Abstract
Leukotrienes (LTs) are powerful proinflammatory lipid mediators that may play a central role in cardiovascular diseases, including arteriosclerosis, myocardial infarction, and stroke. Owing to restricted expression of 5-lipoxygenase (5-LO), the enzyme required for their synthesis, LTs are almost exclusively produced by myeloid cells. Here, we report that human cytomegalovirus (HCMV) infection of human vascular smooth muscle cells (SMCs) increases 5-LO mRNA levels by up to 170-fold in a dose- and time-dependent manner. Infected cells expressed 5-LO protein, as shown by immunohistochemistry, enabling them to synthesize bioactive LTB4. HCMV-infected vascular SMCs expressing 5-LO protein were readily detected in tissue samples from CMV-infected patients with inflammatory bowel disease or AIDS. Thus, pathogen-induced LT production in HCMV-infected tissues may contribute to local inflammation, consistent with the ability of HCMV to control cellular and immunological functions. HCMV-induced LT biosynthesis in SMCs offers a molecular mechanism to explain HCMV-induced pathogenesis in inflammatory diseases.
Leukotrienes (LTs) are powerful proinflammatory and immune-modulating lipid mediators that are synthesized from arachidonic acid. The LT family is divided into two classes, the classical chemoattractant LTB4 and spasmogenic cysteinyl LTs (cys-LTs), such as LTC4, LTD4, and LTE4. LTs are involved in chronic inflammatory conditions, and LTB4 appears to be important in cardiovascular disease, including arteriosclerosis, myocardial infarction, and stroke (1, 2). 5-Lipoxygenase (5-LO), the enzyme that catalyzes the first two steps in the conversion of arachidonic acid to LTs, is a key determinant of LT biosynthesis. Its expression is tightly regulated and essentially restricted to myeloid cells (3–5). Two downstream enzymes, LTA4 hydrolase (LTA4H) and LTC4 synthase (LTC4S), give rise to the two classes of bioactive LTs, LTB4 and cys-LTs.
Human CMV (HCMV) is a member of the β-herpes virus family, which latently infects a majority of the world's population. The infection is generally asymptomatic but may cause severe disease in immunocompromised patients. HCMV is frequently detected in tissues from patients with inflammatory diseases such as autoimmune diseases and vascular diseases (6). The virus establishes latency in myeloid lineage cells and is reactivated by inflammation. The unique ability of HCMV to compromise cellular and immunological functions in the host may be a critical factor in the pathogenesis of inflammatory diseases (6–8). However, a clear cause–effect relationship has yet to be determined.
Here, we report that HCMV infection triggers expression of active 5-LO in human vascular smooth muscle cells (SMCs), enabling them to synthesize LTB4. The ability of HCMV to trigger LT biosynthesis in nonmyeloid cells offers a molecular mechanism to explain HCMV-induced pathogenesis in inflammatory diseases.
RESULTS AND DISCUSSION
HCMV induces expression of LT biosynthetic genes in SMCs
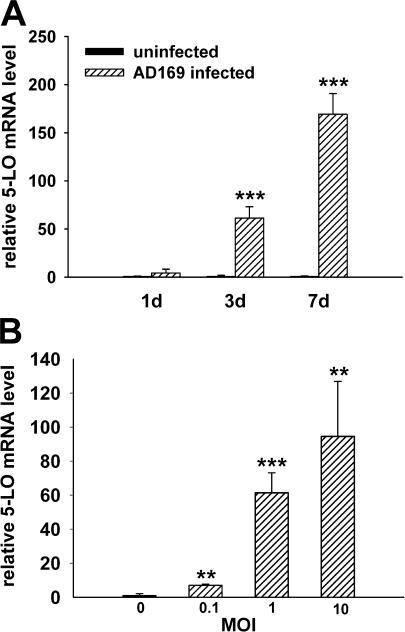
Human pulmonary arterial SMCs (HPASMCs) were infected with the HCMV laboratory strain AD169. At 1, 3, and 7 d after infection, mRNA levels of genes involved in LT biosynthesis were determined by quantitative real-time PCR. Expression of 5-LO mRNA was prominently up-regulated and increased in a time- and dose-dependent manner. The mean 5-LO mRNA levels were more than 60-fold higher at 3 d after infection and nearly 170-fold higher at 7 d after infection than in uninfected controls (Fig. 1 A). In dose–response experiments, 5-LO mRNA levels at 3 d after infection were increased sevenfold by HCMV at a multiplicity of infection (MOI) of 0.1, 61-fold at an MOI of 1, and 95-fold at an MOI of 10 (Fig. 1 B).
Figure 1.
HCMV infection increases 5-LO mRNA expression in HPASMCs. mRNA levels were determined by real-time PCR and normalized to β-2-microglobulin mRNA levels. 5-LO mRNA expression in HPASMCs 1, 3, and 7 d after infection with HCMV at an MOI of 1 (A) and 3 d after infection with HCMV at an MOI of 0.1, 1, and 10 (B). n = 6. Values are mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus uninfected cells.
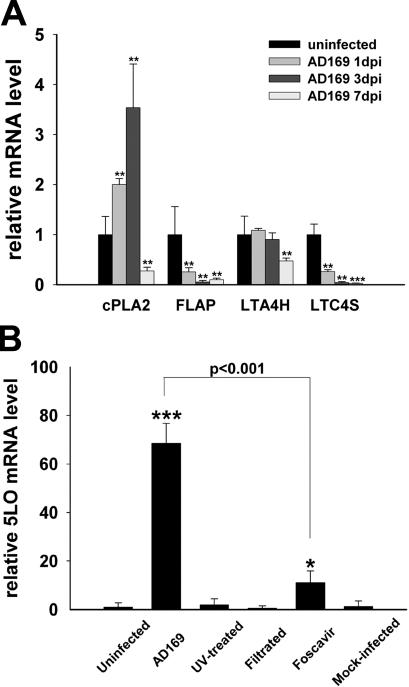
Other genes involved in LT biosynthesis were differentially regulated by HCMV infection, albeit to a lesser extent. Cytosolic phospholipase A2 (cPLA2) mRNA levels were increased twofold at 1 d after infection and 3.5-fold at 3 d after infection but were decreased at 7 d after infection compared with controls. LTA4H was stably expressed at 1 and 3 d after infection but declined to about half the control level at 7 d after infection. mRNA expression of 5-LO activating protein (FLAP) and LTC4S was reduced at all three time points (Fig. 2 A).
Figure 2.
Other genes in LT biosynthesis pathway are differentially regulated by HCMV infection. (A) Expression of cPLA2, LTA4H, FLAP, and LTC4S in HPASMCs 1, 3, and 7 d after infection with HCMV at an MOI of 1. n = 6. (B) Expression of 5-LO mRNA in HPASMCs exposed for 3 d to virus (AD169), UV-irradiated virus, viral inocula filtrated through a 0.1-μm pore filter, virus in the presence of 5 mM Foscavir, or SMC supernatant (Mock-infected). n = 3. Values are mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus uninfected cells.
Late HCMV gene expression mediates the increase in 5-LO mRNA
To determine if viral replication is essential for the induction of 5-LO mRNA, we infected cells with UV-irradiated virus or filtrated viral inocula. None of these treatments induced 5-LO mRNA expression, indicating that this effect was mediated by HCMV gene expression and not by soluble factors in the viral stock preparations. Foscavir, which inhibits late viral gene expression, blocked the induction of 5-LO mRNA by 90% (Fig. 2 B).
HCMV infection induces expression of bioactive 5-LO protein
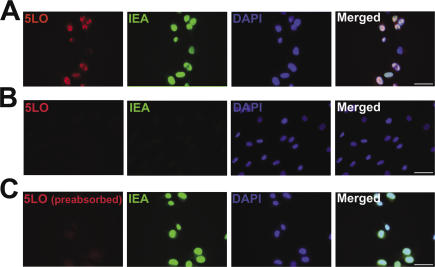
Immunofluorescence staining was performed to determine if the HCMV-induced 5-LO mRNA is translated into 5-LO protein. When HPASMCs were exposed to HCMV (MOI of 10) for 3 d, the majority of infected cells that showed positive staining for HCMV immediate-early antigen (IEA) was also positive for 5-LO protein; however, 5-LO protein was not detected in any of the uninfected cells (Fig. 3).
Figure 3.
HCMV infection induces 5-LO protein expression in HPASMCs. (A) HPASMCs were infected with HCMV at an MOI of 10. At 3 d after infection, the cells were stained simultaneously with antibodies against 5-LO (Cy3, red) and HCMV IEA (FITC, green), and with DAPI (blue) to stain DNA. (B) Uninfected HPASMCs were stained for 5-LO (Cy3, red), HCMV IEA (FITC, green), and DNA (DAPI, blue). (C) HPASMCs were infected with HCMV at an MOI of 10 and stained at 3 d after infection for 5-LO, with primary antibodies pre-absorbed with purified 5-LO protein (Cy3, red), HCMV IEA (FITC, green), and DNA (DAPI, blue). Bar, 50 μm.
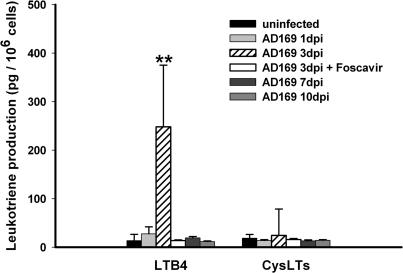
To assess the function of the HCMV-induced 5-LO protein, we measured the cellular production of LTs from infected and uninfected HPASMCs by HPLC and enzyme immunoassay. As expected, neither uninfected nor infected cells could produce a detectable amount of LTs without stimulation (not depicted). However, incubation of the HCMV-infected HPASMCs at 3 d after infection with calcium ionophore led to a significant production of LTB4 (Fig. 4), providing indisputable evidence of 5-LO activity. Most likely, this biosynthetic activity was facilitated by the increased expression of cPLA2, which is critical for mobilizing endogenous arachidonic acid. Production of LTB4 could not be detected 7 d after infection (Fig. 4), possibly due to the down-regulation of both cPLA2 and LTA4H (Fig. 2 A). Although FLAP was down-regulated, it was sufficiently expressed to support 5-LO activity and conversion of arachidonic acid to LTA4 for subsequent hydrolysis by LTA4H into LTB4. It is also possible that in SMCs, FLAP is not required by 5-LO to obtain a fully active enzyme complex for the generation of LTB4. In fact, it was recently reported that 5-LO activity can also be supported by a soluble coactosine-like protein in a manner resembling the effects of FLAP (9). The lack of cys-LTs production reflects the fact that HCMV infection rapidly down-regulated LTC4S, the critical enzyme in the biosynthesis of LTC4, the parent compound of all cys-LTs (Fig. 2 A). We further examined whether Foscavir could affect LTB4 production, and found that exposure of HCMV-infected cells to Foscavir blocked LTB4 production (Fig. 4). These observations suggest a potential therapeutic impact of Foscavir related to prevention of the viral induction of 5-LO and LTB4 biosynthesis.
Figure 4.
Production of LTs from HCMV-infected HPASMCs. HPASMCs were infected with HCMV at an MOI of 10 for 1, 3, 7, and 10 d, collected, washed, and incubated with 2 mM Ca2+ and 2.5 μM ionophore for 5 min at 37°C. LTB4 and cys-LTs were purified by solid-phase extraction and HPLC and measured with an enzyme immunoassay. n = 6. Values are mean ± SD. **, P < 0.01 versus uninfected cells.
Although the 5-LO pathway is considered specific for myeloid cells, it has been reported that 5-LO can be expressed in nonmyeloid cells under certain conditions (10–14). However, in these studies the mechanism of 5-LO induction, its potential impact on LT production, and the relevance for a biological context have not been addressed, and hence, the role of 5-LO outside of myeloid cells remains largely unknown.
HCMV induces 5-LO expression in human tissues
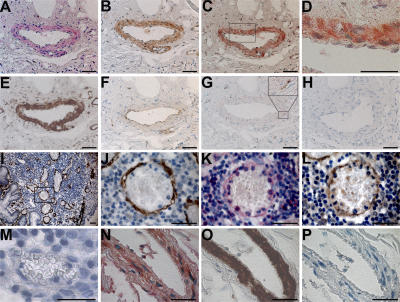
To determine if HCMV infection induces 5-LO expression in vascular SMCs in vivo, we examined tissue from seven patients with active ulcerative colitis (UC) who also had an active HCMV infection in the bowel. In serial sections of intestinal tissue from all seven patients, immunohistochemical staining readily detected multiple cells in the blood vessel wall that coexpressed 5-LO protein, HCMV IEA, and SMC α-actin (Fig. 5, A–L). Vascular SMCs in serial tissue sections from one AIDS patient's adrenal gland were also found to coexpress 5-LO, HCMV IEA, and SMC α-actin (Fig. 5, N–P). Together with our in vitro findings in HPASMCs, these histological data suggest that HCMV infection elicits 5-LO expression and production of LTB4 in vivo.
Figure 5.
HCMV-induced 5-LO expression in vascular SMCs in human tissues. (A–H) Serial sections of a blood vessel in intestinal tissue from an HCMV-infected patient with active UC were stained for the following: (A) 5-LO (FastRed, red), (B) CMV IEA (DAB, brown), (C) 5-LO/IEA double staining (FastRed/DAB), (E) SMC α-actin (DAB, brown), (F) CD31 (DAB, brown), and (G) CD68 (DAB, brown), with an area of adventitia at a higher magnification in the top right corner. (D) Image at a higher magnification of the tissue area indicated with a box in C. (H) Negative control without primary antibody showed no staining with DAB and FastRed. (I–M) Serial sections of intestinal tissue from another HCMV-infected patient with active UC stained for (I) SMC α-actin (DAB, brown), demonstrating an area with multiple small blood vessels surrounded with abundant leukocytic infiltrates. (J) Image at higher magnification of the tissue area indicated with a box in I. The adjacent sections stained for (K) 5-LO (FastRed, red) and (L) IEA (DAB, brown). (M) An HCMV− vessel in the same tissue section showed negative staining for 5-LO (FastRed, red). (N–P) Serial sections of an adrenal gland capsular vessel from one HCMV-infected AIDS patient. (N) Double staining for IEA (DAB, brown) and 5-LO (FastRed, red), showing multiple double-positive cells in the vascular wall. (O) The adjacent section stained for SMC α-actin (DAB, brown). (P) Negative control without primary antibodies showed no staining with DAB and FastRed. Bar, 50 μm.
Over the past decade, novel actions of LTs have been identified, especially in patients with cardiovascular diseases, highlighting the importance of understanding how LT biosynthesis is regulated. According to prevailing dogma, LTs are produced primarily by myeloid cells, a notion supported by the predominant, constitutive, and cell-specific expression of 5-LO. Therefore, the involvement of LTs in cardiovascular disease is thought to represent the function of resident and infiltrated leukocytes, in particular monocytes/macrophages. In this study, however, we provide evidence for a novel and potentially important mechanism of LT biosynthesis in HCMV-infected vascular SMCs. Specifically, HCMV infection enabled these cells to produce LTB4.
LTB4 is one of the most potent chemotactic agents known. In the microenvironment of HCMV-infected SMCs, increased LTB4 levels may promote the infiltration and activation of leukocytes, including monocytes/macrophages and T cells (15–17). In the present study, the proximity between 5-LO induction and leukocytic infiltrates was often observed in HCMV-infected tissues from UC patients (Fig. 5, I–L), indicating that the virus can initiate and sustain a local inflammation in the vasculature, a key element in the development and progression of inflammatory diseases such as atherosclerosis (18, 19), inflammatory bowel disease (20, 21), and allograft rejection (22, 23). In addition, LTB4 stimulates SMC migration and proliferation, which also contribute to atherosclerosis progression and restenosis (24).
HCMV produces up to 250 proteins in an infected cell, most of which are not essential for viral replication. Rather, they control critical cellular and immunological functions, helping the virus to persist and spread in an immunocompetent host. In the case of HCMV-infected SMCs, the consequences of LTB4 production for virus survival in the host may be complex and dependent on multiple factors in the microenvironment. We (8, 9) and others have shown that inflammation leads to reactivation from latency. Hence, the HCMV-induced 5-LO–LTB4 pathway may facilitate the reactivation and spread of the virus by amplifying local inflammation. On the other hand, pharmacological doses of LTB4 have been shown to protect against CMV infection, perhaps via generation of α-defensins and MIP-1α (25, 26). Nonetheless, the expression of 5-LO in HCMV-infected AIDS patients suggests a possible involvement of LTs in the pathological outcomes of this disease. This notion is supported by previous observations that high levels of LTs are produced in co-cultures of HIV-infected monocytes and astroglia and are associated with neuronotoxicity (27).
Our data demonstrate that in human vascular SMCs, HCMV infection induces the expression of 5-LO mRNA and protein through a mechanism that is dependent on viral replication. The induced 5-LO enzyme was active, enabling infected SMCs to produce LTB4 in response to stimulation. The histological evidence of 5-LO expression in HCMV-infected vascular SMCs in vivo with proximal leukocytic infiltrates strongly suggests that SMC-derived 5-LO–LTB4 in the HCMV-infected vasculature is an important component of vascular pathology related to HCMV. Because HCMV is a species-specific virus, and several human LT-dependent pathologies such as asthma and atherosclerosis are poorly reproduced in transgenic mouse models (28), it is unlikely that available mouse or rat models will mimic this biological phenomenon we have discovered in human tissues.
In summary, we identified a novel and potentially important mechanism of LT biosynthesis in vascular SMCs. Our findings provide insights into the biological functions of LTs and suggest that HCMV is an etiological agent, rather than a ubiquitous bystander, in the pathogenesis of inflammatory diseases.
MATERIALS AND METHODS
Culture and infection of HPASMCs.
HPASMCs (Clonetics) were cultured in 75-cm2 cell culture flasks (Costar) with SmGm2 medium containing growth factors (Clonetics). Experiments were performed at passages 8–12. At 80% confluence, cells were exposed to AD169, a laboratory strain of HCMV (VR-538; American Type Culture Collection), at an MOI of 0.1, 1, or 10 for 1–10 d. In some experiments, cells were exposed to UV-irradiated virus (irradiated with the Stratagene UV Stratalinker 1800 set to auto cross-link, repeated six times) or virus-cleared inocula filtered through a 25-mm syringe filter (Acrodisc) with 0.1 μM of nonpyrogenic Supor Membrane (Pall Corporation), mock-infected with SMC supernatant or infected with AD169 in the presence of 5 mM Foscavir (Astra) added 2 h after infection. As shown by real-time PCR, Foscavir treatment resulted in 95% inhibition of the late gene pp150 mRNA compared with uninfected, UV-irradiated, filtered, and SMC supernatant–treated samples, which were negative for pp150 mRNA (not depicted). The Foscavir-treated samples were also positive for immediate-early mRNA by nested PCR (not depicted). Cell-free viral stocks were prepared by centrifugation of cell culture medium from AD169-infected human lung fibroblasts (MRC-5 cells), frozen, and stored at −80°C until use. Viral titers were determined by plaque assays as described previously (29).
Quantitative real-time PCR.
Total RNA from uninfected and HCMV-infected HPASMCs was isolated with RNeasy mini kits and QIAshredder 79654 (QIAGEN). RNA concentrations were measured with an Agilent 2100 Bioanalyzer and 2100 Expert Software (Agilent Technologies). RNA was analyzed with the 6000 Nano LabChip kit (Caliper Technologies) together with RNA 6000 Nano Reagents & Supplies and the RNA 6000 Ladder (Ambion). cDNA was synthesized from 500 ng RNA with the SuperScript III First-Strand Synthesis System for RT-PCR with OligodT20 primers (Invitrogen). Real-time PCR was performed with TaqMan reagents and an ABI Prism 7700 sequence detection system (Applied Biosystems) according to the manufacturer's instructions. Normalizations were made to transcripts of human β-2-microglobulin. The following primer/probe pairs were from Assay-on-Demand (Applied Biosystems): 5-LO (assay ID, Hs00167536_m1); cPLA2 (assay ID, Hs00233352_m1); LTA4H (assay ID, Hs00168505_m1); LTC4S (assay ID, Hs00168529_m1); and β-2-microglobulin (assay ID, Hs00187842_ml). The primer/probe pair for FLAP was from Assay-by-Design (Applied Biosystems): forward primer, GCCTTTGAGCGGGTCTACA; reverse primer, AGAGCACAGCGAGGAAAGTG; reporter sequence, CTGCCAACCAGAACTG.
Immunocytochemistry.
HPASMCs were cultured on sterile eight-well chamber glass slides fixed at 3 d after infection with ice-cold methanol-acetone (1:1) for 10 min at room temperature, rinsed, and incubated with 10% normal goat serum for 30 min at room temperature. The cells were then incubated with rabbit anti–human 5-LO (1:50; provided by O. Rådmark, Karolinska Institutet, Stockholm, Sweden) and mouse anti–HCMV IEA (1:40; Argene) overnight at 4°C. The primary antibody was detected by incubating the cells with Cy3-conjugated goat anti–rabbit IgG (1:400; Jackson ImmunoResearch Laboratories) and FITC-conjugated goat anti–mouse IgG (1:200; Jackson ImmunoResearch Laboratories) at room temperature for 1 h. After staining of DNA with DAPI, slides were mounted with antifade mounting medium (Vector Laboratories) and examined by epifluorescence microscopy. To assess the specificity of 5-LO staining, the primary antibody was adsorbed with a 20-fold excess of purified 5-LO protein at room temperature for 1 h before use.
Immunohistochemistry.
Intestinal tissues from seven HCMV-infected patients with active UC and tissue from one HCMV-infected AIDS patient's adrenal gland were embedded in paraffin. Serial sections of 4 μm were de-waxed and treated with proteinase K (Sigma-Aldrich) at 37°C for 15 min. Nonspecific peroxidase activity was blocked with 3% H2O2, Fc receptors were blocked with Fc receptor blocker (Innovex Sciences), and biotin and avidin were blocked with the Biotin/Avidin Blocking kit (Dako). Adjacent sections were incubated with the following primary antibodies: mouse anti-HCMV IEA (1:70; BioGenex), rabbit anti–human 5-LO (1:50), mouse anti–human SMC α-actin (1:100; Dako), mouse anti–human CD31 (1:20; Dako), and mouse anti–human CD68 (1:50; Dako) in dilution reagent (Innovex Sciences) overnight at 4°C. Sections incubated with dilution reagent without primary antibody served as negative controls. For IEA, α-actin, CD31, and CD68 stainings, colorimetric determination was performed with a three-step horseradish peroxidase detection system (BioGenex) with the chromogen diaminobenzidine (DAB) (Innovex Sciences). 5-LO staining was detected using a streptavidin alkaline phosphatase detection system (Dako) and visualized by FastRed (Dako). In case of double staining, slides were stained for IEA and 5-LO and visualized by DAB and FastRed, respectively. After counterstaining with hematoxylin (Sigma-Aldrich), slides were mounted in permanent mounting medium (Dako). This study was approved by the ethics committees at Huddinge Hospital (diary no. 38/95) and Karolinska Institutet (EPN2006/764-32).
Reverse-phase HPLC coupled to enzyme immunoassay.
HPASMCs were collected in PBS at 1, 3, 7, and 10 d after infection, preincubated at 37°C for 5 min, incubated with 2 mM Ca2+ and 2.5 μM ionophore for 5 min at 37°C, and quenched by adding an equal volume of methanol. After acidification to pH 3–4, samples were purified by solid-phase extraction (Supelclean LC18; Supelco) and analyzed by reverse-phase HPLC. The column (Nova-Pak C18; Waters Instruments) was eluted with acetonitrile/methanol/water/acetic acid (30:35:35:0.01 by vol) at a flow rate of 1.0 ml/min. Absorbance was monitored at 270 nm for LTB4 and at 280 nm for cys-LTs. The eluate fractions corresponding to the retention times of standard LTB4 and cys-LTs (Cayman Chemical) were collected, dried under nitrogen, and resuspended with enzyme immunoassay buffer. Sample LTB4 and cys-LTs levels were determined in triplicate assays with enzyme immunoassay kits for LTB4 or cys-LTs (Cayman Chemical) according to the manufacturer's instructions. Data were derived from dilutions within the linear portion of the standard curve.
Statistical analysis.
Values are presented as mean ± SD. Differences between means were evaluated with the t test or the Mann-Whitney test. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Dr. Olle Rådmark (Karolinska Institutet, Sweden) for providing 5-LO antibodies.
This work was supported by the Swedish Research Council (10350), AFA Health Foundation, Eicosanox and Aineroremo (EC) and CIDaT (Vinnova) (to J.Z. Haeggström), the Swedish Research Council (K2004-16X-12615-07A), the Swedish Children Cancer Research Foundation (project 05/100), the Swedish Cancer Foundation (5044-B05-01XAB), and the Swedish Heart Lung Foundation (20050266; to C. Söderberg-Nauclér). Disclaimer: This report reflects only the author's views, and the European Commission is not liable for any use that may be made of the information herein.
H. Qiu, K. Strååt, C. Söderberg-Nauclér, and J.Z. Haeggström designed the studies; H. Qiu, K. Strååt, A. Rahbar, and M. Wan performed the research; H. Qiu and K. Strååt analyzed the data; and H. Qiu, K. Strååt, C. Söderberg-Nauclér, and J.Z. Haeggström wrote the paper.
The authors have no conflicting financial interests.
H. Qiu and K. Strååt contributed equally to this work.
C. Söderberg-Nauclér and J.Z. Haeggström contributed equally to this work.
References
- 1.Mehrabian, M., H. Allayee, J. Wong, W. Shi, X.P. Wang, Z. Shaposhnik, C.D. Funk, and A.J. Lusis. 2002. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ. Res. 91:120–126. [DOI] [PubMed] [Google Scholar]
- 2.Funk, C.D. 2005. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat. Rev. Drug Discov. 4:664–672. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson, B. 1983. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 220:568–575. [DOI] [PubMed] [Google Scholar]
- 4.Samuelsson, B., S.E. Dahlen, J.A. Lindgren, C.A. Rouzer, and C.N. Serhan. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237:1171–1176. [DOI] [PubMed] [Google Scholar]
- 5.Reid, G.K., S. Kargman, P.J. Vickers, J.A. Mancini, C. Leveille, D. Ethier, D.K. Miller, J.W. Gillard, R.A. Dixon, and J.F. Evans. 1990. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J. Biol. Chem. 265:19818–19823. [PubMed] [Google Scholar]
- 6.Soderberg-Naucler, C. 2006. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J. Intern. Med. 259:219–246. [DOI] [PubMed] [Google Scholar]
- 7.Soderberg-Naucler, C., K.N. Fish, and J.A. Nelson. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Invest. 100:3154–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soderberg-Naucler, C., K.N. Fish, and J.A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 91:119–126. [DOI] [PubMed] [Google Scholar]
- 9.Rakonjac, M., L. Fischer, P. Provost, O. Werz, D. Steinhilber, B. Samuelsson, and O. Radmark. 2006. Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc. Natl. Acad. Sci. USA. 103:13150–13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen-Timmen, U., P.J. Vickers, U. Wittig, W.D. Lehmann, H.J. Stark, N.E. Fusenig, T. Rosenbach, O. Radmark, B. Samuelsson, and A.J. Habenicht. 1995. Expression of 5-lipoxygenase in differentiating human skin keratinocytes. Proc. Natl. Acad. Sci. USA. 92:6966–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lammers, C.H., P. Schweitzer, P. Facchinetti, J.M. Arrang, S.G. Madamba, G.R. Siggins, and D. Piomelli. 1996. Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J. Neurochem. 66:147–152. [DOI] [PubMed] [Google Scholar]
- 12.Gupta, S., M. Srivastava, N. Ahmad, K. Sakamoto, D.G. Bostwick, and H. Mukhtar. 2001. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 91:737–743. [DOI] [PubMed] [Google Scholar]
- 13.Wright, L., R.M. Tuder, J. Wang, C.D. Cool, R.A. Lepley, and N.F. Voelkel. 1998. 5-Lipoxygenase and 5-lipoxygenase activating protein (FLAP) immunoreactivity in lungs from patients with primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 157:219–229. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, Y.Y., J.L. Walker, A. Huang, J.F. Keaney, C.B. Clish, C.N. Serhan, and J. Loscalzo. 2002. Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem. J. 361:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson, E.E. 2004. Mechanisms of leukocyte recruitment to atherosclerotic lesions: future prospects. Curr. Opin. Lipidol. 15:553–558. [DOI] [PubMed] [Google Scholar]
- 16.Aiello, R.J., P.A. Bourassa, S. Lindsey, W. Weng, A. Freeman, and H.J. Showell. 2002. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler. Thromb. Vasc. Biol. 22:443–449. [DOI] [PubMed] [Google Scholar]
- 17.Luster, A.D., and A.M. Tager. 2004. T-cell trafficking in asthma: lipid mediators grease the way. Nat. Rev. Immunol. 4:711–724. [DOI] [PubMed] [Google Scholar]
- 18.Nieto, F.J., E. Adam, P. Sorlie, H. Farzadegan, J.L. Melnick, G.W. Comstock, and M. Szklo. 1996. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 94:922–927. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix, M.G., M.M. Salimans, C.P. van Boven, and C.A. Bruggeman. 1990. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am. J. Pathol. 136:23–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Cottone, M., G. Pietrosi, G. Martorana, A. Casa, G. Pecoraro, L. Oliva, A. Orlando, M. Rosselli, A. Rizzo, and L. Pagliaro. 2001. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am. J. Gastroenterol. 96:773–775. [DOI] [PubMed] [Google Scholar]
- 21.Roberts, W.H., J.M. Sneddon, J. Waldman, and R.E. Stephens. 1989. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleic acid hybridization and immunohistochemistry. Arch. Pathol. Lab. Med. 113:461–464. [PubMed] [Google Scholar]
- 22.Koskinen, P., K. Lemstrom, C. Bruggeman, I. Lautenschlager, and P. Hayry. 1994. Acute cytomegalovirus infection induces a subendothelial inflammation (endothelialitis) in the allograft vascular wall. A possible linkage with enhanced allograft arteriosclerosis. Am. J. Pathol. 144:41–50. [PMC free article] [PubMed] [Google Scholar]
- 23.Li, F.L., G. Grauls, M. Yin, and C.A. Bruggeman. 1996. Correlation between the intensity of cytomegalovirus infection and the amount of perivasculitis in aortic allografts. Transpl. Int. 9:S340–S344. [DOI] [PubMed] [Google Scholar]
- 24.Lee, P.C., G.H. Gibbons, and V.J. Dzau. 1993. Cellular and molecular mechanisms of coronary artery restenosis. Coron. Artery Dis. 4:254–259. [DOI] [PubMed] [Google Scholar]
- 25.Gosselin, J., P. Borgeat, and L. Flamand. 2005. Leukotriene B4 protects latently infected mice against murine cytomegalovirus reactivation following allogeneic transplantation. J. Immunol. 174:1587–1593. [DOI] [PubMed] [Google Scholar]
- 26.Flamand, L., P. Borgeat, R. Lalonde, and J. Gosselin. 2004. Release of anti-HIV mediators after administration of leukotriene B4 to humans. J. Infect. Dis. 189:2001–2009. [DOI] [PubMed] [Google Scholar]
- 27.Genis, P., M. Jett, E.W. Bernton, T. Boyle, H.A. Gelbard, K. Dzenko, R.W. Keane, L. Resnick, Y. Mizrachi, D.J. Volsky, et al. 1992. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage–astroglia interactions: implications for the neuropathogenesis of HIV disease. J. Exp. Med. 176:1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu, H., A. Gabrielsen, H.E. Agardh, M. Wan, A. Wetterholm, C.H. Wong, U. Hedin, J. Swedenborg, G.K. Hansson, B. Samuelsson, et al. 2006. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc. Natl. Acad. Sci. USA. 103:8161–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wentworth, B.B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253–258. [DOI] [PubMed] [Google Scholar]