Abstract
Background
Current understanding of the lymphatic system of the breast is derived mainly from the work of the anatomist Sappey in the 1850s, with many observations made during the development and introduction of breast lymphatic mapping and sentinel node biopsy contributing to our knowledge.
Methods
Twenty four breasts in 14 fresh human cadavers (5 male, 9 female) were studied. Lymph vessels were identified with hydrogen peroxide and injected with a lead oxide mixture and radiographed. The specimens were cross sectioned and radiographed to provide three dimensional images. Lymph (collecting) vessels were traced from the periphery to the first-tier lymph node.
Results
Lymph collecting vessels were found evenly spaced at the periphery of the anterior upper torso draining radially into the axillary lymph nodes. As they reached the breast some passed over and some through the breast parenchyma, as revealed in the cross-section studies. The pathways showed no significant difference between male and female specimens. We found also perforating lymph vessels that coursed beside the branches of the internal mammary vessels, draining into the ipsilateral internal mammary lymphatics. In some studies one sentinel node in the axilla drained almost the entire breast. In most more than one sentinel node was represented.
Conclusion
These anatomical findings are discordant with our current knowledge based on previous studies and demand closer examination by clinicians. These anatomical studies may help explain the percentage of false-negative sentinel node biopsy studies and suggest the peritumoral injection site for accurate sentinel lymph node detection.
Keywords: Sentinel lymph node biopsy, Lymphoscintigraphy, Peritumoral injection, Subareolar injection, Dermal injection, Cadaver study
Management of the lymph nodes has been an important part of breast cancer treatment since the time of Halsted’s radical mastectomy.1 Until the 1970s it was based on the perceived need to remove cancer cells before they spread via the lymphatics beyond the reach of the surgeon. The national surgical adjuvant breast and bowel project B04 study questioned the therapeutic benefit of axillary clearance, however it remained part of the treatment of invasive breast cancer because of the prognostic significance of the axillary lymph nodes status.
Sentinel node biopsy (SNB) has replaced axillary dissection in clinically node negative patients,2–4 with trials confirming a false negative rate of 5–10%. While it is agreed that SNB is appropriate, the optimal injection sites of dye and/or colloid has not been defined.5–7 There are advocates of peritumoral,8–9 dermal and subdermal10–12 or subareolar and periareolar injection13–14 of the tracers. These recommendations regarding injection sites are derived from clinical experience rather than from anatomical studies.
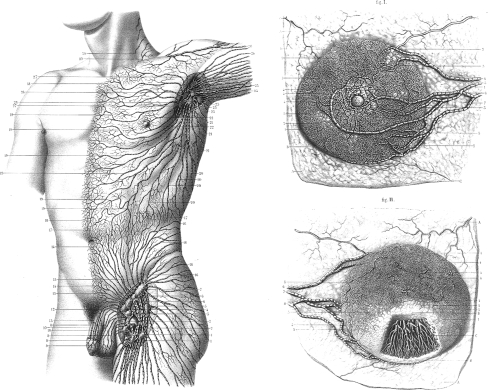
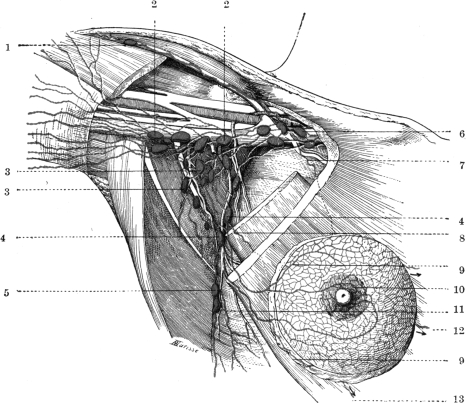
The anatomical basis of axillary clearance was derived from the publications of Sappey in the 1870s15 who showed the breast lymphatics as separate from those of the underlying torso, with a subareolar plexus of lymphatics, and a small number of large lymphatic vessels draining into axillary lymph nodes (Fig. 1). Despite the revolution in the surgical approach, there has been no advance in the underlying anatomical knowledge of the breast lymphatics.
Fig. 1.
Sappey’s drawing of the superficial lymphatics of the upper torso (left) and female breast (right) in 1874.15
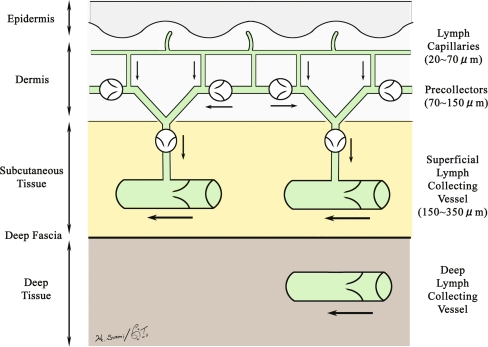
The lymphatics start as an avalvular lymph capillary network. In the skin they commence in the superficial dermis with diameters of 20–70 μm.16 They drain to precollectors that are up to 300 μm diameter, contain valves, have some smooth muscle cells in their wall,17 and drain this network to subcutaneous lymph collecting vessels (Fig. 2). The lymph collecting vessels (lymph collectors) have a smooth muscle layer18 and are the vessels commonly identified as lymphatics. The breast develops as a modified skin gland appendage and expands into the subcutaneous tissues.19
Fig. 2.
Schematic diagram of the relationship between the lymph capillaries, precollectors, and lymph collecting vessels.
Progress in microsurgery has enabled new methods for investigating the lymphatic system. We developed a protocol for injecting radio-opaque contrast medium into lymph vessels of <0.3 mm diameter in cadavers. The contrast agent fills the draining lymph vessels, which are then radiographed.20,21We have previously published detailed investigations of the lymphatics of the upper limb.22–24
This study addresses the lymphatic anatomy of the breast and anterior upper torso. Our findings may explain the clinical experience in lymphatic mapping and sentinel node biopsy, and also the persistence of a false-negative rate of 5–10% irrespective of the experience of the surgeon.
Materials And Methods
Bilateral anterior upper torso specimens, which included both breasts, were harvested from 10 cadavers (4 male and 6 female) with incisions across the root of the neck, down the posterior axillary line and across the abdominal wall, just above the umbilicus. Another four unilateral studies were obtained from separate cadavers (1 male and 3 females) by a midline incision of the sternum, thus resulting in a total of 24 sides. Protocol refinements at the beginning of the study limited early results and there were always time limits to complete the work, especially on the contralateral side of bilateral subjects, before the specimen became putrefied. The injection and dissection for each side of a specimen took 4–5 weeks. The specimens were stored at −20°C prior to dissection and at 4°C during the dissection.
The lymphatic mapping technique has been described previously.20–21 Briefly 6% hydrogen peroxide was injected into the tissue being examined. Bubbles appear in the tissue and the lymphatic collectors and an operating microscope (Stemi 2000, Carl Zeiss Pty. Ltd. Germany) is used to identify and cannulate the lymphatic vessels with a 30-gauge needle (Precision glide needle: Becton Dickinson & Co., USA.) or an extruded glass cannula for very small vessels. Radio-opaque contrast medium containing lead oxide (P3O4 Red lead, AJAX Chemicals, Australia) was injected antegrade with a micromanipulator (UM-3C, Narishige Co., Japan). The contrast stopped after a variable distance because of coagulation requiring further cannulation and injection. This was repeated until the first lymph node for each collector was reached (sentinel node).
The procedure was performed: around the entire periphery of the specimens in the subcutaneous plane; around the internal thoracic artery on the inner aspect of the chest; and in the vicinity of the perforating branches of the internal thoracic artery to identify superficial lymphatics, internal mammary lymphatics, and perforating lymphatics respectively.
Indocyanine Green dye (Pulsion Co., Germany) was injected into the internal mammary artery to demonstrate the artery and its perforating branches, which supply blood to the medial aspect of the breast, thus facilitating the identification of associated deep perforating lymphatics.
A mixture of blue dye (Ultamarine, Educational Colours Pty Ltd., Melbourne) and hydrogen peroxide was used to stain the lymph collecting vessels originating from the nipple and areolar region. In previous studies of the upper limb we have first injected this blue dye into the dermis before injecting hydrogen peroxide into the subcutaneous tissues to help identify the collecting lymphatics.22 This was helpful in identifying lymphatics in the fingertips, where they exist at high concentration. This dye was injected also into the dermis over the breast mound but was only helpful in the nipple areolar area where again the lymphatics were concentrated.
The specimen was radiographed after completion of all injections to give the two-dimensional views of the lymph vessel anatomy. To provide the three-dimensional views, the female specimens were sliced parallel to the lymph vessels as they passed towards the axilla. These slices were then placed on their sides and radiographed to show the position of the lymphatic collectors with respect to the skin and the breast tissue.
Radiographs were photographed (Nikon D100, Nikon Co., Japan) and transferred to the computer, then each lymphatic collector was traced (Adobe Photoshop CS, Adobe System Inc.) to its first-tier node and color coded. All collectors draining to the same first-tier node were assigned the same color after tracing them retrogradely from the sentinel nodes.
Results
Superficial Lymphatic System
Lymphatic collectors were identified in the subcutaneous tissues near the peripheral cut edges of the specimens and the lateral border of the sternum. The collectors often branched in the peripheral region then combined to form larger collectors that remained approximately uniform in diameter until they reached the first lymph node (Figs. 3 and 4). Some collecting lymph vessels joined with others within the same sentinel node territory, rarely with others, before reaching the lymph node. All superficial lymph vessels in these dissections entered a lymph node in the axilla, which was always close to the lateral edge of the pectoralis minor muscle. The findings were similar in both sexes (Fig. 4). In all of the specimens we examined, many of the lymphatic collectors that passed over or through the breast ended by draining into the same first-tier lymph node. In some, almost the entire breast drained to one sentinel node. In others however, there was at least one other node that was the first-tier node for a collecting lymphatic that passed through part of the breast.
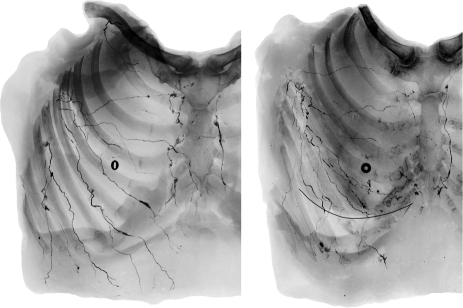
Fig. 3.
Radiographs in antero-posterior views of a male (left) and female (right) specimen after completing injections with the lead oxide mixture. Note that the torso lymph vessels radiate centripetally towards the axilla.
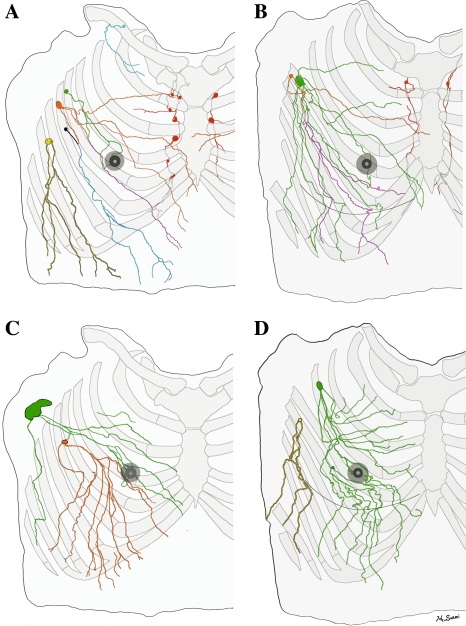
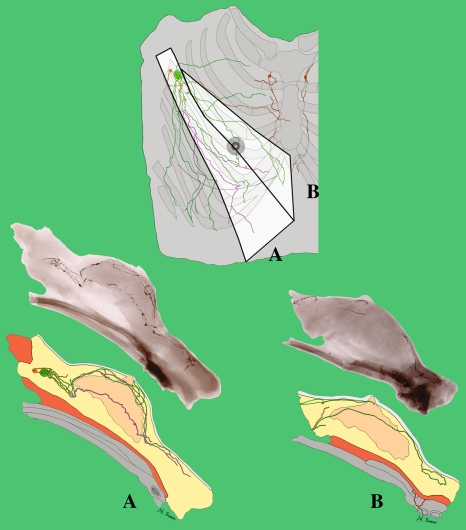
Fig. 4.
Tracing distally of lymphatics of both hemi upper torsos (male: A and C, female: B and D) from each first-tier lymph node colour coded; pectoral node (green, orange, black and yellow), subclavicular node (light blue), and internal mammary node (red). Note (i) that the lymph collecting vessels from the nipple and areolar region on each specimen drain into the green-colored lymph node; (ii) the similar pattern of chest and breast drainage between the male and female studies; (iii) that the breast lies in the pathway of collecting lymphatics that start peripherally and (iv) that, although the majority of the breast drains to one sentinel node in D, every breast area is drained by more than one first-tier node in each study.
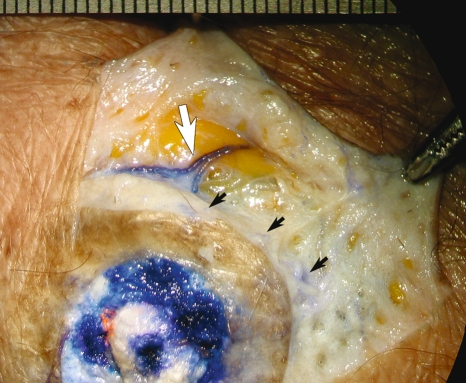
The lymphatics deep to the nipple and areola area were different from those of the other areas we examined. Microscope-assisted dissection of the areolar region revealed a dense network of lymph capillaries and precollectors in the dermis. This structure is presumably the subareolar plexus identified by Sappey. In most areas, dye injected subcutaneously over the breast mound was not taken up by the lymphatics. Dye injected in the nipple and areolar region was taken up by 2 or 3 superficial lymph collecting vessels via lymph capillaries and precollectors (Fig. 5) each side of the areola and these drained into a first tier node in the pectoral group of axillary nodes (shown green in Fig. 4).
Fig. 5.
Photograph of dissection in the areolar region after injecting the mixture of dye and hydrogen peroxide into the nipple. Lymph capillaries and precollectors (black arrows), and lymph collecting vessels (white arrow) were stained with the blue dye.
The radiograph of the cross-sectioned female specimen showed that the lymphatics have a “wavy” path through the superficial part of the subcutaneous tissue of the chest (Fig. 6). In the breast region most of the lymph collectors ran between the dermis and the breast tissue. However, some passed through the breast tissue itself. After traversing the breast all of the collectors passed deeply to reach axillary lymph nodes.
Fig. 6.
The diagram (above) shows how the breast of Fig. 4B was sectioned to provide cross-section radiographs (middle of the breast and its lymphatic drainage (below). Note that some lymph collectors originating from the lower torso run through the breast tissue in both A and B sections.
Internal Mammary Lymphatic System
The internal mammary lymphatic vessels were identified alongside the internal mammary artery and vein, deep to the parietal pleura, with lymph nodes present in the intercostals spaces. Collecting lymphatics were identified beside the perforating branches of the internal mammary arteries that were found in the deep medial aspect of the breast tissue (Fig. 7). A single collector passed though the intercostal fascia and muscle beside each perforating artery to join the internal mammary lymphatic system.
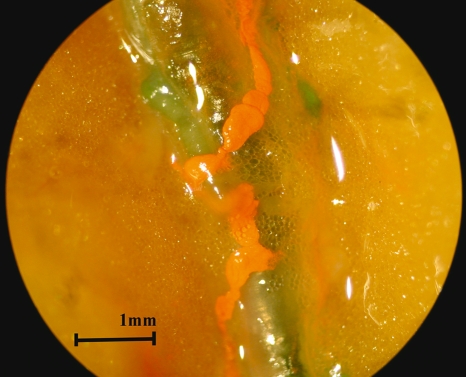
Fig. 7.
The appearance of the perforating lymphatics (orange) along the branch from the internal mammary artery (green) in the right lateral sternal region of the female specimen.
There was no apparent connection between the collecting lymphatics accompanying the branches of the internal mammary artery and the superficial collecting lymphatics. Contrast injected into the superficial collecting lymphatics never flowed into the perforating collecting lymphatics. Even in the subcutaneous tissues overlying the lateral edge of the sternum, the superficial collecting lymphatics passed laterally towards the axilla. We found no evidence of direct anastomosis between the superficial collecting lymphatics and the collectors associated with perforating arterial branches, at the size of the collecting vessels. However, this does not exclude a connection at either the precollector or lymphatic capillary network as we were unable to demonstrate them radiologically.
Discussion
Cruikshank was the first to report the lymphatics of the breast in his book published in 1787.25 He used the cadaver of a pregnant woman injecting mercury into the mammary duct from the nipple. He reported seeing by chance mercury-filled lymphatics but did not make any diagrams of his findings.
In 1874, Sappey applied the same method,15 injecting mercury into the dermis of a semiputrefied cadaver to identify the superficial lymph vessels. A very thin adult cadaver was used and he described the superficial lymphatic drainage of the torso, dividing it into four territories by a sagittal midline and a horizontal line at the L2 level. The upper torso lymphatics always drained into the ipsilateral axilla (Fig. 1). Sappey reported that the lymphatics of the breast collected in a subareolar plexus and then drained towards the axilla via lymph collecting vessels. Sappey’s description of the breast lymphatics was adopted by anatomists and became the theoretical basis for the subareolar injection of dye and/or isotope for lymphatic mapping as part of the sentinel node biopsy for breast cancer more than one century later.13–14
In 1903, Poirer and Cuneo summarized Sappey’s results.26 They added their results from foetal studies using with Gerota’s method27 using oil painting dye to stain the lymphatics, they reviewed other people’s studies and published a comprehensive anatomy book of the lymphatic system. Poirer and Cuneo’s famous picture of the lymphatics of the breast (Fig. 8) was redrawn and is still being used in Gray’s Anatomy.28
Fig. 8.
Poirier and Cuneo’s summary diagram of the breast lymph drainage.26 This diagram was composed based on the anatomical and clinical findings of several people, including Sappey.
In 1959, Turner-Warwick performed photographic and radiographic studies of the collecting lymphatics injecting them with iron Prussian blue or radioisotope gold (Au198) during surgery in breast cancer patients.29 He demonstrated lymphatic pathways that passed direct from the tumor injection site in the breast to the axillary lymph nodes that bypassed the subareolar plexus. He found that all quadrants of the breast drained to the axilla and either the internal mammary or posterior intercostal nodes. He suggested that Sappey had mistaken the mammary duct for a lymphatic vessel, thereby overemphasizing the importance of the subareolar plexus.
The more recent users of lymphoscintigraphy examinations of the breast are also skeptics of the centripetal lymphatic route towards the subareolar plexus. Tanis30 and Uren (Uren RF: personal communication, May 2007) stated that there is no constant route via the subareolar plexus.
Our current anatomical knowledge of the breast lymphatics still depends on Sappey and Poierer and Cuneo’s diagrams. Since Sappey used thin adult cadavers and Poirer and Cuneo used infant cadavers, the relationship between the superficial lymphatics and the adult breast tissue, and also the relationship of the lymphatic drainage of the breast tissue with that of the surrounding superficial tissues, has not been adequately described. Controversy still exists over the role of the subareolar plexus in the lymphatic drainage of the breast. There has been no concrete evidence of a centripetal anatomical lymphatic pathway that drains the breast tissue towards the subareolar plexus and then, via this plexus, towards the sentinel node.
Our direct injection technique, however, gives a comprehensive image of the lymphatic system in individual specimens. Each lymph vessel that enters a lymph node can be traced retrogradely to provide an accurate map of the tissue for which that lymph node is “sentinel.” We have photographed, radiographed, and recorded actual lymphatic pathways. In addition, cross-sectional studies have been performed to obtain three-dimensional images of these lymph collecting vessels.
We have shown that some of the torso vessels pass from the periphery through the breast tissue on their way towards the axilla. This is discordant with the conventional understanding that they run just underneath the skin.
We compared the torso lymphatic vessel pathways and breast tissue lymphatic vessel pathways in adult female cadavers. Although our results do not show conclusively that the lymph drainage of the breast tissue is via the collecting lymphatics that pass through or immediately superficial to the breast substance, this is likely to be the case since (i) the lymph collecting vessel has a microporous nature and drains surrounding tissue fluid along its entire course31 and (ii) lymphoscintigraphy in breast cancer patients after peritumoral injection, coupled with similar studies with radioactive gold by Turner-Warwick, usually reveals a direct pathway from the injection site to the axillary lymph node,29–30 not via a subareolar plexus. Therefore we conclude that the lymph collecting vessels that pass through the breast contribute to breast lymph drainage and must be the same vessels shown in lymphoscintigraphy examinations.
Embryologically, the development of the human peripheral lymphatics is poorly understood. However, the lymphatics of the foetus represent similar arrangements to those of adults.32–33 We assume that some of the lymph vessels are trapped inside the mammary gland as the breast develops around preexisting superficial lymph collectors. We have color coded a diagram of our dissection to simulate various injection sites in lymphatic mapping and sentinel node biopsy for breast cancer. Figure 4 shows that, if the tracer is injected deep around the purple-colored vessel (lower outer quadrant), it reaches both the green and orange lymph node in the pectoral group. However, if the tracer is injected into the subareolar region or into the lower outer quadrant intradermaly near the tumor, it reaches only the green node. This anatomical analysis suggests a mechanism for false negative sentinel node biopsy since more than one sentinel node drains the breast in this example.
False-negative results following sentinel node biopsy are of the order of 5–10% in different series.34–37 Reasons given to this have been technical; either related to:
The surgeons’ experience with the technique or
The size of the radioactive tracer which may not reach the lymph node, especially if sited in a peritumoral position.
Our results now offer an anatomical explanation for these false negative results as a distinct third possibility.
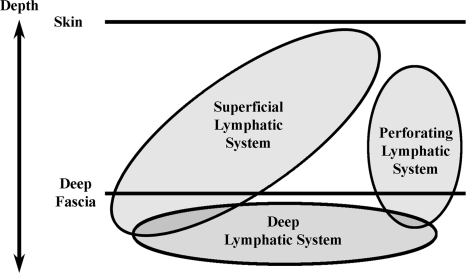
Perforating lymphatics that pierce the deep fascia are critical when discussing breast lymph drainage. The lymphatic system is classified conventionally into the superficial system and the deep system because of their relationship to the deep fascia. However, we subdivided the system draining the tissues above the deep fascia into the superficial system and the perforating system (Fig. 9). The perforating system is connected to the deep lymphatic system and these collecting vessels have the same appearance as the superficial lymphatics as they course with the internal thoracic blood vessels. Variations of the blood supply to the breast have been reported.38–42 Therefore, we hypothesize that the perforating lymphatics may have the same variations. This helps explain the fact that in all quadrants of the breast, cancer has the potential to metastasize via the internal mammary lymphatics especially if the tumor is medial or deep in the breast parenchyma.43–44 It is accepted that intradermal injection of tracer rarely demonstrates the internal mammary lymphatics.10–12 The variable contribution of perforating lymphatics along the branches of the internal mammary artery to lymphatic drainage of the breast cannot be predicted clinically and therefore the likelihood of sentinel nodes being located along the internal mammary artery is also unpredictable. Our anatomical analysis suggests that accurate lymphatic mapping requires peritumoral injection.
Fig. 9.
Our concept of the breast lymph drainage, drained by both the perforating lymphatic system and the conventional horizontal superficial lymphatic system with their relationship to the lymphatic system beneath the deep fascia.
Conclusions
Current anatomical knowledge of the breast lymphatics is derived from the work of Sappey, Poirier, and Cuneo. Our anatomical cadaver study of the relationship of the lymphatics of the torso and the adult breast goes a step further and provides additional information which has special significance for sentinel lymph node biopsy. We have used a refined protocol to accurately record the anterior upper torso lymphatics in adult male and female cadavers. The patterns of the superficial lymphatics were no different between sexes and frequently more than one sentinel node drained the breast. The cross-sectional studies of the female breast showed some lymph vessels of the torso coursing through the breast tissue. We found also perforating lymphatics coursing with similar branches of the internal mammary artery and vein.
This anatomical analysis suggests that (i) peritumoral injection is preferable for identification of the sentinel lymph node for breast cancer treatment and (ii) may help explain the incidence of negative sentinel node studies where only the subareolar plexus is injected with the radioisotope tracer.
Acknowledgements
We thank Mrs Prue Dodwell for her help with the preparation of the manuscript. We are indebted to The Jack Brockhoff Foundation, The Colonial Foundation, and The National Health & Medical Research Foundation for funding this research, The Royal Melbourne Hospital, The Sydney Melanoma Unit at the Sydney Cancer Centre, The Ludwig Institute for Cancer Research, and The Department of Anatomy and Cell Biology at The University of Melbourne for their continuous support.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Halsted WS. The treatment of wounds with especial reference to the value of the blood clot in the management of dead spaces. Johns Hopkins Hosp Rep 1891; 2: 255–314
- 2.Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997; 226: 271–8 [DOI] [PMC free article] [PubMed]
- 3.Hsueh EC, Tuner RR, Glass EC, Brenner RJ, Brennan MB, Giuliano AE. Sentinel node biopsy in breast cancer. J Am Coll Surg 1999; 189: 207–13 [DOI] [PubMed]
- 4.Giuliano AE, Haigh PL, Brennan MB, Hansen NM, Kelley MC, Ye W, Glass EC, Turner RR. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol 2000; 18:2553–9 [DOI] [PubMed]
- 5.Ciesl L, Mann GB. Alternative sites of injection for sentinel lymph node biopsy in breast cancer. ANZ J Surg 2003; 73: 600–4 [DOI] [PubMed]
- 6.Allweis TM, Badriyyah M, Ad VB, Cohen T, Freund HR. Current controversies in sentinel lymph node biopsy for breast cancer. The Breast 2003; 12: 163–71 [DOI] [PubMed]
- 7.Noguchi M. Current controversies concerning sentinel lymph node biopsy for breast cancer. Breast Cancer Res Treat 2004; 84: 261–71 [DOI] [PubMed]
- 8.Krag DN, Weaver DL, Alex JC, Fairbank JT. Surgical resection and radiolocalization of the sentinel node in breast cancer using a gamma probe. Surg Oncol 1993; 2: 335–9 [DOI] [PubMed]
- 9.Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg 1994; 220: 391–8 [DOI] [PMC free article] [PubMed]
- 10.Veronesi U, Paganelli G, Galimberti V, et al. Sentinel-node biopsy to avoid acillary dissection in breast cancer with clinically negative lymph-nodes. Lancet 1997; 349: 1864–7 [DOI] [PubMed]
- 11.Borgstein PJ, Meijer S, Pijpers R. Intradermal blue dye to identify sentinel lymph nodes in breast cancer. Lancet 1997; 349: 1668–9 [DOI] [PubMed]
- 12.Linehan DC, Hill AD, Akhurst T, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimize sentinel node identification in breast cancer patients. Ann Surg Oncol 1999; 6: 450–4 [DOI] [PubMed]
- 13.Kimberg VS, Rubio IT, Henry R, et al. Subareolar versus peritumoral injection for location of the sentinel node. Am Surg 1999; 6: 860–5 [DOI] [PMC free article] [PubMed]
- 14.Kern KA. Sentinel lymph node mapping in breast cancer using subareolar injection of blue dye. J Am Coll Surg 1999; 189: 539–45 [DOI] [PubMed]
- 15.Sappey MPC. Anatomie, Physiologie, Pathologie des vaisseaux Lymphatiques consideres chez L’homme at les Vertebres. Paris: A. Delahaye and E. Lecrosnier, 1874
- 16.Wenzel-Hora BI, Berens von Rautenfeld D, Majewski A, Lubach D, Partsch H. Scanning electron microscopy of the initial lymphatics of the skin after use of the indirect application technique with glutaraldehyde and MERCOX as compared to clinical findings. Lymphology 1987; 20: 126–44 [PubMed]
- 17.Sacchi G, Weber E, Agliano M, Raffaelli N, Comparini L. The structure of superficial lymphatics in the human thigh: precollectors. Anat Rec 1997; 247: 53–62 [DOI] [PubMed]
- 18.Kinmonth JB, Taylor GW. Spontaneous rhythmic contractility in human lymphatics. J Physiol 1956; 133: 3
- 19.Ellis H, Colborn GL, Skandalakis JE. Surgical embryology and anatomy of the breast and its related anatomic structures. Surg Clin North Am 1993; 73: 611–32 [DOI] [PubMed]
- 20.Suami H, Taylor GI, Pan WR. A new radiographic cadaver injection technique for investigating the lymphatic system. Plast Reconstr Surg 2005; 115:2007–13 [DOI] [PubMed]
- 21.Suami H, Taylor GI, O’Neill JK, Pan WR. Refinements of the radiographic cadaver injection technique for investigating minute lymphatic vessels. Plast Reconstr Surg 2007; 120:61–7 [DOI] [PubMed]
- 22.Suami H, Taylor GI, Pan WR. The lymphatic territories of the upper limb: Anatomical Study and clinical implications. Plast Reconstr Surg 2007; 119: 1813–22 [DOI] [PubMed]
- 23.Suami H, Pan WR, Taylor GI. Changes in the lymph structure of the upper limb after axillary dissection — radiographic and anatomical study in a human cadaver. Plast Reconstr Surg 2007;120:982–91 [DOI] [PubMed]
- 24.Suami H, O’Neill JK, Pan WR, Taylor GI. Superficial lymphatic system of the upper torso — preliminary radiographic results in human cadavers. Plast Reconstr Surg (In press) [DOI] [PubMed]
- 25.Cruikshank WC. The anatomy of the absorbing vessels of the human body. London: G. Nicol, 1786
- 26.Delamere G, Poirier P, Cuneo B. The lymphatics. In: Charpy PP eds. A trearise of human anatomy. Westminster: Archibald Constable, 1903
- 27.Gerota D. Zur technik der Lymphgefassinjection. Eine neue Injectionmasse fur Lymphgefasse. Polychrom Injection. Anat Anz 1896; 12: 216
- 28.Susan Standring, Editor in Chief, Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 39th Ed. Elsevier Churchill Livingstone, 2005
- 29.Turner-Warwick RT. The lymphatics of the breast. Br J Surg 1959; 46: 574–82 [DOI] [PubMed]
- 30.Tanis PJ, Nieweg OE, Valdes Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg 2001; 192:399–409 [DOI] [PubMed]
- 31.Leak LV. Electron microscopic observations on lymphatic capillaries and the structural components of the connective tissue-lymph interface. Microvasc Res 1970; 2:361–91 [DOI] [PubMed]
- 32.Rouvière H. Anatomie des lymphatiques de l’Homme. Paris: Masson, 1932
- 33.Bartels P. Das lymph gefasssystem. In: Bardeleben Kv eds. Handbuch der Anatomie des Menschen. Jena: Gustav Fisher, 1909
- 34.Mabry H, Giuliano AE. Sentinel node mapping for breast cancer: progress to date and prospects for the future. Surg Oncol Clin N Am 2007; 16:55–70 [DOI] [PubMed]
- 35.Martin II RCG, Chagpar AC, Scoggins CR, et al. Clicopathologic factors associated with false-negative sentinel lymph-node biopsy in breast cancer. Ann Surg 2005; 241: 1005–15 [DOI] [PMC free article] [PubMed]
- 36.Cox CE, Salud CJ, Cantor A, et al. Learning curves for breast cancer sentinel lymph node mapping based on surgical volume analysis. J Am Coll Surg 2001; 193:593–600 [DOI] [PubMed]
- 37.Morrow M, Rademaker AW, Bethke KP, et al. Learning sentinel node biopsy: results of a prospective randomized trial of two techniques. Surgery 1999; 126: 714–22 [PubMed]
- 38.Cormack GC, Lamberty BG. The arterial anatomy of skin flaps. Second Edition. Edinburgh: Churchill Livingstone, 1994
- 39.Anson BJ, Wright RR. Blood supply of the mammary gland. Surg Gynaecol Obstet 1939; 69: 468–73
- 40.Carr BW, Bishop WE, Anson BJ. Mammary arteries. Q Bull Northwest Uni Med School 1942; 16: 150–4
- 41.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987; 40: 113–41 [DOI] [PubMed]
- 42.Taylor GI, Caddy CM, Watterson PA, Crock JG. The venous territories (venosomes) of the human body: experimental study and clinical applications. Plast Reconstr Surg 1990; 86: 185–213 [DOI] [PubMed]
- 43.Shimazu K, Tamaki Y, Taguchi T, et al. Lymphoscintigraphic visualization of internal mammary nodes with subtumoral injection of radiocolloid in patients with breast cancer. Ann Surg 2003; 237: 390–8 [DOI] [PMC free article] [PubMed]
- 44.Veronesi U, Cascinelli N, Buffalino R, et al. Risk of internal mammary lymph node metastases and its relevance on prognosis of breast cancer patients. Ann Surg 1983; 198: 681–4 [DOI] [PMC free article] [PubMed]