Abstract
Capsaicin ion channels are highly expressed in peripheral nervous terminals and involved in pain and thermal sensations. One characteristic of the cloned VR1 receptor is its multimodal responses to various types of noxious stimuli. The channel is independently activated by capsaicin and related vanilloids at submicromolar range, by heat above 40°C, and by protons at pH below 6.5. Furthermore, simultaneous applications of two or more stimuli lead to cross sensitization of the receptor, with an apparent increase in the sensitivity to any individual stimulus when applied alone. We studied here the mechanism underlying such cross-sensitization; in particular, between capsaicin and pH, two prototypical stimuli for the channel. By analyzing single-channel currents recorded from excised-patches expressing single recombinant VR1 receptors, we examined the effect of pH on burst properties of capsaicin activation at low concentrations and the effect on gating kinetics at high concentrations. Our results indicate that pH has dual effects on both capsaicin binding and channel gating. Lowering pH enhances the apparent binding affinity of capsaicin, promotes the occurrences of long openings and short closures, and stabilizes at least one of the open conformations of the channel. Our data also demonstrate that capsaicin binding and protonation of the receptor interact allosterically, where the effect of one can be offset by the effect of the other. These results provide important basis to further understand the nature of the activation pathways of the channel evoked by different stimuli as well as the general mechanism underling the cross-sensitization of pain.
Keywords: vanilloid receptor, TRP, pain, cross-sensitization, single-channel
INTRODUCTION
Peripheral pain is perceived through activation of nociceptors, mainly the small diameter, unmyelinated, slowly conducting C fibers and the medium-diameter, lightly myelinated, intermediately fast Aδ fibers (Squire et al., 2003). A principal pharmacological trait of a large subpopulation of the nociceptors, most C but also Aδ types, is their high sensitivity to capsaicin, the pungent ingredient of hot peppers (Jancso et al., 1967). The molecular entity of the capsaicin receptors, however, has remained elusive until recent cloning of the VR1 channels (Caterina et al., 1997; Hayes et al., 2000). When expressed in heterologous systems, VR1 exhibits many characteristics of the native capsaicin ion channels, including cationic selectivity, relatively high Ca2+ permeability, outward rectification, vanilloid sensitivity, Ca2+-dependent desensitization, antagonization by capsazepine, and tissue distributions in sensory and nodose ganglia and anterior hypothalamus (Helliwell et al., 1998; Guo et al., 1999; Mezey et al., 2000). The channel consists of six putative transmembrane domains and a pore loop, with a general membrane topology similar to that of the other six transmembrane domain channels, though it doesn't have a highly charged TM4. Since the finding of VR1, several homologues have subsequently been cloned, including a stretch-inhibitable channel (SIC)* (Schumacher et al., 2000) and a vallinoid receptor–like protein (VRL1) (Caterina et al., 1999). Neither of these two proteins exhibits capsaicin sensitivity. Whether VR1 is the sole molecular target of capsaicin in vivo remains uncertain, though there is evidence suggesting the existence of multiple vanilloid receptors (Szolcsanyi, 1990; Colquhoun et al., 1995; Appendino et al., 1996; Griffiths et al., 1996; Petersen et al., 1996; Liu et al., 1998).
One characteristic feature of VR1 is its polymodal functions in response to multiple noxious stimuli. Heat, acid, and capsaicin can each activate the channel separately at conditions that are similar to those found in normal physiological states. Furthermore, simultaneous application of two or more stimuli leads to cross sensitization of the receptor, with an apparent increase in the sensitivity to any individual stimulus when applied alone. The capability of VR1 to detect and integrate information from diverse physical and chemical inputs underscores its potential role in inflammatory pain sensation. Indeed, the gene knock-out experiments suggest that the receptor is essential for the development of thermal hypersensitivity associated with tissue injury, a condition that results in local tissue acidosis with pH as low as 5 and releases of proinflammatory peptides such as substance P (Caterina et al., 2000; Davis et al., 2000). Mice that lacked the VR1 gene showed no increased sensitivity to thermal stimuli after tissue inflammation as wild-type mice normally do. The feature of polymodality is well known at the level of nociceptors, in particular, the C fibers, but it is remarkable that such polymodal functions are also present at a single molecular level.
The mechanism of polymodal functions of VR1 remains largely unknown. Mutagenesis experiments so far suggest that the region between TM2 and TM3 is essential to attain capsaicin activity (Jordt and Julius, 2002). The effect of low pH on the channel is presumably a result of protonation of acidic residues on the extracellular domain. Neutralization experiments suggest that the residues that have the most profound effects are localized around the pore region between TM5 and TM6, including E600, E636, D646, and E648 (Jordt et al., 2000; Welch et al., 2000). In particular, E600 appears to be important for low pH potentiation of heat and capsaicin activations, whereas E648 necessary for proton activation of the channel. By analogy with the pore structure of the Kcsa potassium channel (Doyle et al., 1998), mutagenesis experiments have also led to identification of D646, which is important for modulating the blocking of ruthenium red, and E636 and K639, which are believed to form intrahelical salt bridges to stabilize the interactions between pore helices (Garcia-Martinez et al., 2000). There is an emerging notion that the activation pathways for capsaicin and low pH are different and independent, as supported by a recent study of a triple mutant in the region of TM6, which disrupts capsaicin and resiferatoxin activations but retains the ability to respond to protons (Kuzhikandathil et al., 2001).
We present here a functional study of VR1 at the single-channel level in an attempt to draw insights into the multimodal gating mechanisms of the channel. In particular, we focus on capsaicin and proton activations and their cross talks. Other stimuli might involve different activation mechanisms, but the study of the two representative noxious stimuli offers a starting point to peer into the complex process. We address the problem by exploiting the full promise of single-channel measurements. As opposed to mutagenesis studies, these experiments permit quantitative analysis and comparisons of native gating behavior of the channel at different conditions. We find that low pH has a multitude of effects on capsaicin activation, including the unitary channel conductance, the capsaicin binding affinity and the channel gating efficacy. Our analysis also reveals many common aspects of the gating of the channel involved in both capsaicin and pH activations, suggesting that the two pathways may be more imminently related than the mutagenesis experiments have alluded to.
MATERIALS AND METHODS
Cell Culture and Channel Expression
Whole-cell currents reported in this study were measured from human embryonic kidney cells (HEK 293) (American Type Culture Collection). Cells were maintained with a confluence of 70–80% in standard growth medium (DMEM) at 37°C and 5% CO2. They were transiently transfected usually 24 h after plating by the standard calcium phosphate precipitation method. The VR1 cDNA, provided by Dr. David Julius (Caterina et al., 1997), was added for 6 h, after which the medium was changed. The typical amount of DNA used was 0.5–1 μg per 35-mm tissue culture dish. The channel-expressing plasmid was cotransfected with either the human CD8 lymphocyte antigen (Jurman et al., 1994) or an enhanced GFP (Clontech) as a surface marker. Eectrophysiological recordings took place 12–24 h after transfection. Cells cotransfected with CD8 antigen were identified visually using beads coated with an antibody against CD8 (Dynabeads M-450 CD8; Dynal).
Single-channel experiments were conducted with the oocyte expression system. The cDNAs were transcribed in vitro using T7 RNA polymerase (mMessage Machine; Ambion). The cRNA products were extracted with ethanol and suspended in RNase free water to a final concentration of 1 μg/μl. Defolliculated Xenopus laevis oocytes were prepared and injected with 20–30 ng cRNA, as previously described (Hui et al., 2003). Injected oocytes were incubated at 18°C in ND96 (96 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, pH 7.5) and used for electrophysiological recordings after 1–12 d.
Electrophysiology
Patch pipettes were fabricated from borosilicate glass (Sutter Instrument Co.), yielding a resistance of 0.5–2 MΩ for whole-cell recordings and 6–10 MΩ for single-channel recordings. Data was acquired using Axopatch 200B patch-clamp amplifiers (Axon Instruments, Inc.), low-pass filtered at 10 kHz, digitized at 25 kHz through a BNC-2090/MIO acquisition system (National Instruments), and recorded with custom designed software using Labview 5.1 (National Instruments). Single-channel recordings were performed in the outside-out configuration (Hamill et al., 1981). The presence of single VR1 channels in the patch was confirmed by application of high capsaicin (>1 μM) and block with capsazepine. All experiments were performed at room temperature (20–25°C).
For whole-cell recordings from HEK 293 cells, the standard bath solution contained (mM): 150 NaCl, 10 EGTA, 10 HEPES, pH 7.4 (adjusted with NaOH). No Ca2+ was included in order to avoid desensitization. The internal pipette solution contained (mM): 150 KCl, 5 EGTA, 10 HEPES, pH 7.4 (adjusted with KOH). For single-channel recordings from oocytes, the bath solutions contained 100 mM NaGluconate and 10 mM NaCl instead of 140 mM NaCl, and other components were the same as for HEK 293 cells. Solutions for pipettes were the same as the bath solution. For experiments involving high salt concentrations, 210 mM NaGluconate was used instead. Capsaicin and capsazepine were dissolved to a concentration of 0.1–10 μM and 10 μM, respectively, in the above recording solutions from a 1 mM ethanol-dissolved stock. The final ethanol was between 0.001–0.1%, which is expected to have a negligible effect on our experiments since it was present in all solutions at different pH and its concentration was below the limit at which it may mediate channel functions (Trevisani et al., 2002). Capsaicin was purchased from Fluka through Sigma-Aldrich and has a purity ≥98%, and capsazepine from Precision Biochemicals. Exchange of external solutions was performed using a gravity-driven perfusion system with manually controlled solenoid valves (ALA Scientific Instruments). The perfusion solutions were the same as the bath solutions except for appropriate agonists. For recordings from HEK 293 cells under low pH conditions, the solution also contained 50 μM amiloride as a blocker for the native ASIC channels.
Low pH solutions were buffered differently over different pH ranges. Buffers used were: pH 5.0–6.5 MES (2-(N-morpholino)ethanesulfonic acid); pH 7.0–7.5 HEPES; pH 8.0–8.5 Tris (N-tris [hydroxymethyl] methylglycine), all from Sigma-Aldrich. Solutions were titrated to their nominal pH at room temperature (20–25°C).
Data Analysis
Single-channel currents were analyzed mostly as previously described (Hui et al., 2003). In brief, bursts of openings were identified using a fixed closed criterion, τ crit = 100 ms, with little open activity (Po < 0.01%). The value was chosen so that it could sufficiently distinguish the longest closures (inter-burst gaps) from the others that were concentration independent, as verified by the duration histograms. Idealization of single-channel currents was performed using the segmental k-means algorithm (Juang and Rabiner, 1990), where the Viterbi detection (Forney, 1973) was iteratively applied to obtain a most likely dwell-time sequence and the empirical averaging was used to reestimate parameters of current amplitudes, noise variances, and transition probabilities. The method takes account of background noise explicitly, thereby allowing reliable detections of events at a relatively high bandwidth (10 kHz). The idealized dwell-time sequences were analyzed using a full maximum likelihood approach (Qin et al., 1996, 1997), with the imposition of a fixed dead-time of 40 μs, corresponding to the sampling duration and approximately equal to the rise time of the low-pass Gaussian filter applied (∼33 μs), to account for missed events. A fully connected but uncoupled model was employed, which ensures the complete use of all information available in the data. For each fitting, the conventional open and closed dwell-time distributions were calculated and superimposed with the experimental histograms for visual validation of fitting results. The complexity of the model was increased gradually until little improvement in the final likelihood values as well as a tight superimposition between the model distributions and the histograms. At the end of fitting, various properties of the model such as the time constants and areas of individual closed and open components were determined. The coupling between closed and open components was evaluated as the relative volumes of the corresponding two-dimensional (2D) exponentials in the 2D dwell-time distributions, which were calculated from the final model (Fredkin et al., 1985).
RESULTS
General Effects of pH on Capsaicin Activation
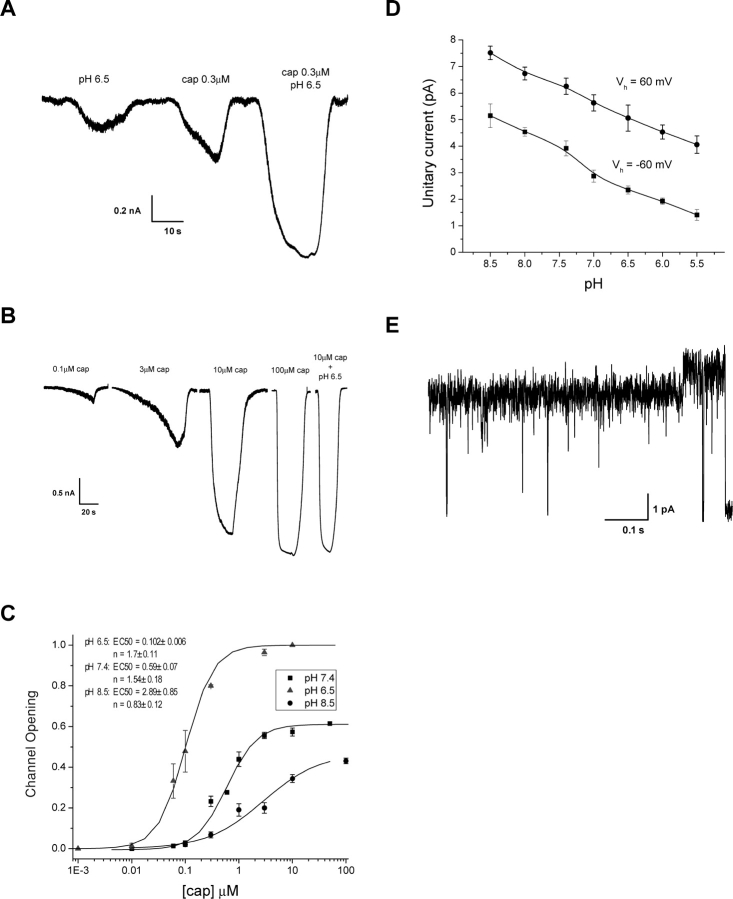
Both capsaicin and proton activate VR1. However, a mild acidification of extracellular solution is usually not sufficient to induce large activity. Instead, it potentiates the activity by other agonists such as capsaicin and heat. Fig. 1 A shows representative currents evoked by capsaicin and low pH alone as well as by the combination of both. The data was recorded from a HEK293 cell expressing VR1. As evident from the figure, lowering pH resulted in a substantial increase in the magnitude of the current at the same capsaicin concentration. The increase cannot be interpreted as a linear superimposition of the currents evoked by the two stimuli alone. Instead, it must arise as a collaborative effect between them. This is also evident from the change in the time course of the activation, which became more rapid at acidic pH.
Figure 1.
Potentiation of capsaicin-activated currents by low pH at the whole-cell level. (A) Application of capsaicin at low pH resulted in a current that was larger than the sum of the currents activated by pH and capsaicin at normal pH alone, suggesting a nonadditive effect between the two stimuli. The activation kinetics of the channel also became noticeably faster at low pH than at normal pH. (B) An example of capsaicin dose–response currents at pH 8.5. A combined stimulus of 10 μM capsaicin and pH 6.5 was applied to elicit the maximal current from the cell, which was used for normalization. (C) Dose–response curves of capsaicin at different pH. The responses were obtained from the actual currents after being normalized by the maximal attainable current for each cell and the unitary current amplitude at the corresponding pH. The solid lines represent the best fits with the Hill equation, corresponding to n H = 1.7 ± 0.11 and EC 50 = 0.1 ± 0.006 at pH 6.5, n H = 1.54 ± 0.18 and EC 50 = 0.59 ± 0.18 at pH 7.4, and n H = 0.83 ± 0.12 and EC 50 = 2.89 ± 0.85. Each point in the figure describes mean ± SEM from 5–8 recordings. (D) Effect of pH on the unitary current amplitude. Lowering pH reduced the currents, and the reduction followed about the same trend for both inward and outward currents. Data were averaged from 3–5 experiments. (E) An example of multiple conductance levels exhibited by the channel. Multiple conductance levels occurred more frequently at acidic pH than at normal pH. All recordings from HEK 293 cells heterogeneously expressing VR1 channels. Recordings made at V h = −60 mV except those indicated.
The effect on the open probability of the channel is more profound than that on the apparent currents. Single-channel recordings revealed that lowering pH reduces the conductance of the channel, consistent with the observation on the native capsaicin receptors in trigeminal neurons (Baumann and Martenson, 2000). Fig. 1 D shows the unitary current amplitude and its dependence on extracellular pH. The conductance decreased at both depolarizing and hyperpolarizing voltages. Interestingly, despite the rectification of amplitudes, the reduction followed approximately a linear trend with about the same slopes for both inward and outward currents, suggesting that it arises from a common mechanism, and that the proton interaction sites are probably outside the membrane electrical field.
Fig. 1 C shows the dose–response curves for capsaicin activation at different pH. The fraction of channel openings was obtained by appropriate normalization of the apparent currents, taking account of the differences in unitary current amplitudes at different pH and the maximal attainable currents from individual cells. The latter was determined by a simultaneous application of high capsaicin and low pH, as illustrated in Fig. 1 B. Single-channel measurements confirmed that the condition suffices to fully open the channel. From the dose–response curves, it is evident that pH not only affects the maximal attainable Po, but also the apparent cooperativity of capsaicin bindings as well as the potency of agonist. Lowering pH resulted in a leftward shift of the dose–responses, reduced capsaicin EC50, and increased the Hill coefficient and the maximal Po. At high pH, capsaicin became a much weaker and essentially a partial agonist.
It was observed previously that the channel could adopt multiple conductance levels, but they occurred rarely (Hui et al., 2003). At low pH, however, they appeared relatively more frequently. Fig. 1 E shows an example at pH 5.5. The change of the conductance was unlikely due to background ASIC channels since the recording had ∼50 μM amiloride in solution. Furthermore, the closures from the full level reached about the same amplitude as those from the partial level, a highly unlikely event if it arose from two different channels. Overall, the partial level appeared to be the dominant conductance of the channel at low pH. The difference between the two levels was small. Moreover, the change of the conductance didn't seem to correlate with any significant change in channel kinetics, as evident from Fig. 1 E.
Since our focus was on the kinetic effects of low pH, we did not pursue any further study on the occurrences of multiple conductance levels. Instead, we treated them as the same type of openings in our analysis. This is likely to have little impact on our results since the gating kinetics appear to be independent of conductance changes.
Potentiation of Capsaicin Binding
The apparent increase in capsaicin activity at low pH can be attributed to changes in either capsaicin binding or channel gating. At the macroscopic level, these individual steps are intrinsically coupled. An increase in either of them can account for many aspects of our observations, including the increase in maximal Po, the shift of dose–response curves, and the acceleration of activations. To determine the specific effects, we resorted to single-channel experiments and studied the single-channel currents in detail.
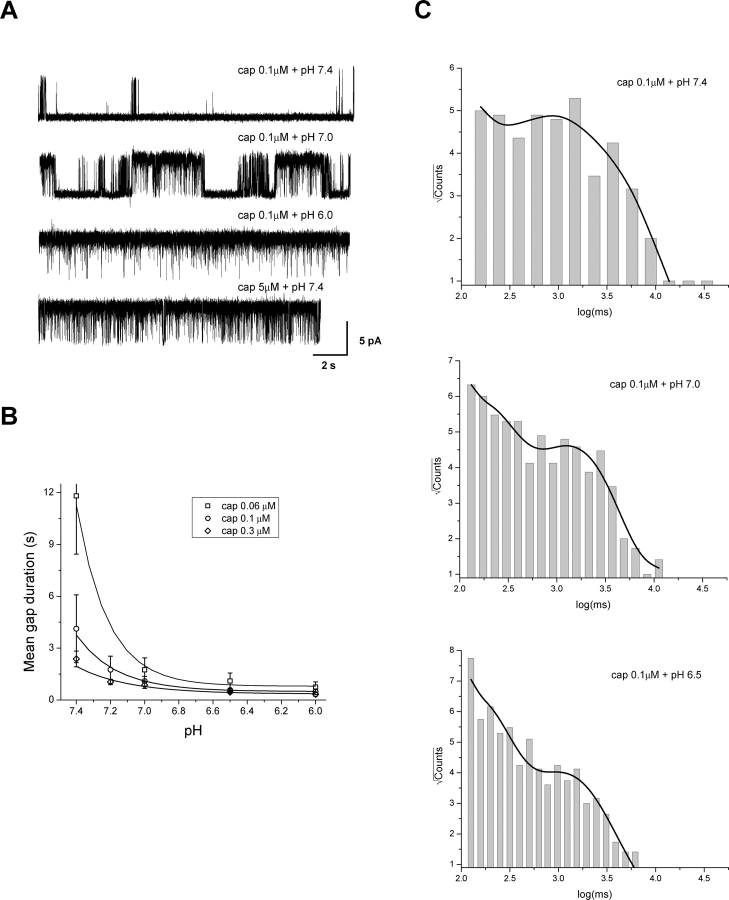
An important clue pertaining to capsaicin binding is its relatively low affinity. Our previous study of capsaicin activation at normal pH suggests that the openings of the channel tend to appear in bursts at low to medium capsaicin concentrations (Hui et al., 2003). The long closures (gaps) that separate the bursts are strongly dependent on the capsaicin concentration. As capsaicin increases, they are significantly shortened, and at high capsaicin level, they virtually disappear leaving bursts indistinguishable. These results demonstrate that the interburst gaps are directly associated with capsaicin binding. They occur because of the absence of capsaicin binding rather than a slow opening rate that prevents the channel from being open.
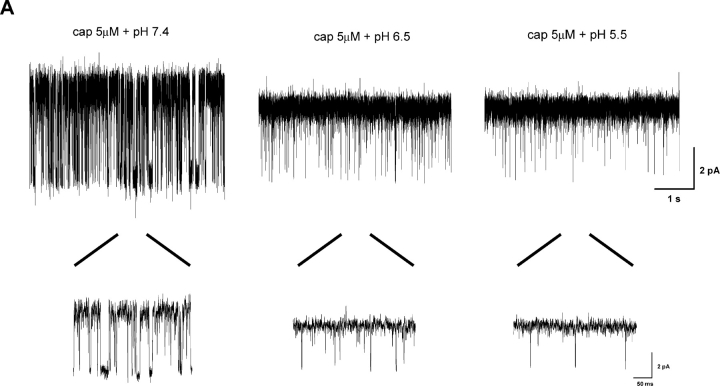
With the interburst gaps as an indicator, we determined the influences of low pH on capsaicin binding. Fig. 2 A shows single-channel recordings from a patch perfused with a constant capsaicin concentration but variable pH. A relatively low capsaicin concentration (0.1 μM) was used so as to maximize the occurrences of long gaps. The activity at the normal pH indeed consisted of short bursts of openings separated by long closures with durations on the order of seconds. As pH was lowered, the openings still appeared in bursts, but the gaps became considerably shorter. At pH 6, the gaps became undetectable, reminiscent of the effect as capsaicin is increased. With capsaicin at normal pH, the gaps disappear at concentrations above 1 μM, as illustrated in the bottom trace of Fig. 2 A.
Figure 2.
Effect of low pH on gap durations. (A) Single-channel currents activated by capsaicin at different pH conditions. The openings of the channel clustered into bursts separated by long closures (gaps) at normal pH. Lowering pH reduced the durations of these gaps. (B) Gap durations plotted against pH for several capsaicin concentrations. Data were averaged from 6–25 experiments. The solid lines refer to fits by exponential kinetics, t gap = t 0 × 10n × pH, assuming the existence of n identical protonation sites responsible for the reduction of gap durations. The fitting gave rise to n = 2.41, 1.89, and 1.45 at 0.06, 0.1, and 0.3 μM capsaicin, respectively. (C) Distributions of individual gap durations at constant capsaicin but different pH. The distributions were fitted with three exponentials for pH 7.4 and two for pH 7.0 and 6.5, respectively. The time constants (in ms) and relative proportions of the components were: τ1 = 109 (∼2%), τ2 = 640 (∼19%), and τ3 = 2,610 (∼79%) for pH 7.4; τ1 = 130 (12%) and τ2 = 1,328 (88%) for pH 7.0; τ1 = 108 (∼20%) and τ2 = 1,059 (∼80%) for pH 6.5. Data were recorded from outside-out patches containing single VR1 channels at V h = 60 mV.
Fig. 2 B quantifies the reduction of gap durations as a function of pH. A rapid decay was observed at the initial phase of acidification. With 0.1 μM capsaicin, the gaps were on the order of 3.5 s at pH 7.4. Lowering pH by less than a half unit reduced them to <1 s (∼500 ms). Since the Po of the channel is largely determined by the long closures, such a change is expected to have a profound impact on the increase of channel openings. The reduction of the gap durations appeared to reach a steady-state between pH 6.5–6, where a further acidification resulted in little changes. However, this saturation could also be an artifact due to the threshold used in gap detections. It is likely that the gap durations were further decreased, but they became shorter than the detection threshold and therefore indistinguishable from the closures within bursts.
Assuming that the pH effect was due to protonation of the channel, the reduction of the gap durations would be determined by the protonation kinetics. Suppose there were n identical protonation sites, the gap durations would be inversely proportional to [H+]n, or equivalently, 10− n × pH. The rate of the decay of the gap durations therefore provided an estimate on the number of the sites that were necessarily to be protonated in order for the channel to open. Indeed, the reduction of the gap durations could be well fitted into an exponential decay, as shown in Fig. 2 B. Interestingly, the number of the protonation sites varied with capsaicin concentration (n = 2.41, 1.89 and 1.45 for [cap] = 0.06, 0.1, and 0.3 μM, respectively). The higher the capsaicin concentration was, the less number of protonation sites was required, suggesting that capsaicin binding and protonation of the channel are probably energetically additive.
The protonation kinetics and the existence of multiple protonation sites were further substantiated by the distributions of gap durations, as shown in Fig. 2 C. Although the plots are somewhat rugged due to the lack of a large number of events, the presence of multiple peaks is evident at all pH conditions. None of the histograms could be satisfactorily fitted by a single exponential. Below pH 7.0, at least two components were well resolvable; while at pH 7.4, there appeared to be an additional one with a long time constant but low occupancy. As pH was lowered, the short components became more favored at the expense of the long ones, leading to a shift of the overall distribution toward the left. These observations suggest that the gap durations may have a complicated composition, involving as many as three and possibly more components that are sensitive to pH.
Cooperativity between Capsaicin Binding and Protonation
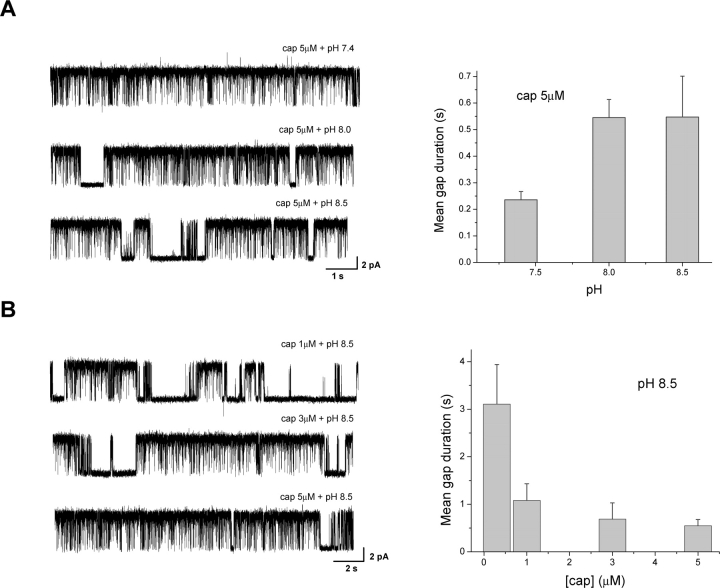
The profound effect of low pH on gap durations of capsaicin activation raises the question whether the protonation of the channel is required for capsaicin binding, or alternatively, capsaicin and proton interact cooperatively to promote channel opening. To address the issue, we studied the activation of the channel at high pH conditions. If protonation was a necessary step for channel opening, one would expect to see a limited channel activity at high pH, and that the reduced activity could only be recovered by lowering pH.
Fig. 3 A shows the effect on single-channel currents as pH was increased over the alkaline range while capsaicin held at constant. With 5 μM capsaicin, the channel activity was saturated at normal pH. The gaps rarely occurred, and their durations were short. As pH was increased, more gaps became visible, and their durations were elongated. The gating of the channel was also noticeably different, involving more spiky short openings and less long ones (see below). The effect of high pH on the gap durations appeared to reach a saturation point around pH 8. A further increase to pH 8.5 resulted in no further elongation of the gaps, which may indicate that the channel has been fully deprotonated.
Figure 3.
Cooperative interactions between capsaicin binding and protonation. (A) Single-channel currents and mean gap durations at high capsaicin (5 μM) and alkaline pH. Capsaicin at 5 μM and normal pH suffices to saturate the channel activity, leaving virtually no detectable gaps. Increasing pH while maintaining the same capsaicin concentration resulted in the occurrences of long gaps. (B) Single-channel currents and mean gap durations at high pH (8.5) and different capsaicin concentrations. The gaps that appeared at high pH could be reduced by further increasing capsaicin concentrations. Results were averaged from 10–15 experiments for A and 4–14 for B.
While the activity was appreciably reduced at high pH, it remained considerably high, indicating that protonation was not a rate-limiting step in the activation of the channel and that capsaicin alone can open the channel. As a further corroboration, we examined whether the reduced activity at high pH could be recovered by further increasing capsaicin concentration. Fig. 3 B shows the results measured at high pH (8.5) but different capsaicin concentrations. Indeed, the long gaps present at high pH could be reduced by increasing the capsaicin concentration, in a way similar to that observed at normal pH. This argues that the gaps are necessarily determined by both capsaicin and pH in an energetically cooperative manner, where the effect from one can be compensated by the effect from the other.
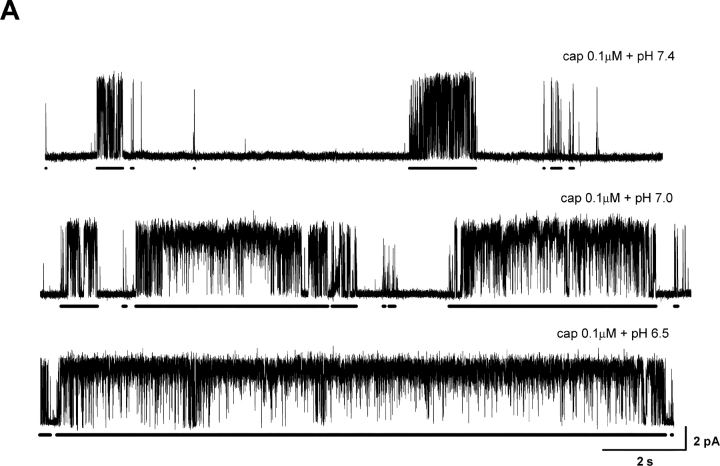
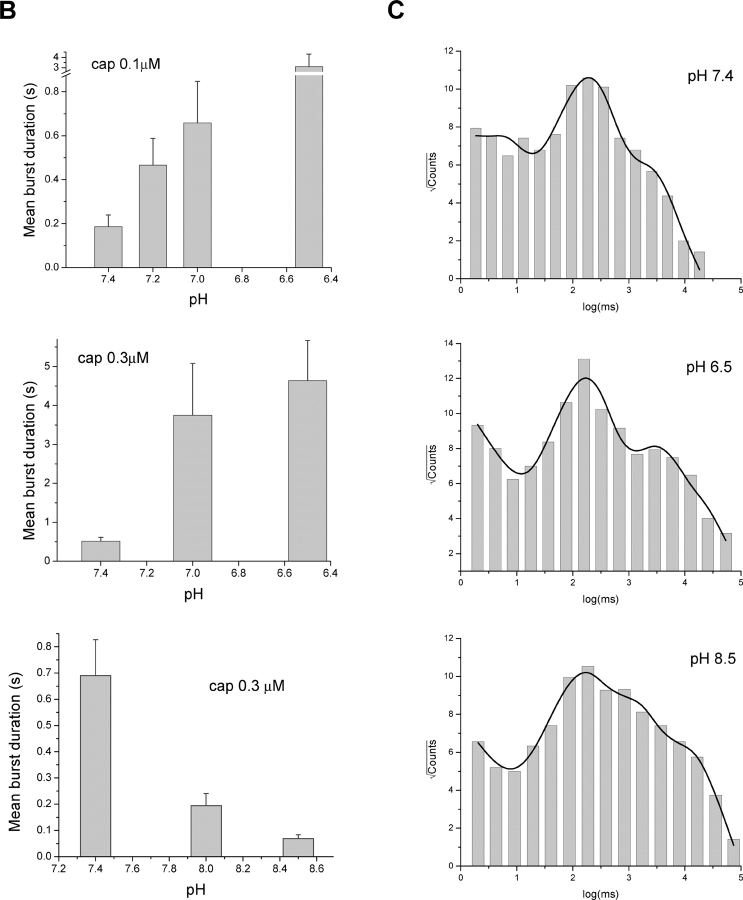
Effect of pH on Burst Durations
The burst durations were affected by pH as dramatically as the gap durations. Fig. 4 A illustrates an example of the detected bursts at 0.1 μM capsaicin and different pH. The bursts are indicated by the bars underneath the traces. On average, the burst durations increased by more than threefold over a half unit acidification from the normal pH, as shown in Fig. 4 B at 0.1 and 0.3 μM capsaicin. The effect was also evident at high pH. With 0.3 μM capsaicin, an increase of pH by a half unit reduced the burst durations to be approximately one-third as long as in the normal condition, which is in a good agreement with the rate of the change when pH was lowered.
Figure 4.
Effect of low pH on burst durations. (A) Bursts of single-channel openings elicited at low capsaicin concentration. Bars below traces indicate detected bursts with the criteria as described in the text. Currents are shown with filtering at 1 kHz. (B) Mean burst durations plotted against pH. Acidic pH prolonged the bursts (top two panels) while alkaline pH shortened them (bottom). Results were averaged from 5–19 experiments, all containing single VR1 channels. (C) Distributions of individual burst durations at representative pH conditions. Bursts from multiple capsaicin concentrations were merged at each pH to minimize the statistical variations. The concentrations used in each pH include (μM): 0.03, 0.06, 0.1, 0.3, and 0.6 for pH 7.4, 0, 0.03, 0.06, 0.1, 0.3, 0.6 for pH 6.5, and 1, 3, 5 for pH 8.5. The solid lines represent fits by sums of exponentials. Three components were used for pH 7.4 and four for pH 6.5 and 8.5, respectively. The resultant time constants (in ms) and component populations are: τ1 = 4.91 (∼0.25%), τ2 = 199 (∼21%), τ3 = 2148 (∼78%) at pH 7.4; τ1 = 2.94 (0.03%), τ2 = 182 (4%), τ3 = 2869 (∼27%), τ4 = 16852 (∼69%) at pH 6.5; τ1 = 2.62 (∼0.01%), τ2 = 139 (∼2%), τ3 = 1136 (∼14%), τ4 = 11425 (∼84%) at pH 8.5.
The populations of the bursts were not homogeneous. Even at the same pH, multiple types of bursts could be discerned, which differed in durations and intraburst openings and closings. This raises the question whether the apparent increase of the averaged burst durations was due to changes in the durations of individual components or their relative populations. To distinguish them, we examined the burst distributions and their pH dependences, as shown in Fig. 4 C. For each pH, the bursts from multiple capsaicin concentrations were combined in order to obtain a reasonable number of events. Such a maneuver should not affect our analysis since capsaicin is expected to alter only the occupancy of the bursts rather than their durations (Hui et al., 2003). Evident from the figure is that the distributions involve multiple peaks, representing different burst components. Fitting the distributions with exponentials revealed three components at pH 7.4 and an additional long one at pH 6.5 and 8.5, as described in the figure legend. The long component appeared to be associated with high channel activity. It was absent at pH 7.4 presumably because the data included there were all from low capsaicin experiments. Despite the absence of the long component at pH 7.4, the other three exhibited comparable time constants across pH, which were on the order of 3–5 ms, ∼200 ms and 1–2 s, respectively. The long components present at pH 6.5 and 8.5 also had a similar time constant. These results suggest that the durations of the individual burst components are relatively invariant to pH. The apparent increase in the averaged duration is most likely due to the changes in their populations. The observation is consistent with our analysis of capsaicin activation, where low capsaicin gives rise to predominantly short bursts while high capsaicin favors long ones (Hui et al., 2003). Lowering pH has a similar effect to alter the burst distributions as increasing capsaicin.
Potentiation of Channel Gating
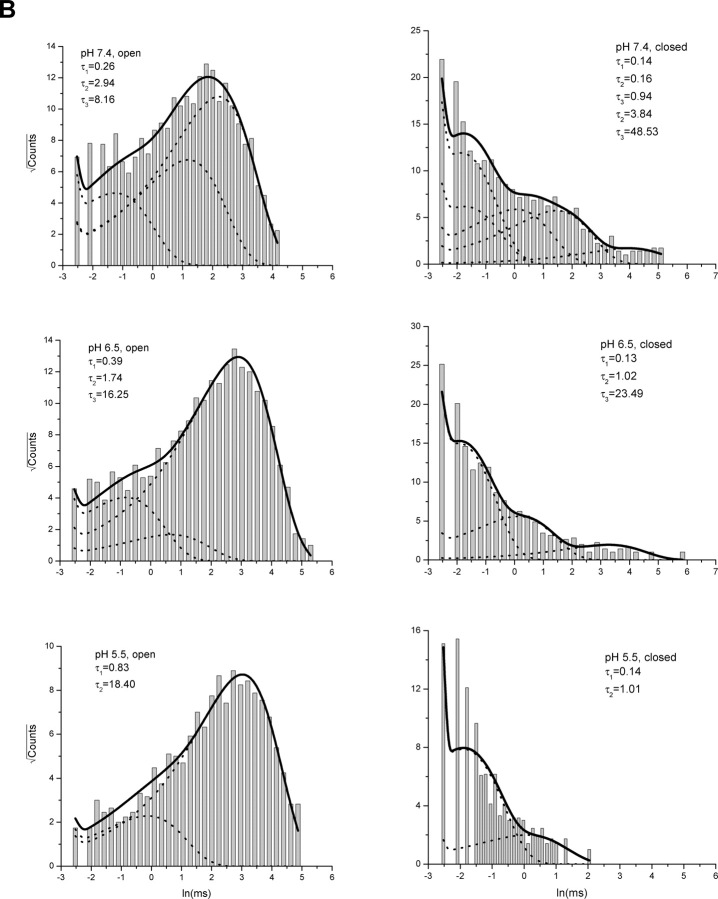
The dose–response curves in Fig. 1 showed that lowering pH increased the maximal attainable current at the whole-cell level. The increase occurred even after the capsaicin concentration was saturated, suggesting that pH also affects the gating of the channel. To understand the underlying mechanism, we studied the single-channel activity at high capsaicin concentrations. Fig. 5 A shows an example of recordings from an outside-out patch perfused with 5 μM capsaicin at different pH. Noticeably, although Po was high in all conditions, the gating of the channel appeared differently. At normal pH, the channel closed frequently and the durations of the closures were relatively long. At low pH, the openings appeared more stable and the closures were briefer. These changes were also evident from the dwell-time distributions, as shown in Fig. 5 B. The closed dwell-time histogram at normal pH exhibited a more significant portion of long closures, while the open histogram contained an excessive number of short openings that were nearly absent at low pH.
Figure 5.
Gating of VR1 at high capsaicin and low pH. (A) Exemplar single-channel currents from an outside-out patch perfused with saturated capsaicin (5μM) at different pH (7.4, 6.5, 5.5). Lowering pH reduced the unitary currents but increased the channel Po. The insets underneath the traces show enlarged views of openings and closings. Data are shown with low-pass filtering at 1 kHz. (B) Dwell-time histograms from the same experiment. The data were low-pass filtered to 10 kHz priori to analysis and idealized using SKM. The resultant dwell-time sequences were subject to a dead time of 40 μs. Superimposed with the histograms are the distributions predicted from the results of maximum likelihood fitting. The dotted lines show the distributions of individual components. Five closed and three open states were found necessary for an adequate fit at pH 7.4, three closed and three open at pH 6.5, and two closed and two open at pH 5.5, respectively. The time constants of the components are indicated in the plots.
Maximum-likelihood fitting of the idealized dwell-times revealed that the gating of the channel involves multiple conformational states, ∼3–5 for closures and 3–4 for openings, in general. Fig. 5 B shows an example of the distributions of these components as superimposed on the experimental histograms. For this particular patch, five closed and three open states were necessary for a satisfactory fit at pH 7.4. All three open components were well populated, and so were the four short closed ones. The long closures were less abundant but well resolvable. At pH 6.5, three open components remained, but the two short ones became less significant, making the long one more dominant. The closed distributions changed too, with the short closures becoming more abundant. Such a trend of favoring long openings and short closures continued with further acidification. At pH 5.5, two closed and two open states became sufficient to describe the gating of the channel.
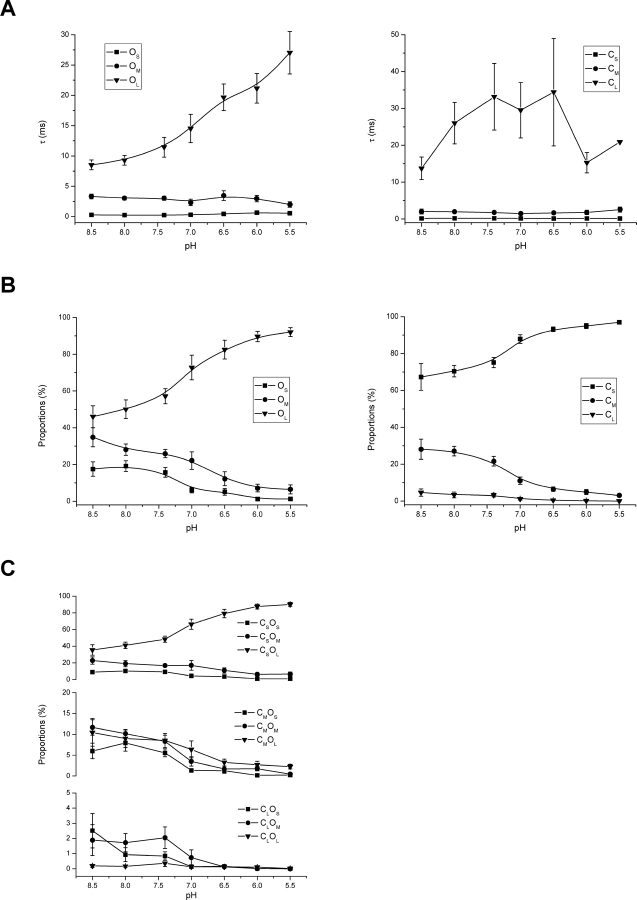
The analysis of 10 other experiments confirmed the above observations. Fig. 6, A and B , plot the time constants and proportions of the individual closed and open components against pH. The number of the components was not always the same across experiments, but they generally fell into three ranges, namely, short, medium, and long. The short closures had time constants on the order <1 ms, the medium ones between 1 and 10 ms, mostly ∼2–5 ms, and the long ones on the order of tens or hundreds of ms. The open components could be categorized similarly, with the short openings <1 ms, the medium ones between 1 and 10 ms, typically ∼4 ms, and the long ones around or above 10 ms. From the figures it is observed that lowering pH had little effect on the time constants of the two short open components but increased that of the long one. It also reduced the populations of the short and medium components while increasing that of the long one. Similarly, lowering pH didn't alter the time constants of the short and medium closures, but increased the occupancy of the short one and reduced that of the long and medium ones. The long closures were relatively insignificant, and their time constants fluctuated. The fluctuation arose partly because of their infrequent occurrences and partly because they were sometimes deliberately excluded from analysis in some experiments in order to focus on the gating kinetics. It is interesting to notice that while pH altered many aspects of the gating of the channel, all these changes occurred in a consistent way to favorably increase the gating efficacy.
Figure 6.
pH dependences of dwell-time components at high capsaicin. (A) Time constants of the components plotted against pH. While the short and medium openings were relatively invariant to pH changes, the long openings exhibited a monotonic increase, which was nearly three times at pH 5.5 as long as at pH 8.5. The closed time constants, except the long one, were also resistant to pH changes. The fluctuation in the long closed time constant was partly due to its rare occurrences at the tested capsaicin concentrations and partly due to the deliberate exclusion of long gaps from fitting. (B) Proportions of the open and closed components, which were determined from the relative areas in the dwell-time distributions. As pH was lowered, the long openings became favored at the expense of the short and medium ones. Similarly, the population of the short closures increased as opposed to that of the medium ones. The population of the long closures was insignificant throughout the entire pH range. At pH 5.5, the long openings and short closures became nearly the exclusive components involved in the gating. (C) Coupling between closed and open components, as measured from the relative volume of the exponential pairs in the 2D dwell-time distributions. The most significant coupling occurred between short closures and long openings. At normal or high pH, strong couplings were present between short closures and medium or short openings as well as medium closures and all three types of openings. Lowering pH further enhanced the coupling between short closures and long openings. Results were averaged from 11 single-channel outside-out patches. In all experiments, capsaicin at 5 μM was used to maximize the gating activity. The fitting of individual experiments sometimes involved a different number of components. For the purpose of comparison, these components were grouped into three categories, short, medium, and long, where the short ones had time constants below 1 ms, the medium ones between 1–10 ms, and the long ones above 10 ms. Components that belonged to the same category within each experiment were averaged according to their proportions.
The simultaneous increase in the occupancy of short closures and long openings implied that they might be mechanistically related. The correlation analysis between adjacent closed and open dwell-times revealed that they were indeed most strongly coupled, as shown in Fig. 6 C. This was the case throughout the entire pH range. Lowering pH enhanced the occurrences of the pair, consistent with the component analysis results. At normal or alkaline pH, strong couplings also existed between long openings (O L) and medium closures (C M), medium openings (O M) and short (C S) and medium (C M) closures, and short openings (O S) and short (C S) and medium (C M) closures. Noticeably, while the population of the short closures increased with acidification, the medium and short openings adjacent to them decreased, suggesting that the short closures are not a single gateway state to enter openings. More likely, all three types of openings are probably accessible from any type of closures.
Specificity of the Elongation of Long Openings to Low pH
The elongation of the long open component at low pH is intriguing as it underlines the differences among the three types of openings and suggests that pH may affect the stability of the open conformations of the channel. The mechanism of this elongation is unclear. To better understand its origin and specificity to pH, we examined the gating of the channel at other conditions.
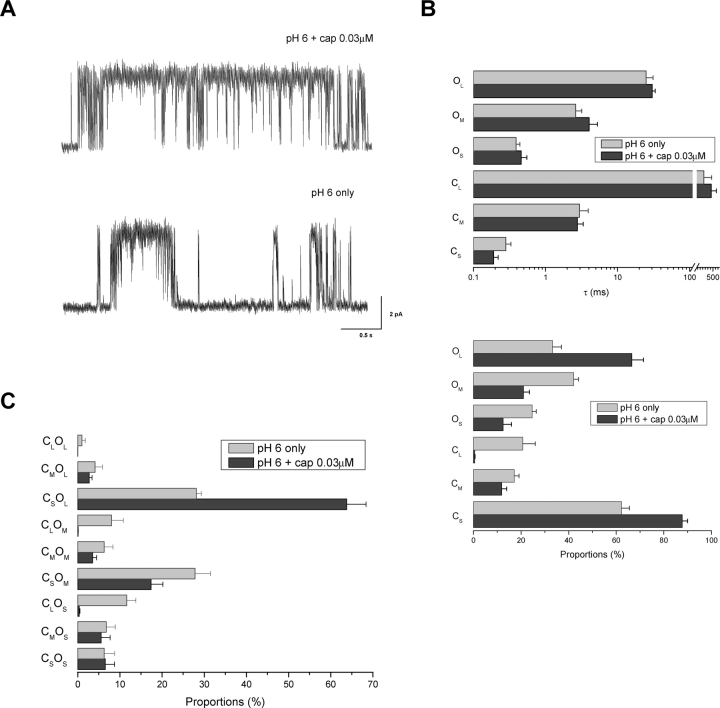
We first investigated whether the elongation of the openings requires the presence of both high capsaicin and low pH, a condition used in the previous experiments. Instead of a saturated capsaicin concentration, we examined the gating of the channel at relatively low capsaicin with low pH. Fig. 7 A (top trace) shows a representative stretch of recordings at pH 6.0 in conjunction with 0.03 μM capsaicin. The activity of the channel under such a condition had a similar appearance to that at high capsaicin and low pH. The most noticeable difference is the more frequent occurrences of long gaps as well as some relatively short bursts. Fig. 7, B and C (dark gray), show the component analysis results. As before, the gating involved mostly short closures and long openings. But unlike at high capsaicin concentrations, the medium closures and the short and medium openings became more significantly populated. This change was likely due to the occurrences of the short bursts. More importantly, the openings of the channel still consisted of three components, and their time constants (0.34, 5.36, and 26.54 ms) remained quite similar to those observed at high capsaicin and low pH. Therefore, reducing capsaicin doesn't appear to alter the elongation of the long open component of the channel.
Figure 7.
Gating at low pH and low capsaicin concentrations. (A) Representative single-channel traces recorded at pH 6.0 with 0.03 and 0 μM capsaicin, respectively. Data were low-pass filtered at 1 kHz for display. (B) Time constants and proportions of components determined from maximum likelihood analysis. Results were averaged from three patches for pH 6.0 only and 9 for pH 6.0 plus 0.03 μM capsaicin. (C) Coupling between closed and open components, obtained from those same experiments.
The single-channel activity at pH 6.0 and 0.03 μM capsaicin remained considerably high. To see whether the elongation of the long open component pertains to a full activity of the channel, we examined the gating at pH 6.0 with no capsaicin. As illustrated in Fig. 7 A (bottom trace), the activity of the channel at this condition appeared somewhat similar to that at low capsaicin and normal pH, comprising bursts of openings separated by long closures. Fig. 7, B and C (light gray), show the analysis results averaged from nine experiments. Many of the components had time constants similar to those previously observed at low pH and high capsaicin. These include the short closures (0.28 ms), the medium closures (2.96 ms), and all three types of openings (0.39, 2.61, 24.96 ms). In particular, the long openings had a time constant that was nearly as doubled as at the normal pH. It was slightly shorter than at saturated capsaicin and low pH, but the differences were within the range of standard errors. Together, these results suggest that the elongation of the long open component is most likely specific to low pH and independent of capsaicin concentrations.
With capsaicin binding, it was observed to be mutually cooperative with protonation, i.e., increasing pH tends to elongate the gaps, but the elongation can be undone by further increasing capsaicin concentration. The results described above suggest that this is not the case for the elongation of the long openings. Increasing capsaicin at the normal pH has little effect on the time constants of all three open components (Hui et al., 2003). Therefore, different mechanisms are probably underlying the pH dependences of the long openings and the interburst gap durations.
DISCUSSION
We investigated the potentiation of capsaicin activation of the VR1 channel by low extracellular pH. To unmask the underlying mechanisms, we exploited the rich characteristics of single-channel kinetics in conjunction with whole-cell measurements. Our results suggest that pH has several effects on the function of the channel. These include a decrease in channel conductance, an increase in capsaicin binding affinity, an increase in gating efficacy, as well as an improvement in stability of channel openings. Our analysis also reveals that the protonation of the channel interacts with capsaicin binding in an allosteric manner. Capsaicin can bind to either protonated or deprotonated channel, but the binding to the protonated channel is energetically facilitated. Similarly, either protonated or deprotonated channel is capable of opening. The protonation, however, enhances the gating efficacy by promoting the occurrences of long openings and short closures. While the conductance is reduced at low pH, the effect is outweighed by the changes on capsaicin binding and channel gating, thereby resulting in an overall increase in the apparent macroscopic current (Baumann and Martenson, 2000).
Given the role of VR1 as a pain receptor, our results highlight the importance of protons as a mediator for pain sensation. The channel must be partially protonated even at the normal physiological condition. A shift of pH in either acidic or alkaline direction can have an impact on channel activity, suggesting that the extracellular pH may play both an analgesic and an algesic role in peripheral pain sensation. Our data also suggests that capsaicin alone is a partial agonist for the VR1 receptor. Its partial agonist nature becomes particularly evident at hyperpolarized voltages with high pH. A full activation of the channel requires a combined application of both capsaicin and low pH.
One previous study on proton potentiation of capsaicin responses of VR1 at the whole-cell level suggested a mere shift of the capsaicin dose–response curve (Tominaga et al., 1998). Our data showed changes in both the EC50 of capsaicin and the Hill coefficient of the curve. The difference may be partly attributed to the different way in which the currents were normalized. Besides to the maximal response, we also normalized the currents by the unitary current amplitude to take account of the effect of protons on the channel conductance. This also explains the open probability in response to capsaicin at low pH exceeds the maximal open probability that is achievable at normal pH, even though the apparent currents are of the same size (Fig. 1 B). Our results are in agreement with those observed on the hVR1 expressed in Xenopus oocytes (Hayes et al., 2000), which also show changes in the maximal currents, the capsaicin EC50 and the slope of the dose–response curve. But they contrast with an early report that protons reduce the Hill slope of the capsaicin concentration-response relationship and do not alter either the single-channel conductance or the maximal whole-cell current. The reasons for this difference are unclear, though it may be related to the differences in cellular environments between the native and cloned receptors. To this extent, it is also interesting to note that the capsaicin receptors in cultured neonatal rat dorsal root ganglion neurons are insensitive to low pH (Oh et al., 1996). On the other hand, single-channel studies of the native capsaicin receptors in trigeminal ganglion neurons show that low pH effects on both unitary conductance and Po (Baumann and Martenson, 2000), suggesting that the cellular environment may not be the only reason for the difference.
Binding Effect
We studied the binding effect of pH based on the sensitivity of long interburst gaps to both capsaicin and protons. The effect is profound; shifting pH by half a unit resulted in a 2–6-fold change in gap durations. It should be emphasized that the effect remains conservatively estimated. There are several technical limitations that may cause this bias. One is the use of a fixed threshold for detection of the gaps. Some of the gaps are inevitably shorter than the threshold and become indistinguishable from the other closures. Exclusion of these short gaps will result in an overestimate of gap durations. The bias becomes more significant at low pH and high capsaicin where the majority of the gaps are short. Another bias arises from the inherent low occurrence of the long closures. In the simplest case where the gaps result from a single binding, the durations of these gaps would follow an exponential distribution. The longer the durations, the lower the frequency of their occurrences. Therefore, given a limited recording time, the long gaps are more likely to be missed, leading to an underestimate of the mean duration of the gaps, especially at high pH where the long gaps tend to be the majority. In either case, the bias won't affect our conclusion, but only the quantitative magnitude of the effect.
The binding effect of pH can be ascribed to a change in either association or disassociation rates of capsaicin. At this point it is uncertain which rates are altered by pH. We had attempted to separate the two by, for example, examining the changes of burst durations. In general, the burst durations are taken as a measure of ligand unbinding rates. By studying the distributions of individual bursts over a large range of capsaicin concentrations and pH, we found that the burst durations are relatively insensitive to pH. This appears to support the idea that pH doesn't affect the disassociation rates of capsaicin. Unfortunately, the argument was compromised by the complexity of the underlying gating mechanism of the channel. There is evidence suggesting that both partial and full level of capsaicin bindings can activate the channel, and each level of binding may lead to a burst of openings (Hui et al., 2003). Given such a scheme, the burst durations would depend on several parameters, including not only the capsaicin disassociation rates, but also the closing rates leaving the bursts as well as the association rates for a higher level of capsaicin binding. At low capsaicin concentrations, the association rates are slow, and the burst durations are mainly determined by the capsaicin dissociation rates and the rates leaving bursts, depending on whichever is more rapid. Therefore, it is possible that the rates leaving bursts are the rate-limiting steps. This would explain the independence of the apparent burst durations on pH, though capsaicin dissociation rates may have changed.
Despite the lack of conclusive evidences, our data are most consistent with a change in capsaicin binding rates. At high capsaicin concentrations, the closures are only on the order of milliseconds. This suggests that the disassociation rate of capsaicin from the fully bound receptor is on the order of hundreds per second. The dissociation rates at low levels of bindings are essentially on the same order if the ligand disassociates independently. At very low capsaicin concentrations (e.g., 0.03 μM), we observed long gaps on the order of many seconds. Given the relatively fast dissociation rates, such long gaps arise most likely from the slow association of capsaicin. As a result, the significant reduction on the gap durations at low pH must result from an increase in the capsaicin binding rates. Another line of support for this argument follows from the observation that both capsaicin and protons are effectors to promote channel openings. Low pH alone can directly activate the channel, suggesting that proton bindings can provide a sufficient amount of activation energy to open the channel. At a mildly acidic pH, proton bindings may not suffice to open the channel, but may substantially lower the energy barrier, making capsaicin more ready to activate the channel. Essential to this rationale is the assumption that the activation pathway by pH is correlated to that by capsaicin, which remains uncertain.
Gating Effect
The primary effect of pH on the gating of the channel arises from the increase of the population of long openings and the reduction of medium and long closures. Our previous study of capsaicin activation at normal pH showed that the gating of the channel involves multiple components (Hui et al., 2003). Even at saturated capsaicin concentrations, there remain at least three closed and three open states. Increasing capsaicin favors the occurrences of the long openings and short closures, but doesn't lead to a complete inhibition of the others. In particular, the significant occurrences of the medium and long closures restrict the attainable channel Po. Here we see that lowering pH is able to diminish these components and keep the channel fully open. This suggests that capsaicin and proton bindings have an additive effect on the gating of the channel. Capsaicin binding alone doesn't provide sufficient energy to maintain the channel in its most stable open conformation. As a consequence, the gating is interrupted frequently by long closures and short openings. Further binding of protons contributes additional energy into the channel in a cooperative way. As a result, the long open state becomes favored while the short ones inhibited.
A secondary gating effect we observed at low pH is the elongation of openings. This effect appears to be specific to the long open component. Assuming a Markovian gating scheme, this would imply that protons could bind directly to the open channel. It is conceivable that some protonation sites may become available only when the channel is in its open conformations, and the protonation of these sites makes the conformations more stable. Alternatively, the elongated long open state may represent a novel gating pathway. The entry to this pathway is inhibited at normal pH, but activated at low pH. This doesn't explain why we observed only a single long open state and not the one that occurs at the normal pH. It is possible that the apparent long open state may represent an aggregate of the two. If their time constants were too close to each other, they would be indistinguishable. In either case, further experiments are needed to understand the underlying molecular mechanisms.
Given that the long openings are mostly coupled to the short closures, we were concerned whether the elongation of the openings was due to inaccurate detection of the short closures. Because of limited time resolution of the system, there are always a certain number of brief events that go undetected during the idealization of the data. At low pH, the channel conductance is reduced while the open noise increased, leading to a decrease in the signal-to-noise ratio. To resolve the issue, experiments were repeated using solutions with high salt concentrations. The motivation was to increase the channel conductance and thereby improve the signal-to-noise ratio. With 210 mM Na+, the single-channel current amplitude at pH 5.5 became comparable to that at normal pH with 150 mM Na+. The results from these experiments were virtually the same, confirming that the elongation of the long open component is unlikely due to inaccurate data idealizations. This is also consistent with the observation that pH has a differential effect on the three open components. If the elongation were due to inappropriate detection, the same effect would be expected on the other two states. But the time constants of those components stay invariant to pH changes.
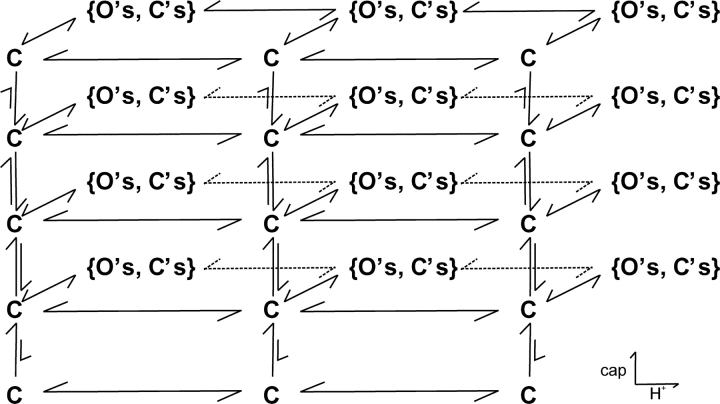
A Possible Model
Based on the cooperative nature of the interaction between capsaicin binding and protonation, we propose the model in Fig. 8 as a possible mechanism for the potentiation of pH on capsaicin activation. The model is extended from the one at normal pH. The vertical branches represent capsaicin bindings, and the horizontal ones describe protonation. Each binding of capsaicin or proton leads to a burst of openings as designated by the brackets. While the exact nature of these burst branches remains unknown, some of the features can be postulated. They are likely to consist of multiple closed and open states, probably involving at least the short and medium closures and all three types of openings since they appeared over all capsaicin and pH conditions. The connectivity between these states must be arranged in a way to favor the long openings and short closures as capsaicin and protons are progressive bound.
Figure 8.
A model for potentiation of capsaicin activation by low pH. The vertical branches represent capsaicin activations, and the horizontal branches correspond to protonation of the channel. Each level of capsaicin and/or proton binding leads to a burst of openings as designated in the brackets. High capsaicin and low pH drive the equilibrium of the system toward the upper-right part of the model, which tends to have a higher Po and longer opening durations. The exact nature of the branches as well as the number of capsaicin binding and protonation steps remains uncertain.
The model explains the major features of our data. The channel is partially protonated at normal pH because it is mostly populated at the central vertical branches. Capsaicin is a partial agonist at high alkaline pH because the equilibrium is favored on the left vertical branches where the bursts have limited openings. The binding effect of low pH results from the shift of the equilibriums toward the right vertical branches, which have a higher capsaicin binding affinity. The gating effect of pH can be explained similarly, i.e., low pH shifts the equilibrium toward the top right, where the bursts are mostly composed of long openings and short closures.
Structural Implications
The channel probably possesses multiple and nonidentical protonation sites. The sites that modulate capsaicin binding and channel gating are likely to be different. The binding effect occurred with either a basic or an acidic change around pH 7.4. The gating effect, however, was observed mainly at acidic pH. The elongation of the long open component didn't saturate until pH 5.5. In addition to these kinetic effects, low pH also reduced the conductance of the channel, suggesting that there may be additional protonation sites along the permeation pore of the channel.
The molecular basis of these protonation sites remains uncertain. Mutagenesis experiments have identified a number of acidic residues on the extracellular pore loops that affect the function of the channel, some of which appear to have a discriminative effect on pH potentiation versus pH activation (Jordt et al., 2000; Welch et al., 2000). This study provides an important basis for future experiments to determine the specific roles of these residues and correlate them to the effects that have been observed at the single-channel level.
Acknowledgments
This work was supported by grants R01-RR11114 and R01-GM65994 from National Institutes of Health.
Lawrence G. Palmer served as editor.
Footnotes
Abbreviations used in this paper: SIC, stretch-inhibitable channel; VR1, capsaicin (vanilloid) receptor.
References
- Appendino, G., G. Cravotto, G. Palmisano, R. Annunziata, and A. Szallasi. 1996. Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 39:3123–3131. [DOI] [PubMed] [Google Scholar]
- Baumann, T.K., and M.E. Martenson. 2000. Extracellular protons both increase the activity and reduce the conductance of capsaicin-gated channels. J. Neurosci. 20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M.J., M.A. Schumacher, M. Tominaga, T.A. Rosen, J.D. Levine, and D. Julius. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 389:816–824. [DOI] [PubMed] [Google Scholar]
- Caterina, M.J., T.A. Rosen, M. Tominaga, A.J. Brake, and D. Julius. 1999. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 398:436–441. [DOI] [PubMed] [Google Scholar]
- Caterina, M.J., A. Leffler, A.B. Malmberg, W.J. Martin, J. Trafton, K.R. Petersen-Zeitz, M. Koltzenburg, A.I. Basbaum, and D. Julius. 2000. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 288:306–313. [DOI] [PubMed] [Google Scholar]
- Colquhoun, E.Q., T.P. Eldershaw, K.L. Bennett, J.L. Hall, K.A. Dora, and M.G. Clark. 1995. Functional and metabolic evidence for two different vanilloid (VN1 and VN2) receptors in perfused rat hindlimb. Life Sci. 57:91–102. [DOI] [PubMed] [Google Scholar]
- Davis, J., J. Gray, M. Gunthorpe, J. Hatcher, P. Davey, P. Overend, J. Latcham, C. Clapham, K. Atkinson, K. Rance, et al. 2000. Abolition of hyperalgesia, but not algesia, in mice lacking VR1. Eur. J. Neurosci. 12:171. [Google Scholar]
- Doyle, D.A., C.J. Morais, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Forney, G.D. 1973. The Viterbi algorithm. Proc. IEEE. 61:268–278. [Google Scholar]
- Fredkin, D.R., M. Montal, and J.A. Rice. Identification of aggregated Markovian models: application to the nicotinic acetylcholine receptor. 1985. Proc. Berkeley Conference in Honor of Jerzy Neymann and Jack Kiefer. 269–289.
- Garcia-Martinez, C., C. Morenilla-Palao, R. Planells-Cases, J.M. Merino, and A. Ferrer-Montiel. 2000. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J. Biol. Chem. 275:32552–32558. [DOI] [PubMed] [Google Scholar]
- Griffiths, C.D., T.P. Eldershaw, D.P. Geraghty, J.L. Hall, and E.Q. Colquhoun. 1996. Capsaicin-induced biphasic oxygen uptake in rat muscle: antagonism by capsazepine and ruthenium red provides further evidence for peripheral vanilloid receptor subtypes (VN1/VN2). Life Sci. 59:105–117. [DOI] [PubMed] [Google Scholar]
- Guo, A., L. Vulchanova, J. Wang, X. Li, and R. Elde. 1999. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 11:946–958. [DOI] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Hayes, P., H.J. Meadows, M.J. Gunthorpe, M.H. Harries, D.M. Duckworth, W. Cairns, D.C. Harrison, C.E. Clarke, K. Ellington, R.K. Prinjha, et al. 2000. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 88:205–215. [DOI] [PubMed] [Google Scholar]
- Helliwell, R.J., L.M. McLatchie, M. Clarke, J. Winter, S. Bevan, and P. McIntyre. 1998. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 250:177–180. [DOI] [PubMed] [Google Scholar]
- Hui, K.Y., B.Y. Liu, and F. Qin. 2003. Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. Biophys. J. 84:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancso, N., A. Jancso-Gabor, and J. Szolcsanyi. 1967. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. 31:138–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt, S.E., and D. Julius. 2002. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 108:421–430. [DOI] [PubMed] [Google Scholar]
- Jordt, S.E., M. Tominaga, and D. Julius. 2000. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA. 97:8134–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang, B.H., and L.R. Rabiner. 1990. The segmental k-means algorithm for estimating parameters of hidden Markov models. IEEE Trans on Acoustic, Speech, and Signal Processing. 38:1639–1641. [Google Scholar]
- Jurman, M.E., L.M. Boland, Y. Liu, and G. Yellen. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881. [PubMed] [Google Scholar]
- Kuzhikandathil, E.V., H. Wang, T. Szabo, N. Morozova, P.M. Blumberg, and G.S. Oxford. 2001. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J. Neurosci. 21:8697–8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., A. Szallasi, and S.A. Simon. 1998. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13 acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience. 84:569–581. [DOI] [PubMed] [Google Scholar]
- Mezey, E., Z.E. Toth, D.N. Cortright, M.K. Arzubi, J.E. Krause, R. Elde, A. Guo, P.M. Blumberg, and A. Szallasi. 2000. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA. 97:3655–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, U., S.W. Hwang, and D. Kim. 1996. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 16:1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, M., R.H. Lamotte, A. Klusch, and K.D. Kniffki. 1996. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neuroscience. 75:495–505. [DOI] [PubMed] [Google Scholar]
- Qin, F., A. Auerbach, and F. Sachs. 1996. Estimating single channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys. J. 70:264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, F., A. Auerbach, and F. Sachs. 1997. Maximum likelihood estimation of aggregated Markov processes. Proc. R. Soc. Lond. B. Biol. Sci. 264:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher, M.A., B.E. Jong, S.L. Frey, S.P. Sudanagunta, N.F. Capra, and J.D. Levine. 2000. The stretch-inactivated channel, a vanilloid receptor variant, is expressed in small-diameter sensory neurons in the rat. Neurosci. Lett. 287:215–218. [DOI] [PubMed] [Google Scholar]
- Squire, L.R., F.E. Bloom, S.K. McConnell, J.L. Roberts, N.C. Spitzer, and M.J. Zigmond. 2003. Fundamental Neuroscience. 2nd ed. Academic Press.
- Szolcsanyi, J. 1990. Capsaicin-sensitive chemoceptive B-afferents - a neural system with dual sensory-efferent function. Behav. Brain Sci. 13:316. [Google Scholar]
- Tominaga, M., M.J. Caterina, A.B. Malmberg, T.A. Rosen, H. Gilbert, K. Skinner, B.E. Raumann, A.I. Basbaum, and D. Julius. 1998. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21:531–543. [DOI] [PubMed] [Google Scholar]
- Trevisani, M., D. Smart, M.J. Gunthorpe, M. Tognetto, M. Barbieri, B. Campi, S. Amadesi, J. Gray, J.C. Jerman, S.J. Brough, et al. 2002. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 5:546–551. [DOI] [PubMed] [Google Scholar]
- Welch, J.M., S.A. Simon, and P.H. Reinhart. 2000. The activation mechanism of rat vanilloid receptor 1 by capsaicin involves the pore domain and differs from the activation by either acid or heat. Proc. Natl. Acad. Sci. USA. 97:13889–13894. [DOI] [PMC free article] [PubMed] [Google Scholar]