cycler (Bio-Rad, Hercules, CA) using SYBR green (Applied Bioscience, Foster City, CA). The data were normalized with a GAPDH internal control. The primers used for mouse species-specific quantitative PCR were:
Sca-1 Forward 5’-CCATCAATTACCTGCCCCTA-3’
Sca-1 Reverse 5’-GGCAGATGGGTAAGCAAAGA-3’
CD133 Forward 5’-GAAAAGTTGCTCTGCGAACC-3’
CD133 Reverse 5’-TCTCAAGCTGAAAAGCAGCA-3’
α6-Integrin Forward 5’-AGCCCCAGGGACTTACAACT-3’
α6-Integrin Reverse 5’-CTTCATAGGGCCCATCTTCA-3’
Spermine binding protein Forward 5’- AGAGCCCAGAATGTCCTGGG-3’
Spermine binding protein Reverse 5’-TTATCACGTGCTCTCCGTCC-3’
Beta-microseminoprotein (PSP94) Forward 5’-TGG CTG GGC AGT CTC TTA TT-3’
Beta-microseminoprotein PSP94 Reverse 5’-AGC ATC CAT GCA GTC ATC AG-3’
mGAPDH Forward 5’- GCAGTGGCAAAGTGGAGATT-3’
mGAPDH Reverse 5’-CGTTGAATTTGCCGTGAGTG-3’
mProbasin Forward 5’- GGA GGA GAT GAC GGA GTT CA-3’
mProbasin Reverse 5’- ACA GTT GTC CGT GTC CAT GA-3’
mNKX3.1 Forward 5’- GAA AGC AGC TGT CGG AAG AC-3’
mNKX3.1 Reverse 5’- CGG AGA CCA AGG AGG TAC TG-3’
Statistical analysis
Statistical analysis of prostasphere generation, prostasphere growth and gene expression data were performed using unpaired t-test with assistance from the UW Madison Department of Surgery’s Statistical Core.
Results
The epithelial layer of the E16 CD-1 mouse UGS was mechanically and enzymatically isolated, dissociated and sieved to obtain a single cell suspension. Each UGS yielded approximately 2000 - 4,000 epithelial cells. When 10,000 cells were plated at low density in ultralow attachment plates in EGF and bFGF supplemented serum free media without DHT, they formed floating spheres of proliferating cells with an average of 60 spheres being generated from 10,000 cells. Histological examination of spheres revealed nearly solid spheres of viable, undifferentiated cells that increased progressively in diameter (Figure 1). After 15 days growth in culture, the prostaspheres were collected by gentle centrifugation, dissociated and sieved and re-plated at low density. The efficiency of sphere formation from the passaged cells was approximately 300 spheres from 10,000 cells, indicating an enhanced capacity for sphere formation among the cultured cells (Figure 1D). This enhanced capacity for sphere formation persisted for several passages. There was a progressive decrease in sphere-forming efficiency and sphere growth rate after the 5th generation (not shown). Even so, spheres could be serially passaged for up to 10 generations.
Figure 1.
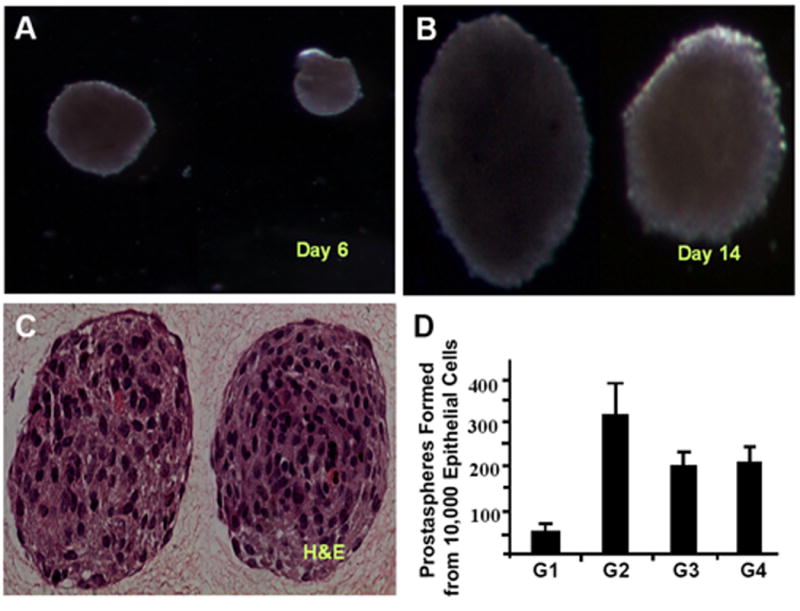
A single cell suspension of E16 epithelial cells cultured in anchorage-independent conditions exhibited growth in spheres. (A, B) Serial photographs during culture demonstrated progressive increase in prostasphere diameter. (C) Second generation prostaspheres from the E16 UGS were fixed in 4% paraformaldehyde, coated with collagen, paraffin embedded, and sectioned (4 um) and stained with H&E. (D) Efficiency of sphere formation on serial passage, for example from first generation to second generation (G1-G2), plotted as the number of prostaspheres generated from 10,000 epithelial cells.
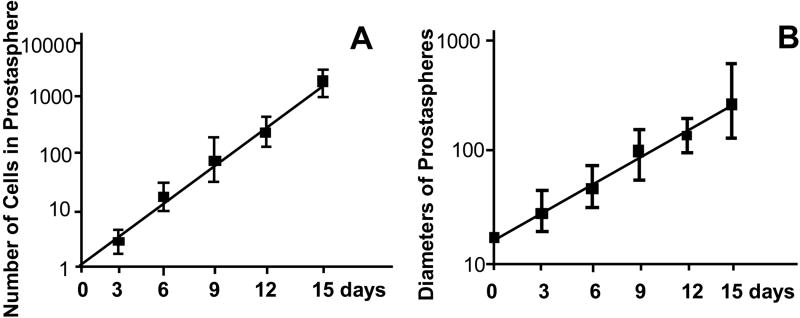
We next examined the effect of plating density on sphere formation and growth. First generation prostaspheres were dissociated into single cells and plated at densities of 100, 500, 1000, 2500, 5000, 10,000 cells per ml of culture media. Cells and spheres were observed daily, and the increase in average cell number and diameter of prostaspheres measured over the time course of culture. When cells were plated at low density (500 cells per ml culture media), microscopic examination performed within 24 hours revealed mostly single cells. Multicellular bodies appeared on day two, and there was a progressive increase in mean sphere diameter over 15 days. Measurements of sphere diameter and average number of cells/sphere produced linear curves on a semi-log plot. These curves closely approximated the predicted curves for an increase in sphere diameter from cell proliferation using a doubling time of 30 hours and the formula (4/3πR3)/(4/3πr3), where R is the diameter of prostasphere and r (25 μm) is the estimated diameter of an epithelial cell (Figure 2). These data indicate that sphere growth under these conditions results primarily from cell proliferation rather than amalgamation between spheres. The ability of single cells to form clonal spheres was confirmed by plating cells at low density in methylcellulose based colony assay media to prevent cell movement. We observed comparable efficiencies of sphere formation under these restrictive conditions (data not shown). Significant amalgamation can occur when cells are plated in anchorage-independent conditions at high-density (> 4000 cells/ml). Under these conditions, we observed multicellular bodies within 24 hours of plating, and both sphere diameter and average number of cells/sphere increased at a faster rate than predicted (data not shown). Since these data indicate plating at low density favors clonal sphere formation and discourages amalgamation, cells were plated at 500 cells per ml culture media for all subsequent experiments.
Figure 2.
Cells were plated at in anchorage independent conditions at a density of 500 cells per ml culture media and the increase in average cell number and diameter of prostaspheres measured daily. (A) Average number of cells per prostasphere. (B) Average diameter of prostaspheres. Data represent average of three independent experiments.
To determine whether culture of epithelial cells in anchorage independent conditions enriches for cells expressing putative stem cell markers as compared to culture of cells in monolayer dishes, we examined the expression of several putative prostate stem cell markers including Sca-1, CD-133 and α6-integrin (Richardson et al., 2004; Xin et al., 2005; Burger et al., 2005; Lawson et al., 2007). A single cell suspension made from enzymatically dissociated second generation (G2) prostaspheres was plated on either ultralow attachment plates or mammalian tissue culture plates and grown for 14 days. Comparison of gene expression by RT-PCR showed significantly higher expression of Sca-1 and CD-133 in cells grown in anchorage independent conditions (Figure 3). The average expression level of α6-integrin also trended higher in prostasphere-cultured cells but the difference was not statistically significant.
Figure 3.
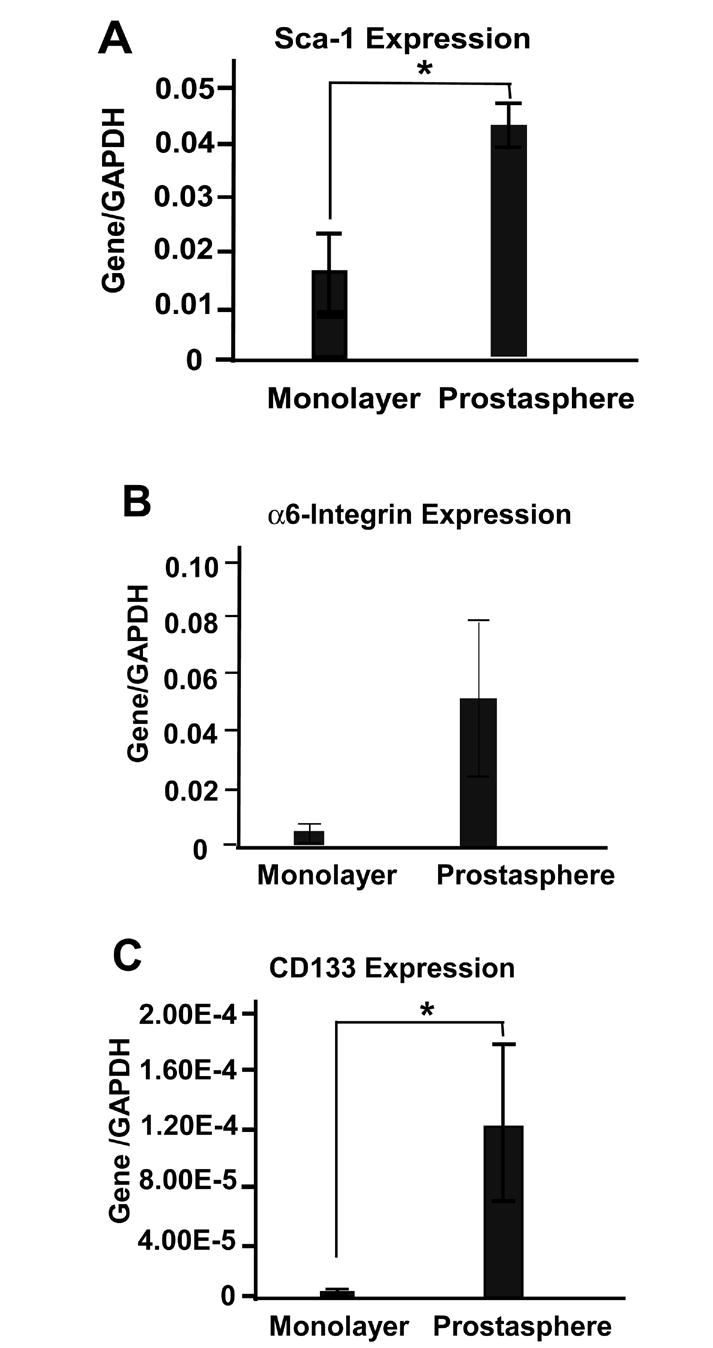
Cells isolated from second generation (G2) prostaspheres were cultured in either ultralow attachment plates or mammalian monolayer cell culture plates and gene expression determined by RT-PCR. * P<0.05
We next compared the prostasphere-forming ability of epithelial cells isolated from prostate tissue of various ages. Epithelial cells isolated from the E16 UGS, the P1 prostate and adult prostate formed spheres in anchorage independent culture that were morphologically and histologically indistinguishable (data not shown). Cells derived from the E16 UGS showed modestly higher (< 2 fold; P<0.05) prostasphere forming ability than cells isolated from either the P1 or adult prostate (Figure 4A). Examination of gene expression by RT-PCR in the developing and adult prostate showed a marked increase in Sca-1 expression from E14 to P5 and robust expression in all three lobes of the adult prostate. In contrast, there was little change in the expression of CD-133 and α6-integrin through most of the developmental stage, but the expression of both was modestly increased in the adult anterior prostate (AP) and dorsal-lateral prostate (DLP) (Figure 4 B-D).
Figure 4.
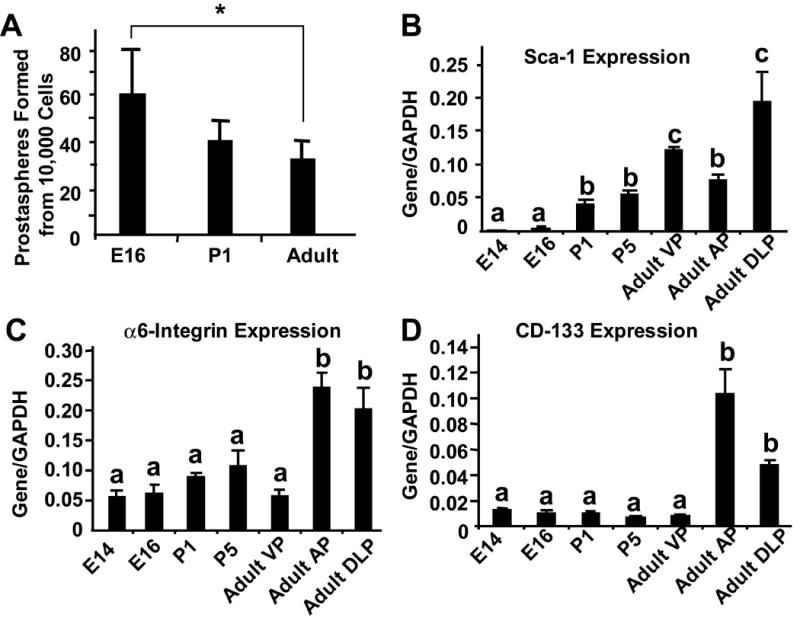
(A) Prostasphere forming ability epithelial cells of the E16 UGS, P1 and adult prostate was determined by plating 10,000 epithelial cells and counting the number of spheres formed after 9 days. * P<0.05. (B, C, D) Stem cell marker mRNA expression in the E14 and E16 UGS and the P1, P5 and adult prostate determined by RT-PCR. a,b,c denote groups with significantly different levels of expression (a and b: P<0.05; b and c: P< 0.05; a and c: P<0.01).
The adult prostate exhibits widespread epithelial apoptosis and involution in response to castration and robust re-growth in response to subsequent androgen supplementation. Tissue specific stem cells are postulated to be androgen independent and to serve as the basis for epithelial repopulation during androgen induced re-growth. When we examined the effect of castration on expression of Sca-1, CD-133 and α6-integrin, we did not observe any consistent pattern of changes (Figure 5 A-C). However, when we compared the prostasphere forming ability of epithelial cells isolated from the three lobes of the prostate in castrated and sham operated mice, we observed a significantly higher efficiency of prostasphere formation in cells isolated from the castrate mouse prostate (Figure 5D). The magnitude of the observed increase is comparable to the enrichment for stem cells as calculated by other methods, including in vivo grafting assays and soft agar cloning assays (Lawson, et al., 2007), suggesting that prostasphere forming efficiency may serve as a surrogate measure of stem cell activity.
Figure 5.
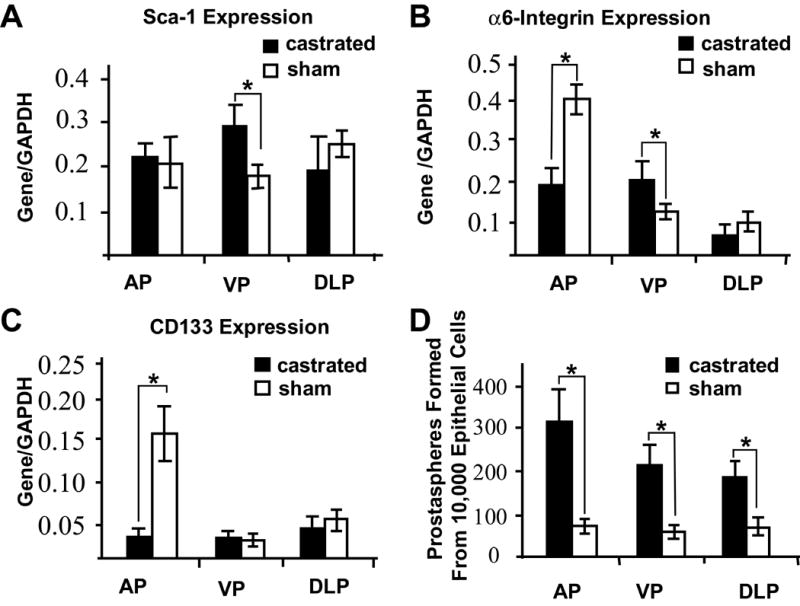
Stem cell marker gene expression level is not a good indicator for stem cell activity. Adult mice were castrated or sham-castration as control. AP, VP and DLP were isolated to assess stem cell marker gene expression or prostasphere forming ability assay. (A-C) Stem cell marker expression in the castrated and sham-operated adult prostate. (D) Sphere-forming ability of epithelial cells isolated from the different prostate lobes of the intact adult and 14 days after castration.
* P<0.01.
To determine whether prostaspheres contain bona fide epithelial progenitor cells, we examined their regenerative potential in vivo. We first assayed the ability of prostaspheres alone to regenerate prostate tissue. Approximately 30 G2 prostaspheres (equivalent to 100,000 cells) derived from either the E16 UGS (n=3) or adult prostate (n=4) were mixed with 30 μl rat-tail collagen and grafted under the renal capsule of a male nude mouse. Histological examination of the grafts 4 weeks later showed no evidence of prostatic duct formation and RT-PCR assay revealed no expression of prostate specific markers (data not shown).
We then tested the ability of prostaspheres to regenerate prostate tissue when grafted together with E17 rUGM. G2 prostaspheres derived from either E16 mouse UGS or adult mouse prostate epithelial cells were grafted with embryonic rUGM to the renal capsule of male adult nude mouse, and the grafted tissue was retrieved after 4, 8 and 12 weeks growth in vivo. After 4 weeks in vivo growth, the grafts exhibited ductal development with some canalized lumina. Glandular lumina were larger in renal grafts grown for 8 and 12-weeks and contained large amounts of luminal secretion. Grafting of G2 prostaspheres derived from E16 mouse UGS (n= 5) or adult mouse prostate epithelial cells (n=10) together with rUGM consistently yielded recombinant grafts with characteristic prostate glandular morphology (Figure 6A, C, D). Neither grafts of rUGM alone (not shown) nor grafts of rUGM combined with G1 cells cultured in monolayer for 14 days (n=7) generated any tissues with prostate-like morphology (Figure 6 E, F). Several other investigators have found that prostate epithelial cells can maintain the ability to regenerate prostate ducts in recombination assays. Some of these studies utilized an established epithelial cell line, while others used epithelial cells isolated from the retinoblastoma null mouse or transformed cancer cell lines (Hayward et al., 1999, Day et al., 2002, and Gu et al., 2007). The reason for the discrepancy with our observations using primary epithelial cells cultured in monolayer are unknown, but may relate to separation of the primary epithelial cells from stromal cells or the monolayer culture conditions. The mouse origin of the glandular epithelium in recombinant grafts was demonstrated by three independent methods. We stained grafts with Hoechst 33258 to distinguish cells of mouse and rat origin (Cunha and Vanderslice 1984), and observed brightly speckled staining of the nuclei in the glandular epithelium consistent with mouse origin (data not shown). We made prostaspheres from the adult prostate of the GFP mouse, recombined G2 prostaspheres with rUGM and immunostained the matured grafts for GFP (Figure 7A). Finally, we performed RT-PCR for the prostate epithelial markers Nkx3.1 and Probasin (Doles et al., 2005) using mouse species-specific primers (Figure 7B). Immunostaining was performed for several epithelial and stromal markers, confirming the expression of androgen receptor in the glandular epithelium, expression of smooth muscle actin in the periglandular stroma, showing the presence of neuroendocrine cells, demonstrating the presence of the basal cell markers p63 and CK14 and showing robust staining for probasin peptide in the luminal epithelium (Figure 8). This expression was not observed in grafts made by combining cells cultured in monolayer and subsequently grown in vivo with rUGM. To determine if the regenerative potential of cells grown in anchorage independent conditions was maintained through serial passage, we performed tissue recombination assays using third (G3) and fourth (G4) generation prostaspheres. The result demonstrated that prostaspheres maintain their regenerative capacity through 4 passages, but with a diminishing rate of success at later passages (Table 1).
Figure 6.
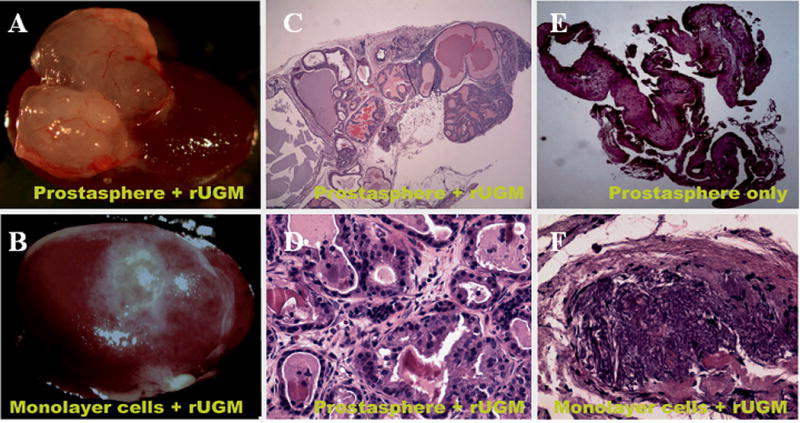
Second generation prostaspheres or monolayer-cultured cells were mixed with E17 rUGM and grafted under renal capsule of an adult male nude mouse. Grafted tissues were retrieved 8 weeks later. The representative photomicrograph of retrieved tissue from (A) prostasphere and (B) monolayer-cultured prostate epithelial cells grafted with rUGM. H&E stained tissue sections show mouse glandular epithelial ducts developed from prostasphere cultured epithelial cells with rUGM (C and D) but not from prostasphere only (E) or monolayer cultured cells with rUGM (F).
Figure 7.
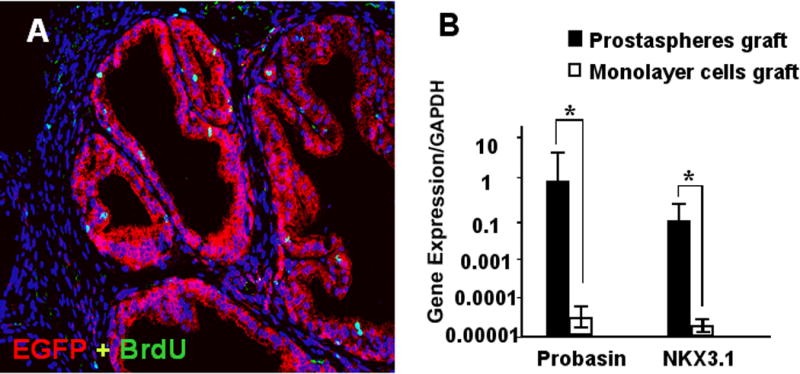
(A) Prostaspheres generated from adult GFP transgenic mice were recombined with E17 rUGM and grafted. BrdU labeling was performed prior to harvest 8 weeks later. Immunostaining with GFP (red) demonstrated that glandular epithelial ducts were derived from prostasphere cultured mouse prostate epithelial cells. A small number of stromal cells also stain positive for GFP. BrdU staining (green) indicated proliferation of both epithelial and stromal cells. (B) Real-time RT-PCR was performed using mouse species -specific primers to probasin and NKX3.1. The expression levels of Probasin and Nkx3.1 are >1000-fold higher in grafts made from prostaspheres combined with rUGM than in grafts made with monolayer cells.
Figure 8.
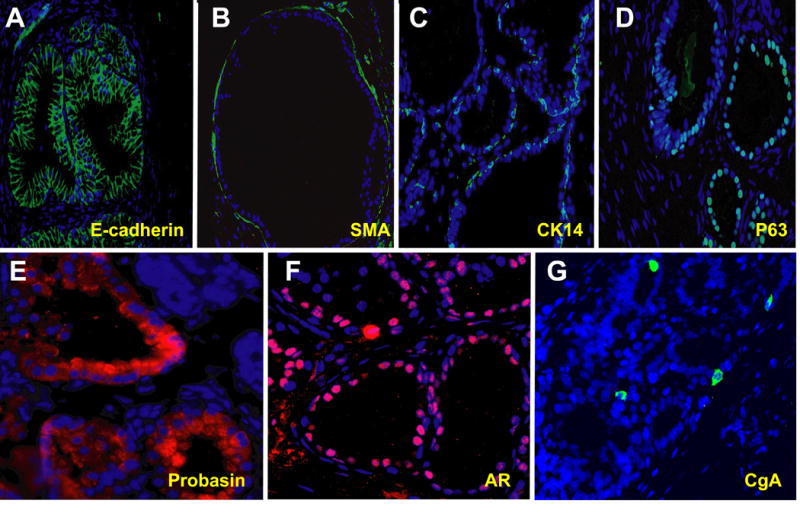
Immunostaining of grafts made with second generation prostaspheres for markers of prostate stromal and epithelial differentiaation, including (A) E-cadherin, (B) Smooth muscle actin, (C) Cytokeratin 14 (CK14), (D) p63, (E) Probasin, (F) Androgen receptor and (G) Chromogranin A.
Table 1.
Prostasphere Cultured Prostate Epithelial Cells Tissue Regenerative Ability
| Passage of Prostatspheres | Number of Tissue Renal Grafted (n) | Glandular Epithelial Ducts Developed* (n) | Rate (%) |
|---|---|---|---|
| G2 | 15 | 14 | 93.3 |
| G3 | 12 | 9 | 75.0 |
| G4 | 6 | 4 | 66.7 |
Based on histology, RT-PCR, and immunostaining
No glandular epithelial ducts developed in control groups
We next tested whether cells grown in prostaspheres exhibit the plasticity expected of primitive prostate progenitor cells by determining whether cells isolated from one lobe can be re-programmed to express genes specific for another lobe. Prostate epithelial cells were isolated from either ventral or dorsal-lateral prostate, cultured as prostaspheres, then mixed with E17 rUGM and grafted to the renal capsule of a male nude mouse. The expression level of VP marker spermine binding protein (SBP) and lateral prostate marker beta-microseminoprotein (PSP94) was determined by RT-PCR (Lin et al., 2002), and probasin expression was used to normalize terminal differentiation and overall growth and development of grafted tissue. The results showed that there is no statistical significance in expression levels of SBP and PSP94 in grafts made with cells isolated from VP or DLP (Figure 9). In adult mouse prostate, DLP does not express SBP, however, prostasphere cultured epithelial cells isolated from DLP express SBP when combined with embryonic rUGM and grown in vivo. This result demonstrates that progenitor cells isolated from DLP maintained the ability to express genes specific to VP. This is in agreement with previous studies that UGM is both a ventral and a dorsal inducer (Kinbra et al., 1996).
Figure 9.
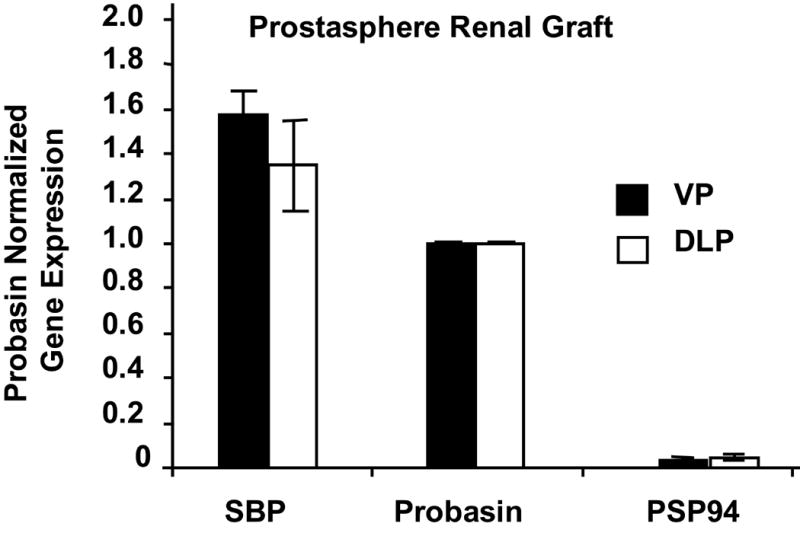
Prostaspheres generated from epithelial cells isolated from VP or DLP grafted with E17 rUGM generate prostate tissue expressing equivalent levels of VP- and DLP-specific markers SBP and PSP94 respectively. SBP and PSP94 gene expression were normalized to probasin expression for each grafts
This experiment demonstrated that cells isolated from the adult prostate and cultured in anchorage independent conditions exhibit the pleuripotent differentiation phenotype of the urogenital sinus epithelium. This observation prompted us to explore the possibility that slow cycling (label-retaining) cells of the adult prostate, which have been implicated as quiescent stem or progenitor cells (Tjusimura et al., 2002) are descendents of urogenital sinus epithelial cells that undergo limited proliferation during early prostate development. We labeled the developing prostate in utero by BrdU administration at E16. Prostate tissues were harvested at selected time points during development (P1, P5, P15, P30 and adult), sectioned and immunostained for BrdU. There was generous labeling of both epithelial and mesenchymal cells at early time points (not shown). The percentage of labeled epithelial cells progressively diminished during postnatal development but a small number of strongly labeled cells persisted in the ductal epithelium of the adult prostate and usually co-stained for the basal cell marker p63 (Figure 10).
Figure 10.
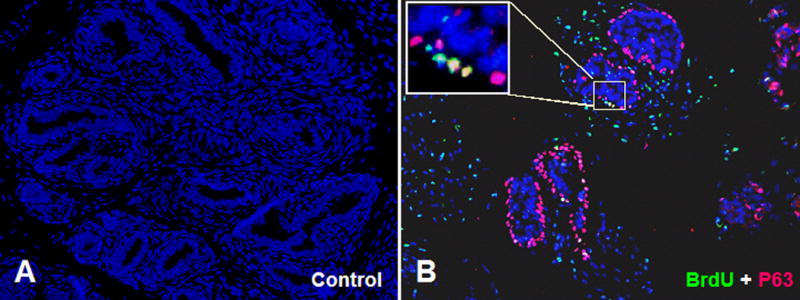
In utero BrdU labeling was performed at E16. BrdU labeled prostate tissue was collected 8 weeks later, paraffin embedded, sectioned and immunostained for BrdU and p63. (A) Control without any primary antibody. (B) Tissue stained for both BrdU (green) and p63 (red). Small numbers of label-retaining epithelial cells are observed that co-stain for BrdU and p63 (inset).
Discussion
The use of grafting of the UGS to the renal capsule for the study of prostate development was pioneered by Cunha (1972) and Turner et al. (1990) who reported that adult mouse prostate epithelial cells grown in collagen can regenerate prostate when combined with rUGM. The studies reported here validate anchorage independent culture as a reliable method to maintain and grow prostate progenitor epithelial cells in vitro. Our studies provide a sorely needed methodology to enable detailed studies of prostate stem cell biology. We recently showed that grafting of the UGS under the renal capsule faithfully recapitulates the canonical process of prostatic differentiation (Doles et al., 2005) and variations of this technique have been used previously by others to evaluate stem cell function in isolated prostate epithelial preparations (Xin et al., 2005; Burger et al., 2005). We employed a combined strategy including immunohistochemical staining and RT-PCR using species-specific primers to demonstrate that prostasphere-derived epithelial cells generated prostate glandular epithelium containing both basal and luminal cells and exhibiting robust expression of terminal differentiation markers. Physical separation of the E16 UGS epithelium and mesenchyme is an efficient way to obtain a highly purified epithelial cell preparation. We began our studies of anchorage-independent culture with fetal epithelial cells obtained in this way so as to minimize stromal cell contamination. We subsequently developed the methodology to create a highly purified population of epithelial cells from the P1 and adult prostate and demonstrated that cells from all three ages generated prostaspheres with comparable efficiency and with the same capacity to generate fully differentiated prostate epithelium when recombined with fetal mesenchyme. Despite our efforts to eliminate contaminating stromal cells, immunostaining for stromal and epithelial markers showed that some prostaspheres contain both epithelial and stromal cells (data not shown). This may not be unexpected since the growth of stromal cells would be more robust than the epithelial component. The separation of stromal from epithelial cells is never complete and so some stromal cells may contain in the epithelial portion. One might also speculate that progenitor cells can give rise to both stromal and epithelial lineage. Indeed, GFP staining of prostasphere-derived cells from the GFP mouse recombined with rUGM and grafted into a nude host mouse showed a small number of GFP+ stromal cells in the resulting graft (Figure 7). Stromal cell contamination is the most probable explanation for this finding. We cannot exclude the possibility of a common progenitor for epithelial and stromal cell lineages; however, our finding that prostaspheres grafted without rUGM do not generate prostate glandular tissue argues against this possibility. Our studies, like other recent studies of neurosphere formation (Singec, et al., 2006), suggest that prostaspheres can be formed either from a single cell or by a process of amalgamation depending on the density at which the cells are plated. We speculate that some amalgamation occurs, even when cells are plated under dilute conditions, producing some spheres of multi-cellular origin including both epithelial and stromal cells.
Previous studies have suggested that the stem cell or primitive progenitor cells of the adult mouse prostate are concentrated in the proximal duct segments (Tsujimura et al., 2002; Burger et al., 2005; Salm et al., 2005; Goto, et al., 2006; Xin et al., 2005; Lawson et al., 2007). Tsujimura et al. (2002) utilized BrdU pulse labeling at 3 weeks postnatal followed by multiple rounds of castration-induced regression and testosterone-induced regeneration to demonstrate the presence of slow-cycling cells in the prostate ductal epithelium. This population of cells, which localized preferentially to the proximal duct segments, was postulated to include the primitive progenitor cells of the adult prostate epithelium. Subsequent studies appear to have confirmed this inference. The epithelium of the proximal duct segments has been shown to be enriched in regenerative potential. Sca-1+ cells have been shown to contain a subpopulation of cells coexpressing α6-integrin (Burger et al., 2005). FACS sorting for Sca-1 and α6-integrin enriches for epithelial cells with regenerative potential in tissue recombination assays and soft agar cloning assay (Burger et al., 2005; Lawson et al., 2007). CD133 has also been proposed as prostate epithelial stem cell marker, but the relationship between CD133 and other prostate stem cell markers has not been fully explored (Richardson et al., 2004). Although prostate epithelial cells quickly lose their regenerative potential when placed into monolayer, our studies suggest that sphere culture allows the survival and proliferation of epithelial cells that includes a population of bona fide progenitor cells. We noted that culture under these conditions is associated with maintained expression of the putative stem cell markers Sca-1, α6-integrin and CD-133.
However, our studies show that stem cell marker mRNA expression by itself is an unreliable index for progenitor cell activity. Castration of the adult mouse induces apoptosis of terminally differentiated, androgen-dependent prostate luminal epithelial cells while sparing the androgen independent cells that include basal cells and some luminal cells (Reuter, 1997). Rapid regeneration of the glandular epithelium occurs in response to administration of exogenous testosterone and several different assays have shown that epithelial cells of the castrate prostate possess higher regenerative potential compared to cells from intact adult prostate (Xin et al., 2005; Lawson et al., 2007). We found castration-induced regression of the adult prostate was associated with only a moderate change in the mRNA expression levels of stem cell markers, even though we observed significantly increased efficiency of sphere formation by cells isolated from all three prostate lobes of the castrated mouse suggesting that sphere formation can be used as a surrogate index of progenitor cell content (Figure 5). In profiling expression of Sca-1, α6-integrin and CD133 expression from E14 to adult, we noted a progressive rise in Sca-1 expression along with modest increases in CD-133 and α6-integrin expression (Figure 4). In contrast, sphere-forming efficiency was actually slightly lower for epithelial cells isolated from either the P1 or adult prostate than for cells isolated from the E16 UGS (Figure 4). These findings indicate that global measures of stem cell marker mRNA expression do not correlate with progenitor cell activity and reinforce the notion that it is demonstrated epithelial co-expression of surface markers such as Sca-1 and α6-integrin that is the distinguishing feature of prostate epithelial progenitor cells.
Our studies revealed that some of the UGS epithelial cells pulse-labeled at E16 take up residence as slow-cycling cells in the adult prostate. This observation prompts us to speculate that these slow-cycling cells are a component of the adult prostate epithelial stem cell population (Figure 10). This concept that resonates with previous studies by Wang et al., (2001) suggesting the presence of functionally equivalent stem cells in the UGS epithelium and adult. It also is consistent with the shared capacity, demonstrated here, for epithelial cells from the E16 UGS and adult prostate to form spheres in anchorage independent culture that exhibit an equivalent potential for lobe-specific differentiation in tissue recombination studies. Following the studies of Cunha (1972), Norman et al. 1986 demonstrated that isolated epithelium from the adult prostate combined with embryonic UGM can form prostate tissue in graft. These studies suggested an equivalence between cells in UGS and adult prostate tissue. Several studies have highlighted the role of stem cells in brain and mammary gland development (Fricker-Gates et al., 2004; Liu et al 2005; Chepko and Smith 1999), but our studies provide the first evidence that suggests equivalence between stem cells in the developing and adult prostate and, as such, opens the way to using the well characterized and tractable system of prostate development for the study of stem cell regulation and function.
Acknowledgments
This work was supported in part by grant from National Institutes of Health (NIDDK R01 DK056238-05), a Prostate Cancer Foundation Research Award, and an American Urological Association Foundation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL. Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue. Proc Natl Acad Sci U S A. 2005;102:7180–5. doi: 10.1073/pnas.0502761102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepko G, Smith GH. Mammary epithelial stem cells: our current understanding. J Mammary Gland Biol Neoplasia. 1999;4(1):35–52. doi: 10.1023/a:1018752519356. [DOI] [PubMed] [Google Scholar]
- Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114:3865–72. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Epithelio-mesenchymal interactions in primordial gland structures which become responsive to androgenic stimulation. Anat Rec. 1972;172:179–95. doi: 10.1002/ar.1091720206. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7–12. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- Cussenot O, Villette JM, Cochand-Priollet B, Berthon P. Evaluation and clinical value of neuroendocrine differentiation in human prostatic tumors. Prostate Suppl. 1998;8:43–51. Review. [PubMed] [Google Scholar]
- Day KC, McCabe MT, Zhao X, Wang Y, Davis JN, Phillips J, Von Geldern M, Ried T, KuKuruga MA, Cunha GR, Hayward SW, Day ML. Rescue of embryonic epithelium reveals that the homozygous deletion of the retinoblastoma gene confers growth factor independence and immortality but does not influence epithelial differentiation or tissue morphogenesis. J Biol Chem. 2002;277:44475–84. doi: 10.1074/jbc.M205361200. [DOI] [PubMed] [Google Scholar]
- Doles JD, Vezina CM, Lipinski RJ, Peterson RE, Bushman W. Growth, morphogenesis, and differentiation during mouse prostate development in situ, in renal grafts, and in vitro. Prostate. 2005;65(4):390–9. doi: 10.1002/pros.20321. [DOI] [PubMed] [Google Scholar]
- Foster CS, Dodson A, Karavana V, Smith PH, Ke Y. Prostatic stem cells. J Pathol. 2002;197(4):551–65. doi: 10.1002/path.1194. [DOI] [PubMed] [Google Scholar]
- Fricker-Gates RA, White A, Gates MA, Dunnett SB. Striatal neurons in striatal grafts are derived from both post-mitotic cells and dividing progenitors. Eur J Neurosci. 2004;19(3):513–20. doi: 10.1111/j.1460-9568.2004.03149.x. [DOI] [PubMed] [Google Scholar]
- Goto K, Salm SN, Coetzee S, Xiong X, Burger PE, Shapiro E, Lepor H, Moscatelli D, Wilson EL. Proximal prostatic stem cells are programmed to regenerate a proximal-distal ductal axis. Stem Cells. 2006;24(8):1859–68. doi: 10.1634/stemcells.2005-0585. [DOI] [PubMed] [Google Scholar]
- Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–15. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Baskin LS, Haughney PC, Cunha AR, Foster BA, Dahiya R, Prins GS, Cunha GR. Epithelial development in the rat ventral prostate, anterior prostate and seminal vesicle. Acta Anat (Basel) 1996;155(2):81–93. doi: 10.1159/000147793. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Lopes ES, Danielpour D, Cunha GR. The rat prostatic epithelial cell line NRP-152 can differentiate in vivo in response to its stromal environment. Prostate. 1999;39:205–12. doi: 10.1002/(sici)1097-0045(19990515)39:3<205::aid-pros9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Hudson DL, O’Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80(8):1243–50. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Hernandez I, Wei Y, Greenberg N. Isolation and characterization of mouse probasin: An androgen-regulated protein specifically expressed in the differentiated prostate. Prostate. 2000;43(4):255–62. doi: 10.1002/1097-0045(20000601)43:4<255::aid-pros4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kinbara H, Cunha GR, Boutin E, Hayashi N, Kawamura J. Evidence of stem cells in the adult prostatic epithelium based upon responsiveness to mesenchymal inductors. Prostate. 1996;29(2):107–16. doi: 10.1002/(SICI)1097-0045(199608)29:2<107::AID-PROS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–64. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Lamm ML, Podlasek CA, Barnett DH, Lee J, Clemens JQ, Hebner CM, Bushman W. Mesenchymal factor bone morphogenetic protein 4 restricts ductal budding and branching morphogenesis in the developing prostate. Dev Biol. 2001;232:301–14. doi: 10.1006/dbio.2001.0187. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007;104(1):181–6. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Ko K, Moore RW, Simanainen U, Oberley TD, Peterson RE. Effects of aryl hydrocarbon receptor null mutation and in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on prostate and seminal vesicle development in C57BL/6 mice. Toxicol Sci. 2002;68(2):479–87. doi: 10.1093/toxsci/68.2.479. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol. 2005;283(2):437–45. doi: 10.1016/j.ydbio.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Miki J, Furusato B, Li H, Gu Y, Takahashi H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S, Rhim JS. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67(7):3153–61. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- Norman JT, Cunha GR, Sugimura Y. The induction of new ductal growth in adult prostatic epithelium in response to an embryonic prostatic inductor. Prostate. 1986;8:209–20. doi: 10.1002/pros.2990080302. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Leung GK, Wong ST. Keratin expression: a measure of phenotypic modulation of human prostatic epithelial cells by growth inhibitory factors. Cell Tissue Res. 1994;277(1):11–8. doi: 10.1007/BF00303075. [DOI] [PubMed] [Google Scholar]
- Price D. Comparative aspects of development and structure in the prostate. Natl Cancer Inst Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- Ravindranath N, Dym M. Isolation of rat ventral prostate basal and luminal epithelial cells by the STAPUT technique. Prostate. 1999;41:173–80. doi: 10.1002/(sici)1097-0045(19991101)41:3<173::aid-pros4>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Reuter VE. Pathological changes in benign and malignant prostatic tissue following androgen deprivation therapy. Urology. 1997;49:16–22. doi: 10.1016/s0090-4295(97)00164-7. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–74. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117(Pt 16):3539–45. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37(3):149–60. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. J Cell Biol. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A, Papadopoulos J, Johnson C, Bushman W. Isolation and characterization of an immortalized mouse urogenital sinus mesenchyme cell line. Prostate. 2006;66(13):1347–58. doi: 10.1002/pros.20357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, Dhar S, Majumder P, McKeon F, Kantoff PW, Sellers WR, Loda M. p63 regulates commitment to the prostate cell lineage. Proc Natl Acad Sci U S A. 2005;102(32):11355–60. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3(10):801–6. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34(5):973–83. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–65. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, Bern HA, Young P, Cunha GR. Serum-free culture of enriched mouse anterior and ventral prostatic epithelial cells in collagen gel. In Vitro Cell Dev Biol. 1990;26:722–30. doi: 10.1007/BF02624429. [DOI] [PubMed] [Google Scholar]
- Verhagen AP, Aalders TW, Ramaekers FC, Debruyne FM, Schalken JA. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13(1):25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68(45):270–9. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci U S A. 2005;102:6942–7. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2(3):305–16. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]