Abstract
Background
In addition to the regulation of blood pressure, α2- and β-adrenoceptor (AR) subtypes play an important role in the modulation of noradrenergic neurotransmission in the human CNS and PNS. Several studies suggest that the α2-AR responsiveness in cells and tissues after chronic epinephrine (EPI) or norepinephrine (NE) exposure may vary, depending on the β-AR activity present there. Recently, we reported that in BE(2)-C human neuroblastoma cells (endogenously expressing α2A- and β2-AR), chronic EPI treatment (300 nM) produced a dramatic β-adrenoceptor-dependent desensitization of the α2A-AR response. The aim of this study is to determine if stable addition of a β2-AR to a second neuroblastoma cell line (SH-SY5Y), that normally expresses only α2A-ARs that are not sensitive to 300 nM EPI exposure, would suddenly render α2A-ARs in that cell line sensitive to treatment with the same EPI concentration.
Methods
These studies employed RT-PCR, receptor binding and inhibition of cAMP accumulation to confirm α2-AR subtype expression. Stable clones of SH-SY5Y cells transfected to stably express functional β2-ARs (SHβ2AR4) were selected to compare sensitivity of α2-AR to EPI in the presence or absence of β2-ARs.
Results
A series of molecular, biochemical and pharmacological studies indicated that the difference between the cell lines could not be attributed to α2-AR heterogeneity. We now report that after transfection of functional β2-AR into SH-SY5Y cells (SHβ2AR4), chronic treatment with modest levels of EPI desensitizes the α2A-AR. This effect results from a β2-AR dependent down-regulation of native α2A-ARs by EPI accompanied by enhanced translocation of GRK2 and GRK3 to the membrane (required for GRK-mediated phosphorylation of agonist-occupied receptors).
Conclusion
This study further supports the hypothesis that the presence of the β-AR renders the α2A-AR more susceptible to desensitization with physiological levels of EPI.
Background
Studying changes in α2-adrenoceptor (AR) signaling is important for understanding the development and/or manifestation for several CNS (cerebral ischemia, pain, depression) and PNS disorders (hypertension and cardiac dysfunction). Under physiological conditions, norepinephrine and epinephrine (NE and EPI, respectively) activate the α2-AR along with other members of the AR family, which also includes α1- and β-ARs. The α2- and β-ARs are often co-expressed on the same cell surface. Upon activation by NE and EPI, the independent signals initiated by the α2- and β-ARs often converge to regulate specific physiological endpoints such as insulin release [1], maintenance of uterine smooth muscle tone [2], and noradrenergic transmission in the CNS and PNS [3,4]. The α2- and β-ARs regulate many of these physiological mechanisms by mediating opposing actions on adenylyl cyclase; α2-AR inhibits while β-AR stimulates the adenylyl cyclase pathway.
Continuous exposure to catecholamines leads to a declining receptor response, a phenomenon called desensitization. The process of desensitization generally includes receptor phosphorylation, internalization, and down-regulation. Unlike other members of the AR family, the α2A-AR subtype does not readily down-regulate. Since this subtype is the dominant α2-AR in the CNS and mediates the "classical effects" of α2-ARs which include hypotension, sedation, and antinociception [5,6], numerous studies have focused on the regulatory mechanisms of the α2A-AR. In cultured cell lines expressing either native α2A-AR [7] or recombinantly over-expressed α2A-AR [8,9], supra-physiological concentrations of EPI (100 μM) and NE (30 μM) were required to produce long-term α2A-AR desensitization. The waning α2A-AR signal is attributed primarily to down-regulation of the receptor and/or phosphorylation of the agonist occupied receptor by G-protein coupled receptor kinases (GRK), specifically GRK2 and GRK3 [10,11]. Previous studies suggest that either of these two α2A-AR desensitization mechanisms require supra-physiological (μM) concentrations of agonist [10,12-14].
However, our recent studies in the BE(2)-C human neuroblastoma cell line suggest that when β-ARs are present on the same cells lower, more physiologically relevant, concentrations of EPI (300 nM) are able to desensitize the α2A-AR following chronic (24 hr) treatment [15]. In the absence of β-ARs, α2A-AR desensitization occurs only with supra-physiological concentrations of EPI, if it occurs at all [15]. Concurrent activation of the β-AR and α2A-AR also prompts down-regulation of cell surface α2A-ARs while specifically up-regulating the expression of GRK3 within BE(2)-C cells [15]. Enhanced GRK3 expression plays a prominent role, as it is required for both β-AR-dependent α2A-AR desensitization and down-regulation [15,16]. Recently we reported similar findings for the α2B-AR subtype in mouse neuroblastoma cells [17-19].
Since both α2- and β-ARs are often co-localized and share the same endogenous ligands, it is reasonable that the α2A-AR response is regulated differently in the presence and absence of the β-AR. Indeed, evidence suggests that the α2-AR responsiveness in cells and tissues after chronic EPI or NE vary, depending on the β-AR activity present there [2,15,20-23]. The aim of the present study is to compare α2A-AR responsiveness after chronic EPI and NE treatment in non-β-AR expressing (wild-type SH-SY5Y, wt) human neuronal cells to α2A-AR responsiveness in SH-SY5Y cells that have been stably transfected to express β2-AR (SHβ2AR4). In doing so, we hope to determine whether co-expression of the two ARs intrinsically produced this differential α2A-AR regulation and whether enhanced expression of GRK3 is required for this regulation.
Results
Characterization of the model system and establishment of the SHβ2AR4 cell line
Our first goal was to find a second model system that was similar to the BE(2)-C human neuroblastoma cell line (expressing modest levels of α2A-AR), but that didn't express β-ARs. Kazmi and Mishra previously identified the SH-SY5Y cell line as expressing two α2-AR binding sites [24], while Parsley et al.[25] reported that it expressed a single AR subtype, α2C, based upon functional and molecular studies. Since receptor expression varies depending on differentiation state and passage number, it was necessary to determine which α2-AR subtypes were expressed in our population of SH-SY5Y cells, using a combination of binding, functional, and molecular approaches.
SH-SY5Y cells expressed α2-AR levels slightly greater than the level detectable in BE(2)-C cells (Bmax: SH-SY5Y, 67.6 ± 8.2 ; BE(2)-C, 40.8 ± 7.0 fmol/mg protein). According to nonlinear and linear regression analysis of saturation binding, the data best fit a single-site model in SH-SY5Y cells, as observed previously in BE(2)-C cells. Rauwolscine and yohimbine competed for specific [3H]rauwolscine binding to SH-SY5Y cell membranes with higher affinity than prazosin, the α2B/C-selective antagonist (Table 1; [24]). Apparent Ki values of agonists and antagonists against [3H]rauwolscine binding were determined for comparison with previously reported values in cells natively expressing α2A-, α2B, or α2C-ARs (HT29 and BE(2)-C, NG108-15, OK; [15,26,27]) or cell lines expressing cloned α2C10, α2C2, and α2C4 [28]. Values obtained from binding studies in SH-SY5Y cells correlated only to values from BE(2)-C cells and showed the greatest similarity with those derived from native and cloned α2A-AR-containing cell membranes (Table 2). These results are consistent with binding of [3H]rauwolscine to an α2A-AR in SH-SY5Y cells.
Table 1.
Pharmacological characteristics of adrenoceptors in SH-SH5Y and SHβ2AR4 cells.
| SH-SY5Y | SHβ2AR4 | ||
| Agonist: | log(Ki) | log(EC50) | log(EC50) |
| EPI | -7.38 ± .04 | -8.83 ± .06 | -8.22 ± 0.21 |
| UK 14,304 | -7.38 ± .12 | -7.22 ± .36 | -7.72 ± 0.77 |
| Oxymetazoline (OXY) | -8.85a | -8.35 ± .47 | N.D. |
| Isoproterenol | N.A. | N.A. | -7.02 ± 0.28 |
| Antagonist: | log(Ki) | Ki Ratio with OXY | |
| Rauwolscine | -8.82 ± .15 | 1.07 | N.A |
| Yohimbine | -8.56 ± .17 | 1.95 | N.A |
| Prasozin | -6.98a | 74.4 | N.A |
Binding inhibition and cAMP accumulation studies were performed as described in Methods. The values of the apparent affinity constants Log(Ki) for each competitor were derived from their IC50 values (n = 3–9) using the equation of Cheng and Prusoff [40]. The Log(EC50) values (concentration of the drug that produces 50% of the maximal inhibitory/stimulatory effect of that drug) were calculated by nonlinear regression analysis of the agonist concentration-response curves (n = 3–9) of each agonist. aValues from Kazmi and Mishra [24]. N.D., not determined; N.A., not applicable.
Table 2.
Correlation of SH-SY5Y cell α2-AR pKi values with those of native and cloned α2-AR subtypes.
| Comparison | Reference | # of Values Compared | Correlation Coefficient | Slope | p value |
| v. HT29 | 21,22 | 6 | 0.93 | 1.48 ± 0.41 | 0.07 |
| v. NG108-15 | 21 | 6 | 0.13 | 0.17 ± 0.91 | 0.87 |
| v. OK | 21,22 | 6 | 0.62 | 0.99 ± 0.73 | 0.27 |
| v. α2C10 | 23 | 6 | 0.80 | 1.04 ± 0.45 | 0.10 |
| v. α2C2 | 23 | 6 | 0.40 | 0.48 ± 0.65 | 0.50 |
| v. α2C4 | 23 | 6 | 0.70 | 0.94 ± 0.52 | 0.16 |
| v. BE(2)-C | 6 | 6 | 0.98* | 1.38 ± 0.18 | 0.01 |
Correlation coefficient values (r) were generated by comparing pKi values from Table 1 with previously published values for one-site models using Pearson correlation analysis (GraphPad Prism). The slope of the linear regression line is also included. Correlations were considered significant (*) if p ≤ 0.05.
Functional studies were performed by measuring the ability of various α2-AR agonists to inhibit forskolin (10 μM)-stimulated cAMP accumulation in intact cells. All α2-AR agonists inhibited forskolin-stimulated cAMP accumulation in a concentration-dependent manner; no stimulation of cAMP accumulation was noted in the absence of forskolin. Inhibition of cAMP accumulation by the α2-AR agonist UK14,304 (30 nM; Fig. 1) was completely reversed by 10 nM yohimbine, whereas the α2B/C-selective antagonist ARC-239, at a concentration over 30-fold higher than that of the agonist, failed to reverse the actions of UK14,304. Thus, both binding and functional data support the classification of the α2-AR subtype in this neuroblastoma cell line as α2A.
Figure 1.
Reversal of the inhibitory effect of UK 14,304 on forskolin-stimulated cAMP accumulation. Yohimbine significantly antagonized the ability of UK14,304 (30 nM) to inhibit cAMP accumulation using an unpaired Student's t-test (GraphPad Prism, San Diego, CA), while the α2B/C-selective antagonist, ARC-239, had no effect. The results represent the mean ± S.E. of 2–9 experiments, performed in duplicate.
Since Parsley et al.[25] were unable to detect α2A-AR RNA by performing RT-PCR with total RNA extract, we optimized our chances for detecting α2A-AR RNA by generating RT-PCR products from SH-SY5Y mRNA using primer pairs selective for individual α2-AR subtypes (Table 3; [29,30]) or a primer pair that recognizes two α2-AR receptor subtypes distinguished by their restriction nuclease digestion products (Table 3; [30]). RT-PCR with α2C10/C4 primers gave a 233 bp product specific for α2A- and α2C-ARs; restriction digestion of this fragment with BglII, that would specifically cleave α2A-AR, resulted in two fragments of 117 bp and thereby established expression of α2A-AR mRNA in SH-SY5Y cells. RT-PCR with α2C4 primers gave a 630 bp fragment, which was successfully digested by BstXI to produce three fragments of 271, 225, and 78 bp, consistent with the presence of an α2C-AR gene product (Fig. 2). RT-PCR products were neither noted in samples lacking reverse transcriptase (-), nor were they produced with primers selective for α2C2 (α2B-AR; data not shown). While SH-SY5Y cells express mRNA for both α2A- and α2C-ARs, it appears that the predominant functional α2-AR in our cell line is the α2A-AR.
Table 3.
Molecular characteristics of α2-AR RT-PCR products
| PCR Product | Primer: | Receptor | Expected size (bp) | Restriction Enzyme | Digestion Products (bp) |
| α2A/C-AR | α2C10/C4 | α2A | 233 | BglII | 117 (2) |
| α2C | 233 | SacI | 153, 80 | ||
| α2C-AR | α2C4 | α2C | 630 | BstXI | 271,225,78 |
Figure 2.
RT-PCR Products obtained from SH-SY5Y RNA using α2-AR subtype selective primers. RT-PCR experiments were performed as described in "Methods" using primer pairs recognizing α2C10/C4 (corresponding to α2A and α2C) and α2C4 (corresponding to α2C) gene products (Table 3). The reactions amplified fragments of the expected size from each set of primers. α2C10/C4 primers amplified 233 bp products from SH-SY5Y mRNA that were sensitive to digestion by BglII (specific for the α2A product). Restriction digestion with BstXI of the 630 bp product of α 2C4 primer amplification gave three fragments of 271, 225 and 78 bp. All reactions were performed in the presence (+) or absence (-) of reverse transcriptase (RT) to rule out the possibility of DNA contamination. Lane M designates the 100 bp ladder; the 500 bp fragment is indicated by an arrow in each panel.
Since these cells appear to express α2A-ARs with properties similar to those in BE(2)-C cells [15] but lack a β-AR, pcDNA 3.0 plasmid vector containing the human β2-AR gene was transfected into SH-SY5Y cells. Colonies of stable transfectants were selected and maintained by their resistance to G418 (600 μg/mL) and subsequently clonal populations of β2-AR-expressing SH-SY5Y cells (SHβ2AR) were screened for β-AR expression using [3H]CGP-12177 for binding studies as described in Methods. Since BE(2)-C cells express very low levels of β2-AR (Bmax: 18.5 ± 6.2 fmol/mg protein), the SHβ2AR4 cell line that expressed 14.78 ± 4.19 fmol/mg protein of the β2-AR was selected for the subsequent studies. To ensure that the β-ARs were functional, the ability of isoproteranol (ISO) to stimulate cAMP accumulation was assessed (Table 1). The α2A-AR responses were also tested in this new cell line to confirm that α2A-AR function had not been altered by the expression of the β2-AR (Table 1).
Chronic 300 nM EPI exposure induces α2A-AR desensitization only in SH-SY5Y cells transfected with functional β-AR
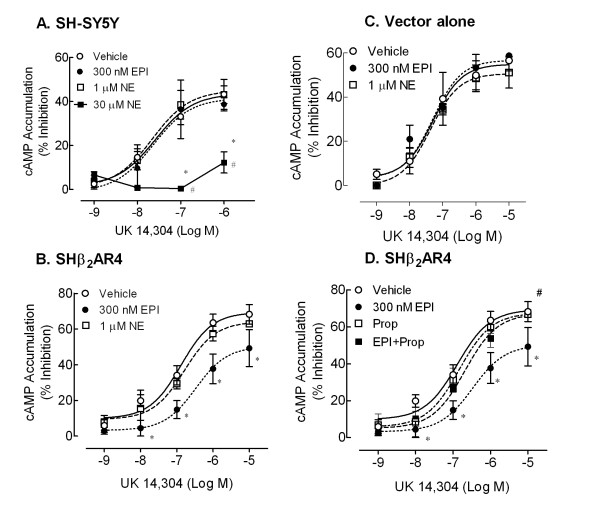
To determine whether the presence of the β-AR influences α2A-AR signaling, the ability UK14,304 to inhibit forskolin-stimulated cAMP accumulation was evaluated after wildtype (wt) and SHβ2AR4 cells were exposed to vehicle or the indicated concentration of agonist for 16–24 hr. Wt SH-SY5Y cells (Fig 3A) require a 30-fold higher concentration of NE (30 μM) to desensitize the α2A-AR signal than SHβ2AR4 cells (1 μM; Fig 3B). Both the potency (-Log EC50 (M): 5.2 ± 0.1) and efficacy (Imax (%): 17.0 ± 1.6; P < 0.05 Fig. 3A) of UK14,304 were reduced by 30 μM NE compared to vehicle treatment in wt cells (-7.6 ± 0.2 M and 43.2 ± 6.8%); modest concentrations of NE (1 μM) and EPI (300 nM) are insufficient to alter the α2A-AR signal in the wt SH-SY5Y cell line. In contrast, chronic treatment of the β-AR-expressing SHβ2AR4 cells with 300 nM EPI desensitized the α2A-AR signal causing loss of UK14,304 potency (-Log EC50 (M): Vehicle 6.9 ± 0.2; EPI 6.3 ± 0.2) and efficacy (Imax (%): Vehicle 68.2 ± 5.4; EPI 49.3 ± 10.4; p < 0.05; Fig. 3B). Unlike EPI, which co-activates both ARs, NE, at the concentrations employed activates only α2A-ARs and does not alter α2A-AR signaling. We concluded that the difference in α2-AR signaling following EPI treatment between the transfected and wt SH-SY5Y was attributable to the presence of functional β2-ARs, respectively. To ensure that the vector was not responsible for the observed difference between the wt and SHβ2AR4 cells, similar experiments were conducted in SH-SY5Y cells transfected with the vector alone (minus the β2-AR gene). These vector only-expressing clones responded to EPI (300 nM) and NE (1 μM) pretreatments as the parent SH-SY5Y cells did (Fig. 3C).
Figure 3.
Pretreatment with a modest concentration of EPI produces α2A-AR desensitization in SH-SY5Y cells only when the β2-AR is present. Wild-type SH-SY5Y cells (A), cells expressing recombinant β2-AR (SHβ2AR4, B and D), or SH-SY5Y cells expressing the vector alone (C) were pretreated 16–24 hr with any or all of the following: EPI (300 nM), NE (1 μM or 30 μM), EPI + Prop (30 nM), Prop (30 nM) alone or vehicle (0.1 mM ascorbate). Following pretreatment, the ability of UK14,304 to inhibit forskolin-stimulated cAMP accumulation was evaluated. A) Neither chronic EPI nor 1 μM NE pretreatments were sufficient to alter the α2A-AR signal (n = 6) in native SH-SY5Y cells. The α2A-AR signal in these cells desensitized only when exposed to higher agonist concentrations (30 μM NE, n = 3; 100 μM EPI, n = 3, data not shown). B) Unlike native SH-SY5Y cells, pretreatment with 300 nM EPI is sufficient to desensitize the α2A-AR signal in SHβ2AR4 cells (n = 6; p < 0.05). NE (1 μM), acting predominantly at α2A-AR with little affinity for the β2-AR, does not produce α2A-AR desensitization. C) In SH-SY5Y cells transfected with the vector alone, neither EPI nor NE pretreatments altered α2A-AR signal (n = 4).D) Addition of propranolol (30 nM) prevents EPI-induced α2A-AR desensitization, suggesting a β2-AR-dependent process (# p < 0.05 as compared to EPI treatment).
To validate the importance of the β2-AR in the desensitization of the α2-AR signal, we included the β-AR selective antagonist propranolol (30 nM) with the chronic 300 nM EPI treatment. Addition of propranolol blocks EPI-induced α2A-AR desensitization resulting in UK14,304 concentration-response curves indistinguishable from control (-Log EC50 (M) for EPI + Prop 6.7 ± 0.1; Imax (%) for EPI + Prop 67.9 ± 0.4; p < 0.05; Fig. 3D). Propranolol treatment alone did not alter UK14,304 potency or efficacy.
β2-AR signal is desensitized following exposure to 300 nM EPI
To ensure that the β2-AR is functioning properly following catecholamine treatment, we evaluated the ability of ISO to stimulate cAMP accumulation over basal in SHβ2AR4 cells. The β2-AR signal is desensitized following chronic EPI but not NE treatment, consistent with the fact that NE has a low affinity for the β2-AR. Inclusion of propranolol (30 nM) inhibited EPI-induced β2-AR desensitization (p < 0.05; Fig. 4), but had no effect in the absence of EPI.
Figure 4.

Chronic EPI, but not NE, treatment desensitizes the β2-AR signal in SHβ2AR4 cells. SHβ2AR4 cells were treated for 16–24 hr with the vehicle (ascorbate, 1 μM), EPI (300 nM), NE (1 μM), EPI + Prop (30 nM), or Prop (30 nM) alone. Intact cells were assessed for ISO-stimulated (250 nM) cAMP accumulation. Chronic 300 nM EPI (n = 6; *P < 0.05), but not 1 μM NE (n = 3), pretreatment desensitized the β-AR response to ISO compared to the corresponding vehicle-treated control. The β-AR antagonist propranolol blocked EPI-induced β2-AR desensitization. Data represent mean ± S.E. of at least 3 independent determinations; comparisons were made by ANOVA with Dunnett's post-hoc test.
Chronic EPI-induces down-regulation of the α2A-AR in SHβ2AR4, but not wt SH-SY5Y cells
Our study in BE(2)-C cells suggests that β2-AR-induced α2A-AR desensitization following long-term EPI exposure is due in part to down-regulation of the α2-ARs. To determine if the same mechanism is responsible for the EPI-induced α2A-AR desensitization in SHβ2AR4 cells, changes in α2A-AR expression following catecholamine treatment were evaluated. Specific binding was measured with a single concentration of radioligand. We, and others, have shown that this is sufficient for accurate assessment of changes in receptor number for the α2A-AR [9,15]. Chronic exposure of SHβ2AR4 cells to 300 nM EPI down-regulates the α2A-ARs by 20% (p < 0.05; Fig. 5). The α2A-AR down-regulation in this cell line, as in BE(2)-C cells, requires β2-AR co-activation since loss of α2A-ARs is prevented when 30 nM propranolol is included with EPI. Down-regulation of the α2A-AR is not observed following chronic activation of α2A-AR alone by 1 μM NE. Further, 300 nM EPI does not alter the expression of α2A-AR in wt SH-SY5Y cells as compared to vehicle-treated cells (% of vehicle: 88.6 ± 25.9; n = 2) consistent with a lack of α2A-AR desensitization. Hence, it can be concluded that chronic EPI treatment induces a loss of α2A-AR response via β2-AR-dependent down-regulation of α2A-ARs in SHβ2AR4, but not in wt SH-SY5Y cells.
Figure 5.

Chronic 300 nM EPI down-regulates α2A-AR in β2-AR-transfected, but not native, SH-SY5Y cells. Wt SH-SY5Y or SHβ2AR4 cells were incubated for 16–24 hr with vehicle (ascorbate, 0.1 mM), 1 μM NE, 300 nM EPI, EPI + Propranolol (30 nM), or 30 nM Propranolol alone. Cell membrane homogenates were generated as described in Methods. Specific binding (8084 ± 609 cpm/mg protein in vehicle-treated cells) was calculated by subtracting the binding of a single concentration of radioligand (2 nM) in the presence of phentolamine (10 μM) from the binding in its absence. Unlike in native cells, chronic EPI treatment reduced α2A-AR levels as compared to vehicle (*p < 0.05); inclusion of propranolol blocked the EPI-induced α2A-AR down-regulation (#p < 0.05 as compared to EPI treatment) in SHβ2AR4 cells. Data represent mean ± S.E., n = 2–4; comparisons were made by ANOVA with Tukey's post-hoc test.
Chronic EPI exposure does not alter GRK2 or GRK3 levels in whole cells but instead enhances GRK2 and GRK3 expression at the membrane in SHβ2AR4 cells
We previously established that EPI-induced α2A-AR desensitization and down-regulation in BE(2)-C cells is mediated via β2-AR-dependent GRK3 up-regulation [15]. Therefore, GRK3 levels in whole cell SHβ2AR4 lysates were evaluated following 24 hr catecholamine treatments. Chronic EPI exposure altered neither GRK3 nor GRK2 levels in the transfected SH-SY5Y cell line (Table 4). Therefore, unlike results in BE(2)-C cells, increases in whole cell GRK3 levels do not contribute to the modest α2A-AR desensitization or down-regulation observed in the SHβ2AR4 cells.
Table 4.
Total GRK levels are unaltered in SHβ2AR4 cells with catecholamine treatment.
| Catecholamine Treatment* | ||||
| EPI | EPI+P | Prop | NE | |
| GRK3 | 94 ± 8 (7) | 84 ± 15 (7) | 80 ± 20 (7) | 84 ± 20 (5) |
| GRK2 | 101 ± 10 (7) | 97 ± 16 (7) | 105 ± 14 (7) | 83 ± 23 (4) |
SHβ2AR4 cells were treated with vehicle (0.1 mM ascorbate), EPI (300 nM), propranolol, EPI + propranolol, or 1 μM NE for 24 hr. Approximately 25 μg of whole cell lysate from each treatment group was resolved by SDS-PAGE through a 10% gel. Immunoreactive bands were normalized to the GAPDH loading control and the GRK/GAPDH ratio was calculated.* Data represent % of expression levels noted in vehicle-treated cells (mean ± s.e.m.); number of independent determinations is given in parentheses following the values.
Although GRK3 levels in whole cell lysates remain unaltered in SHβ2AR4 cells, it is not known whether GRK3 recruitment to the membrane is regulated via chronic EPI treatment in that cell line. Since GRK2 and GRK3 have been shown to regulate α2A-AR signaling [10], we wanted to determine whether the membrane recruitment of either GRK isoform was changed following chronic EPI exposure in SHβ2AR4 cells. GRK2 and GRK3 are cytosolic proteins that anchor to the membrane via interaction with free Gβγ subunits; thus both kinases translocate from the cytosol to the membrane to regulate receptor signaling upon activation. Taking this characteristic of GRK2 and GRK3 into account, the levels of both kinases in membrane fractions following chronic EPI exposure were evaluated. SHβ2AR4 cells exhibit an increase in membrane-associated GRK2 and GRK3 with 24 hr EPI treatment compared to vehicle (P < 0.05; Fig. 6). In SHβ2AR4 cells, the same propranolol concentration (30 nM) that inhibited EPI-induced α2A-AR desensitization and down-regulation also attenuated EPI-induced increase in GRK2 and GRK3 content in the membrane fraction (P < 0.05; Fig. 6). In contrast, no increased translocation of GRKs by EPI treatment was observed in wt SH-SY5Y cells that do not express β2-ARs. Therefore, this increased GRK2 and GRK3 translocation to the membrane following prolonged EPI treatment in SHβ2AR4 cells is β2-AR dependent.
Figure 6.
Chronic 300 nM EPI enhances expression of GRK3 and GRK2 at the membrane of SHβ2AR4 cells via β2-AR-dependent mechanism. Wildtype SH-SY5Y (Wt SH) and SHβ2AR4 cells were subjected to catecholamine treatment in the presence or absence of 30 nM propranolol. Isolation of the membrane fraction and immunoblotting for GRK2 and GRK3 was conducted as described in Methods. EPI exposure significantly increased the level of GRK3 and GRK2 expressed in the membrane fractions from SHβ2AR4 cells compared to vehicle-treated controls (*P < 0.05; n = 3). Inclusion of propranolol (P) with EPI treatment prevented the increased translocation of both GRK isoforms (#P < 0.01 as compared to EPI treatment), while propranolol treatment alone was without effect. In contrast, EPI failed to increase mobilization of GRK to the plasma membrane of wt SH cells (n = 4–7). Data represent mean ± S.E.; comparisons were made by ANOVA with Tukey's post-hoc test.
Discussion
The major finding of the present study is the confirmation (using a different approach) that sensitivity of α2A-AR to desensitization following exposure to relatively low levels of epinephrine is significantly increased in cells expressing both α2A- and β2-AR. The first evidence for this was recently reported in a human neuronal cell line endogenously expressing α2A- and β2-ARs. Alpha2A- and β-ARs in BE(2)-C cells desensitized after chronic EPI (300 nM), but not NE (1 μM), treatment [15]. Interestingly, the α2A-AR responsiveness in SH-SY5Y cells (an alternative human neuroblastoma cell line that does not express β-ARs) is not desensitized after chronic treatment with 300 nM EPI or 1 μM NE (Fig. 3).
Obviously, the difference in α2-AR sensitivity to lower concentrations of EPI could be due to several factors, including differences in the α2-AR subtypes expressed in each cell line. Since it is difficult to demonstrate with great certainty what α2-AR subtypes are present in a given cell or tissue by biochemical or pharmacological means only, we took a molecular approach to ascertain which subtypes might potentially be expressed based on the presence of mRNA encoding each subtype. SH-SY5Y cells contained mRNA for α2A- and α2C-ARs (Fig. 2). As we noted and as reported by others [25], no evidence for α2B mRNA was found. This was further confirmed by Northern blot analysis (data not shown). Initially, total RNA isolated from SH-SY5Y cells did not produce the α2A-AR PCR products using the antisense primer selective for α2A-AR previously described [30]. Instead α2A-AR RT-PCR product was obtained only with poly(A) mRNA. However, employing poly(A)-enriched mRNA in the RT-PCR did not yield an α2B-AR RT-PCR product. Parsley et al. [25]) identified only α2C-AR mRNA using total RNA isolated from SH-SY5Y cells; this observation may reflect the limitation associated with the α2A-AR primers used for RT-PCR of total RNA, similar to what we encountered.
The rank order binding affinity of the various agonists and antagonists tested is in agreement with that previously reported in cells expressing recombinant [3,28,31] or native α2A-ARs [26,32]. When we compared apparent pKi values for various α2-AR agonists and antagonists against binding to [3H]rauwolscine in SH-SY5Y membrane homogenates with previously reported values, we saw a correlation only with those cells that expressed α2A-ARs (Table 2). Another means of distinguishing between various α2-AR subtypes involves comparing the prazosin/oxymetazoline (OXY) or OXY/yohimbine affinity ratios (Table 1; [4]). Prazosin/OXY (74.4) and OXY/yohimbine (1.95) ratios were within the range reported for native and recombinant α2A-ARs, and differ by at least 10-fold from values reported for α2C-AR (from [4]). The agonist potency series in SH-SY5Y cells also most closely parallels that reported for the α2A [26-28,32]. The inhibitory effect of α2-AR agonists on cAMP production in SH-SY5Y cells is readily reversed in a concentration-dependent fashion by the antagonist yohimbine (Fig. 1); the failure of the selective α2B/C antagonist ARC-239 to antagonize UK 14,304 is consistent with activation of α2A-ARs in SH-SY5Y cells. Therefore, our results strongly support the designation of the functional α2-AR in SH-SY5Y cells as α2A.
The present study supports our previous findings that pretreatment with a modest EPI concentration readily desensitizes the α2A-AR signal in the presence, but not in the absence, of the β2-AR. This conclusion is based on several results. First, in wt SH-SY5Y cells (no β2-AR), the α2A-AR signal is not desensitized following 24 hr treatment with modest concentrations of EPI or NE (300 nM and 1 μM, respectively). Instead wt cells required chronic exposure to supra-physiological concentrations of catecholamines (30 μM NE and 100 μM EPI; data not shown) for desensitization of the α2A-AR signal; supporting the fact that α2A-ARs do not desensitize and/or down-regulate readily in response to low to moderate levels of EPI. Second, 300 nM EPI induces α2A-AR desensitization only in SHβ2AR4 cells which express functional β2-AR. Finally, EPI-generated waning of the α2A-AR response is not observed in transfected cells expressing the pcDNA plasmid vector minus the β2-AR gene. This observation suggests that introduction of the β2-AR, and not the vector, is responsible for the difference in the α2A-AR signal between wt and SHβ2AR4 cells exposed chronically to modest EPI concentrations.
As previously observed in BE(2)-C cells [15], desensitization of α2A-AR signal with 24 hr EPI exposure is due, in part, to down-regulation of the receptor in SHβ2AR4 cells. Chronic co-activation of both α2A- and β2-AR is required for desensitization and down-regulation of the α2A-AR in SHβ2AR4 cells as indicated by the following results. First, 300 nM EPI, but not 1 μM NE, produces α2A-AR desensitization and down-regulation in the recombinant cell line. Lands et al. [33] established that EPI has equal affinity for α2A- and β2-AR while NE has a higher affinity for the α2-AR than β2-AR; therefore, EPI activates both α2A- and β2-ARs simultaneously while NE activates the α2A-AR alone. It is evident that the modest EPI concentration readily activates the β2-AR since chronic pretreatment with 300 nM EPI, but not 1 μM NE, desensitized the β2-AR response. Second, the inclusion of the β2-AR blocker propranolol prevented EPI-induced α2A-AR desensitization and down-regulation in β2-AR-transfected SH-SY5Y cells. This propranolol concentration (30 nM) is sufficient to prevent EPI activation of β2-AR as indicated by the inhibition of EPI-induced β2-AR desensitization.
Although chronic EPI treatment desensitized and down-regulated α2A-AR in both BE(2)-C and SHβ2AR4 cells, several differences were observed. First, a more profound loss of efficacy is observed following 24 hr EPI exposure in BE(2)-C cells as compared to SHβ2AR4 cells. The maximal inhibition of forskolin-stimulated cAMP accumulation by UK14,340 was reduced 54% in BE(2)-C, but only 27% in SHβ2AR4, cells following EPI treatment (Fig. 3). The greater down-regulation of α2A-ARs observed in BE(2)-C versus SHβ2AR4 cells most likely accounts for the greater change in efficacy: in SHβ2AR4, chronic EPI treatment produces a 20% loss of α2A-ARs while in BE(2)-C cells, there is a 60% α2A-AR down-regulation (Fig. 5). This more profound α2A-AR desensitization and down-regulation observed in BE(2)-C is mediated via the up-regulation of GRK3 [15]. The lack of GRK3 up-regulation in the SHβ2AR4 cells is the second major difference between the two cell lines. At present, it is unknown what prompts GRK3 up-regulation in BE(2)-C cells but not in the SHβ2AR4 cells. However, we have observed that ERK1/2 activation is required for this induction of GRK3 following chronic exposure of BE(2)-C cells to EPI [16]. Moreover, while α2A-ARs do not readily activate this pathway in neuronal cells, and β-AR activation by ISO can activate ERK1/2 at high concentrations, we have observed that ERK1/2 activation by EPI at concentrations that up-regulate GRK3 appears to require the simultaneous activation of both α2A- and β2-ARs. Conversely, the inability of transfected β-ARs to prompt ERK1/2 activation in SH-SY5Y cells could explain the lack of GRK3 up-regulation in SHβ2AR4 cells.
Even though total GRK3 levels are unaltered, GRKs play a role in β2-AR-regulated α2A-AR signaling in SHβ2AR4 cells as indicated by several results. First chronic EPI treatment enhances localization of GRK2 and GRK3 to the membrane. As indicated previously, translocation of these two cytosolic kinases to the membrane is required for phosphorylation and subsequent desensitization of its receptor substrate, which in this study is the α2A-AR. Second, addition of propranolol attenuated EPI-mediated translocation of both GRK isoforms. This same propranolol concentration also inhibited α2A-AR desensitization and down-regulation as discussed above. Therefore, β2-AR co-activation with α2A-AR is required for enhanced GRK2 and GRK3 translocation to the membrane and subsequent α2A-AR desensitization and down-regulation.
Translocation of both GRK2 and GRK3 to the membrane following chronic EPI treatment in SHβ2AR4 cells differs from the selective translocation of GRK3 (but not GRK2) observed in BE(2)-C cells following the same treatment (unpublished observations). The selective GRK3 up-regulation in BE(2)-C cells could account for the enhanced GRK3 levels at the membrane in these cells since in a previous study increase in total GRK2 levels promoted increased GRK2 expression at the membrane [34]. It is unknown at present why chronic EPI treatment translocates both GRK2 and GRK3 in SHβ2AR4 cells, and not in BE(2)-C cells. A possible explanation for the difference in the GRK isoform translocation between the two cell lines is differences in the β subunit expressed and/or released upon EPI exposure. GRK2 and GRK3 require the βγ subunit of the G proteins to anchor to the membrane but GRK2 and GRK3 exhibit distinct binding preferences for individual β subunits [35]. The β3 isoform preferentially binds GRK3 but not GRK2, whereas β1 and β2 bind equally to both GRK3 and GRK2 [35,36].
Conclusion
Based on results obtained in this series of experiments, we conclude that exposure to modest EPI concentrations readily desensitizes and down-regulates α2A-ARs in the presence, but not in the absence, of a functional β-AR. The β-AR-dependent down-regulation of α2A-ARs is modulated via GRKs. In BE(2)-C cells, chronic co-activation of β- and α2A-AR prompts enhanced expression of GRK3, but not GRK2, in whole cells [15] and membrane fractions. In contrast, EPI pretreatment of SH-SY5Y cells transfected with functional β2-ARs does not increase either GRK3 or GRK2 expression per se, but does increase translocation of GRK2 and GRK3 to the plasma membrane. Like α2A-AR desensitization and down-regulation, this translocation of GRK2 and GRK3 in SHβ2AR4 cells is β-AR-dependent and thus presents an alternate mechanism for the regulation of the α2A-ARs by β-ARs.
Methods
Materials
The following drugs were purchased or obtained from the indicated sources: (-) epinephrine (EPI), (±)norepinephrine (NE), sodium ascorbate, UK14,304 (Sigma-Aldrich, St. Louis, MO.); cell culture media (Gibco, Grand Island, NY); fetal bovine serum (Atlanta Biologicals, Norcross, GA); and antibiotics (Mediatech, Inc., Herndon, VA). GRK2 (C-15) and GRK3 (C-14) primary antibodies and horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA); anti-glyceraldehyde-3-phosphate dehydrogenase (GADPH, Research Diagnostics, Inc., Flanders, NJ).
Cell culture
SH-SY5Y (passages 37–55) human neuroblastoma cells (Dr. Robert A. Ross, Fordham University, Bronx, NY) were maintained in a humidified atmosphere (6% CO2:94% air) in a 1:1 mixture of Eagle's minimum essential medium with non-essential amino acids and Ham's F-12 that contains 10% fetal bovine serum, 100 U/ml penicillin G and 0.1 mg/ml streptomycin sulfate. Plates of cells greater than 60% confluence were used throughout the study.
Transfection
Plasmid cDNA with the human β2-AR gene (provided by Dr. Brian Knoll; University of Houston, Houston, TX) or vector alone was stably transfected into SH-SY5Y cells with the fuGENE 6 Transfecting Reagent (Roche). Ten positive clones were isolated by their resistance to 800 μg/mL of G418 and maintained in media containing 600 μg/mL of G418. SHβ2AR4 was selected for use in all experiments because it expressed similar levels of β2-ARs as that expressed natively in BE(2)-C cells; SHβ2AR4 expressed 14.78 ± 4.19 fmol/mg protein while BE(2)-C express 18.5 ± 6.2 fmol/mg protein [15]. This β2-AR level remained consistent to passage 12 in SHβ2AR4 cells. After passage 12, SHβ2ARs neither expressed β2-ARs nor maintained resistance to G418, suggesting that the cells no longer expressed the transfected plasmid.
RNA isolation and RT-PCR
Total RNA was isolated from several different passages of freshly harvested SH-SY5Y cells by the guanidinium isothiocyanate/phenol-chloroform extraction method [37]. Total RNA concentrations were determined by UV spectroscopy; integrity of each isolate was determined by electrophoresis through a 1% agarose gel in the presence of 0.01 M sodium phosphate buffer. Poly(A) mRNA was isolated using a Dynabead oligo(dT)25 Kit (Dynal, Oslo, Norway) and was used for RT-PCR reactions. Each RT reaction (20 μL) contained 5–10 μg total or poly(A) RNA preincubated with 5 ng/μL oligo(dT)12–18, for 10 min at 70°C. The reaction mixture contained 80 μM each of deoxynucleotides (dATP, dCTP, dGTP and dTTP), RT buffer (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2), and 5 mM dithiothreitol, and was preincubated for 2 min at 42°C before the addition of Moloney Murine Leukemia Virus reverse transcriptase (200 U/μl) for 60 min at 42°C; a 5 min incubation at 95°C terminated the reactions.
ODNs [29,30] corresponded to sequences for the various human α2-AR (α2A antisense: 5'-AGA CGA GCT CTC CTC CAG GT-3'; sense: 5'-AAA CCT CTT CCT GGT GTC TC-3'), α2A/2C-(antisense: 5'-GTG CGC TTC AGG TTG TAC TC-3'; sense: 5'-AAA CCT CTT CCT GGT GTC TC-3'), or α2C-AR (antisense: 5'-CGT TTT CGG TAG TCG GGG AC-3'; sense: 5'-GTG GTG ATC GCC GTG CTG AC-3'). The contents of each RT reaction tube were diluted to a final volume of 50 μL with 10% DMSO, 80 μM each of dATP, dCTP, dGTP and dTTP, 8 μM each of the appropriate sense/antisense primer pair, 1.5 mM MgCl2, and magnesium free buffer [containing 10 mM Tris-HCl (pH 9.0), 0.1% Triton X-100, 50 mM KCl] in sterile distilled water. Reaction mixtures were overlayed with mineral oil and subjected to a hot start for 5 min at 95°C. DNA polymerase (2.5 U taq, 5 U/μl, Promega, Madison, WI) was added to each reaction tube after the hot start, and the tubes were subjected to a PCR reaction of 30 cycles in a thermal cycler (MJ Research Inc., Watertown, MA) for 1 min at 94°C, 1.5 min at 55°C, and 2 min at 72°C with a final elongation step at 72°C for 7 min. Reaction products were separated by electrophoresis through 2% agarose gels and visualized by ethidium bromide staining. PCR products were isolated from the gel using a DNA extraction kit (Amicon Inc., Bedford, MA). Identity of the purified PCR products was confirmed by their susceptibility to digestion with restriction enzymes specific for each reaction product (see Table 1; [30]).
cAMP accumulation
To determine the effects of α2-AR agonists on forskolin-induced cAMP accumulation, intact cells were incubated for 5 minutes at 37°C in HBSS buffer (in mM): NaCl (137), KCl (5), Na2HPO4 (0.6), KH2PO4 (0.4), NaHCO3 (4), D-glucose (6), MgCl2 (0.5), MgSO4 (0.4) and CaCl2 (1), containing the phosphodiesterase inhibitor IBMX (0.5 mM). In some experiments, antagonists also were included in this step. To prohibit oxidation, sodium ascorbate (0.11 mM) was included when assaying catecholamines. Upon addition of forskolin (10 μM) and agonist, assay tubes were incubated for an additional 10 min at 37°C. Removing the tubes to a boiling water bath for 5 min terminated the assay. All assays were performed in duplicate in a total volume of 0.5 ml. After boiling, samples were centrifuged for 5 min at 14000 × g, and cAMP levels from the supernatant fractions were determined in a [3H]cAMP (0.8 pmol) binding assay as previously described [38]. β-AR-mediated stimulation of cAMP accumulation was performed in the same manner except that forskolin was not included in the assay mixture. Forskolin (10 μM) stimulated cAMP accumulation to 587 ± 88 pmol/mg protein (n = 46), 15-fold over basal levels (40.5 ± 2 pmol/mg protein).
Receptor binding
Preparation of cell membranes
Cells were homogenized in 20 volumes of Tris-HCl buffer (50 mM, pH 7.4) containing NaCl (100 mM), Na2 EDTA (1 mM) and PMSF (0.1 mM), and the membranes sedimented by centrifugation for 30 minutes at 34000 × g at 4°C. Pellets were resuspended in 0.32 M sucrose, and aliquots of the membrane fractions were stored frozen (-80°C) until use.
Saturation experiments
The level of α2-ARs in SH-SY5Y cell membranes (0.5 mg/ml) was determined with various concentrations of [3H]rauwolscine (60–80 Ci/mmol, 0.3 – 12 nM) in a total volume of 1–2 ml in potassium phosphate buffer (50 mM, pH 7.4) containing MgSO4 (5 mM) at 37°C for 45 min. Thereafter, 2 ml Tris-HCl (5 mM, pH 7.4, 4°C) was added to the homogenate to terminate the binding reaction and the contents of the tubes was filtered over #32 glass fiber filter strips (Schleicher & Schuell, Keene, NH) using a PHD cell harvester (Cambridge Technology, Cambridge, MA). The reaction tubes and the filter strips were rinsed twice with a further 2–3 ml of buffer. Levels of radioactivity were determined by scintillation spectroscopy in a Beckman LS6000 liquid scintillation counter. All assays were performed in triplicate, and specific binding was determined by subtracting the binding in the presence of yohimbine or phentolamine (10 μM; nonspecific) from the binding in its absence.
Previously we have shown that agonist treatments do not alter the Kd of the ligand for the α2-AR [15]. Therefore, levels of α2-ARs in SHβ2AR4 cell membranes (0.1 – 0.2 mg/mL) were determined using a single concentration (2 nM) of either [3H]rauwolscine or [3H]RX821002 following catecholamine treatment.
β2-AR binding was performed with [3H]CGP-12177. For saturation studies, cell membranes (0.5 mg/mL) were incubated with [3H]CGP-12177 (0.2 to 40 nM) in Tris-HCl buffer (50 mM, pH 7.5) containing MgCl2 (0.5 mM) at 37°C for 30 min. Specific binding was determined by subtracting the binding in the presence and absence of propranolol (1 μM).
Competition experiments
Cell membrane fractions were incubated as described above except that the concentration of [3H]rauwolscine was fixed (1 nM), and various (4–9) concentrations of unlabeled drugs were included.
Immunoblotting
Membrane proteins were separated from cytosolic proteins by centrifugation, were resolved by SDS-PAGE through 10% gels and relative levels of GRK2 and GRK3 determined by immunoblotting as described previously [15]. Briefly, proteins were transferred to PVDF membrane, blocked with 5% nonfat dried milk in PBS containing 0.1% Tween (PBS/T) and incubated overnight at 4°C with dilutions of a rabbit polyclonal antibody directed against GRK2 (1:1000), GRK3 (1:1000), or both GRK2 and GRK3 (GRK2/3; 1:1000; wt SH-SY5Y). Blots were subjected to 4 washes before incubating for 60 min at room temperature with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:2000) in PBS/T. Immunoreactive bands were visualized by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL or Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The intensity of each immunoreactive band was determined using a Nucleovision Imaging Workstation (Nucleotech Corp., San Carlos, CA), and normalized to the GAPDH loading control (1:5000).
Protein determination
Bovine serum albumin was used as a standard in the determination of protein levels in intact cells and cell membranes as described [39].
Data analysis
Kd, Bmax, IC50 and LogEC50 values were determined by nonlinear regression analysis using GraphPad Prism (GraphPad Software http://www.graphpad.com). The Ki values were calculated according to the Cheng-Prusoff equation [40] in which Ki = (IC50)/(1+S), where S = [concentration of radioligand]/[KD of radioligand]. Comparisons between groups were made by two-way Student's t-tests or ANOVA and Tukey's or Dunnett's post hoc test (where appropriate; GraphPad Software, San Diego, CA), and groups were considered significantly different if p ≤ 0.05.
Abbreviations
IBMX, 3-isobutyl-1-methylxanthine; HBSS, Hank's balanced salt solution; UK 14,304, 5-Bromo-N-(4,5-dihydro-1H-imidazole-2-yl)-6-quinoxalinamine; ARC-239, 2-(2,4-(O-methoxyphenyl)-piperazin-1-yl)-ethyl-4,4dimethyl-1,3-(2H,4H)-isoquinolindione, AR, adrenoceptor; ISO, isoproterenol; EPI, epinephrine; NE, norepinephrine; wt, wild-type.
Authors' contributions
TB-K participated in the design of the study, generated and selected stable SHβ2AR4-expressing clones, carried out all chronic treatment experiments, performed the statistical analyses and immunoblotting experiments, and drafted the manuscript. GFA carried out the binding and functional assays characterizing the α2-AR subtype. CDM conducted the molecular analysis studies. DCE participated in the conception and design of the study and helped draft the manuscript. LAS participated in the design and coordination of the molecular studies. KMS conceived of the study, participated in the design and coordination of all experiments and helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by grants from the Department of Health and Human Services (DA10738 and DA01738) and Texas Advanced Research Program (003652-0114-1999 and 003652-0182-2001) to KMS and the American Heart Association, Texas Affiliate, Inc (Grant 0555032Y) to DCE. We gratefully acknowledge Dr. Brian Knoll (University of Houston, Houston, TX) for the gift of the plasmid cDNA with the human β2-AR gene and Ms. Vanessa Ramirez and Dr. Hibah Awwad for their excellent technical expertise.
Contributor Information
Tasneem Bawa-Khalfe, Email: Tasneem.Bawa-Khalfe@uth.tmc.edu.
Ghazi F Altememi, Email: gtememi@hotmail.com.
Chitra D Mandyam, Email: cmandyam@scripps.edu.
Lindsay A Schwarz, Email: lschwarz@uh.edu.
Douglas C Eikenburg, Email: deikenbu@central.uh.edu.
Kelly M Standifer, Email: kelly-standifer@ouhsc.edu.
References
- John GW, Doxey JC, Walter DS, Reid JL. The role of alpha- and beta-adrenoceptor subtypes in mediating the effects of catecholamines on fasting glucose and insulin concentrations in the rat. Br J Pharmacol. 1990;100:699–704. doi: 10.1111/j.1476-5381.1990.tb14078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrivain JL, Mhaouty-Kodja S, Cohen-Tannoudji J, Maltier JP, Legrand C. In vivo stimulation of the beta(2)-adrenergic pathway increases expression of the Gi proteins and the alpha(2)A-adrenergic receptor genes in the pregnant rat myometrium. J Endocrinol. 1998;156:379–387. doi: 10.1677/joe.0.1560379. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/S0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Hieble JP., Ruffolo, R.R. Jr. Starke, K. An overview in alpha2-adrenergic receptors: structure, function and therapeutic implications. In: Lanier SMLLE, editor. Identification, characterization and subclassification of alpha2-adrenoceptors. Amsterdam , Harwood Academic Publishers; 1997. pp. 1–18. [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science. 1996;273:801–803. doi: 10.1126/science.273.5276.801. [DOI] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J Pharmacol Exp Ther. 2000;293:1–7. [PubMed] [Google Scholar]
- Jones SB, Halenda SP, Bylund DB. Alpha 2-adrenergic receptor stimulation of phospholipase A2 and of adenylate cyclase in transfected Chinese hamster ovary cells is mediated by different mechanisms. Mol Pharmacol. 1991;39:239–245. [PubMed] [Google Scholar]
- Jones SB, Leone SL, Bylund DB. Desensitization of the alpha-2 adrenergic receptor in HT29 and opossum kidney cell lines. J Pharmacol Exp Ther. 1990;254:294–300. [PubMed] [Google Scholar]
- Eason MG, Liggett SB. Subtype-selective desensitization of alpha 2-adrenergic receptors. Different mechanisms control short and long term agonist-promoted desensitization of alpha 2C10, alpha 2C4, and alpha 2C2. J Biol Chem. 1992;267:25473–25479. [PubMed] [Google Scholar]
- Jewell-Motz EA, Liggett SB. G protein-coupled receptor kinase specificity for phosphorylation and desensitization of alpha2-adrenergic receptor subtypes. J Biol Chem. 1996;271:18082–18087. doi: 10.1074/jbc.271.30.18082. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Regan JW, Matsui H, Mayor F, Jr., Cotecchia S, Leeb-Lundberg LM, Caron MG, Lefkowitz RJ. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987;262:17251–17253. [PubMed] [Google Scholar]
- Heck DA, Bylund DB. Mechanism of down-regulation of alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther. 1997;282:1219–1227. [PubMed] [Google Scholar]
- Jewell-Motz EA, Donnelly ET, Eason MG, Liggett SB. Role of the amino terminus of the third intracellular loop in agonist-promoted downregulation of the alpha2A-adrenergic receptor. Biochemistry. 1997;36:8858–8863. doi: 10.1021/bi970487x. [DOI] [PubMed] [Google Scholar]
- Eason MG, Moreira SP, Liggett SB. Four consecutive serines in the third intracellular loop are the sites for beta-adrenergic receptor kinase-mediated phosphorylation and desensitization of the alpha 2A-adrenergic receptor. J Biol Chem. 1995;270:4681–4688. doi: 10.1074/jbc.270.9.4681. [DOI] [PubMed] [Google Scholar]
- Bawa T, Altememi GF, Eikenburg DC, Standifer KM. Desensitization of alpha 2A-adrenoceptor signalling by modest levels of adrenaline is facilitated by beta 2-adrenoceptor-dependent GRK3 up-regulation. Br J Pharmacol. 2003;138:921–931. doi: 10.1038/sj.bjp.0705127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Standifer KM, Eikenburg DC. Extracellular signal-regulated kinase 1/2-mediated transcriptional regulation of g-protein-coupled receptor kinase 3 expression in neuronal cells. J Pharmacol Exp Ther. 2007;321:51–59. doi: 10.1124/jpet.106.116921. [DOI] [PubMed] [Google Scholar]
- Desai AN, Standifer KM, Eikenburg DC. Cellular G protein-coupled receptor kinase levels regulate sensitivity of the {alpha}2b-adrenergic receptor to undergo agonist-induced down-regulation. J Pharmacol Exp Ther. 2005;312:767–773. doi: 10.1124/jpet.104.076042. [DOI] [PubMed] [Google Scholar]
- Desai AN, Salim S, Standifer KM, Eikenburg DC. Involvement of G protein-coupled receptor kinase (GRK) 3 and GRK2 in down-regulation of the alpha2B-adrenoceptor. J Pharmacol Exp Ther. 2006;317:1027–1035. doi: 10.1124/jpet.105.098996. [DOI] [PubMed] [Google Scholar]
- Desai AN, Standifer KM, Eikenburg DC. Simultaneous alpha2B- and beta2-adrenoceptor activation sensitizes the alpha2B-adrenoceptor for agonist-induced down-regulation. J Pharmacol Exp Ther. 2004;311:794–802. doi: 10.1124/jpet.104.069674. [DOI] [PubMed] [Google Scholar]
- Apparsundaram S, Eikenburg DC. Prejunctional alpha adrenoceptor desensitization in rat heart after chronic epinephrine treatment. J Pharmacol Exp Ther. 1996;278:862–870. [PubMed] [Google Scholar]
- Schwartz DD, Eikenburg DC. Enhanced endogenous neurotransmitter overflow in the isolated perfused rat kidney after chronic epinephrine administration: lack of a prejunctional beta adrenoceptor influence. J Pharmacol Exp Ther. 1988;244:11–18. [PubMed] [Google Scholar]
- Sadeghi HM, Eikenburg DC. Chronic epinephrine treatment fails to alter prejunctional adrenoceptor modulation of sympathetic neurotransmission in the rat mesentery. J Pharmacol Exp Ther. 1992;261:924–930. [PubMed] [Google Scholar]
- Shivachar AC, Eikenburg DC. Differential effects of epinephrine and norepinephrine on cAMP response and g(i3)alpha protein expression in cultured sympathetic neurons. J Pharmacol Exp Ther. 1999;291:258–264. [PubMed] [Google Scholar]
- Kazmi SM, Mishra RK. Identification of alpha 2-adrenergic receptor sites in human retinoblastoma (Y-79) and neuroblastoma (SH-SY5Y) cells. Biochem Biophys Res Commun. 1989;158:921–928. doi: 10.1016/0006-291X(89)92810-6. [DOI] [PubMed] [Google Scholar]
- Parsley S, Gazi L, Bobirnac I, Loetscher E, Schoeffter P. Functional alpha2C-adrenoceptors in human neuroblastoma SH-SY5Y cells. Eur J Pharmacol. 1999;372:109–115. doi: 10.1016/S0014-2999(99)00190-9. [DOI] [PubMed] [Google Scholar]
- Gleason MM, Hieble JP. Ability of SK&F 104078 and SK&F 104856 to identify alpha-2 adrenoceptor subtypes in NCB20 cells and guinea pig lung. J Pharmacol Exp Ther. 1991;259:1124–1132. [PubMed] [Google Scholar]
- Blaxall HS, Murphy TJ, Baker JC, Ray C, Bylund DB. Characterization of the alpha-2C adrenergic receptor subtype in the opossum kidney and in the OK cell line. J Pharmacol Exp Ther. 1991;259:323–329. [PubMed] [Google Scholar]
- Jansson CC, Marjamaki A, Luomala K, Savola JM, Scheinin M, Akerman KE. Coupling of human alpha 2-adrenoceptor subtypes to regulation of cAMP production in transfected S115 cells. Eur J Pharmacol. 1994;266:165–174. doi: 10.1016/0922-4106(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Eason MG, Liggett SB. Human alpha 2-adrenergic receptor subtype distribution: widespread and subtype-selective expression of alpha 2C10, alpha 2C4, and alpha 2C2 mRNA in multiple tissues. Mol Pharmacol. 1993;44:70–75. [PubMed] [Google Scholar]
- Schaak S, Cayla C, Blaise R, Quinchon F, Paris H. HepG2 and SK-N-MC: two human models to study alpha-2 adrenergic receptors of the alpha-2C subtype. J Pharmacol Exp Ther. 1997;281:983–991. [PubMed] [Google Scholar]
- Piletz JE, Zhu H, Chikkala DN. Comparison of ligand binding affinities at human I1-imidazoline binding sites and the high affinity state of alpha-2 adrenoceptor subtypes. J Pharmacol Exp Ther. 1996;279:694–702. [PubMed] [Google Scholar]
- Lomasney JW, Cotecchia S, Lefkowitz RJ, Caron MG. Molecular biology of alpha-adrenergic receptors: implications for receptor classification and for structure-function relationships. Biochim Biophys Acta. 1991;1095:127–139. doi: 10.1016/0167-4889(91)90075-9. [DOI] [PubMed] [Google Scholar]
- Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG., Jr. Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967;214:597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Escriba PV, Ventayol P, Murga C, Mayor F, Jr., Garcia-Sevilla JA. Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem. 1998;70:1249–1257. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Standifer KM, Cheng J, Brooks AI, Honrado CP, Su W, Visconti LM, Biedler JL, Pasternak GW. Biochemical and pharmacological characterization of mu, delta and kappa 3 opioid receptors expressed in BE(2)-C neuroblastoma cells. J Pharmacol Exp Ther. 1994;270:1246–1255. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]