Abstract
The liver protects the host from gut-derived pathogens yet is tolerant of antigenic challenge from food and commensal sources. Innate responses involving liver macrophages (Kupffer cells) and effector liver natural killer (NK) cells form the first line in this defense. We address the impact of Toll-like receptor (TLR) signaling on the cross talk between these two cells, and reveal how the liver displays a down-regulated inflammatory response to constitutive bacterial elements through the secretion of interleukin (IL) 10 yet retains a vigorous response to viral challenge. The data support the model that (a) human liver Kupffer cells respond to TLR ligands and indirectly activate NK cells; (b) the activation depends on cell–cell contact; (c) the Kupffer cells synthesize NK cell activating signals, among which IL-18 is critical, and NK cell inhibitory factors, including IL-10; (d) ligands that signal via myeloid differentiation factor 88 induce IL-10, giving a blunted response in the NK cells; and (e) ligands that signal via the Toll–IL-1 receptor domain–containing adaptor inducing interferon (IFN) β–IFN regulatory factor 3 pathway induce less IL-10, and also directly potentiate the stimulatory effect of IL-18 on NK cells, resulting in enhanced activation. Subversion of cellular mechanisms of innate immune response against viruses may be important for hepatotropic viruses (e.g., hepatitis B and C) to develop persistence.
Kupffer cells, the resident macrophages of the liver, are poised to initiate innate immune responses through reciprocal interactions with local NK cells when primed by pathogen-derived products. The integrated macrophage–NK response is an important first-line defense against a variety of infectious agents, including bacteria (1, 2), viruses (3), fungi (4), and parasites (5). Innate immune defenses can be similarly activated by neoplasms (6) or even systemic inflammation. This particular activation of macrophages and NK cells and their tight reciprocal regulation occurs through ligation of Toll-like receptors (TLRs) (7, 8). The ligands include cell wall derivatives of bacteria, double-stranded RNA, and viral DNA, as well as flagella and other molecular components derived from pathogens (9). TLRs are expressed on macrophages and dendritic cells that indirectly stimulate NK cells through the secretion of cytokines, particularly IL-12 and IL-18 (10, 11) for activation and IL-15 for maintenance of activated NK cells (12, 13). More recent work demonstrates that NK cells can be directly activated by pathogen-associated molecules (14–16). An alternative path of NK cell activation is through expression of the NK receptor G2D (NKG2D) ligand by macrophages or tumor cells and its interaction with NKG2D (17).
The liver is continuously exposed to nonpathogenic antigens (from food) and to gut-derived LPS. The LPS is a powerful stimulus for innate immunity through TLR ligation and similarly activates professional APCs. Thus, the liver environment would seem optimized to promote immunity to food antigens, raising the question of how the liver avoids making a strong immune response to harmless food antigens in the context of TLR ligation (18, 19). The elaboration of IL-10 by the Kupffer cell is one mechanism of modulating the host response to the proinflammatory cytokines (IL-12, IL-15, and IL-18) also secreted by the Kupffer cells (20–22). With this suppression of the immune response within the liver caused by the continual exposure to LPS, how does the liver mount an appropriate response to threats by viral-encoded antigens? The liver antiviral responses require IFN-γ and NK cell activation, and this occurs despite the protective hyporesponsiveness of LPS-triggered IL-10 secretion. In this study, we analyze this issue and show that although Kupffer cells elaborate IL-10 in response to activation by bacterial cell wall products, a full-fledged inflammatory response occurs when they are exposed to viral components.
TLRs activate two different intracellular signaling pathways: one via myeloid differentiation factor 88 (MyD88), resulting in NF-κB activation and inflammatory cytokine secretion, and an MyD88-independent pathway via Toll–IL-1 receptor domain–containing adaptor inducing IFN-β (TRIF)–IFN regulatory factor 3 (IRF-3) responsible for type I IFN synthesis as well as inflammatory cytokines through associated NF-κB activation (8, 23, 24). Both MyD88 and TRIF are capable of stimulating proinflammatory cytokines through NF-κB, but type I IFN synthesis is limited to signaling through the TRIF–IRF-3 pathway. Bacterial cell wall products engage TLR2 (Gram-positive bacteria) and TLR4 (Gram-negative bacteria). TLR2 signaling relies solely on the MyD88-dependent pathway, whereas TLR4 can signal through both the MyD88-dependent and the TRIF–IRF-3, MyD88-independent pathway. Viral products (e.g., double-stranded RNA) engage TLR3, which utilizes the TRIF–IRF-3 pathway exclusively (25, 26). We hypothesize that it is the differential use of the MyD88-dependent and -independent pathways that allows the liver to exhibit a diminished inflammatory response to its constitutive bacterial stimulation (MyD88 dependent), and a robust inflammatory response to viral stimulation (MyD88 independent), which generally occurs only under pathogenic circumstances.
To define the subtle regulation of responsiveness within the liver, we examined TLR2, 3, and 4 pathways as stimulated by their respective natural or synthetic ligands: lipoteichoic acid (LTA), polyinosinic:polycytodylic acid (poly I:C), and LPS. These molecules represent bacterial cell wall derivatives, LTA (Gram positive) and LPS (Gram negative), and double-stranded RNA, a common product of the replication of RNA viruses. TLR2 relies solely on the MyD88 signaling pathway, whereas TLR4 uses both the MyD88-dependent pathway and the MyD88-independent pathway involving TRIF–IRF-3. TLR3 signals through the TRIF–IRF3 pathway only (for review see reference 27). A cell culture of freshly isolated liver sinusoidal leukocytes from healthy living donors of liver allografts was used to study the effects of TLR stimulation. Our findings strongly support the concept that IL-10 elaborated through the MyD88-dependent pathways used by liver Kupffer cells and NK cells leads to an attenuation of IFN-γ secreted by NK cells. The viral double-stranded RNA analogue poly I:C, which engages the TRIF–IRF-3 (MyD88-independent) pathway, does not lead to substantial IL-10 synthesis and causes a robust secretion of IFN-γ by the liver NK cells. This blunting of inflammation through IL-10 elaboration represents the liver's adaptation to constant exposure of gut-derived bacterial products.
RESULTS
Kupffer cell responses to TLR ligands
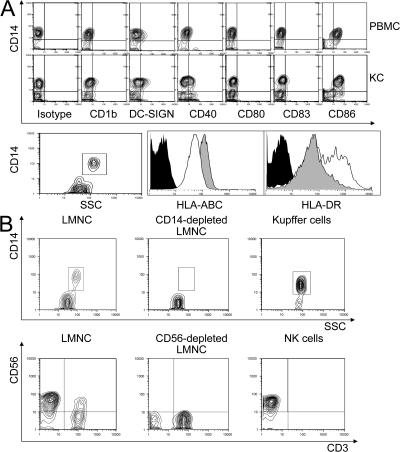
Simple perfusion of a liver graft via the portal vein with an organ preservation solution yields an abundant number of liver sinusoidal mononuclear cells (28). These cells are free of peripheral blood contamination and are phenotypically comparable to cells derived by mechanical digestion of whole liver tissue (unpublished data). From such intrahepatic leukocytes, individual cell populations were isolated by negative selection with immunomagnetic beads. We obtained NK and Kupffer cell populations ranging from 85–97% purity as confirmed by flow cytometry. To confirm that these cells were distinct from blood monocytes, the phenotypes of isolated Kupffer cells and blood monocytes were compared. Fig. 1 A shows the differences between these two monocyte cell populations, obtained from a single donor. The two cell populations were distinct in CD1b, DC-SIGN, CD40, and CD83 cell-surface expression and, most markedly, in the lower expression of class I HLA and up-regulation of class II HLA in Kupffer cells compared with peripheral blood monocytes. Fig. 1 B shows the relatively pure population of Kupffer cells (top) and NK cells (bottom) that can be obtained using magnetic bead isolation. There are also distinct differences in NK cells derived from the liver. Liver-derived NK cells are predominantly CD16−, whereas those derived from peripheral blood are predominantly CD16+ (unpublished data).
Figure 1.
Kupffer cells from healthy living donors are phenotypically different from PBMCs. (A) There were differences in cell-surface expression of CD1b, DC-SIGN, CD40, and CD83, and most strikingly in the lower expression of HLA class I and greater expression of HLA class II in the Kupffer cells (open). (B) Kupffer cells were negatively selected from liver sinusoidal mononuclear cells before immunomagnetic bead separation. The CD14− cells that were depleted as a by-product of Kupffer cell isolation, and the negatively selected (purified) CD14+ cells are shown (top). NK cells (bottom) were purified by positive selection. Contour plots show the whole liver leukocyte population, the discarded CD56− cells, and the purified NK cells (positive selection). SSC, side scatter.
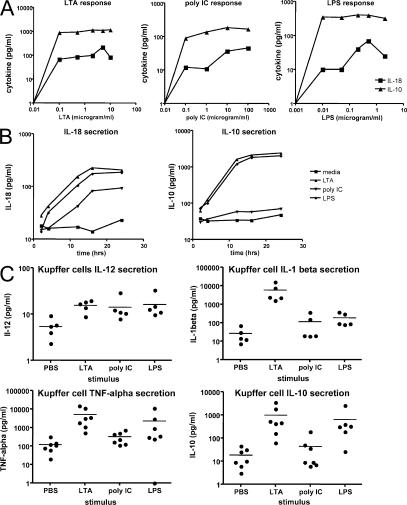
After the isolation and identification of pure Kupffer cells, the functional responses of these cells were optimized to subsequently examine Kupffer cell–NK cell cross talk. The function of the two TLR pathways was initially tested by culturing human liver Kupffer cells with ligands for TLR2 (LTA), TLR3 (poly I:C), and TLR4 (LPS). These engage, respectively, the MyD88 pathway, the TRIF–IRF-3 pathway, and both pathways. To select as an appropriate dose for ligands, dose–response curves were generated for the three ligands. The liver, by virtue of its constant exposure to a low level of endotoxin, makes IL-10 to down-regulate the inflammatory response (20). The liver also releases IL-18 in response to inflammatory stimuli, and this cytokine is important in the activation of liver NK cells in mice (29). We chose these two monokines as the readout for dose–response curves, as they represent key inhibitory and stimulatory signals. The dose–response curves of Kupffer cells to TLR2 (LTA), TLR3 (poly I:C), TLR4 (LPS) for output of IL-10 and IL-18 are shown in Fig. 2 A. To define optimal time points for signal detection, the kinetics of the IL-18 and IL-10 output were examined (Fig. 2 B). Using these curves we selected TLR ligand doses of 5 μg/ml (LTA), 100 μg/ml (poly I:C), and 0.5 μg/ml (LPS). The 16-h time point was used to harvest cells and supernatants for cytokine analysis.
Figure 2.
Kupffer cell cytokine elaboration in response to TLR ligands. (A) TLR ligands stimulate Kupffer cells to secrete IL-10 and IL-18 in a dose-dependent manner. Kupffer cells from a living liver donor were cultured at 106 cells/ml for 16 h in medium supplemented with the various concentrations of the TLR ligands. (B) The time course of IL-10 and IL-18 cytokine secretion in response to TLR ligands. Kupffer cells from a living liver donor were cultured at 106 cells/ml in medium supplemented with the TLR ligands (5 μg/ml LTA, 100 μg/ml poly I:C, and 0.5 μg/ml LPS). Cell-culture supernatants were harvested at various time points. All samples were assayed in triplicate. IL-18 concentration was measured by ELISA; IL-10 was measured by CBA. (C) Consistent pattern of Kupffer cell cytokine secretion across multiple donors. All three TLR ligands (LTA, poly I:C, and LPS) cause IL-12, TNF-α, and IL-10 secretion; however, poly I:C caused less IL-10 secretion than the other two ligands. Only LTA causes IL-1β secretion. Horizontal bars represent means.
The secretion of IL-12, IL-1β, TNF-α, and IL-10 by Kupffer cells from a series of living donor livers is shown in Fig. 2 C. All three ligands caused significant increases in IL-12 and TNF-α secretion, whereas only LTA was able to stimulate IL-1β secretion. The inhibitory cytokine IL-10 was stimulated strongly by LTA and LPS but less so by poly I:C stimulation. However, IL-10 was significantly induced by all three stimuli (P = 0.0391, 0.0078, and 0.0047 for poly I:C, LTA, and LPS stimulation, respectively).
Kupffer cells as transducers of NK activation
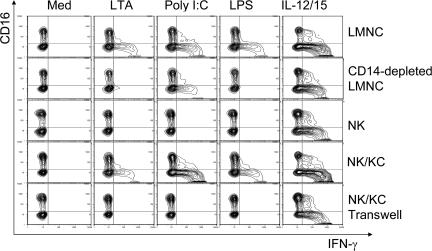
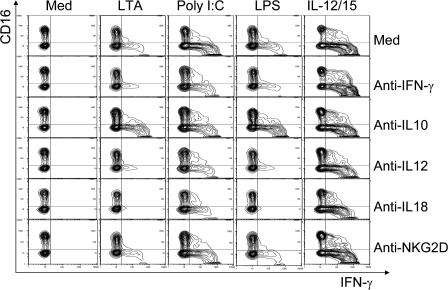
To test the function of the two TLR pathways in Kupffer cell–NK cell cross talk, human liver leukocytes were cultured with ligands for TLR2, 3, and 4. The effects of the TLR2 ligand (LTA), the TLR3 ligand (poly I:C), and the TLR4 ligand (LPS) on IFN-γ production by NK cells in the presence or absence of Kupffer cells is represented in Fig. 3. A mixture of IL-12 and IL-15 cytokines, known inducers of IFN-γ production, served as a positive control that acts directly on the NK cells. This experiment shows that TLR2, 3, and 4 ligands can all cause NK cell activation manifested as induced IFN-γ production, but this requires Kupffer cells. Thus CD14-depleted liver leukocytes responded poorly, and pure NK cells failed to respond to these stimuli. In contrast, NK cells co-cultured with purified Kupffer cells responded, but co-cultures separated in a transwell did not. This argues that NK cell activation by TLR ligands is dependent on direct contact between the Kupffer and the NK cells. It also appears from the intracellular staining in this representative experiment that the TLR3 ligand poly I:C leads to the highest frequency of NK cells synthesizing IFN-γ (observed in six separate experiments). We considered the possibility that this may be related to the fact that poly I:C also caused the least amount of IL-10 to be secreted by Kupffer cells (Fig. 2 C, top).
Figure 3.
IFN-γ production in co-culture experiments. The data shown are representative examples from six separate living donor experiments. Freshly isolated human LMNCs, LMNCs depleted of CD14+ cells, NK cells, and NK cells co-cultured with Kupffer cells together and separated by a transwell were stimulated by TLR agonists for 16 h. Medium and IL-12/15 served as negative and positive control, respectively. Cells were stained with antibodies for cell-surface CD3, CD56, and CD16 and for intracellular IFN-γ. Results show intracellular IFN-γ in CD3−, CD56+ NK cells. The synthesis of IFN-γ in NK cells was dependent on direct contact with Kupffer cells.
IL-10 production in co-culture
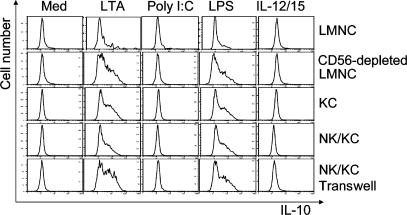
Although IL-10 secretion was augmented by all three ligands, poly I:C stimulation of its secretion was the least effective in pure Kupffer cell culture (Fig. 2 C). We examined this effect in co-culture and a similar observation was made. IL-10 secretion was limited to Kupffer cells after stimulation by all three ligands, as seen in the representative experiment in Fig. 4. Although IL-10 was detectable after poly I:C stimulation, it was present at a very low level. This phenomenon was seen in six independent experiments, and we interpret this as support for the idea that diminished inhibition by IL-10 leads to increased IFN-γ secretion by NK cells.
Figure 4.
IL-10 production in co-cultures. Freshly isolated human LMNCs, LMNCs depleted of CD14+ cells, NK cells, and NK cells co-cultured with Kupffer cells together and separated by a transwell were stimulated by TLR agonists for 16 h. Antibodies to cell-surface CD14 and intracellular IL-10 were used to detect IL-10 expression in Kupffer cells. The results shown are from one representative donor out of six separate experiments. In the co-cultures, cytoplasmic IL-10 staining was weak in Kupffer cells stimulated with poly I:C.
Inhibition of NK cells by IL-10 and activation by IL-18
To directly test the premise that IL-10 acts as an inhibitory cytokine blunting NK activation, we tested the effect of anti–IL-10 antibody in co-culture. A representative experiment is shown in Fig. 5; we observed the augmentation of the IFN-γ synthesis within NK cells after stimulation by LTA and LPS but not poly I:C. As expected from the earlier experiments, there was little poly I:C–stimulated IL-10 to inhibit and, consequently, no effect on cross talk. We examined the effects of inhibiting two known stimulants of NK-cell IFN-γ, namely IL-12 and IL-18, by addition of their respective antibodies. Although some effect was seen after the IL-12 blockade, the blockade of IL-18 was much more effective (Fig. 5). Surprisingly anti-NKG2D had no effect on NK IFN-γ production (P = 0.2812, 0.1562, and 0.4219 for LTA, poly I:C, and LPS stimulations, respectively, as measured in culture supernatants).
Figure 5.
IFN-γ production in co-culture experiments treated with blocking antibodies to mediators of NK cell function. Freshly isolated NK cells were co-cultured with Kupffer cells and stimulated by TLR agonists for 16 h in the presence or absence of anticytokine antibody. Medium and IL-12/15 served as negative and positive controls, respectively. Shown is a co-culture experiment of one representative donor showing IFN-γ production in CD3−, CD56+ NK cells. The most striking inhibitory effect was obtained using anti–IL-18, with a more modest effect of blocking IL-12, a minimal effect of blocking with anti-NKG2D, and a dramatic increase in IFN-γ after IL-10 blockade in the case of LTA- and LPS-stimulated cells. The data are representative examples taken from one out of six experiments.
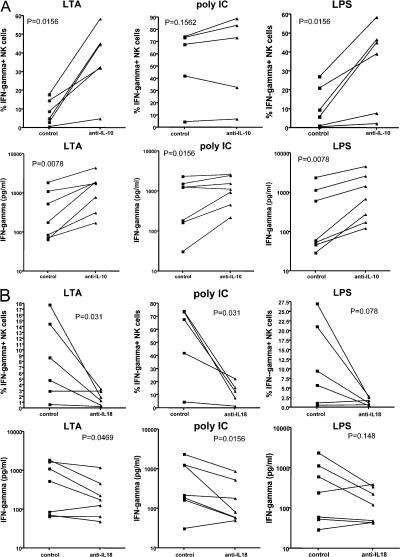
The effect of IL-10 blockade in each liver cell donor was quantified by two methods, namely the estimation of the percentage of IFN-γ–positive NK cells by intracellular staining and IFN-γ protein detected in the culture supernatants by bead-based fluorescent ELISA (cytokine bead assay [CBA]). The results are shown in Fig. 6 A. There was dramatic and significant augmentation of IFN-γ after IL-10 blockade of both of the MyD88 pathway–dependent TLR ligands (LTA and LPS). The effects for the TRIF pathway–dependent ligand (poly I:C) were more modest, with no significant effect on the percentage of IFN-γ–positive NK cells, and a lesser though significant effect on the IFN-γ concentration in culture supernatants. The effect of IL-18 blockade was similarly quantified by two methods (Fig. 6 B). There was a dramatic inhibition of IFN-γ synthesis in response to both poly I:C and LTA. The effects on LPS were not significant because of several low responders, but the trend was in the same direction.
Figure 6.
IFN-γ production in co-culture experiments treated with IL-10 and IL-18 blockade. Freshly isolated human liver sinusoidal NK cells and Kupffer cells from adult live donors were co-cultured and stimulated by TLR agonists for 16 h in the presence or absence of anti–IL-10 (A) or anti– IL-18 (B) antibodies. Media and IL-12/15 served as negative and positive controls, respectively. The percentages of IFN-γ–positive cells were measured by flow cytometry (A and B, top). Supernatants were collected, and cytokine protein content was measured by CBA (A and B, bottom). The dramatic effects of IL-10 blockade are seen in LTA- and LPS-stimulated cells (A). The effects on poly I:C–stimulated cells were not significant in terms of the percentage of NK cells synthesizing IFN-γ, whereas they were significant in terms of IFN-γ secretion. On the other hand, IL-18 blockade significantly inhibited IFN-γ synthesis induced by all three ligands, and such inhibition was also significant in terms of secreted IFN-γ for LTA and poly I:C (B).
These data support the model that (a) human liver Kupffer cells respond to TLR ligands and indirectly activate NK cells; (b) the activation depends on cell–cell contact; (c) the Kupffer cells synthesize NK cell–activating signals, among which IL-18 is critical, and NK cell inhibitory factors, among which IL-10 is important; (d) ligands that signal via MyD88 induce more IL-10, giving a blunted response in the NK cells; and (d) ligands that signal via the TRIF–IRF-3 pathway do not induce IL-10 as strongly, resulting in enhanced NK cell activation.
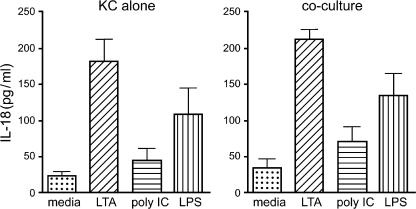
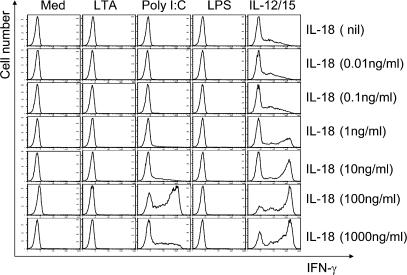
Because the described blocking experiments identified IL-18 as the key mediator of NK cell activation, we assessed IL-18 synthesis in response to TLR ligation. Fig. 7 depicts the IL-18 secretion by Kupffer cells caused by ligation of all three TLRs. Although the Kupffer cells were able to secrete IL-18, the amounts were in the 50–200 pg/ml range. There appeared to be no modulation of this Kupffer cell IL-18 synthesis because of reciprocal cross talk by NK cells as seen in Fig. 7. From these data it appears that IL-18 is a likely candidate for mediating the events after TLR3 ligation of Kupffer cells. This hypothesis was tested in the experiment depicted in Fig. 8 in which we examined the effect of increasing IL-18 doses on IFN-γ production by NK cells. It is evident that NK cells did not elaborate IFN-γ in response to TLR stimulation directly, but poly I:C, the TLR3 ligand, caused IFN-γ production by NK cells in the presence of IL-18, thus demonstrating that TLR3 acts directly on the NK cell to sensitize it to IL-18 stimulation.
Figure 7.
IL-18 secretion by Kupffer cells alone and in co-culture. IL-18 secretion was assessed by ELISA in culture supernatants from six separate experiments. IL-18 increased in response to all three TLR ligands and was similar whether the Kupffer cells were cultured in isolation or in co-culture without or with the intervening transwell membrane. Note that IL-18 secretion was in the range of 50–200 pg/ml. Error bars are means ± SEM.
Figure 8.
Recombinant human IL-18 induced IFN-γ expression of purified NK cells in the presence of TLR agonists at nanogram concentrations. Freshly isolated human liver sinusoidal NK cells from adult live donors were stimulated by TLR agonists for 16 h in the presence of different concentrations of IL-18. Intracellular IFN-γ expression was assessed in CD3−, CD56+ NK cells by flow cytometry. Only poly I:C synergistically induced an NK cell IFN-γ response together with IL-18. However, note that the effective concentration of IL-18 was in the range of 100 ng/ml.
Strikingly, the concentration of IL-18 required for a strong response was 100 ng/ml, which was 500-fold higher than what was detected by ELISA in our cell cultures (Fig. 7). These data are consistent with the concept that an effective cytokine response occurs through signaling via close cell-to-cell contact, greatly increasing the effective concentration of the cytokine. This concept is consistent with three elements of our data: first, the cross talk response was abolished in a transwell culture; second, it could be restored by a soluble cytokine; and third, the requirement for soluble cytokine was many-fold higher (∼500) than the concentration actually observed in cell culture. In conclusion, the effective cytokine concentration at which IL-18 affects NK cells is high, and we argue that this can only be achieved with soluble cytokines acting at very close ranges, which is achieved in the co-culture but not in the transwell.
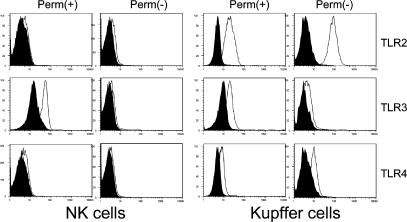
These collective data are consistent with the model that IL-18 is the main positive signal for NK cell IFN-γ production after TLR ligation of monocytes. This observation relies primarily on the blocking experiment in which antibody to IL-18 was capable of abrogating the response to TLR2, 3, or 4 ligation in Kupffer cell–NK cell co-culture (Fig. 5 and Fig. 6 B). The IL-18 dose–response experiments demonstrated a unique sensitivity of NK cells to TLR3 stimulation, but these experiments must be interpreted carefully because, as demonstrated by the dose–response experiment (Fig. 8), this occurred at a nonphysiological IL-18 concentration. We examined the intracellular and extracellular expression of the three TLRs on NK and Kupffer cells. The results in Fig. 9 reveal that, as expected, TLR3 in Kupffer cells was primarily intracellular in contrast to TLR2 and 4. These data also show that there was significant detectable expression of the TLR3 receptor intracellularly in NK cells. This supports the sensitizing effect of TLR3 ligation on the stimulation of IL-18 directly on isolated NK cells.
Figure 9.
Detection of TLR expression intracellularly and on the cell surface of NK and Kupffer cells. Isolated NK and Kupffer cells were stained (open) to detect TLR on the cell surface (Perm −) or permeabilized to detect intracellular TLR. Results revealed the intracellular expression of TLR3 in both NK and Kupffer cells, whereas the expression of TLR2 and TLR4 was limited to the Kupffer cells.
DISCUSSION
There is a growing recognition that the immune response at any tissue site is best analyzed using locally harvested cells. For example, the responses of cells derived from the healthy spleen, a microbiologically sterile site, are dramatically different from those of the lung or lamina propria of the gut (30). This difference is in part conditioned by the plethora of stimulants to innate immunity in an environment where microbes are abundant (i.e., the lung or gut) compared with an environment where they are not (spleen). In this study, we have analyzed the response to TLR ligands of Kupffer cells derived from resting, resident liver mononuclear cells (LMNCs). We term these Kupffer cells, with the provisos that the population of cells that are given that name in intact liver tissue may be heterogeneous and that our harvested cells may not equally represent all Kupffer cell subsets. Liver-derived mononuclear cells responded to TLR2, 3, and 4 ligands by secreting the monokines IL-12 and IL-18. When Kupffer cells were co-cultured with liver-derived NK cells in the presence of TLR ligands, NK cell activation and IFN-γ synthesis ensued. Although antibodies to both IL-12 and IL-18 inhibited this interaction (Fig. 5), anti–IL-18 had the more profound inhibitory effect. In addition, supraphysiological concentrations of IL-18 substituted for the otherwise essential role of Kupffer cells in sensitizing the NK cells to TLR3 ligation. We therefore conclude that IL-18 is the most important Kupffer cell–derived signal promoting NK cell activation.
This central importance of IL-18 contrasts with the key role of IL-12 in encounters between NK cells and dendritic cells. Previous work has demonstrated that IFN-γ secretion by human NK cells is secondary to IL-12 secretion from peripheral lymphoid dendritic cells (31, 32). IL-12 has been shown to be important for IFN-γ secretion, whereas IL-15 is important for NK cell proliferation (33). In contrast, liver NK cells respond to IL-18, which also augments their cytotoxic activity (29). IL-18 may represent the first cytokine released as immature dendritic cells promote NK cell activation because of its constitutive production by these cells (34). In contrast, IL-12 is produced after maturation of dendritic cells (35). Our studies demonstrate a unique susceptibility of NK cells' capacity for IFN-γ secretion to IL-18 blockade, suggesting that in Kupffer cells, IL-18 plays a role in NK cell activation similar to that played by IL-12 in dendritic cells. Similar dependence of NK cells' IFN-γ synthesis on monocyte or dendritic cell IL-18 has also been observed in a mouse model (36).
Activated Kupffer cells synthesized and released IL-18 in the range of hundreds of picograms/milliliter, whereas exogenous soluble IL-18 only promoted NK cell activation at concentrations in the ten or hundreds of nanograms/milliliter. Consistent with this, the effect of TLR ligation in promoting NK cell activation was only evident in co-cultures in which the NK cells and the Kupffer cells were in contact, but not in transwell co-cultures. We therefore conclude that IL-18 acts in the interface between LMNCs and NK cells. This restriction of IL-18 secretion to a synaptic cleft has been demonstrated for immature dendritic cells and adherent NK cells, preventing the potential spread of this activating signal, and, thus, the inappropriate bystander activation of other cells (37).
However, the mononuclear cells respond to TLR2 and 4 ligands by making IL-10, which suppresses the activation of the NK cells. These two ligands both act via the MyD88 signaling pathway, whereas ligation of TLR3 acts only via the TRIF–IRF-3 pathway. This signaling pathway is relatively ineffective in IL-10 induction (Fig. 2, A and C; and Fig. 4). The concomitant release of IL-10 by MyD88-dependent TLR engagement in dendritic cells has been previously demonstrated for TLR2 (38), and the secretion of IL-10 by Kupffer cells is well documented after TLR4 (LPS) stimulation (20, 39).
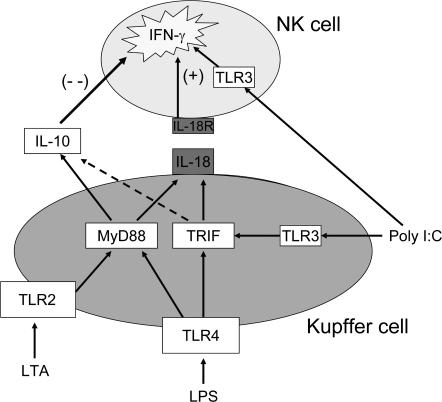
These data support the model illustrated in Fig. 10. This model encapsulates the idea that innate immunity, exemplified in this case by the interaction of liver NK cells with liver-derived Kupffer cells, can be shaped by elements within the local environment to make a nuanced response to pathogen-associated molecular patterns (PAMPs). This mechanism is optimized to maintain the balance between self-tolerance and host defense. Because the liver is exposed constitutively to bacterial products, including TLR2 and 4 ligands, it is inappropriate for these signals to promote either inflammation or innate immunity. In contrast, ligation of TLR3 does not occur in response to harmless signals from the intestinal bacteria but only to viral infection. Therefore, it is highly appropriate for this pattern of TLR engagement to trigger innate immunity. The feedback between the MyD88-driven expression of IL-10 and the synthesis of IL-18 driven by either TLR pathway results in immune unresponsiveness to harmless commensal bacteria but a strong response to viral signals. This prevalence of IL-10 signaling by LMNCs (this study and reference 20) and liver sinusoidal endothelial cells (40) creates a baseline state of immune tolerance that may be helpful in the context of organ transplantation but could create a window of vulnerability in terms of liver infections.
Figure 10.
Model of TLR ligand activation of Kupffer cell–NK cell cross talk in co-culture. Salient features of this model, consistent with the experimental data, are the dependence of NK activation by Kupffer cells on cell-to-cell contact, IL-18 as the critical activating signal, and IL-10 as the modulating inhibitory signal induced mainly through the MyD88 signaling cascade. The model predicts that bacteria-associated TLR agonists signaling through TLR2 and TLR4 result in down-modulated NK cell activation caused by IL-10, whereas a virus-associated TLR agonist (double-stranded RNA) acting through the MyD88-independent pathway results in stronger activation of NK cells. The model also recognizes the intracellular location of TLR3 in NK cells, as well as Kupffer cells, with the possibility of direct NK cell activation through this channel.
The explanation offered by the augmented IL-10 output by the MyD88 pathway compared with the TRIF pathway is probably not the sole explanation for the differential NK cell response to stimulation. The direct effects of TLR3 ligand on the NK cells in sensitizing it to a stimulatory response (Fig. 8 and Fig. 9) also plays a role. It is noteworthy that Kupffer cells elaborate less IL-18 in response to TLR3 ligation than to optimal doses of TLR2 and 3 ligands, yet the NK IFN-γ response is more robust. We believe this is caused by a different balance between potentiating and inhibiting signals, in this case the lesser amount of IL-10 and the potentiation of the IL-18 stimulus by the effect of TLR3 ligation on the NK cell.
Understanding innate immunity, particularly as it relates to host defense against hepatotropic viral infections, is immensely important. Although this study points to the conservation of an antiviral response of NK cells despite the bias of liver immune cells toward tolerance to Gram-negative bacteria, it is well established that certain infections of the liver effectively evade the innate immune response. Hepatitis C virus (HCV) gives rise to chronic infection and viral persistence in 85% of infected individuals (41) and is a major risk factor for the development of cirrhosis and hepatocellular cancer (42). There are numerous reports of deficient innate and adaptive immune responses in HCV infection. Examples include reduced dendritic cell function in chronic HCV infection (43–45), defective NK responses (46–49), and altered CD4+ T cell responses (50). Noteworthy is the study of Dolganiuc in which binding of HCV proteins, core and NS3, were associated with the production of IL-10 and TNF-α by human monocytes, with consequent inhibition of IFN-a production (51). Interestingly, their follow-up study revealed binding of these proteins to TLR2 and activation of the MyD88 pathway (52). Although this latter study focused on the inflammatory component of this interaction, it is intriguing to speculate on an associated elaboration of the inhibitory IL-10, as would be expected from the data presented in this paper. Most exciting is the potential role for the inhibition of IL-10 in the resolution of this chronic viral infection. This kind of immune invigoration therapy was conceptually demonstrated by two recent studies on chronic lymphocytic choriomeningitis virus infection in a mouse model (53, 54). Although these latter two studies specifically evaluated recovery of exhausted T cell function, the source of suppressing IL-10 was the dendritic cell. In the model presented in this paper, the source of suppressant IL-10 would be the Kupffer cell, and one would expect suppression of both innate and adaptive immune responses from IL-10 generated in response to TLR2 ligation by HCV proteins, as noted by Dolganiuc et al. (51, 52). The interaction of the suppressive viral protein effects on Kupffer cells with any stimulatory engagement of MyD88-independent pathways by viral nucleic acids will require examination. The findings of this study, however, point to a local immune state within the liver that is biased toward tolerance via TLR2- and TLR4-mediated IL-10 secretion, with a conserved antiviral response of the TLR3 pathway operating through MyD88-independent signal transduction. If this conclusion is correct, the MyD88-mediated induction of IL-10 in Kupffer cells will become an attractive therapeutic target.
MATERIALS AND METHODS
Patients and collection of samples.
The study protocol was approved by the Institutional Review Board at the University of Rochester Medical Center. All tissues were obtained with informed consent from living donors who volunteered to participate. Liver-associated leukocytes were collected from the hepatic vein after portal flush using cold (4°C) preservation solution by previously established methods (28). After discarding the first aliquot, these cells have little peripheral blood contamination.
Isolation of LMNCs.
Liver perfusates were centrifuged and resuspended in RPMI 1640 medium containing FCS, Pen-Strep (Invitrogen), and glutamine. The cells were then filtered through a 40-μm mesh and centrifuged at 2,800 g for 30 min. The cell pellets were resuspended and subjected to Ficoll-Hypaque density gradient (1.077–1.08) centrifugation before staining and FACS analysis.
Depletion of NK and Kupffer cells from LMNCs.
NK and Kupffer cells were depleted from LMNCs by human CD56 and CD14 microbeads (Miltenyi Biotec), respectively. The depletion purity was analyzed by flow cytometry.
Isolation of pure populations of NK and Kupffer cells.
To obtain pure populations of NK and Kupffer cells from mononuclear cells separated from liver perfusion and peripheral blood of live donors in liver transplantation, NK and Kupffer cells were sorted by magnetic cell sorting, respectively, with human NK cell isolation kit II and human monocyte isolation kit II (Miltenyi Biotec). The purity of all sorted populations was confirmed using FACS analysis and was from 85–97%.
Co-culture of NK and Kupffer cells.
NK and Kupffer cells were co-cultured for 16 h at a 1:1 ratio in complete medium (RPMI 1640, 10% FCS, penicillin, streptomycin, and glutamine) with or without TLR ligands (LTA, poly I:C, and LPS). NK cell induction using the cytokines IL-12 and IL-15 (R&D Systems) were used as positive control, with both at a concentration of 10 ng/ml. Brefeldin A (Sigma-Aldrich) was subsequently added at a final concentration of 5 μg/ml. After cell harvesting, NK cells were stained for surface expression of CD25 and CD56 and intracellular expression of IFN-γ. Kupffer cells were stained for surface expression of CD14 and intracellular expression of IL-10 and TNF-α using anti–IL-10–PE and anti–TNF-α-allophycocyanin (BD Biosciences). Mouse IgG1-PE and -allophycocyanin (BD Biosciences) were used as isotype-matched control antibodies. To investigate the contact dependence of the interaction, Kupffer and NK cells were separated by a membrane (0.4-μm pore size) in TRANSWELL plates (Costar; Corning) in some experiments. To investigate the involvement of selected molecules, blocking experiments were performed by adding the following mAbs: anti–IFN-γ (clone 4S.B3, IgG1; BD Biosciences), anti–IL-10 (clone JES3-19F1, IgG2a; BD Biosciences), anti–IL-12 (clone C11.5, IgG1; BD Biosciences), anti–IL-18 (clone 125-2H; MBL International), and anti-NKG2D (clone 1D11, IgG1; BD Biosciences). Control experiments were performed using isotype-matched mouse antibodies (Sigma-Aldrich). All mAbs were used at a final concentration of 10 μg/ml.
Detection of intracellular cytokine expression by Kupffer and NK cells.
Paired unfractionated and fractionated mononuclear cells from LMNCs were incubated in culture medium alone or in culture medium supplemented with low concentrations of LTA, poly I:C, LPS, and IL-12/15 in 96-well plates for 16 h at 37°C in the presence of 5% CO2. Brefeldin A was subsequently added at a final concentration of 5 μg/ml. The cells were washed, stained with anti-CD56 and anti-CD3 mAbs to gate NK cells and anti-CD14 for Kupffer cells (as described in the previous section), fixed, permeabilized, and stained with 0.5 μg of anti–IFN-γ (eBioscience) and anti–IL-10 (eBioscience). Cells were subsequently washed, resuspended in PBS, and analyzed on a FACSCalibur (BD Biosciences) as described.
CBA.
All sample identification and pipetting was performed by a barcode-enabled, high-speed pipetting robot (Tecan). Similar to our multiplexed serum cytokine assay kits (55), we developed an 11-plex sandwich capture assay kit by coupling 500 μg of the following detection antibodies: IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IFN-γ, and TNF-α (BD Biosciences) to 6.25 × 107 microspheres from unique bead regions, according to the manufacturer's instructions (Luminex). Beads were prepared and pooled as a single lot and aliquoted into single-use tubes and stored at 4°C. Standards (BD Biosciences) were pooled at appropriate concentrations in a single lot and were aliquoted into single-use tubes and stored at −80°C. Biotinylated detection antibodies (BD Biosciences) were pooled into single-use aliquots and stored at −80°C. High and low (500 and 0 pg/ml, respectively, for each cytokine) controls were pooled and stored in single-use aliquots at −80°C. These controls were assayed on all plates. The assay kits demonstrated <4% interanalyte interference, and the median interassay coefficient of variation for all analytes, as assessed by the use of 40 replicate high controls, was 14.3%. The lower limit of detection was consistently <2.4 pg/ml for all cytokines.
ELISA.
IL-18 in culture supernatants was measured by ELISA. Samples at a 1:10 dilution were tested for the 18-kD bioactive isoform of IL-18 (MBL International) using dedicated kits according to the manufacturer's instructions.
Flow cytometry.
In brief, an aliquot (1–5 × 105) of freshly isolated LMNCs or cultured NK and/or Kupffer cells was resuspended in staining buffer (0.5% bovine serum albumin, 0.05% sodium azide in PBS) and preincubated with FcR blocking reagent (Miltenyi Biotec) for 15 min at 4°C. The cells were then simultaneously stained with FITC-conjugated anti–human CD3 (eBioscience), PE-conjugated anti–human CD56 (eBioscience), PE-Cy5–conjugated anti–human CD25 (BD Biosciences), allophycocyanin-conjugated anti–human CD14 (BD Biosciences). In addition, for the determination of myeloid dendritic cell contamination in positively and negatively selected Kupffer cells, purified CD14+ cells were stained with FITC-conjugated anti–human CD11c (Caltag Laboratories), PE-conjugated anti–human HLA-DR (eBioscience), and tricolor-conjugated anti–human CD14 (Caltag Laboratories). To detect contamination with plasmacytoid dendritic cells, Kupffer cells derived from positive and negative selections were stained with FITC-conjugated anti–human blood dendritic cell antigen 2 (Miltenyi Biotec), PE-conjugated anti–human CD123 (eBioscience), and tricolor-conjugated anti–human CD14. The cells were then washed with staining buffer, resuspended in PBS containing 1% paraformaldehyde, and analyzed on a flow cytometer (FACScan; BD Biosciences). The data acquired were analyzed with FlowJo software (BD Biosciences).
Statistics.
Statistics were performed using Prism statistical software (GraphPad). All analyses were unpaired, one-way, nonparametric Mann-Whitney U tests.
Acknowledgments
This work was supported by the Department of Surgery, Division of Solid Organ Transplantation and Hepatobiliary Surgery, University of Rochester School of Medicine (to M.S. Orloff), National Institutes of Health (NIH) grant AI064463 (to I.N. Crispe), and NIH/National Institute of Allergy and Infectious Diseases grant R01-AI48123 (to J. Kurtis).
The authors have no conflicting financial interests.
Abbreviations used: CBA, cytokine bead assay; HCV, hepatitis C virus; IRF-3, IFN regulatory factor 3; LMNC, liver mononuclear cell; LTA, lipoteichoic acid; MyD88, myeloid differentiation factor 88; NKG2D, NK receptor G2D; PAMP, pathogen-associated molecular pattern; poly I:C, polyinosinic:polycytodylic acid; TLR, Toll-like receptor; TRIF, Toll–IL-1 receptor domain–containing adaptor inducing IFN-β.
References
- 1.Sporri, R., N. Joller, U. Albers, H. Hilbi, and A. Oxenius. 2006. MyD88-dependent IFN-gamma production by NK cells is key for control of Legionella pneumophila infection. J. Immunol. 176:6162–6171. [DOI] [PubMed] [Google Scholar]
- 2.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20–26. [DOI] [PubMed] [Google Scholar]
- 3.Salazar-Mather, T.P., T.A. Hamilton, and C.A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Invest. 105:985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaforio, J.J., E. Ortega, I. Algarra, M.J. Serrano, and G. Alvarez de Cienfuegos. 2002. NK cells mediate increase of phagocytic activity but not of proinflammatory cytokine (interleukin-6 [IL-6], tumor necrosis factor alpha, and IL-12) production elicited in splenic macrophages by tilorone treatment of mice during acute systemic candidiasis. Clin. Diagn. Lab. Immunol. 9:1282–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baratin, M., S. Roetynck, C. Lepolard, C. Falk, S. Sawadogo, S. Uematsu, S. Akira, B. Ryffel, J.G. Tiraby, L. Alexopoulou, et al. 2005. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 102:14747–14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalinski, P., A. Giermasz, Y. Nakamura, P. Basse, W.J. Storkus, J.M. Kirkwood, and R.B. Mailliard. 2005. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol. Immunol. 42:535–539. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov, R., and C. Janeway Jr. 2000. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 173:89–97. [DOI] [PubMed] [Google Scholar]
- 8.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell. 124:783–801. [DOI] [PubMed] [Google Scholar]
- 9.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335–376. [DOI] [PubMed] [Google Scholar]
- 10.Biron, C.A. 1999. Initial and innate responses to viral infections–pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374–381. [DOI] [PubMed] [Google Scholar]
- 11.Pien, G.C., A.R. Satoskar, K. Takeda, S. Akira, and C.A. Biron. 2000. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 165:4787–4791. [DOI] [PubMed] [Google Scholar]
- 12.Carson, W.E., and M.A. Caligiuri. 1996. Interleukin 15: a potential player during the innate immune response to infection. Exp. Parasitol. 84:291–294. [DOI] [PubMed] [Google Scholar]
- 13.Carson, W.E., T.A. Fehniger, S. Haldar, K. Eckhert, M.J. Lindemann, C.F. Lai, C.M. Croce, H. Baumann, and M.A. Caligiuri. 1997. A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, M.G., A.O. Dokun, J.W. Heusel, H.R. Smith, D.L. Beckman, E.A. Blattenberger, C.E. Dubbelde, L.R. Stone, A.A. Scalzo, and W.M. Yokoyama. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 292:934–937. [DOI] [PubMed] [Google Scholar]
- 15.Arase, H., E.S. Mocarski, A.E. Campbell, A.B. Hill, and L.L. Lanier. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296:1323–1326. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt, K.N., B. Leung, M. Kwong, K.A. Zarember, S. Satyal, T.A. Navas, F. Wang, and P.J. Godowski. 2004. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J. Immunol. 172:138–143. [DOI] [PubMed] [Google Scholar]
- 17.Diefenbach, A., A.M. Jamieson, S.D. Liu, N. Shastri, and D.H. Raulet. 2000. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 1:119–126. [DOI] [PubMed] [Google Scholar]
- 18.van Oosten, M., E. van de Bilt, T.J. van Berkel, and J. Kuiper. 1998. New scavenger receptor-like receptors for the binding of lipopolysaccharide to liver endothelial and Kupffer cells. Infect. Immun. 66:5107–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock, H.W., M. Frankenberger, and A. Wedel. 1995. Tolerance to lipopolysaccharide in human blood monocytes. Immunobiology. 193:217–223. [DOI] [PubMed] [Google Scholar]
- 20.Knoll, P., J. Schlaak, A. Uhrig, P. Kempf, K.H. Meyer zum Buschenfelde, and G. Gerken. 1995. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J. Hepatol. 22:226–229. [DOI] [PubMed] [Google Scholar]
- 21.Randow, F., U. Syrbe, C. Meisel, D. Krausch, H. Zuckermann, C. Platzer, and H.D. Volk. 1995. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor β. J. Exp. Med. 181:1887–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santucci, L., S. Fiorucci, M. Chiorean, P.M. Brunori, F.M. Di Matteo, A. Sidoni, G. Migliorati, and A. Morelli. 1996. Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology. 111:736–744. [DOI] [PubMed] [Google Scholar]
- 23.Moynagh, P.N. 2005. TLR signalling and activation of IRFs: revisiting old friends from the NF-kappaB pathway. Trends Immunol. 26:469–476. [DOI] [PubMed] [Google Scholar]
- 24.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 437:1167–1172. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 11:115–122. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill, L.A. 2006. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18:3–9. [DOI] [PubMed] [Google Scholar]
- 28.Tu, Z., A. Bozorgzadeh, I.N. Crispe, and M.S. Orloff. 2007. The activation state of human intrahepatic lymphocytes. Clin. Exp. Immunol. 149:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dao, T., W.Z. Mehal, and I.N. Crispe. 1998. IL-18 augments perforin-dependent cytotoxicity of liver NK-T cells. J. Immunol. 161:2217–2222. [PubMed] [Google Scholar]
- 30.Raz, E. 2007. Organ-specific regulation of innate immunity. Nat. Immunol. 8:3–4. [DOI] [PubMed] [Google Scholar]
- 31.Borg, C., A. Jalil, D. Laderach, K. Maruyama, H. Wakasugi, S. Charrier, B. Ryffel, A. Cambi, C. Figdor, W. Vainchenker, et al. 2004. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 104:3267–3275. [DOI] [PubMed] [Google Scholar]
- 32.Vitale, M., M. Della Chiesa, S. Carlomagno, C. Romagnani, A. Thiel, L. Moretta, and A. Moretta. 2004. The small subset of CD56brightCD16− natural killer cells is selectively responsible for both cell proliferation and interferon-gamma production upon interaction with dendritic cells. Eur. J. Immunol. 34:1715–1722. [DOI] [PubMed] [Google Scholar]
- 33.Ferlazzo, G., M. Pack, D. Thomas, C. Paludan, D. Schmid, T. Strowig, G. Bougras, W.A. Muller, L. Moretta, and C. Munz. 2004. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 101:16606–16611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardella, S., C. Andrei, S. Costigliolo, A. Poggi, M.R. Zocchi, and A. Rubartelli. 1999. Interleukin-18 synthesis and secretion by dendritic cells are modulated by interaction with antigen-specific T cells. J. Leukoc. Biol. 66:237–241. [PubMed] [Google Scholar]
- 35.Schulz, O., A.D. Edwards, M. Schito, J. Aliberti, S. Manickasingham, A. Sher, and C. Reis e Sousa. 2000. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 13:453–462. [DOI] [PubMed] [Google Scholar]
- 36.Andoniou, C.E., S.L.H. van Dommelen, V. Voigt, D.M. Andrews, G. Brizard, C. Asselin-Paturel, T. Delale, K.J. Stacey, G. Trinchieri, and M.A. Degli-Esposti. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6:1011–1019. [DOI] [PubMed] [Google Scholar]
- 37.Semino, C., G. Angelini, A. Poggi, and A. Rubartelli. 2005. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 106:609–616. [DOI] [PubMed] [Google Scholar]
- 38.Re, F., and J.L. Strominger. 2004. IL-10 released by concomitant TLR2 stimulation blocks the induction of a subset of Th1 cytokines that are specifically induced by TLR4 or TLR3 in human dendritic cells. J. Immunol. 173:7548–7555. [DOI] [PubMed] [Google Scholar]
- 39.de Waal Malefyt, R., J. Abrams, B. Bennett, C.G. Figdor, and J.E. de Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knolle, P.A., E. Schmitt, S. Jin, T. Germann, R. Duchmann, S. Hegenbarth, G. Gerken, and A.W. Lohse. 1999. Induction of cytokine production in naive CD4(+) T cells by antigen-presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward Th1 cells. Gastroenterology. 116:1428–1440. [DOI] [PubMed] [Google Scholar]
- 41.Boyer, N., and P. Marcellin. 2000. Pathogenesis, diagnosis and management of hepatitis C. J. Hepatol. 32:98–112. [DOI] [PubMed] [Google Scholar]
- 42.El-Serag, H.B. 2004. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 127:S27–S34. [DOI] [PubMed] [Google Scholar]
- 43.Bain, C., A. Fatmi, F. Zoulim, J.P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 120:512–524. [DOI] [PubMed] [Google Scholar]
- 44.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 162:5584–5591. [PubMed] [Google Scholar]
- 45.Auffermann-Gretzinger, S., E.B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 97:3171–3176. [DOI] [PubMed] [Google Scholar]
- 46.Corado, J., F. Toro, H. Rivera, N.E. Bianco, L. Deibis, and J.B. De Sanctis. 1997. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin. Exp. Immunol. 109:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonavita, M.S., A. Franco, M. Paroli, I. Santilio, R. Benvenuto, G. De Petrillo, M. Levrero, A. Perrone, C. Balsano, and V. Barnaba. 1993. Normalization of depressed natural killer activity after interferon-alpha therapy is associated with a low frequency of relapse in patients with chronic hepatitis C. Int. J. Tissue React. 15:11–16. [PubMed] [Google Scholar]
- 48.Deignan, T., M.P. Curry, D.G. Doherty, L. Golden-Mason, Y. Volkov, S. Norris, N. Nolan, O. Traynor, G. McEntee, J.E. Hegarty, and C. O'Farrelly. 2002. Decrease in hepatic CD56(+) T cells and V alpha 24(+) natural killer T cells in chronic hepatitis C viral infection. J. Hepatol. 37:101–108. [DOI] [PubMed] [Google Scholar]
- 49.Kawarabayashi, N., S. Seki, K. Hatsuse, T. Ohkawa, Y. Koike, T. Aihara, Y. Habu, R. Nakagawa, K. Ami, H. Hiraide, and H. Mochizuki. 2000. Decrease of CD56(+) T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 32:962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godkin, A., N. Jeanguet, M. Thursz, P. Openshaw, and H. Thomas. 2001. Characterization of novel HLA-DR11-restricted HCV epitopes reveals both qualitative and quantitative differences in HCV-specific CD4+ T cell responses in chronically infected and non-viremic patients. Eur. J. Immunol. 31:1438–1446. [DOI] [PubMed] [Google Scholar]
- 51.Dolganiuc, A., K. Kodys, A. Kopasz, C. Marshall, T. Do, L. Romics Jr., P. Mandrekar, M. Zapp, and G. Szabo. 2003. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J. Immunol. 170:5615–5624. [DOI] [PubMed] [Google Scholar]
- 52.Dolganiuc, A., S. Oak, K. Kodys, D.T. Golenbock, R.W. Finberg, E. Kurt-Jones, and G. Szabo. 2004. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 127:1513–1524. [DOI] [PubMed] [Google Scholar]
- 53.Ejrnaes, M., C.M. Filippi, M.M. Martinic, E.M. Ling, L.M. Togher, S. Crotty, and M.G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks, D.G., M.S. Trifilo, K.H. Edelmann, L. Teyton, D.B. McGavern, and M.B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coutinho, H.M., S.T. McGarvey, L.P. Acosta, D.L. Manalo, G.C. Langdon, T. Leenstra, H.K. Kanzaria, J. Solomon, H. Wu, R.M. Olveda, et al. 2005. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192:528–536. [DOI] [PubMed] [Google Scholar]