Abstract
Both wild-type (WT) and nonconducting W472F mutant (NCM) Kv1.5 channels are able to conduct Na+ in their inactivated states when K+ is absent. Replacement of K+ with Na+ or NMG+ allows rapid and complete inactivation in both WT and W472F mutant channels upon depolarization, and on return to negative potentials, transition of inactivated channels to closed-inactivated states is the first step in the recovery of the channels from inactivation. The time constant for immobilized gating charge recovery at −100 mV was 11.1 ± 0.4 ms (n = 10) and increased to 19.0 ± 1.6 ms (n = 3) when NMG+ o was replaced by Na+ o. However, the decay of the Na+ tail currents through inactivated channels at −100 mV had a time constant of 129 ± 26 ms (n = 18), much slower than the time required for gating charge recovery. Further experiments revealed that the voltage-dependence of gating charge recovery and of the decay of Na+ tail currents did not match over a 60 mV range of repolarization potentials. A faster recovery of gating charge than pore closure was also observed in WT Kv1.5 channels. These results provide evidence that the recovery of the gating elements is uncoupled from that of the pore in Na+-conducting inactivated channels. The dissociation of the gating charge movements and the pore closure could also be observed in the presence of symmetrical Na+ but not symmetrical Cs+. This difference probably stems from the difference in the respective abilities of the two ions to limit inactivation to the P-type state or prevent it altogether.
Keywords: potassium channel, Kv1.5, gating, Na+ permeation, inactivation
INTRODUCTION
From early studies, when gating charges were first predicted (Hodgkin and Huxley, 1952) and measured (Armstrong and Bezanilla, 1973; Keynes and Rojas, 1974), biophysicists have been interested in the temporal relationship between ionic current movement and that of gating charge, as this constrains quantitative aspects of models used to simulate channel activation and deactivation (Bezanilla et al., 1994; Zagotta et al., 1994a). The gating models for Kv channels that have been proposed for depolarizations to positive potentials include transitions among a number of closed states that carry most gating charge and a concerted rearrangement of subunits involving one or two concerted transitions just before the open state that carries differing amounts of charge (Bezanilla et al., 1994; McCormack et al., 1994; Zagotta et al., 1994a; Ledwell and Aldrich, 1999; Schoppa and Sigworth, 1998b). In general, gating models are forced to show a departure from schemes involving identical and independent transitions because deactivating currents show slower kinetics than predicted by any model involving independently gating subunits. Such models would predict very fast ionic tail currents and somewhat less fast off-gating currents. As a consequence, it is suggested that the first closing transition is very slow and some sort of cooperative stabilization between subunits is required for this (Schoppa and Sigworth, 1998a; Kanevsky and Aldrich, 1999; Bezanilla, 2000; Fedida and Hesketh, 2001). Further support for an initial closing transition that is very slow comes from data showing that after depolarizations of more than a few milliseconds, returning gating charge and ionic tails superimpose (White and Bezanilla, 1985; Zagotta et al., 1994b). All workers agree that the deactivation time course is dominated by transitions near the open state and that a fundamental aspect of deactivation is that the gating charge movements and the pore conformation changes are tightly coupled to each other in time course during channel closing as a result of some nonindependent first-closing transition (Bezanilla et al., 1994; Zagotta et al., 1994a; Schoppa and Sigworth, 1998b).
Given the fundamental importance of matching of channel closure to gating charge recovery, we have asked whether the movement of the activation voltage sensor in potassium channels is inevitably coupled to motion of the pore, or whether there are situations when movement of the two may be uncoupled. In slow inactivated Na+ channels, it was found that the full recovery of gating charge after repolarization at −160 mV did not correlate with the time required for recovery of the inactivated Na+ conductance (Bezanilla et al., 1982). Very recently, it has also been noted that in T-type Ca2+ channels the gating current recovery precedes ionic current reavailability after prolonged depolarization (Burgess et al., 2002). Both of these observations suggest that charge can become fully available before the pore has recovered its resting conformation. Since Na+ channel slow inactivation is quite analogous to C-type inactivation in K+ channels in numerous aspects of its regulation (Townsend and Horn, 1997), we have focused on examining the possibility of uncoupling between the gating machinery and the pore closure in K+ channels during recovery from inactivation. At this time it is possible to follow the closure of inactivated K+ channels as a change in the relative permeabilities to Na+ and K+ of the inactivated states, in particular the Na+ conductance of the inactivated states appears as a tail current at negative potentials (Starkus et al., 1997, 1998; Wang et al., 2000a). In addition, the Na+ tail currents have the advantage of being quite slow, having a very prominent initial rising phase followed by a slow decay process that reflects the transition of inactivated channels to closed-inactivated states in Shaker channels (Starkus et al., 1998) and in Kv1.5 (Wang et al., 2000a).
Gating currents are the electrical manifestations of the displacement of the charged domains within voltage-gated channels (Larsson et al., 1996; Yang et al., 1996) sensing the transmembrane electric field (Sigworth, 1994; Goldstein, 1996; Bezanilla, 2000). The expression of cloned channels in oocytes and mammalian cells has facilitated these measurements (Perozo et al., 1993; Chen et al., 1997) and it is now often possible to measure both gating and ionic current in the same experimental protocol (Starkus et al., 1998; Wang and Fedida, 2001). The measurement of ionic current informs conduction through the open or inactivated pore, whereas gating currents give additional information on the channel transitions occurring among closed states. We have measured the Na+ conductance of inactivated Kv1.5 channels as an index of the pore conformation change during the initial closing step during recovery from inactivation and correlated it with the time course of gating charge recovery. It appears that the return of gating charge in the inactivated channels is much more rapid than the recovery of the pore to its closed-inactivated state, both in wild-type (WT)* and nonconducting mutant (NCM) Kv1.5 channels. This temporal dissociation can be observed during the initial recovery from both P- and C-type inactivated states, but is abolished by using Cs+ to prevent the onset of inactivation. Our data suggest that the conformational changes associated with P- and C-type inactivation in Kv1.5 might disrupt the connection between the internal activation gate and the voltage sensing domains, and as a result, uncouple the two systems.
MATERIALS AND METHODS
Cells and Solutions
Two forms of human Kv1.5 were used in the present experiments, the WT (Fedida et al., 1993) and an NCM channel, W472F, separately expressed in HEK 293 cells to form stable lines. The nonconducting mutation is analogous to the ShH4-IR W434F (Perozo et al., 1993). For gating current recordings from HEK cells, patch pipette solutions contained (in mM): N-Methyl D-Glucamine (NMG+), 135; EGTA, 5; MgCl2, 1; and HEPES, 10; and were adjusted to pH 7.2 with HCl. The bath solution contained (in mM): NMG+, 135; HEPES, 10; MgCl2, 1; CaCl2, 1; and was adjusted to pH 7.4 with HCl. When 135 mM Na+ o solution was used, NMG+ was omitted and the pH was adjusted to 7.4 with NaOH. All chemicals were from Sigma-Aldrich.
Electrophysiological Procedures
Coverslips containing cells were removed from the incubator before experiments and placed in a superfusion chamber (volume 250 μl) containing the control bath solution at 22–23°C. Current recording and data analysis were done using an Axopatch 200A amplifier and pClamp 6 software (Axon Instruments, Inc.). Patch electrodes were fabricated using thin-walled borosilicate glass (World Precision Instruments). Capacitance compensation and leak subtraction using a P/6 protocol from a holding potential of −100 mV were routinely used during gating current recordings. Data were sampled at 10–50 kHz and filtered at 2–10 kHz. Membrane potentials have been corrected for the junction potentials (<10 mV) that arose between pipette and bath solutions. All Qon measurements were obtained by integrating the on-gating currents during 12 ms depolarizations. This is sufficient time to allow gating currents to return to the baseline. In the presence of Na+, the baseline for Qon measurements was adjusted to the current level at the end of pulse (Fig. 6) or as the holding potential level before the pulses (Fig. 3) . The channel closure was measured by the decay of tail charge or disappearance of tail current (Figs. 3, 6, and 8) . The tail charge was integrated and tail amplitude measured at 5-ms intervals, and normalized to the total tail charge that was obtained by integrating the tail currents to baseline (1.2 s), or to the peak tail currents, respectively. Data are shown as mean ± SEM throughout.
Figure 6.
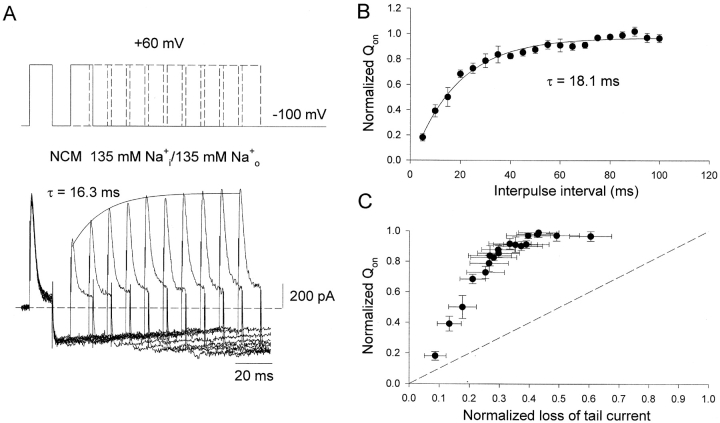
Relationship between the gating charge recovery and the channel closure in Kv1.5 NCM channels in the presence of 135 mM Na+ i/o. (A) Top, the same double-pulse protocol as shown in Fig. 3 A. Bottom, superimposed whole cell current traces elicited by the protocol shown at the top. Currents were recorded with symmetrical 135 mM Na+ i/o solutions. The solid line is the single exponential fitting to the peak of on-gating current with a time constant (τ) of 16.3 ms. The dotted line represents the zero current level. (B) Fractional recovery of Qon (Qon test/Qon prepulse) plotted as a function of interpulse interval. Data points represent means ± SEM (n = 3). The solid line is the best fit to data points using a single exponential function with τ = 18.1 ± 1.3 ms (n = 3). (C) Fractional recovery of Qon is plotted as a function of the loss of Na+ tail currents, the latter is normalized to the peak inward current. The dotted line represents a linear relationship between charge recovery and channel closing, with a slope of 1.0.
Figure 3.
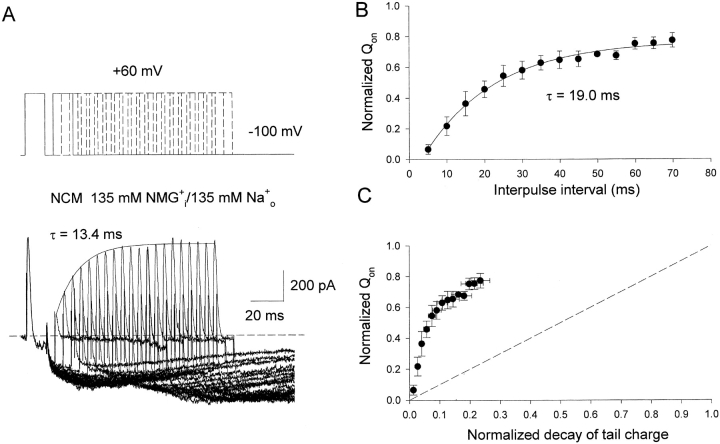
Effects of 135 mM Na+ o on the relation between charge recovery and pore closure in inactivated Kv1.5 NCM channels. (A) Top, double-pulse protocol includes two identical 12-ms pulses to 60 mV separated by a variable interpulse interval from 5 to 100ms with increments of 5 ms. Cells were held at −100 mV for 2 s between pairs of pulses. Bottom, superimposed whole cell current traces elicited by the protocol shown at the top. Currents were recorded with 135 mM NMG+ i/135 mM Na+ o solutions to record on-gating currents and inward Na+ tail currents at −100 mV. Solid line is the single exponential fitting to the peak of on-gating currents (τ = 13.4 ms). The dotted line represents the zero current level. (B) Fractional recovery of Qon (Qon test/Qon prepulse) plotted as a function of interpulse interval. Data points represent means ± SEM from four cells, and the solid line is the best fit to data points using a single exponential function with τ = 19.0 ± 1.6 ms. (C) Fractional recovery of Qon is plotted as a function of the loss of Na+ tail charge. The Na+ tail charge was obtained by integration of Na+ tail currents shown in Fig. 2 A. Data points represent means ± SEM from 4–5 cells. Dotted line represents a linear relationship between the charge recovery and the channel closure, with a slope of 1.0.
Figure 8.
The relationship between gating charge recovery and channel closure in the presence of 135 mM Cs+ i/o. Fractional recovery of Qon is plotted as a function of the loss of Cs+ tail currents using data from experiments as shown on Fig. 7. Tail current loss was normalized to the peak inward Cs+ tail current, at different times during recovery, and at different repolarization potentials in the three panels. Dotted lines depict a linear relationship between charge recovery and channel pore closure. Data points represent mean ± SEM (n = 4–6), with a slope of 1.0.
Gating Scheme Used to Interpret Results in the Present Study
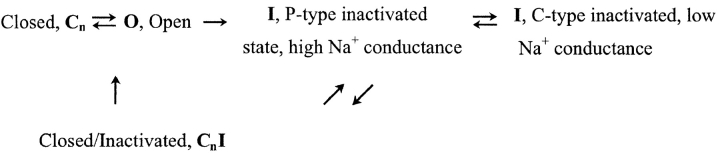
Our present understanding of the pathway by which Kv1.5 and a number of other channels, including Kv2.1, recover from inactivation has been established by previous work from our laboratory (Wang et al., 2000a; Wang and Fedida, 2001) and others (Kiss et al., 1999). Only the open state (O) is potassium conducting.
Briefly, the evidence for this kind of scheme of inactivation has been obtained from fluorescence and conventional voltage clamp studies of C-type inactivation that have revealed the presence of at least two non-K+–conducting states in the inactivation pathway (Loots and Isacoff, 1998; Kiss et al., 1999; Loots and Isacoff, 2000). They show that the P-type state, the first state reached after the open, has a higher Na+ permeability and results from a relatively local conformational change in the outer pore mouth. The second, further state from the open state is fully C-type inactivated and shows both a lower Na+ permeability (Kiss et al., 1999) and more extreme conformational changes (Loots and Isacoff, 1998). Na+ conductance of the inactivated state(s) appears as a sustained current during prolonged depolarization or as slow tails on repolarization (Starkus et al., 1997), with a very prominent initial rising phase followed by a slow decay (e.g., Fig, 2 A). The initial rising phase is due to the transition of the inactivated channels out of the C-type inactivated state into a higher Na+-conductance state, which is either the P-type state or a very analogous state in the recovery pathway, an R-state (Wang et al., 2000a). The slow decay process reflects the closure of inactivated channels to closed-inactivated states in Shaker channels (Starkus et al., 1998) and in Kv1.5 (Wang et al., 2000a). Note that inactivated WT channels generally recover through the higher Na+ conductance state, to close in the inactivated form, and return back to resting closed states via a slow vertical transition. The time constant of this slower recovery to resting closed states in WT channels is of the order of 3–4 s (Wang and Fedida, 2001).
RESULTS
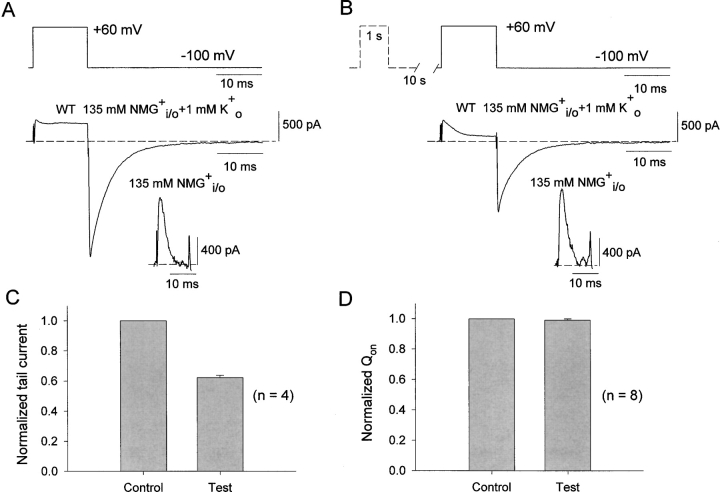
It has been observed during slow inactivation in Na+ channels (Bezanilla et al., 1982) and in T-type Ca2+ channels (Burgess et al., 2002) that gating charge is fully reavailable after an inactivating pulse well before the conductance has reprimed. We have tested whether this phenomenon can also be observed in K+ channels. In WT Kv1.5 channels, 1 mM K+ o was added to the standard NMG+ bath solution to allow K+ tail currents to be measured. Test pulses of 12-ms duration were given alone (Fig. 1 A) or after a 1-s pulse to 60 mV (Fig. 1 B) to inactivate the channels and a 10-s rest to allow partial recovery of the conductance. The smaller K+ tail current apparent on repolarization in Fig. 1 B compared with Fig. 1 A indicates that the 10-s interpulse period at −100 mV was insufficient to allow the inactivated channels to fully recover their resting pore conformation. However, using the same protocol, on-gating charge is fully recovered after a 10 s repolarization period (Fig. 1, A and B, inset). Mean data in Fig. 1, C and D, summarizes results from 4–8 experiments. After a 10-s interpulse interval, 99.1 ± 0.9% of charge has recovered, compared with only 62.2 ± 1.4% of ionic current. These data demonstrate an uncoupling between the recovery of gating charge and full reavailability of the conducting pore after inactivation in WT Kv1.5 channels. This result is not entirely unexpected since transitions through closed-inactivated states are expected to occur before full reversal of the pore changes that accompany inactivation (Wang et al., 2000a). However, in the experiments described below we show that much of this more rapid reavailability of gating charge in Kv1.5 channels occurs extremely quickly, even before the initial pore closure of inactivated channels.
Figure 1.
Uncoupling between the recoveries of gating charge and channel conductance in the inactivated WT Kv1.5 channels. (A and B) Top, voltage protocols used in the experiments shown at bottom of A and B, respectively. Dotted line in protocol in B depicts a 1-s prepulse and 10-s interpulse interval given before the 12-ms test pulse. Whole cell current traces were recorded with symmetrical 135 mM NMG+ i/o and 1 mM K+ o to record K+ tail currents at −100 mV. Note that the K+ tail current in B is significantly reduced, indicating a failure to fully recover from inactivation during the 10-s interval. Inset panels show on-gating current tracings recorded in symmetrical 135 mM NMG+ i/o from the same cells. They were subjected to the same protocol as ionic currents above. Note that in this case there is no difference between on-gating current amplitudes in A and B, indicating full recovery of gating charge in B. (C) Reduction in ionic tail current during the test depolarizations compared with controls (n = 4). (D) No change in normalized on-gating charge obtained by integrating the on-gating currents during control depolarizations without a prepulse and test depolarizations after a prepulse (n = 8). The dotted lines in A and B represent the zero current level.
Recovery of Gating Charge and Closure of Inactivated Channels Occur at Different Rates
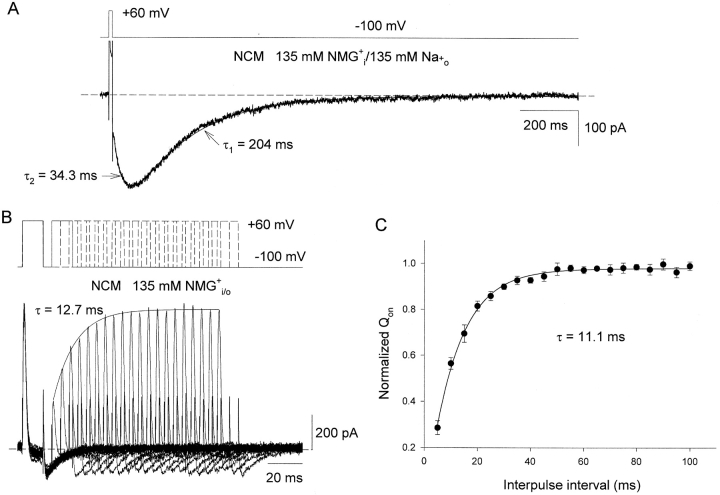
The most significant finding of the present study is illustrated in Fig. 2 . Previous work has shown that Shaker (Starkus et al., 1997) and a mammalian homologue Kv1.5 (Wang et al., 2000a), as well as Kv2.1 (Kiss et al., 1999) undergo a change of relative permeability during inactivation with the result that K+ conduction disappears but Na+ conduction becomes more significant. No Na+ current is seen unless K+ o and most of the K+ i is omitted from the solutions, as K+ can prevent the Na+ from entering the selectivity filter of the K+ channel (Kiss et al., 1998; Starkus et al., 1997). The presence of inactivation is illustrated, in both WT and nonconducting channels by the appearance of inward Na+ tail currents when Na+ is included in the extracellular medium (Starkus et al., 1997; Wang and Fedida, 2001). We have shown previously that Kv1.5 channels inactivate extremely rapidly on depolarization in the absence of K+ in the intracellular and extracellular solutions (Wang and Fedida, 2001), so the tail current can be seen after only a 12-ms depolarization (Fig. 2 A). Channels are inactivated by a strong but brief pulse to 60 mV and then repolarized to −100 mV to observe tail currents. With 135 mM Na+ o an instantaneous inward tail current reflects conduction through C-type inactivated channels (a driving force effect). The tail first increases substantially in the inward direction, which reveals an intermediate, higher Na+ conductance state (Wang et al., 2000a), and then decays slowly as inactivated channels close. The time constant of this channel closing is quite slow, with a time constant in this example of 204 ms (mean = 129 ± 26 ms, n = 18).
Figure 2.
Rates of pore closure and gating charge recovery in inactivated Kv1.5 NCM channels. (A) Na+ tail current through Kv1.5 NCM channels after a 12-ms depolarization. Voltage protocol is shown at the top. The current was recorded with 135 mM NMG+ i/135 mM Na+ o solutions. The decay of the tail current was fitted by single exponential function with a time constant (τ1) of 204 ms. The dotted line represents the zero current level. (B) Superimposed on- and off-gating current traces were elicited by a double pulse to 60 mV (top) separated by a variable interpulse interval from 5 to 100 ms with increments of 5 ms each cycle. The duration of both prepulse and the test pulse was 12 ms, and the interval between pairs of pulses was 2 s at −100 mV. Currents were recorded with symmetrical 135 mM NMG+ i / NMG+ o solutions. The solid line is the best fit to the peak of On-gating currents using a single exponential function with a time constant (τ) of 12.7 ms. The dotted line represents zero current level. (C) Fractional recovery of Qon after a 12-ms prepulse. Qon was obtained by integrating on-gating currents during 12-ms pulses, normalized to that during the prepulse, and plotted as a function of interpulse interval. Data points represent means ± SEM from 10 cells. Solid line is the best fit to data points using single exponential function with a time constant (τ) of 11.1 ± 0.4 ms.
Closing and recovery from inactivation of K+ channels is associated with the return of channels to their resting conformations—which also implies the eventual return of the voltage-sensing domains to their resting positions. This process can be tracked by measuring the reavailability of on-gating current after brief depolarizations to inactivate the channels and variable recovery periods (Fig. 2 B). Charge was moved by fully activating the channels with a 12-ms depolarization to 60 mV. Then, variable duration repolarizations (5–100 ms) to −100 mV allowed recovery of charge. The amount of charge recovery was assessed by retesting the ability of charge to move during a subsequent 12-ms depolarization to 60 mV. The recovery of peak on-gating current is rapid, and monoexponential with a time constant of 12.7 ms, as indicated by the solid line on the recovery data. The mean recovery time constant for the peak of on-gating current was 11.2 ± 0.4 ms (n = 10). On-gating charge reavailability was also rapid with recovery time constant for Qon 11.1 ± 0.4 ms (n = 10, Fig. 2 C). This time course was not changed by longer depolarizing prepulses of up to 10 s duration (unpublished data). The data in Fig. 2 clearly show a 10–20-fold separation of the time course of closing of inactivated Kv1.5 channels, as measured by the decay of inward Na+ tail currents (Fig. 2 A), and the recovery of gating charge after similar duration depolarizations (Fig. 2, B and C). The result suggests that reavailability of gating charge can occur without substantial pore closure when channels are inactivated.
To ensure that the presence of 135 mM external Na+ in the tail current experiment did not alter the time course of gating charge recovery, the experiment in Fig. 2, B and C, was repeated in the presence of external Na+ rather than NMG+. Using the same experimental protocol, the peak gating current recovery was a little slower, at 13.4 ms, but still far more rapid than the decay of the Na+ tails to the baseline. This is clearly seen in Fig. 3 A where, with external 135 mM Na+, inward currents through closing inactivated channels are observed on repolarization. The decay phase of these tails far outlasts the time taken for on-gating currents to become fully available again. Peak gating current recovery time is illustrated in Fig. 3 A and can be fit to a single exponential process with a time constant of 13.4 ms (14.3 ± 0.6 ms, n = 4). Charge recovery occurs with a time constant of 19.0 ± 1.6 ms (n = 4, Fig. 3 B). In the presence of external Na+, the gating charge only showed 80% recovery after a 100-ms interpulse interval (Fig. 3 B), although it appears that the peak of on-gating currents reaches a saturated level. The explanation for peak current saturation below the level of control currents is the downward current displacement by inward Na+ currents both during the test pulses and also on repolarization. This contamination also affects the measurement of Qon. Even if we admit the possibility that external Na+ might slow the charge return, the time course was only a little slower compared with that in the presence of symmetrical NMG+ (Fig. 2 C).
The well-known linear models of deactivation of open channels, based largely on observations from Shaker channels, propose that the first channel-closing step carries little charge and is faster than the subsequent steps between closed states that carry most charge (Bezanilla et al., 1994). This model predicts that charge recovery should be no faster than channel closure, which must precede it. This seems to be the case in Shaker channels, where the time course of charge movement matches channel closing (Starkus et al., 1997). However, in the inactivated Kv1.5 channels that we have studied here, the relationship between the gating charge recovery and the channel closure is clearly inconsistent with a linear deactivation model. We plotted the fraction of total charge recovered as a function of the fraction of channels closed (normalized decay of tail charge) in Fig. 3 C. The proportion of the channels that closed during the repolarizing pulse was calculated from the decay of the charge carried by the Na+ tail currents. The ratio of gating charge during the test pulse to that during the prepulse yielded the fraction of gating charge returned during the interpulse interval. The relationship between the two deviates markedly from linearity. For inactivated NCM channels, a significant fraction of gating charge recovers before any significant channel closure, such that ∼80% of gating charge recovers while only ∼20% of channels closed. Thus, our observations clearly show an uncoupling between the gating machinery and the conducting pore during the recovery from inactivation.
Full Recovery of Gating Charge Occurs while Channels Are Still Inactivated in WT Kv1.5 Channels
Although the W472F mutant faithfully reproduces many aspects of WT channel gating, both in Shaker channels (Perozo et al., 1993) and in Kv1.5 (Wang and Fedida, 2001), it is possible that the mutation somehow allows gating element recovery to proceed in advance of pore closure. Therefore, in WT Kv1.5 channels we repeated the recovery experiments already described in NCM channels. In WT Kv1.5, in the presence of symmetrical NMG+, the time constant of recovery of peak on-gating current was 13.1 ± 0.8 ms (n = 21). In contrast, decaying Na+ tail currents recorded under the same experimental conditions as in Fig. 1 had a time constant of 162 ± 5.8 ms (n = 28).
Correlation between the Voltage Dependence of Gating Charge Recovery and Decay of Na+ Conductance
At the particular potential that we have used (−100 mV), we have observed a significant disparity between the time course of charge recovery and Na+ tail current decay. Our previous studies provided evidence that on repolarization the inactivated Kv1.5 channels initially recover to a P-type inactivated state with high Na+ conductance before transition to closed-inactivated states. This is the explanation for the initial rising phase followed by a slow decay phase of the Na+ tail currents seen in Fig. 2 (Wang et al., 2000a). This, and other evidence (Loots and Isacoff, 1998), supports the existence of more than one inactivated state in Kv channels (Scheme I) . Since the access to these states is highly voltage dependent (Wang et al., 2000a), we asked if there was a link between either of these inactivated states and the gating charge recovery by testing the voltage dependence of the recovery from the separate inactivated states, and gating charge return (Fig. 4) .
SCHEME I.
Figure 4.
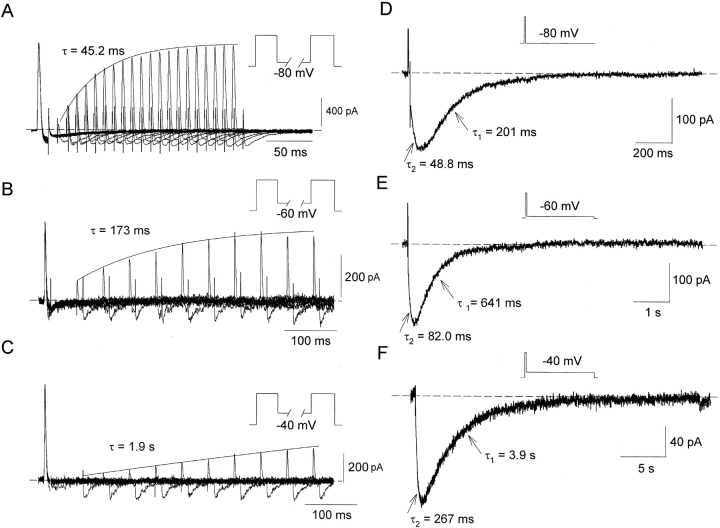
Voltage-dependence of the gating charge recovery and channel closure in the inactivated Kv1.5 NCM channels. (A–C) Superimposed gating current traces were elicited by a double pulse to 60 mV separated by a variable interpulse interval (protocol above each record). The duration of both prepulse and the test pulse was 12 ms and the interval between pairs of pulses was 2 s at –80 mV, 10 s at −60 mV and 15 s at −40 mV, respectively. Currents were recorded with symmetrical 135 mM NMG+ i/o solutions. Solid lines are the best fits to the peak of on-gating currents using a single exponential function with time constants (τ) shown on tracings. (D–F) After a 12-ms pulse to 60 mV, Na+ tail currents were recorded at −80, −60, and −40 mV, respectively. Voltage protocol is shown above each record. The current was recorded with 135 mM NMG+ i/135 mM Na+ o solutions. The tail currents were fitted by double exponential functions with time constants (τ1 and τ2) shown on tracings. Dotted lines represent zero current level.
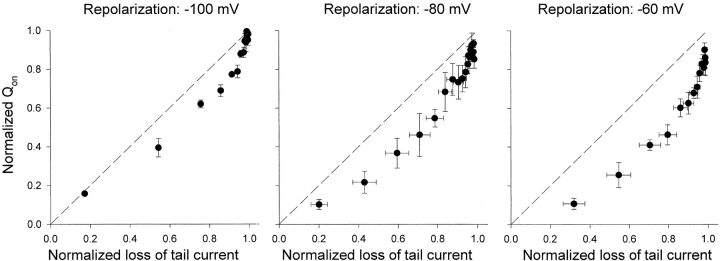
We found that the dissociation of charge recovery and channel closing was maintained at different potentials. The voltage dependence of charge recovery is illustrated in Fig. 4, A–C. Using the three-pulse protocol, but varying the repolarization potential during the middle pulse, changes the recovery time constant of the gating charge. The recovery time constants for the peak of on-gating current increased from 11.2 ± 0.4 ms (n = 10) at −100 mV to a mean of 42.3 ± 1.4 ms (n = 5) at −80 mV, 204 ± 28 ms (n = 10) at −60 mV, and 952 ± 98 ms (n = 13) at −40 mV. The reavailability of charge was also changed at different repolarization potentials, with recovery time constants for Qon ranging from 11.1 ± 0.4 ms (n = 10) at −100 mV to a mean of 50.6 ± 2.6 ms (n = 5) at −80 mV, 235 ± 25 ms (n = 10) at −60 mV and 1513 ± 410 ms (n = 13) at −40 mV. The time course of channel closing as measured from the decay of Na+ tail currents (τ1) also slowed at more positive potentials (Fig. 4, D–F), from 129 ± 26 ms (n = 18) at −100 mV to a mean of 208 ± 44 ms (n = 11) at −80 mV, 748 ± 105 ms (n = 9) at −60 mV and 3805 ± 239 ms (n = 7) at −40 mV. Clearly, at every potential tested the pore closure of the inactivated channels is slower than the recovery of gating charge.
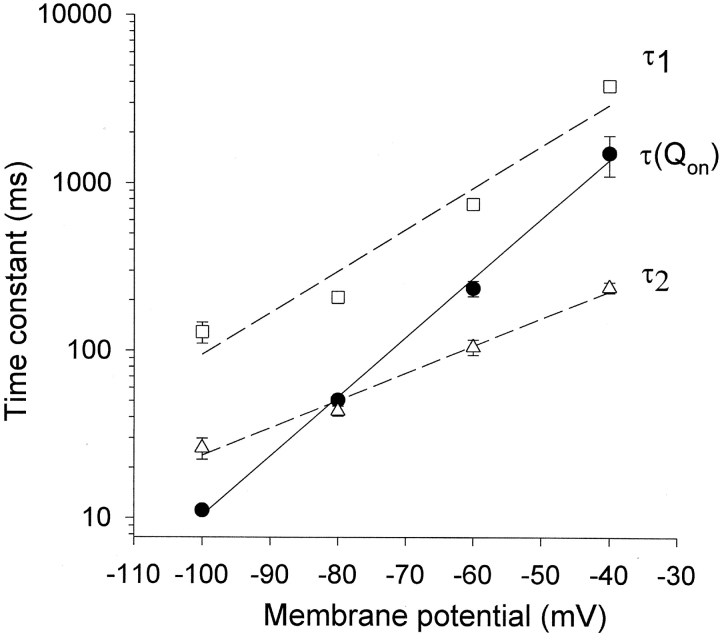
To quantify the relationship between inactivated states and the gating charge recovery, we fitted the inward Na+ tail currents in Fig. 4, D–F with a double exponential function. The time constant (τ1) fitted to the decaying phase of the Na+ tail represents the transition of inactivated channels from the intermediate high Na+-conducting state (P-type state) to closed-inactivated states and τ2 fitted to the rising phase of the Na+ tail represents the transition from the fully C-type inactivated state to the P-type state (Wang et al., 2000a). We plotted τ1, τ2, and the time constant for gating charge recovery (τQon) as functions of repolarization potential (Fig. 5) . The voltage dependence of τ1 and τ2 shows an almost parallel relationship with a 10-fold change in time constant over ∼41 mV for τ1 and ∼60 mV for τ2, respectively. These data are consistent with our previous observations (Wang et al., 2000a) in suggesting that a single voltage-sensitive process (i.e., the transition from the C-type inactivated state to the P-type state) is rate-limiting in the recovery pathway of inactivated channels. In comparison, it is clear that the time constant of gating charge recovery (τQon) is much more voltage-dependent than τ1 and τ2. The slope gives a 10-fold change in τQon over ∼30 mV, significantly less than that for τ1 and τ2.
Figure 5.
Time constants of Na+ current decay and gating charge recovery versus repolarization potential. τ1 was fitted to the slower decay phase of the Na+ tail current, whereas τ2 was fitted to the rising phase, as indicated in Fig. 4. Data points represent mean ± SEM (n = 5–13). Dotted and solid lines represent the best linear fitting to data points. Qon time constants were from exponential fits to the time course of charge recovery at different potentials, obtained from data as in Fig. 4, A–C.
Limiting Inactivation to the P-type State Does Not Synchronize Gating Charge Recovery and Pore Closure
Further confirmation of this dissociation was obtained in the presence of symmetrical 135 mM Na+ i/o. In this ionic situation the channels can only enter the P-type inactivated state (Wang and Fedida, 2001), with the result that the Na+ tail currents on repolarization decay slowly but have no rising phase (Fig. 6 A). Using the same protocol as used to collect data in Fig. 3 A, in the presence of symmetrical Na+ i/o, it is clear that the gating charge still recovered more rapidly than the tail decay (Fig. 6 A). In this case an outward Na+ current is apparent during the depolarizations, which is a result of Na+ efflux through P-type inactivated channels. In this situation, on-gating charge was obtained by integrating only the transient component of on-gating current. Peak gating current recovery is illustrated in Fig. 6 A and was fitted to a single exponential process with a time constant of 16.3 ms, whereas charge recovery occurs with a mean time constant of 18.1 ± 1.3 ms (n = 3, Fig. 6 B). We also plotted the fraction of total charge recovered as a function of the fraction of channels closed (normalized loss of tail current) in Fig. 6 C. As before, (Fig. 3) we determined the proportion of the channels that closed during the repolarizing pulse from the time-dependent decay of the Na+ tail currents. As in Fig. 3 C, it is clearly seen that most of the gating charge recovers before a significant fraction of channels close. The results in Figs. 4–6 suggest that no one particular state in the pathway of recovery from inactivation is permissive for the return of gating charge, but rather that charge recovery in inactivated Kv1.5 channels is quite independent of the kinetics of pore closure.
Cs+ i Prevents Inactivation and Synchronizes Pore Recovery and Gating Charge Return
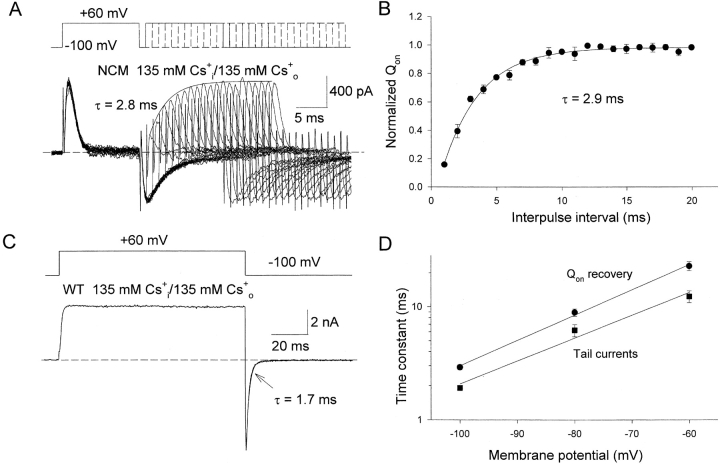
We have shown previously, in both WT and nonconducting channels, that the inactivation of Kv1.5 channels on depolarization can be slowed or prevented in the presence of intracellular and extracellular Cs+ (Chen et al., 1997; Wang et al., 1999; Wang and Fedida, 2001). Thus, we tested the temporal relationship between charge recovery and channel pore closure in the presence of Cs+. In the presence of symmetrical 135 mM Cs+ i/o, the recovery of charge is much more rapid than described earlier (Fig. 7) . Using the same protocol as in Fig. 3 A, peak on-gating current recovery was measured and fitted to a single exponential process with a time constant of 2.8 ms (Fig. 7 A). Charge recovery occurs with a time constant of 2.9 ± 0.1 ms (n = 4, Fig. 7 B). To compare the time course of charge recovery and channel closing, we measured WT Kv1.5 channel deactivation in symmetrical 135 mM Cs+ i/o because the NCM mutant is nonconducting to Cs+. As shown in Fig. 7 C, the tail deactivation is fit by single exponential function with a time constant of 1.7 ms (1.9 ± 0.03 ms, n = 23), which is close to that of charge recovery (Fig. 7 B). The time constants of charge recovery and channel deactivation at various repolarization potentials are shown in Fig. 7 D. Over the range of potentials studied, between −60 and −100 mV, the time constants for charge recovery are all slower than those of ionic current deactivation, but both show an identical voltage dependence. The result suggests that in a situation where channel inactivation is prevented, the time course of recovery of gating charge is closely coupled to channel deactivation.
Figure 7.
Rate of gating charge recovery and channel closure in WT Kv1.5 and NCM channels in the presence of 135 mM Cs+ i/o. (A) Top, the same double-pulse protocol as shown in Fig. 3 A. Bottom, superimposed gating current traces elicited by the protocol shown at the top. Currents were recorded with symmetrical 135 mM Cs+ i/o solutions from NCM channels. Solid line is the single exponential fitting to the peak of on-gating currents with τ = 2.8 ms. The dotted line represents the zero current level. (B) Fractional recovery of Qon (Qon test/Qon prepulse) is plotted as a function of the interpulse interval. Data points represent means ± SEM (n = 4). The solid line is the best fit to data points using a single exponential function with τ = 2.9 ± 0.1 ms. (C) Top, voltage protocol used to elicit the current below. Bottom, whole cell current through WT Kv1.5 channels with symmetrical 135 mM Cs+ i/o solutions. The tail was fitted by a single exponential function with a time constant (τ) of 1.7 ms. The dotted lines represent the zero current level. (D) Time constants of voltage-dependent deactivation (tail currents) and gating charge recovery (Qon recovery) of Kv1.5 channels in the presence of Cs+ i/o, plotted as functions of repolarization potential. Data points represent mean ± SEM (n = 4–6). Solid lines represent the linear fitting to data points.
We quantified the temporal relationship between charge recovery and pore closure in the presence of Cs+ i/o by plotting the fraction of total charge recovered as a function of the fraction of channels closed (normalized loss of tail current) at various potentials (Fig. 8). Channel closure during the repolarizing pulse was determined from the decay of the Cs+ tail currents. Compared with Figs. 3 C and 6 C, charge recovery now much more closely parallels pore closure. In fact, complete pore closure occurs at ∼80% charge recovery at all potentials. These observations are consistent with the linear deactivation model that predicts that the channel closing step carries significant charge but that later transitions between closed states carry more of the charge. Based on our experiments with Cs+, we conclude that the uncoupling of charge recovery and channel pore closure in the presence of NMG+ i/o (Fig. 2) or Na+ i/o (Fig. 6) results from the inactivation in the Kv1.5 channel.
DISCUSSION
The main observation that we have made in this study is that the movement of the activation voltage sensor on channel closing is not always rigidly coupled to changes in pore conformation, and there appear to be situations when movement of the two may be temporally uncoupled. Most existing gating models (Bezanilla et al., 1994; Zagotta et al., 1994a; Schoppa and Sigworth, 1998b) describe a tight coupling between the gating system and the conducting pore. However, in none of these models was information on recovery from slow inactivation incorporated. This is mainly because inactivated channels cannot conduct K+, so a study of the process of pore recovery cannot easily be made. However, Bezanilla et al. (1982) observed in sodium channels of the squid giant axon that the recovery of all gating charge after repolarization at −160 mV preceded full recovery of the Na+ conductance from inactivation. Recently, it has also been reported in T-type Ca2+ channels that gating current recovery can precede ionic current recovery from inactivation (Burgess et al., 2002). This observation suggests that a temporal separation of gating charge recovery and the pore conformation can occur when channels of the voltage-gated superfamily are recovering from inactivated states.
In this study, to compare the return of gating charge and recovery of the conducting pore in inactivated channels, we used the changing Na+ conductance to follow the transition of inactivated channels back to closed-inactivated states. We determined that with 135 mM NMG+ i/135 mM Na+ o both WT and NCM channels inactivated very quickly even during a brief 12-ms depolarization to 60 mV (Wang and Fedida, 2001). On repolarization to −100 mV, an inward Na+ tail current with a rising phase and slow decay represents the transitions of inactivated channels from the C-type to the P-type inactivated state and then to closed-inactivated states. What is so striking is that these transitions take a very long time compared with the time course of the gating charge recovery (Figs. 2 and 3). Although the return of charge is slower in the absence of permeant cations (∼11 ms in Fig. 2 and ∼2.9 ms with Cs+ in Fig. 7) due to inactivation (Wang and Fedida, 2001), the time course for decay of Na+ conductance is still about ∼15-fold slower than that for gating charge return. A comparison of pore closure, measured as decay of Na+ conductance, and gating charge return showed ∼80% charge recovery in the face of only ∼20% pore closure (Fig. 3). This is quite different from models of deactivation from the open state that predict charge recovery to be slower than pore closure. This phenomenon is not limited to NCM channels, as we found that full recovery of gating charge precedes the reavailability of K+ conductance in WT Kv1.5 channels as well. This property may explain in part why gating charge is always reavailable much earlier than the ionic conductance in WT channels, both in Kv1.5 (Fig. 1) and as reported in squid axon Na+ channels (Bezanilla et al., 1982) and T-type Ca2+ channels. Together, the studies suggest that full reavailability of gating charge can occur in inactivated Kv1.5 channels without substantial pore recovery.
It was noted that when the Na+ tail current and gating charge return were directly measured in the same experiment (requiring the presence of a high concentration of external Na+), the gating charge only recovered to ∼80% of the control value after a 100-ms interpulse interval (Fig. 3, A and B). It is likely that Qon during the test pulse is underestimated because the inward Na+ tail makes it difficult to find the zero current level for on-gating currents elicited by test pulses. But we cannot exclude the possibility that external Na+ might play a role in slowing the charge return. However, our observation that the time course of gating charge return was only a little slower in the presence of inward Na+ currents, compared with that in the presence of symmetrical NMG+ (19.0 vs. 11.1 ms) argues against the idea that internal NMG+ can enter the pore and slow charge return (Melishchuk and Armstrong, 2001). If the presence of NMG+ in the internal cavity of the pore produced charge immobilization, one would expect that the inward Na+ during the tail influx should have knocked off the NMG+ and accelerated charge return.
In nonconducting W434F mutant Shaker K+ channels, the decay of inward Na+ tail currents on repolarization showed a similar time course to gating charge recovery (Starkus et al., 1998), quite different from what we have observed in Kv1.5 NCM channels. We do not have a good explanation for the contrasting results, except that differences in the channel used or in the expression system, oocytes versus HEK cells, may play a role. With regard to the channels, evidence suggests that the outer pore conformation change during inactivation is probably quite different in Shaker than in Kv1.5 channels. First, during prolonged depolarizations, Shaker channels show a higher Na+ conductance of the C-type inactivated state than Kv1.5 (Starkus et al., 1997). Thus, when channels recover from inactivation at negative potentials, less of a rising Na+ tail is seen in Shaker than in Kv1.5 (Wang et al., 2000a). Furthermore, mutation of T449 to valine in Shaker abolishes K+ current inactivation (Olcese et al., 2001). However, an analogous mutation, R487V in Kv1.5, only abolishes Na+ current inactivation, not K+ current inactivation (Wang et al., 2000a,b). Together, these different behaviors suggest a more significant conformational change during inactivation in Kv1.5 channels than in Shaker. We speculate that this may narrow the outer pore more in Kv1.5, so that C-type inactivated channels have a lower Na+ conductance and a clearer rising phase to the Na+ tail on repolarization than Shaker channels. In Kv1.5 R487V channels, the mutation allows a minor enlargement of the outer pore that is only sufficient to allow small cations like Na+ to pass through, abolishing Na+ current inactivation but not K+ current inactivation. The mutation of T449 to valine in Shaker channels may enlarge the outer pore enough to allow K+ to pass through, and, as a result, prevent K+ current inactivation as well. The conformational changes during C-type inactivation, coupled with the subtle but important differences in pore structure between Kv1.5 and Shaker may explain the ability of the pore to uncouple from the voltage sensor in Kv1.5 but not, apparently, in Shaker. As a point to note though, in the model proposed by Starkus et al. (1997)(1998) for inactivated Shaker channels, the slow decay of Na+ conductance represents the transition of inactivated channels to closed-inactivated states, because the recovery of K+ conductance is much slower than the time course of pore closing. Thus, similar to Kv1.5 (Fig. 1) and Na+ channels (Bezanilla et al., 1982), completion of the Na+ tail decay does not correspond to full recovery of the inactivated K+ conductance in Shaker potassium channels either.
Transition between Inactivated States Does Not Affect the Dissociation between Pore and Gating Charge Recovery
Above we have summarized evidence showing that slow inactivation of Kv channels includes at least two inactivated states (Yang et al., 1997; Loots and Isacoff, 1998; Loots and Isacoff, 2000; Wang et al., 2000a). Upon repolarization, the transitions from the C-type state to the P-type state and then to closed-inactivated states generate slow Na+ tail currents with a prominent initial rising phase followed by a slow decay process in Shaker channels (Starkus et al., 1998) and in Kv1.5 (Wang et al., 2000a). In the present study we asked if the temporal dissociation of gating charge recovery and the recovery of resting pore conformation could be accounted for by any of these transitions between the inactivated states. We tested the voltage dependence of the time course of gating charge return and the time course of the rising phase and slow decay of Na+ currents (Fig. 4). Although all rates showed voltage-dependent variation, no clear relationship was observed between the recovery of gating charge and any of the transitions of the inactivated states (Fig. 5).
Similarly, in the presence of symmetrical 135 mM Na+ i/o, the NCM Kv1.5 channels can only enter an initial P-type inactivated state (Wang and Fedida, 2001). Thus, the Na+ tail currents on repolarization decay slowly but have no rising phase (Fig. 6 A). This experimental condition allowed us to isolate channels in the P-type inactivated state, but even so a similar pattern of dissociation of the time course of gating charge and pore closure was observed to that seen in the absence of Na+ i (Fig. 6). Thus, it seems that once channels have undergone just the initial inactivating transition, pore closure and gating charge recovery become temporally dissociated.
To demonstrate that inactivation is responsible for this dissociation of pore and gating element recovery, and to validate that we could actually correlate gating charge return and pore closure under some conditions, we replaced NMG+ with 135 mM Cs+. We knew from our previous experiments that Cs+ accelerates the channel closing transition to such an extent that inactivation is prevented (Wang and Fedida, 2001). We found that symmetrical 135 mM Cs+ could synchronize the recovery of gating charge and of the pore (Fig. 7), so that the time course of gating charge return correlated closely with Cs+ current deactivation in a voltage-dependent manner (Fig. 8). Such a correlation between two the systems is quite consistent with the linear models for activation and deactivation of Kv channels where some kind of initial closing transition is slow and rate-limiting for both processes (Bezanilla et al., 1994; Zagotta et al., 1994b; Schoppa and Sigworth, 1998b). The Cs+ experiment seems to confirm that inactivation is a special case in the Kv1.5 channel, in that it allows an uncoupling of the normal time and voltage dependence of gating element recovery on pore closure.
The phenomenon of gating current reavailability after inactivation preceding ionic current reavailability has now been noted in slow inactivated Na+ channels (Bezanilla et al., 1982), T-type Ca2+ channels (Burgess et al., 2002), and Kv1.5 channels in the present study. Here, we have additionally described that the return of gating charge is much faster even than pore closure in the inactivated Kv1.5 channels, which thus appears to be a strongly rate-limiting early step in the recovery of conductance from inactivation. It is possible that conformational changes associated with the inactivation process (Loots and Isacoff, 1998) somehow disrupt the connection between the voltage-sensing domains and the activation gate, so that each system shows its own time- and voltage-dependent recovery from inactivation. The slow decay of Na+ tail current provides evidence to support the idea that the activation gate is affected by the conformational changes resulting from inactivation, since a normally functioning activation gate might be expected to generate Na+ tail currents with a more rapid decay (Wang et al., 2000a,b). Thus, we think the slow decay of Na+ tail current not only reflects a change in the energetics of the selectivity filter but also of the closing of the activation gate in the inactivated Kv1.5 channels. Further experiments, perhaps using fluorescent labels of outer pore residues are required to conclusively demonstrate the structural basis for the phenomenon we have described.
Conclusion
The data indicate that in Kv1.5, after inactivation, gating charge return is rapid and dissociated from pore recovery, which proceeds much more slowly. Extracellular Na+ is not able to inhibit the development of the inactivation process in a significant way, so changes in the Na+ conductance can be used to track the closing of inactivated channels. There is a significant discrepancy between time course of recovery of gating charge and the pore on repolarization to a number of different potentials. Even when inactivation is limited to the P-type state, the gating and pore systems recovered independently. Intracellular Cs+, which can effectively prevent inactivation, allows gating charge return and pore recovery to be synchronized.
Acknowledgments
We thank Dr. S. Kehl for his careful reading of the text and Qin Wang and Linda Sui for their help with cell culture.
Supported by grants from the Heart and Stroke Foundations of British Columbia and Yukon, and the CIHR to D. Fedida.
Footnotes
Abbreviations used in this paper: NCM, nonconducting mutant; WT, wild-type.
References
- Armstrong, C.M., and F. Bezanilla. 1973. Currents related to movement of the gating particles of the sodium channels. Nature. 242:459–461. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F., E. Perozo, and E. Stefani. 1994. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys. J. 66:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla, F., R.E. Taylor, and J.M. Fernández. 1982. Distribution and kinetics of membrane dielectric polarization. 1. Long-term inactivation of gating currents. J. Gen. Physiol. 79:21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, D.E., O. Crawford, B.P. Delisle, and J. Satin. 2002. Mechanism of inactivation of human T-type (low-voltage actviated) calcium channels. Biophys. J. 82:1894–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F.S.P., D. Steele, and D. Fedida. 1997. Allosteric effects of permeating cations on gating currents during K+ channel deactivation. J. Gen. Physiol. 110:87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida, D., and J.C. Hesketh. 2001. Gating of voltage-dependent potassium channels. Prog. Biophys. Mol. Biol. 75:165–199. [DOI] [PubMed] [Google Scholar]
- Fedida, D., B. Wible, Z. Wang, B. Fermini, F. Faust, S. Nattel, and A.M. Brown. 1993. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ. Res. 73:210–216. [DOI] [PubMed] [Google Scholar]
- Goldstein, S.N.A. 1996. A structural vignette common to voltage sensors and conduction pores: canaliculi. Neuron. 16:717–722. [DOI] [PubMed] [Google Scholar]
- Hodgkin, A.L., and A.F. Huxley. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Camb). 117:500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevsky, M., and R.W. Aldrich. 1999. Determinants of voltage-dependent gating and open-state stability in the S5 segment of Shaker potassium channels. J. Gen. Physiol. 114:215–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes, R.D., and E. Rojas. 1974. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J. Physiol. (Camb). 239:393–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, L., D. Immke, J. LoTurco, and S.J. Korn. 1998. The interaction of Na+ and K+ in voltage-gated potassium channels - Evidence for cation binding sites of different affinity. J. Gen. Physiol. 111:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, L., J. LoTurco, and S.J. Korn. 1999. Contribution of the selectivity filter to inactivation in potassium channels. Biophys. J. 76:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, H.P., O.S. Baker, D.S. Dhillon, and E.Y. Isacoff. 1996. Transmembrane movement of the Shaker K+ channel S4. Neuron. 16:387–397. [DOI] [PubMed] [Google Scholar]
- Ledwell, J.L., and R.W. Aldrich. 1999. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 113:389–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots, E., and E.Y. Isacoff. 1998. Protein rearrangements underlying slow inactivation of the Shaker K+ channel. J. Gen. Physiol. 112:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots, E., and E.Y. Isacoff. 2000. Molecular coupling of S4 to a K+ channel's slow inactivation gate. J. Gen. Physiol. 116:623–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack, K., W.J. Joiner, and S.H. Heinemann. 1994. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+ channels. Neuron. 12:301–315. [DOI] [PubMed] [Google Scholar]
- Melishchuk, A., and C.M. Armstrong. 2001. Mechanism underlying slow kinetics of the OFF gating current in Shaker potassium channel. Biophys. J. 80:2167–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese, R., D. Sigg, R. Latorre, F. Bezanilla, and E. Stefani. 2001. A conducting state with properties of a slow inactivated state in a Shaker K+ channel mutant. J. Gen. Physiol. 117:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., R. MacKinnon, F. Bezanilla, and E. Stefani. 1993. Gating currents from a non-conducting mutant reveal open-closed conformation in Shaker K+ channels. Neuron. 11:353–358. [DOI] [PubMed] [Google Scholar]
- Schoppa, N.E., and F.J. Sigworth. 1998. a. Activation of Shaker potassium channels II. Kinetics of the V2 mutant channel. J. Gen. Physiol. 111:295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa, N.E., and F.J. Sigworth. 1998. b. Activation of Shaker potassium channels III. An activation gating model for wild-type and V2 mutant channels. J. Gen. Physiol. 111:313–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F.J. 1994. Voltage gating of ion channels. Q. Rev. Biophys. 27:1–40. [DOI] [PubMed] [Google Scholar]
- Starkus, J.G., L. Kuschel, M.D. Rayner, and S.H. Heinemann. 1997. Ion conduction through C-type inactivated Shaker channels. J. Gen. Physiol. 110:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus, J.G., L. Kuschel, M.D. Rayner, and S.H. Heinemann. 1998. Macroscopic Na+ currents in the “nonconducting” Shaker potassium channel mutant W434F. J. Gen. Physiol. 112:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, C., and R. Horn. 1997. Effect of alkali metal cations on slow inactivation of cardiac Na+ channels. J. Gen. Physiol. 110:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., and D. Fedida. 2001. Gating charge immobilization caused by the transition between inactivated states in the Kv1.5 channel. Biophys. J. 81:2614–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z., X. Zhang, and D. Fedida. 1999. Gating current studies reveal both intra- and extra-cellular cation modulation of K+ channel deactivation. J. Physiol. (Camb). 515:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.R., J.C. Hesketh, and D. Fedida. 2000. a. A high-Na+ conduction state during recovery from inactivation in the K+ channel Kv1.5. Biophys. J. 79:2416–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.R., X. Zhang, and D. Fedida. 2000. b. Regulation of transient Na+ conductance by intra- and extracellular K+ in the human delayed rectifier K+ channel Kv1.5. J. Physiol. (Camb). 523:575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, M.M., and F. Bezanilla. 1985. Activation of squid axon K+ channels. Ionic and gating current studies. J. Gen. Physiol. 85:539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N.B., A.L. George, Jr., and R. Horn. 1996. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 16:113–122. [DOI] [PubMed] [Google Scholar]
- Yang, Y.S., Y.Y. Yan, and F.J. Sigworth. 1997. How does the W434F mutation block current in Shaker potassium channels. J. Gen. Physiol. 109:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W.N., T. Hoshi, and R.W. Aldrich. 1994. a. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W.N., T. Hoshi, J. Dittman, and R.W. Aldrich. 1994. b. Shaker potassium channel gating. II: Transitions in the activation pathway. J. Gen. Physiol. 103:279–319. [DOI] [PMC free article] [PubMed] [Google Scholar]