Abstract
Objective
Marijuana abuse is associated with neurological changes including increases in frontal EEG alpha during abstinence. Research is needed to assess to what extent these EEG patterns are indicative of cerebral perfusion deficits.
Methods
We recorded the resting eyes closed EEG of 75 abstinent marijuana users and 33 control subjects. Fifty-six marijuana users used marijuana for less than eight years and 19 used for eight years or more. The EEG evaluation occurred within 72 hours of admission to an inpatient unit. Fifty-nine marijuana users remained abstinent for a month and were tested twice. Supplemental psychological and physiological data were also collected.
Results
Log alpha2 and beta2 power at posterior sites were significantly lower for the marijuana abusers that used eight years or more than the other marijuana abusers and the control subjects. These EEG changes continued for the month of abstinence. The marijuana users who used marijuana for more than eight years, also, had lower heart rates and thyroid function (T4) compared to the other marijuana users and the control subjects.
Conclusions
Chronic marijuana use was also associated with reduced EEG power in alpha and beta bands at posterior sites. These reductions in EEG power appear to be related to cerebral perfusion deficits and/or thyroid function in marijuana abusers.
Significance
Our results suggest EEG, cerebral blood flow velocity, cardiovascular and thyroid function alterations in marijuana abuser with an extended period of use. These alterations reflect under arousal in these systems.
Keywords: Marijuana, cardiovascular function, cerebral blood flow velocity, resting EEG, thyroid function
Introduction
Illicit marijuana abuse is a major public health problem among young persons (Gruber & Pope, 2002; Compton et al., 2004). Most problematic among young marijuana users is the view that marijuana was not harmful (Gruber & Pope, 2002; Hall & Degenhardt, 2006) even though cognitive and psychiatric impairments have been documented in heavy marijuana users (Fletcher et al., 1996; Pope et al., 1996, 2001; Ehrenreich et al., 1999; Bolla et al., 2002; Solowij et al., 2002; Fergussion et al., 2006). Strokes in relatively young individuals have also been reported (Cooles & Michaud, 1987; Zarchariah, 1991; Barnes et al., 1992; Lawson & Rees, 1996; MacCarron, 1997; White et al., 2000; Marinella, 2001; Mesec et al., 2001; Alvaro et al., 2002). Related to the cognitive impairments and strokes, altered cerebral blood flow has also been observed in chronic marijuana users during abstinence (Tunving et al., 1986; Amen, & Waugh, 1998; Lundqvist et al., 2001; Block et al, 2002; Herning et al., 2005; Sneider et al., 2006).
In addition, chronic marijuana users have an increase in frontal EEG alpha power during abstinence (Struve et al., 1999). The 1999 paper also summarizes two earlier studies (Struve et al., 1989, 1994) of the EEG of abstinent mental patients who used marijuana for an extended period of time. The data collected in the 1999 paper were not collected from mental patients, but represent a well designed study of marijuana abusers and control subjects. However, these EEG alterations in abstinent marijuana abusers are not clearly indicative of cognitive or cerebral perfusion deficits previously observed in marijuana abusers and only one research group has reported it. The increased EEG alpha power observed in marijuana users (Struve et al., 1999) might have resulted from subliminal depression and/or anxiety. On the other hand, a mild withdrawal syndrome has been documented in chronic marijuana users (Jones et al., 1981; Budney et al., 2001; Haney et al., 1999, 2005). The increase in frontal EEG alpha power might be related to these withdrawal symptoms such as irritability, restlessness, aggression, anger, loss of appetite, insomnia and tremor. Thus, it is important to replicate the increase in frontal EEG alpha (Struve et al, 1999) and to link the EEG changes to other changes during marijuana abstinence. To do this, we recorded the resting EEG and battery of physiological and psychological measures that were sensitive to marijuana abstinence from 75 abstinent marijuana users within 72 hours of admission to our closed clinical research ward and compared them with 33 control subjects. Fifty-nine of the marijuana users completed the study and were tested again after 28 to 30 days of monitored abstinence to determine whether the changes observed during early marijuana withdrawal persist. Changes in measures that persist for a month might not be acute marijuana withdrawal symptoms, but more permanent changes resulting from prolonged marijuana abuse. Two marijuana using groups were formed. One group matched the use patterns (almost daily for about nine years) of the Struve and associates’ study (1999) and the other group used marijuana for a shorter period of time (almost daily for about four years). We hypothesized that the group using for a longer period of time would have EEG alterations while the group with a shorter period of use would be similar to the control group. The hypothesis was based on studies of cognitive function in marijuana users that suggested that cognitive deficits occurred after heavy long term use (Gonzalez et al., 2001; Pope et al. 2003; Messimis et al., 2006). In these studies, the shorter term marijuana use did not always produce cognitive deficits.
Methods
Subjects
Seventy-five marijuana users that reported using marijuana at least 15 of the last 30 days and thirty-three control subjects (CS) were studied. These subjects were selected by the specific criteria listed below from a larger sample of over 600 subjects tested at NIDA that were recruited through ads in the community. The marijuana abusers were not seeking treatment for drug abuse or any other psychiatric disorder. The marijuana abusers were divided into two groups based on the number of years that they reported using marijuana. Fifty-six individuals that reported smoking marijuana less than eight years comprised the short duration group (MJ-short). Nineteen individuals that reported using marijuana for eight or more years comprised the long duration group (MJ-Long). The division at eight years of use allowed us to have one group of marijuana abusers with similar marijuana use history as in the Struve and associates’ (1999) study. Both the number of days of marijuana use in the last thirty days and the number of years of marijuana use were obtained from the Addiction Severity Index (ASI: McLellan et al., 1986). Before undergoing the EEG assessment, all volunteers had undergone medical, neurological, psychological (ASI, SCL-90R (Derogatis, 1992), Buss-Durkey Hostility Index, BDHI (Buss et at., 1952), DIS-IV (Robins, et al., 1995)) and medical laboratory evaluations. Exclusion criteria that applied to all subjects were: 1) major medical and psychiatric illnesses including history of hypertension, 2) head injuries with loss of consciousness for greater than five minutes, 3) evidence of any neurological abnormalities by history or examination, 4) HIV seropositivity, and 5) illicit drug use (cocaine, heroin etc.) or excessive alcohol use by DSM-IV criteria for abuse or dependence using the DIS-IV. The research protocol was approved by the National Institute on Drug Abuse and Johns Hopkins Bayview Medical Center Institutional Review Boards for Human Research. Informed consent was obtained from all subjects.
Demographic information and drug use history information were obtained from the ASI. Subjects with drug use other than marijuana use were screened out of the present study. Thus other than alcohol, tobacco and marijuana use reported in Table 1, illicit drug use was not self-reported nor observed in urine toxicologies obtained during the screening process.
Table 1.
Demographic Measures and Drug History
| Control | Marijuana Abusers | |||||
|---|---|---|---|---|---|---|
| MJ-Short | MJ-Long | |||||
| Demographic Measures | Mean | SD | Mean | SD | Mean | SD |
| Age: years | 22.8 | 5.3 | 21.4 | 3.4 | 24.3 | 4.1 |
| Education: years | 12.7 | 1.3 | 11.5 | 1.4 | 12.0 | 1.7 |
| Shipley: IQ | 101.3 | 9.0 | 94.3 | 10.0 | 97.5 | 10.0 |
| Family History of Alcoholism # | 36.1% | 54.8% | 65.2% | |||
| ASPD symptoms | 1.5 | 1.6 | 3.9 | 2.2 | 4.2 | 2.7 |
| Women: % | 53.0% | 32.3% | 39.1% | |||
| African Americans: % | 75.0% | 86.2% | 87.0% | |||
| Drug History Measures | ||||||
| Alcohol: days/30 days& | 0.7 | 1.8 | 4.1 | 5.0 | 6.1 | 6.5 |
| Alcohol: years% | 1.1 | 2.6 | 1.7 | 2.6 | 3.2 | 4.6 |
| Marijuana: days/30 days | 26.0 | 5.1 | 26.4 | 4.9 | ||
| Marijuana: years | 4.4 | 1.5 | 9.6 | 1.8 | ||
| Marijuana: age at first use# | 17.2 | 3.7 | 14.6 | 3.5 | ||
| Cigarettes/day | 2.1 | 6.4 | 6.7 | 7.2 | 5.8 | 6.6 |
| Cigarettes: years | 1.1 | 3.4 | 3.1 | 3.0 | 4.7 | 4.3 |
The number of days of substance use in the last 30 days from the ASI.
Years of substance use calculated from ASI.
Determined from ASI.
Procedures
EEG, cardiovascular, and cerebral blood flow velocity measurements for the marijuana abusers were made within 72 hours of admission to our closed clinical research ward. A second set of measurements was made 28 to 30 days of monitored abstinence on the research ward on 59 marijuana abusers that completed the study. Our closed research ward was assessable only to authorized staff and no visitors were permitted. Random urine samples were collected for toxicologies. The CS group was tested once during an outpatient visit.
EEG Recording
A three minute period of EEG was recorded during resting eyes-closed condition from sixteen electrodes (Fp1, F7, F3, C3, T3, T5, P3, O1, Fp2, F8, F4, C4, T4, T6, P4, and O2). The reference electrodes were on the ear tips. Eye movement was recorded from above and to side of the left eye. The EEG was amplified with Grass (Model 7P511) amplifiers and processed with a 1 to 50 Hz half-amplitude band pass and notch filter at 60 Hz. The EEG was sampled at the rate 104 samples per second per channel. EEG artifact was removed by blind computer-assisted visual inspection (RIH). The EEG was converted to spectral power using a fast Fourier transform (algorithm by Chamberlin, 1985) with 256 points per epoch and the spectra were averaged over epochs. Absolute power was converted to logarithm base ten (log) of power to allow for a more normal distribution of the EEG power measures (Gasser et al., 1982). Log and relative (percent) power was divided into six frequency bands (delta: 0.4-3.9 Hz, theta: 4.0-7.9 Hz, alpha1:8.0-9.9 Hz, alpha2: 10.0-13.9 Hz, beta1: 14.0-24.9 Hz and beta2: 25.0-40.0 Hz). Power in each band was the mean of the discrete spectral resolutions in that band. The absolute and relative EEG power for each band from the 16 electrodes was grouped into five regions: frontal pole (mean of Fp1, Fp2 sites), frontal (mean of F3, F4, F7, and F8 sites), central (mean of C3, C4, T3, and T4 sites), parietal (mean of P3, P4, T5, T6 sites) and occipital (mean of O1 and O2 sites). The peak frequency of alpha power over both alpha1 and alpha2 bands was determined from mean of the peaks of O1 and O2 sites since a similar measure was used in Struve and associates study (1999). Log power at the peak alpha frequency was also determined.
Supplemental Measures
To more accurately describe the subjects in this study, additional psychological and physiological measures were obtained. It was hoped that these additional measures might provide insight into what the EEG changes observed in marijuana abusers in the present study might represent since these measures were observed in individuals during marijuana abstinence. The psychological tests given during early abstinence included: Symptom Check List 90 Revised (SCL-90R: Derogatis, 1992), Buss-Durkee Hostilely Inventory (BDHI: Buss et al., 1957), Ellison Wellness Questionnaire (Ellison, 1991), and the Beck Hopelessness Scale (Beck, 1974). These psychological tests measure in part anger, depression, anxiety, aggression irritability and restlessness known to be increased during marijuana abstinence (Jones et al., 1981; Budney et al., 2001; Haney et al., 1999, 2005). Physiological measures included: resting cardiovascular measures (heart rate and blood pressure), middle cerebral artery (MCA) blood flow velocity measures (mean velocity (Vm) and pulsatility index (PI)) and thyroid function test (TSH, T3 uptake and T4). Cerebral blood flow velocity measures have been previously found to be altered in chronic marijuana abusers (Herning et al., 2005) and may be related to EEG changes observed in this study. The thyroid function measures were selected as a possible way to access possible metabolic changes resulting from the loss of appetite observed in abstinent marijuana abusers (Budney et al., 2001; Haney et al., 1999, 2005). Blood flow velocity was determined using a temporal window (zygomatic arch) for right and left middle (MCA), cerebral arteries using pulsed transcranial Doppler sonography (Nicolet, Model TC2000). The evaluation of the two arteries took from ten to fifteen minutes. Mean velocity (Vm: cm/s), systolic velocity (Vs: cm/s), diastolic velocity (Vd: cm/s), and pulsatility index (PI=(Vs-Vd)/Vm) were determined for each artery. The mean of both arties was used in the analyses. The cardiovascular and cerebral blood flow measures were obtained early (<72 hours) and late (28-30 days) during monitored abstinence for the marijuana abusers. The blood draw for the standard medical thyroid function test was obtained after 10 to 14 days of monitored abstinence.
Statistical Analysis
A between groups (CS, MJ-Short, MJ-Long) by gender analysis of variance was performed on supplemental psychological and physiological measures. A between groups (CS, MJ-Short, MJ-Long) by gender by region analysis of covariance was performed for each of the six EEG bands. A group (MJ-Short, MJ-Long) gender by region by test time (< 72 hours and > 28 days of abstinence) analysis of covariance was performed for each of the six EEG bands for log power to determine whether the EEG changed over a month of monitored abstinence. The covariates were age, lifetime ASPD symptoms (ASPSX) and family history of alcoholism (FHALC). In addition to the standard tests of the assumptions of homogeneity of between group variance, the Greenhouse-Geisser correction for deviations from the assumptions of the repeated measures model were applied and the corrections are reflected in the p-values given for a particular test (Geisser &.Greenhouse, 1959). When assumptions of the model were violated as revealed by the Mauchly=s test the Greenhouse-Geisser corrected p value (pG-G) is given. When assumptions of the model were not violated the unadjusted p-value (p) is presented. Statistical testing was performed with SPSS, Version 13 (Chicago Il.). A planned comparison tested (Winer et al., 1991) whether the mean of one group (MJ-Long) was different than the other two group means (CS, MJ-Short) as a direct test of our hypothese.
Results
EEG Power of Marijuana Users in Early Abstinence
Figure 1 shows the topographical maps of absolute log power bands for the control and marijuana groups. Table 2 lists mean power over all electrodes for each band for the three groups. Since the topographical maps suggest that the MJ-Long group differed from the other two groups, a single planned comparison tests that hypothesis for each band. These results are listed in Table 2. These comparisons report that mean alpha2 power for the MJ-long group was significantly lower than the other two groups and mean alpha power at the peak alpha frequency for the MJ-long group was significantly lower than the other two groups. Also included in Table 2 are the correlations of lifetime ASPD symptoms and the family history of alcoholism with each of the EEG variables. Lifetime ASPD symptoms and the family history of alcoholism did not correlate with any of the EEG measures. Likewise, lifetime ASPD symptoms and the family history of alcoholism when were used as covariates in the analyses in this Table, these covariates were not significant and results appear unrelated to these covariates.
Figure 1.
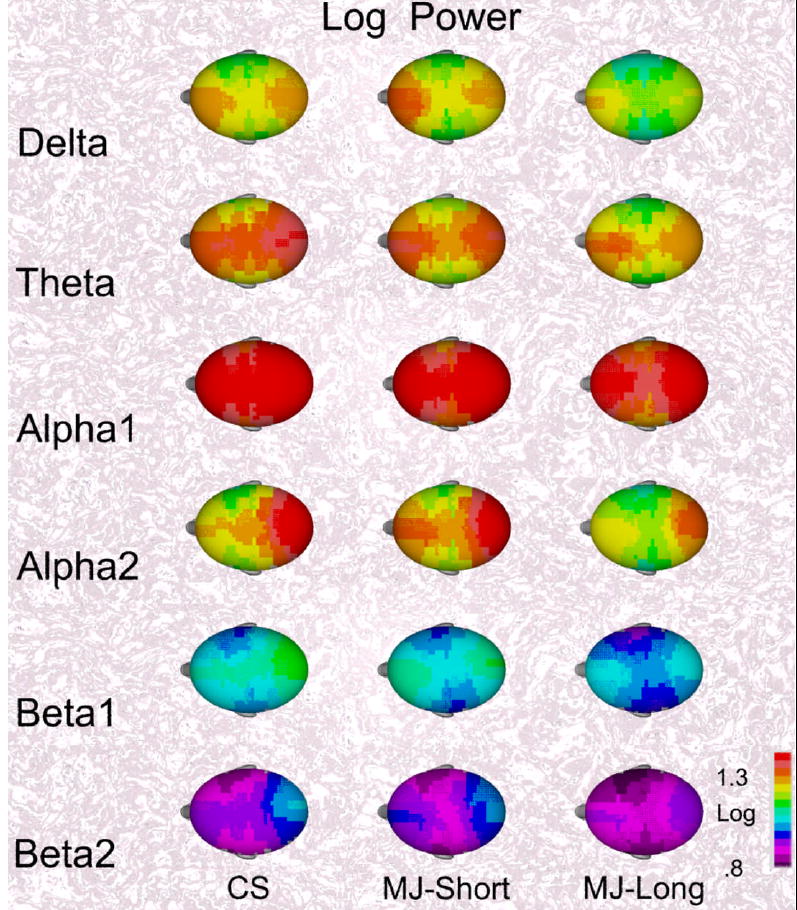
The mean topographical maps of log power for the six EEG bands are shown for the three groups. The mean maps for the control subjects (CS) and marijuana subjects using for less than eight years (MJ-Short) appear similar while the maps of the marijuana subjects using more than eight years appear to have less EEG power.
Table 2.
Mean and SD of Log Power Measures for the Three Groups
| Band | CS | MJ-Short | MJ-Long | Planned Test* | Correlation with ASP Symptoms | Correlation with FH-ALC# | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(1,102) | p | |||||||||||
| Delta | 1.42 | .17 | 1.41 | .15 | 1.35 | .17 | 2.84 | NS | -.067 | NS | .064 | NS |
| Theta | 1.52 | .17 | 1.48 | .22 | 1.44 | .17 | 2.74 | NS | -.070 | NS | .017 | NS |
| Alpha1 | 1.63 | .24 | 1.59 | .26 | 1.55 | .25 | 1.35 | NS | -.013 | NS | -.048 | NS |
| Alpha2 | 1.42 | .18 | 1.44 | .20 | 1.34 | .19 | 3.72 | p<0.05 | -.083 | NS | .011 | NS |
| Peak Alpha Frequency | 9.58 | .77 | 9.63 | .87 | 9.29 | .18 | 2.19 | NS | -.082 | NS | -.013 | NS |
| Alpha Power - Peak Frequency | 1.82 | .25 | 1.83 | .22 | 1.70 | .23 | 4.57 | p<0.04 | -.006 | NS | .071 | NS |
| Beta1 | 1.18 | .17 | 1.17 | .18 | 1.11 | .17 | 1.83 | NS | -.125 | NS | .028 | NS |
| Beta2 | 1.02 | .16 | 1.02 | .18 | .96 | .17 | 3.11 | NS | -.143 | NS | .020 | NS |
The planned comparison (Winer et al., 1991) tests whether the MJ-Long mean is less than the other two means.
Family History of Alcoholism from ASI.
A between groups (CS, MJ-Short, MJ-Long) by gender by region analysis of variance was performed for each of the six EEG bands. These tests examined whether the EEG of CS and marijuana groups differed during early abstinence. The group by region interaction was significant for alpha2 (F(8, 404)= 2.92, pG-G =0.012; covariates: age F(1,99) =2.95, p=0.089; ASPSX: F(1,99) = 0.68, p=0.413; FHALC F(1,99) =0.09, p=0.783) and beta2 (F(8 404)= 2.67, pG-G = 0.019; covariates: age F(1,99) =1.47, p=0.228; ASPSX: F(1,99) = 0.69, p=0.408; FHALC F(1,99) = 0.06, p=0.815) suggesting possible significant differences between groups in some regions of the brain. It is important to note that none of the covariates were significant in these analyses. These regional differences are shown in Figure 2. For alpha2 and beta2 power in the occipital regions, the MJ-Long group had less power than that of the CS and MJ-Short groups. There were no gender by group differences. While beta1 power showed a similar trend, the assumption homogeneity of between group variances did not hold. Thus, the corresponding analysis for beta1 power was not valid.
Figure 2.
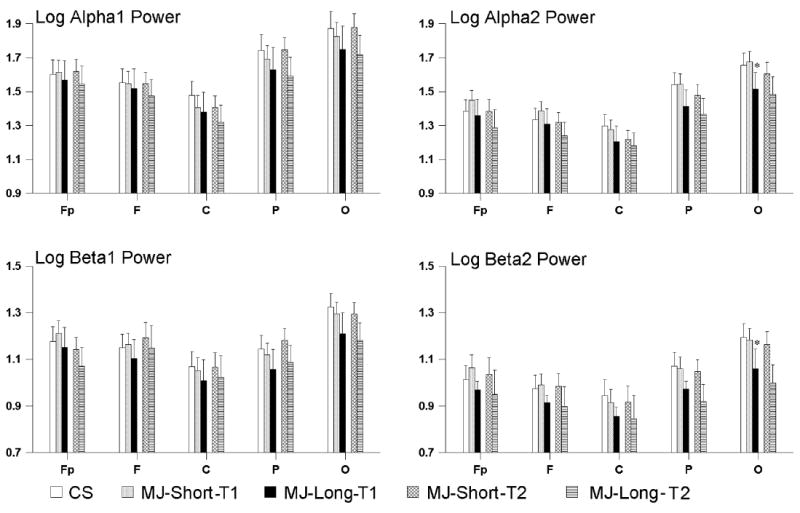
Means and ninety-five confidence intervals of log power for the alpha and beta EEG bands are shown for the control subjects (CS) and marijuana users (MJ-Short, and MJ-Long). The regions are indicated along the x axis as Fp (frontal pole), F(frontal), (C) central, P (parietal) and O (occipital). A single planned comparison was used to test whether MJ-long value differed from the CS and MJ-Short values for each region (Winer et al., 1991). Significantly lower values for the MJ-Long Group as compared to the CS and the MJ-Short groups at the 0.05 Bonferroni corrected probability level are indicated by an asterisk (*). Note that MJ-long group means are lower than CS and MJ-Short values for occipital sites for alpha2 and beta2 bands.
Similar analyses of variance were performed for relative power in each of the EEG bands. No group effects or group by region interactions were observed for relative power in any band.
EEG Power during Monitored Abstinence
Since fifty-nine marijuana abusers (42 in the MJ-Short group and 17 in the MJ-Long group) completed the study, we were able to compare their EEG at early (< 72 hours) and late (28-30 days) time points during monitored abstinence. The EEG power for two groups of marijuana abusers late in abstinence is, also, shown in Figure 2. The time main effect, the time by group (MJ-Short versus MJ-Long) was not significant for any EEG band. The MJ-Long group had lower overall alpha2 (F(1,54)= 7.54, p <0.009; covariates: age F(1,52) =0.15, p=0.705; ASPSX: F(1,52) = 0.00, p=0.998; FHALC F(1,52) =0.72, p=0.402) and beta2 (F(1,52)= 4.89, p <0.032; covariates: age F(1,52) =1.20, p=0.279; ASPSX: F(1,52) = 0.03, p=0.87; FHALC F(1,52) =0.05, p=0.830) power than the MJ-Short group throughout abstinence. There were no gender by group by time differences.
Similar analyses of variance were performed for relative power in each of the EEG bands over the two times during abstinence. No time effects and no group by time by region interactions were observed for relative power.
Peak EEG Alpha Frequency and Power
Peak alpha frequency was analyzed by an ANOVA with group and gender factors. No differences among the three groups (F(2,117) = 1.07, p > 0.30) or group by gender interaction observed. Peak alpha frequency was, also, analyzed over the month period of monitored abstinence with a group by gender by time (< 72 hours by > 28 days) ANOVA. Time effect (F(1,55) = 2.46, p > 0.20) or group by time interaction (F(1,55) = 1.56, p > 0.20) were not significant for peak alpha frequency. EEG power at the peak alpha power for the occipital electrodes for the MJ-long group was significantly lower than the other two groups. No changes in this measure were observed over time or between genders. Table 2 summarizes the power results.
Supplemental Measures
Table 3 lists the means and standard deviations for the psychological measures for the three groups. For most scales of the SCL-90R, both marijuana groups had higher scores than the CS group, but the two marijuana groups did not differ. Both marijuana groups had slightly higher scores on the BDHI scales than the control group, but the MJ-Long group had significantly higher levels of BDHI assault than the other two groups. Table 4 lists the means and standard deviations for the physiological measures for the three groups. Diastolic blood pressure was lower for the marijuana groups than for the CS group, but the values for marijuana groups did not differ. Heart rate was significantly lower for the MJ-Long group as compared to the MJ-Short and CS groups. MCA PI was significantly higher for the marijuana groups than for the CS group. The PI values for marijuana groups did not differ. Diastolic blood pressure and the MCA PI cerebral blood flow measures did not change over the 30 days of monitored abstinence. Heart rate and systolic blood pressure significantly increased over the same abstinence period. The MJ-Long group had significantly higher T3 percent uptake than the MJ-Short and CS groups. The MJ-Long subjects had significantly lower T4 values (two subjects below the normal range) than the other two groups.
Table 3.
Psychological Measures
| Control | MJ-Short | MJ-Long | ||||||
|---|---|---|---|---|---|---|---|---|
| SCL-90R | Mean | SD | Mean | SD | Mean | SD | Planned Test* | |
| Somatization | 40.3 | 5.9 | 44.8 | 8.2 | 46.0 | 7.8 | 3.29 | NS |
| Obsessive-Compulsive | 42.5 | 5.7 | 53.9 | 8.9 | 53.0 | 11.7 | 1.92 | NS |
| Interpersonal | 44.8 | 5.9 | 52.0 | 9.4 | 50.8 | 7.2 | 1.30 | NS |
| Depression | 43.7 | 6.8 | 52.0 | 11.6 | 47.5 | 8.5 | 0.02 | NS |
| Anxiety | 41.1 | 4.6 | 47.3 | 9.4 | 48.0 | 8.2 | 3.50 | NS |
| Hostility | 43.0 | 5.0 | 51.4 | 10.2 | 49.7 | 8.4 | 1.27 | NS |
| Phobic Anxiety | 46.1 | 2.5 | 48.6 | 6.0 | 48.3 | 6.7 | 2.08 | NS |
| Paranoid Ideation | 46.5 | 7.0 | 57.0 | 10.9 | 53.9 | 11.9 | 0.69 | NS |
| Psychoticism | 47.2 | 6.6 | 53.5 | 10.2 | 48.8 | 9.4 | 0.46 | NS |
| BDHI | ||||||||
| Assault | 3.3 | 2.1 | 4.4 | 1.8 | 5.2 | 2.4 | 6.58 | 0.02 |
| Indirect | 3.7 | 2.0 | 3.6 | 1.9 | 3.0 | 1.9 | 1.56 | NS |
| Irritable | 2.9 | 2.6 | 2.8 | 2.1 | 2.9 | 2.6 | 0.01 | NS |
| Negative | 1.5 | 1.2 | 2.5 | 1.3 | 2.1 | 1.5 | 0.96 | NS |
| Resentment | 2.2 | 2.0 | 2.3 | 1.9 | 2.5 | 1.9 | 0.36 | NS |
| Suspicious | 3.2 | 2.3 | 4.3 | 1.8 | 3.9 | 2.3 | 0.09 | NS |
| Verbal | 6.9 | 2.6 | 6.7 | 2.2 | 7.3 | 2.5 | 0.67 | NS |
| Guilt | 3.0 | 2.1 | 4.0 | 2.2 | 2.6 | 1.6 | 3.13 | NS |
| Total | 23.6 | 10.9 | 26.5 | 9.0 | 26.3 | 9.9 | 0.16 | NS |
| Elison | ||||||||
| General Well-Being | 49.9 | 7.7 | 46.3 | 7.9 | 48.4 | 11.2 | 0.02 | NS |
| Spiritual Well-Being | 46.8 | 13.1 | 45.1 | 12.7 | 47.8 | 10.7 | 0.36 | NS |
| Beck Hopelessness | 2.0 | 1.8 | 2.4 | 2.3 | 3.0 | 2.6 | 2.45 | NS |
The planned comparison tests (Winer et al., 1991) whether the MJ-Long mean is different than the other two means, a F value with 1 and 102 degrees of freedom.
Table 4.
Physiological Measures
| Control | MJ-Short | MJ-Long | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Planned Test | ||
| Cardiovascular | ||||||||
| Heart Rate: BPM | 75.8 | 10.2 | 72.1 | 10.4 | 66.6 | 11.2 | 8.32* | 0.03 |
| Heart Rate 28 Days | 78.1 | 11.8 | 80.5 | 8.3 | 0.77$ | NS | ||
| Planned Test | 2.91& | 0.001 | 3.46& | 0.003 | ||||
| Systolic BP: mm Hg | 117.3 | 12.5 | 120.2 | 14.2 | 117.1 | 15.1 | 0.78* | NS |
| Systolic BP 28 Days | 128.2 | 12.7 | 124.6 | 13.6 | 1.11$ | NS | ||
| Planned Test | 2.29& | 0.027 | 3.14& | 0.001 | ||||
| Diastolic BP: mm Hg | 71.0 | 9.9 | 66.8 | 11.4 | 65.9 | 8.5 | 4.06# | 0.05 |
| Diastolic BP 28 Days | 69.9 | 9.4 | 67.9 | 8.5 | 0.76$ | NS | ||
| Planned Test | 0.60& | NS | 0.83& | NS | ||||
| TCD Blood Flow | ||||||||
| MCA PI | 0.94 | 0.11 | 1.01 | 0.11 | 0.99 | 0.11 | 5.62# | 0.03 |
| MCA PI 28 Days | 0.99 | 0.12 | 0.95 | 0.12 | 0.97$ | NS | ||
| Planned Test | 0.33& | NS | 2.14& | NS | ||||
| MCA Vm cm/s | 63.1 | 12.1 | 65.0 | 10.2 | 67.7 | 10.9 | 1.90# | NS |
| MCA Vm 28 Days | 69.2 | 9.8 | 67.0 | 9.0 | 1.18$ | NS | ||
| Planned Test | 0.35& | NS | 1.56& | NS | ||||
| Thyroid Function | ||||||||
| TSH micro IU/dL norms: 0.30 to 3.04 | 1.30 | 0.69 | 1.55 | 0.74 | 1.69 | 1.13 | 1.68* | NS |
| T4 microgram/dL norms: 4.5 to 12.0 | 8.06 | 1.34 | 7.42 | 1.36 | 6.68 | 1.33 | 9.41* | p<0.01 |
| T3 Uptake % norms: 28.0 to 4.1.0 | 33.75 | 3.10 | 35.51 | 3.12 | 36.80 | 5.14 | 5.49* | p<0.05 |
The planned comparison (Winer et al., 1991) tests whether the MJ-Long mean is different than the other two means, F value with 1,102 degrees of freedom.
The planned comparison tests whether the CS mean is different than the other two means, F value with 1,102 degrees of freedom. This alternate comparison was made based on our previous findings with these measures (Herning et al., 2005).
The comparison tests whether MJ-Short differs from MJ-Long, t value with 68 degrees of freedom.
The comparison tests whether the test early in abstinence differs from the 28 day test, paired t value with 39 (MJ-Short) or 18 (MJ-Long) degrees of freedom.
Marijuana Use, EEG Power, Cerebral Blood Flow and Thyroid Function
In view of the group differences in EEG power, thyroid function and the MCA PI measure, correlations were calculated among EEG alpha2 power at Oz, EEG beta2 power at Oz, the thyroid measures, MCA PI and the marijuana use measures from the ASI. Both T3 uptake and T4 significantly correlated with both marijuana use measures (see Table 5). It is interesting to note that MCA PI was only correlated with recent marijuana use where as EEG power measures were only correlated with the years of marijuana use. TSH was correlated with cerebral perfusion (MCA PI) and the EEG (beta2 power).
Table 5.
Correlations between Marijuana Use, Perfusion Measures, Thyroid Function and EEG
| Marijuana
Days |
Marijuana
Years |
Thyroid
T4 |
Thyroid
T3 uptake |
Thyroid
TSH |
TCD
MCA PI |
EEG Oz
Alpha2 |
|
|---|---|---|---|---|---|---|---|
| Marijuana
Years |
0.713
p<0.001 |
||||||
| Thyroid
T4 |
-0.243
p<0.020 |
-0.303
p<0.003 |
|||||
| Thyroid
T3 uptake |
0.223
p<0.035 |
0.265
p<0.014 |
-0.099
NS |
||||
| Thyroid
TSH |
0.091
NS |
0.163
NS |
-0.070
NS |
0.084
NS |
|||
| TCD
MCA PI |
0.237
p<0.015 |
0.163
NS |
-0.018
NS |
0.183
NS |
0.210
p<0.033 |
||
| EEG Oz
Alpha2 |
0.017
NS |
-0.185
p<0.050 |
0.045
NS |
-0.095
NS |
-0.175
NS |
-0.082
NS |
|
| EEG Oz
Beta2 |
-.0.046
NS |
-0.236
p<0.014 |
0.070
NS |
-0.089
NS |
-0.263
p<0.006 |
-0.104
NS |
0.803
p<0.001 |
Discussion
The main observations of the present study are: (1) the marijuana abusers that used for a longer time had significantly less alpha2 and beta2 power at posterior sites than the control subjects and marijuana abusers that used less long and (2) these changes in alpha2 and beta2 power of marijuana abusers persisted over the month of monitored abstinence. The finding of reduced EEG power at posterior sites in abstinent marijuana users is novel and might reflect changes in cerebral perfusion or thyroid function. While the marijuana groups differed from the control subjects on a number of psychological and physiological measures, the marijuana users that used marijuana for more than eight years had higher BDHI assault ratings, lower heart rates, lower T4 and higher T3 uptake levels compared to the marijuana users that used for a shorter period and the control subjects. Heart rate and systolic blood pressure increased over the month of abstinence for both marijuana groups.
The reductions in alpha2 and beta2 EEG power at posterior sites might be related to subtle diminutions in cerebral perfusion. Cerebral perfusion and cognitive deficits were observed in abstinent marijuana users during resting and active cognitive tasks (Tunving et al., 1986; Amen, & Waugh, 1998; Lundqvist et al., 2001; Block et al, 2002; Herning et al., 2005). Decreases in alpha power were observed during transient ischemic attacks (Madkour et al., 1993; Juhasz et al., 1997). While increases in delta and theta EEG activity accompany larger deficits in cerebral perfusion, subtle deficits in perfusion were accompanied by decreases in alpha and beta activity (Jordan, 2004) as we have observed in the present study. In a similar sample of young marijuana abusers, cerebral resistance measured by the transcranial Doppler parameter, Pulsatilty Index (PI), was similar to that observed in healthy individuals over 60 years of age (Herning et al., 2005; Krejza et al. 1999). Cerebral resistance as measured by PI remained elevated over the month of monitored abstinence in the heavy marijuana abusers (Herning et al., 2005). However, in the present study, PI was elevated to a similar extent for both groups of marijuana users, while the EEG changes occurred only in the marijuana users that used marijuana for more than eight years. The EEG changes in the MJ-Long group might be due to an approximately five year longer period of increased cerebral resistance in this group compared to the MJ-Short group. That is, the EEG changes in the posterior regions might to be due the cumulative effects of subtle perfusion deficits. Decreased alpha power was, also, observed in abstinent chronic alcoholics (Enoch et al., 1999; Saletu-Zyhlar et al., 2004), but there was little alcohol use in marijuana users in the present study. Thus, the decreases in alpha2 and beta2 power observed in this study appear to be due to an extended period increased cerebral resistance.
Marijuana users who used marijuana on the average of 26 days out of the last 30 days did not have increased alpha power at frontal sites during recording in abstinence. The findings of Struve and associates’ study (1999) for EEG alpha power were not replicated. They found increased frontal alpha power in recently abstinent (about 24 hours) daily marijuana users who used marijuana on an average of ten years. One of our groups of marijuana abusers, that reported using marijuana regularly for an average of nine and a half years, had no significant increases in frontal alpha power. Struve at al. (1999) also reported that delta, theta and beta EEG power was elevated at most electrode sites for their marijuana abusers as compared to their control subjects. We also failed to replicate these latter observations. Finally, we did not find a reduction in alpha frequency in either of the two groups of marijuana abusers as was reported by Struve and his associates (1999).
The most notable differences between the present study and the Struve study (Struve et al., 1999) were the duration of EEG recording session and selection of EEG epochs for spectral analysis. We recorded three minutes of resting eyes closed EEG. If the operator observed mostly artifacts in that sample, the session was stopped; the subject was informed how best to avoid producing artifacts and the recording session was repeated. The remaining artifacts, if any, were removed by computer-assisted artifacting procedure. Struve and his associates (1999) collected 30 to 45 minutes of EEG while the subjects reclined with their eyes closed. Subjects were aroused when indications of drowsiness appeared in the EEG. Up to sixty 2.5 second artifact free epochs were selected from this extended recording period for spectral and Neurometric analysis (John et al., 1988). Less than ten percent of the EEG sample was used in the analysis. Our subjects were clearly awake for the EEG recording. The subjects in the Struve study might have been drowsy because of the extended nature of the recording procedure. Thus, it is difficult to directly compare our findings with the Struve study (1999).
Increases in frontal EEG alpha have been observed during the transition from wakefulness to sleep (De Gennaro et al., 2001) and in non-REM sleep (Finelli, 2001). Increases in delta, theta and beta power were also observed during such transitions (De Gennaro et al., 2001). Such increases would more likely occur in a 35 to 45 minute recording session than in a three minute session. Marijuana users might be sleepier than the control subjects since marijuana users have reported problems sleeping during early abstinence (Jones et al., 1981). Marijuana users, also, have increased slow wave with reduced REM sleep during sub-chronic THC administration and increased REM sleep during early abstinence (Feinberg et al., 1976; Freemon, 1982). Thus, the increase in EEG frontal alpha and the general increases in other frequency bands observed in daily marijuana abusers by Struve and associates (1999) might reflect increased day time sleepiness.
In addition, little is known about the subjects in the Struve study (Struve et al., 1999) other than age, gender and possible tobacco use. The present study provides extensive demographic information as well as supplement psychological and physiological information about the subjects in each of the groups including a full DIS-IV interview to ruler out psychiatric problems.
We find that marijuana abusers have less alpha2 and beta2 power than control subjects at posterior sites. The reductions in alpha2 and beta2 bands were only seen in marijuana abusers that used marijuana for eight years or longer. These latter marijuana users had lower heart rate lower thyroid T4 and higher T3 uptake levels than the control subjects and the marijuana users that used less than eight years. It is possible that the lower heart rate and thyroid values contributed in some way to the lower alpha2 and beta2 power observed in the marijuana users that used for more than eight years. Heart rate significantly increased for both marijuana groups at the late in abstinence while alpha2 and beta2 power remained the same. Thus, it is unlikely that cardiovascular factors contributed to the EEG power changes observed during abstinence.
In addition to our earlier suggestion that the decrease in power in the alpha2 and beta2 bands may due to an extended period of subtle cerebral perfusion deficits, the role of subtle changes in thyroid function can not be ruled out. There was only one report of a relationship between EEG during cognitive tasks and thyroid function in control subjects (Tucker et al., 1984). A significant positive relationship between marijuana use and T3 uptake and a significant negative correlation between marijuana use and T4 levels was noted in the present study. While thyroid function measures T3 and T4 were related to marijuana use, TSH levels are related to both EEG beta2 power and cerebral blood flow resistance. The relationship suggests that as TSH levels increase cerebral resistance increases and EEG beta2 power decreases. Thus, the EEG changes observed in the present study might be due to subtle changes in thyroid function in marijuana abusers with an extended of period of abuse. These marijuana abusers appear to be under aroused in early abstinence with low heart rate, low diastolic blood pressure, decreased thyroid function (T4), increased cerebral blood flow resistance and decreased EEG alpha2, and beta2 power. Diastolic blood pressure, the cerebral blood flow resistance and the EEG changes (reduced alpha2 and beta 2 power) did not change over the 30 days of monitored abstinence suggesting continued hypo arousal. Marijuana abusers that used less often had only decreased diastolic blood pressure and increased cerebral blood flow resistance during early abstinence. Likewise the diastolic blood pressure and cerebral blood flow resistance for this group did not change over a month of monitored abstinence.
Our findings appear consistent with previous observations that antisocial individuals have decreased EEG alpha power (Fishbein et al., 1989; Deckel et al., 1996; Lindberg et al., 2005) and have signs of autonomic under arousal (Ortiz & Raine, 2004). The marijuana abusers that used marijuana for more than eight years had significantly lower ahpha2 power, heart rate, diastolic blood pressure while having higher scores on the aggression scale of BDHI and more lifetime ASPD symptoms on the DIS-IV. Thus in this sample of marijuana abusers, these signs of under arousal on the thyroid, cardiovascular and cortical level might be related to ASPD. However, a strong case can not be made for this interpretation since the sample of marijuana abusers that used regularly for less than eight years had lifetime ASPD symptom counts similar to the abusers that used more than eight years, but did not have decreases in alpha2 power.
Low voltage alpha, high voltage alpha (Elhers and Phillps, 2003) and increased beta power (Rangaswamy el al., 2004) were observed in individuals with a family history of alcoholism. The both groups of marijuana abusers in the present study had a greater percent of subjects with a family history of alcoholism than the control group, but only the marijuana abusers that used more than eight years had a deceased alpha2 power compared to the control group. Thus, the suggestion, that the reduction in alpha2 power was due to pre-morbid factors such as a family history of alcoholism or ASPD, is not supported by the present study. If the reduction in alpha2 power was related to family history of alcoholism or ASPD, the reduction should have been observed in both marijuana groups since the level of ASPD and family history of alcoholism was similar for both marijuana groups. In addition, these pre-morbid factors did not correlate with any of the EEG measures and were not meaningful covariates when used in the statistical analyses of the EEG measures.
A limitation of this study is that marijuana use is based on subjective reports rather than blood or urine levels of THC and its metabolites. Likewise, the thyroid function tests were not made at the time of EEG testing as were the cardiovascular and cerebral blood flow velocity recordings. However, taken together our results suggest EEG, cerebral blood flow velocity, cardiovascular and thyroid function alterations occur during early abstinence in marijuana users that used regularly for more than eight years. These measures in marijuana abusers require future study during extended abstinence to determine whether they resolve over time or are permanent.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvaro L, Iriondo CI, Villaverde FJ. Sexual headache and stroke in a heavy cannabis smoker. Headache. 2002;42:224–226. doi: 10.1046/j.1526-4610.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- Amen DG, Waugh M. High resolution brain SPECT imaging of marijuana smoker with AD/AD. J Psychoactive Drugs. 1998;30:209–214. doi: 10.1080/02791072.1998.10399692. [DOI] [PubMed] [Google Scholar]
- Barnes D, Palace J, O=Brien MD. Stroke following marijuana smoking. Stroke. 1992;23:1381. doi: 10.1161/01.str.23.9.1381. [DOI] [PubMed] [Google Scholar]
- Beck AR, Weissman A Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. J Consult Clin Psychol. 1974;42:861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A, Baer MB. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bolla K, Herning RI. Neurological assessments of marijuana users. Methods Mol Med. 2006;123:255–268. doi: 10.1385/1-59259-999-0:255. [DOI] [PubMed] [Google Scholar]
- Chamberlin H. Musical applications of microprocessors. Hasbrouck Heights, NJ: Hayden; 1984. pp. 435–464. [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. Prevalence of marijuana use disorders in the United States: 1991-1992 and 2001-2002. JAMA. 2004;291:2114–21. doi: 10.1001/jama.291.17.2114. [DOI] [PubMed] [Google Scholar]
- Cooles P, Michaud R. Stroke after heavy cannabis smoking. Postgrad Med J. 1987;63:511. doi: 10.1136/pgmj.63.740.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Curcio G, Cristiani R. Antero-posterior EEG changes during the wakefulness-sleep transition. Clin Neurophysiol. 2001;112:1901–1911. doi: 10.1016/s1388-2457(01)00649-6. [DOI] [PubMed] [Google Scholar]
- Deckel AW, Hesselbrock V, Bauer L. Antisocial personality disorder, childhood delinquency, and frontal brain functioning: EEG and neuropsychological findings. J Clinical Psychology. 1996;52:639–650. doi: 10.1002/(SICI)1097-4679(199611)52:6<639::AID-JCLP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The SCL-90R: Administration Scoring and Procedures Manual II. Baltimore: Clinical Psychometric Research; 1992. [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Elison CG. Religious involvement and subjective well-being. J Health Soc Behav. 1991;32:80–99. [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. EEG low-voltage alpha and alpha power in African American young adults: relation to family history of alcoholism. Alcohol Clin Exp Res. 2003;27:765–772. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White Kenneth V, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcoholism: Clinical & Experimental Research. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Feinberg I, Jones R, Walker J, Cavness C, Floyd T. Effects of marijuana extract and tetrahydrocannabinol on electroencephalographic sleep patterns. Clin Pharmacol Ther. 1976;19:782–794. doi: 10.1002/cpt1976196782. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Poulton R, Smith PF, Boden JM. Cannabis and psychosis. British Medical J. 2006;332:172–175. doi: 10.1136/bmj.332.7534.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli LA, Achermann P, Borbely AA. Individual Afingerprints= in human sleep EEG topography. Neuropsychopharmacology. 2001;25:S57–S62. doi: 10.1016/S0893-133X(01)00320-7. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Herning RI, Pickworth WB, Haertzen CA, Hickey JE, Jaffe JH. EEG and brainstem auditory evoked response potential in adult male drug abusers with self-reported histories of aggressive behavior. Biological Psychiatry. 1989;26:595–611. doi: 10.1016/0006-3223(89)90085-1. [DOI] [PubMed] [Google Scholar]
- Freemon FR. The effect of chronically administered delta-9-tetrahydrocannabinol upon the polygraphically monitored sleep of normal volunteers. Drug Alcohol Depend. 1982;10:45–353. doi: 10.1016/0376-8716(82)90036-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Carey C, Grant I. Nonacute (residual) neuropsychological effects of cannabis use: a qualitative analysis and systematic review. J Clin Pharmacol. 2002;42:48S–57S. doi: 10.1002/j.1552-4604.2002.tb06003.x. [DOI] [PubMed] [Google Scholar]
- Gasser T, Bacher P, Mocks J. Transformations towards the normal distribution of broad band spectral parameters of the EEG. EEG Clin Neurophysiol. 1982;53:119–124. doi: 10.1016/0013-4694(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Pope HG. Marijuana use among adolescents. Pediatr Clin North Am. 2002;49:389–413. doi: 10.1016/s0031-3955(01)00011-6. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. What are the policy implications of the evidence on cannabis and psychosis? Can J Psychiatry. 2006;51:566–5674. doi: 10.1177/070674370605100904. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychpharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. 2005;44:488–493. doi: 10.1212/01.WNL.0000150882.69371.DD. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Friedman J, Easton P. Neurometrics: computer-assisted differential diagnoses of brain dysfunction. Science. 1988;239:162–169. doi: 10.1126/science.3336779. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz Nl, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Jordan K. Emergency EEG and continuous EEG monitoring in ischemic stroke. J Clin Neurophysiology. 2004;21:341–352. [PubMed] [Google Scholar]
- Juhasz C, Kamondi A, Szirmai I. Spectral EEG analysis following hemispheric stroke: evidences of transhemispheric diaschisis. Acta Neurol Scand. 1997;96:397–400. doi: 10.1111/j.1600-0404.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Krejza J, Kariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. Am J Radiology. 1999;172:213–218. doi: 10.2214/ajr.172.1.9888770. [DOI] [PubMed] [Google Scholar]
- Lawson TM, Rees A. Stroke and transient ischemic attacks in association with substance abuse in a young man. Postgrad Med J. 1996;72:693–693. doi: 10.1136/pgmj.72.853.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg N, Tani P, Virkkunen M, Heiskanen T, Appelberg B, Naukkarinen H, Salmi T. Quantitative electroencephalographic measures in homicidal men with antisocial personality disorder. Psychiatry Res. 2005;136:7–15. doi: 10.1016/j.psychres.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Lundqvist T, Jonsson S, Warkentin S. Frontal lobe dysfunction in long-term cannabis users. Neurotoxicol Teratol. 2001;23:437–443. doi: 10.1016/s0892-0362(01)00165-9. [DOI] [PubMed] [Google Scholar]
- Madkour O, Elwan O, Hamdy H, Elwan H, Abbas A, Taher M, Abdel-Kader A. Transient ischemic attacks: electrophysiological (conventional and topographic EEG) and radiological (CCT) evaluation. J Neurol Sci. 1993;119:8–17. doi: 10.1016/0022-510x(93)90186-3. [DOI] [PubMed] [Google Scholar]
- MacCarron MO, Thomas AM. Cannabis and alcohol in stroke. Postgrad Med J. 1997;73:448. doi: 10.1136/pgmj.73.861.448-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinella MA. Stroke after marijuana smoking in a teenager with factor V Leiden mutation. South Med J. 2001;72:692–693. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, McGaham P, O=Brien CP. Guide to the Addiction Severity Index: Background, administration, and field testing results; Treatment Research Reports; Rockville MD: National Institute on Drug Abuse; 1986. [Google Scholar]
- Mesec A, Rot U, Grad A. Cerebrovascular disease associated with marijuana abuse: a case report. Cerebrovasc Dis. 2001;11:284–285. doi: 10.1159/000047653. [DOI] [PubMed] [Google Scholar]
- Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;23:521–527. [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis M, Yurgelun-Todd D. Earjy-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alc Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Reich T, Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. Int J Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV. St Louis: Washington University Press; 1995. [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, Boning J, Saletu B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol. 2004;39:233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Pope G, Silveri MM, Simpson NS, Gruder SA, Yurgelum-Todd DA. Altered regional blood volume in chronic cannabis smokers. Exp Clin Psychopharmacology. 2006;14:422–428. doi: 10.1037/1064-1297.14.4.422. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Miller M, Chrisitiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis user seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G. Persistent topographic quantitative EEG sequelae of chronic marihuana use: a replication study and initial discriminant function analysis. Clin Electroenceph. 1994;25:63–75. doi: 10.1177/155005949402500207. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Leavitt J, Manno JE, Manno BR. Topographic quantitative EEG sequella of chronic marijuana use: a replication using medically and psychiatrically screened normal subjects. Drug Alcohol Depend. 1999;56:167–179. doi: 10.1016/s0376-8716(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Price L. Topographic mapping of quantitative EEG variables in chronic heavy marihuana users: empirical findings with psychiatric patients. Clin Electroenceph. 1989;20:6–23. doi: 10.1177/155005948902000106. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Peland JG, Beckwith BE, Sandstead HH. Thyroid function in normals: Influences on electroencephalogrgram and cognitive performance. Psychophysiology. 1984;21:72–78. doi: 10.1111/j.1469-8986.1984.tb02320.x. [DOI] [PubMed] [Google Scholar]
- Tunving K, Thulin SO, Risberg J, Warkentin S. Regional cerebral blood flow in long-term heavy cannabis use. Psychiatry Res. 1986;17:15–21. doi: 10.1016/0165-1781(86)90037-5. [DOI] [PubMed] [Google Scholar]
- White D, Martin D, Gellar T, Pittmann T. Stroke associated with marijuana abuse. Pediatr Neurosurg. 2000;32:92–94. doi: 10.1159/000028906. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Mickels KM. Statistical Principles in Experimental Design. Third. New York: McGraw-Hill; 1991. pp. 141–153. [Google Scholar]
- Zarchariah SB. Stroke after heavy marijuana smoking. Stroke. 1991;22:406–409. doi: 10.1161/01.str.22.3.406. [DOI] [PubMed] [Google Scholar]


