Abstract
Activation of phospholipase C (PLC)-mediated signaling pathways in nonexcitable cells causes the release of Ca2+ from intracellular Ca2+ stores and activation of Ca2+ influx across the plasma membrane. Two types of Ca2+ channels, highly Ca2+–selective ICRAC and moderately Ca2+–selective ISOC, support store-operated Ca2+ entry process. In previous patch-clamp experiments with a human carcinoma A431 cell line we described store-operated Imin/ICRACL plasma membrane Ca2+ influx channels. In the present paper we use whole-cell and single-channel recordings to further characterize store-operated Ca2+ influx pathways in A431 cells. We discovered that (a) ICRAC and ISOC are present in A431 cells; (b) ICRAC currents are highly selective for divalent cations and fully activate within 150 s after initiation of Ca2+ store depletion; (c) ISOC currents are moderately selective for divalent cations (PBa/PCs = 14.5) and require at least 300 s for full activation; (d) ICRAC and ISOC currents are activated by PLC-coupled receptor agonists; (e) ISOC currents are supported by Imin/ICRACL channels that display 8.5–10 pS conductance for sodium; (f) ICRAC single channel conductance for sodium is estimated at 0.9 pS by the noise analysis; (g) Imin/ICRACL channels are activated in excised patches by an amino-terminal fragment of InsP3R1 (InsP3R1N); and (h) InsP3 binding to InsP3R1N is necessary for activation of Imin/ICRACL channels. Our findings provide novel information about store-operated Ca2+ influx pathways in A431 cells.
Keywords: calcium signaling; patch-clamp; inositol 1,4,5-trisphosphate; calcium channels; whole-cell
INTRODUCTION
Activation of PLC-mediated signaling pathways in nonexcitable cells causes the release of Ca2+ from intracellular Ca2+ stores and promotes Ca2+ influx across the plasma membrane via capacitative Ca2+ entry (CCE)* or store-operated Ca2+ entry (SOC) processes (Berridge, 1995; Parekh and Penner, 1997; Putney et al., 2001; Venkatachalam et al., 2002). Two types of Ca2+ channels have been implicated in store-operated Ca2+ entry in nonexcitable cells. Highly Ca2+-selective channels (PD/M > 1,000) named “Ca2+ release activated channels” (ICRAC) have been initially discovered in studies of Jurkat and RBL cells (Hoth and Penner, 1992; Zweifach and Lewis, 1993; Premack et al., 1994). Moderately Ca2+-selective channels have been later identified in a number of cells and grouped under the name ISOC (Berridge, 1995; Parekh and Penner, 1997; Nilius and Droogmans, 2001; Putney et al., 2001; Venkatachalam et al., 2002). When compared with ICRAC, ISOC channels display lower selectivity for divalent cations (PD/M ∼10), at least 10-fold higher single channel conductance for divalent and monovalent cations, and different kinetic and pharmacological properties (Berridge, 1995; Parekh and Penner, 1997; Nilius and Droogmans, 2001; Putney et al., 2001; Venkatachalam et al., 2002). The molecular identity of ICRAC remains unclear (Clapham, 1996; Parekh and Penner, 1997; Putney et al., 2001; Venkatachalam et al., 2002). Mammalian trp channels of TRPC family are the most likely candidates for the role of ISOC channels (Birnbaumer et al., 1996; Clapham et al., 2001; Nilius and Droogmans, 2001; Montell et al., 2002; Venkatachalam et al., 2002; Zitt et al., 2002).
The mechanisms of ISOC and ICRAC channel activation have been under intense investigation (Birnbaumer et al., 1996; Clapham et al., 2001; Nilius and Droogmans, 2001; Montell et al., 2002; Venkatachalam et al., 2002; Zitt et al., 2002). Activation of ISOC/ICRAC channels by a diffusible messenger CIF (Ca2+-influx factor) released by depleted Ca2+ stores (Putney and Bird, 1993; Randriamampita and Tsien, 1993; Kim et al., 1995; Csutora et al., 1999; Trepakova et al., 2000), via “conformational coupling” with the intracellular InsP3R (Irvine, 1990; Kiselyov et al., 1998; Zubov et al., 1999), by cleavage of PIP2 (Kaznacheyeva et al., 2000; Estacion et al., 2001), and by regulated insertion of channels into plasma membrane (Patterson et al., 1999; Yao et al., 1999) have been considered. Direct association of TrpC3 and TrpC4 with the InsP3R amino terminus (Boulay et al., 1999; Tang et al., 2001) provided a biochemical support to conformational-coupling model of ISOC activation. The mechanisms of ICRAC activation remain poorly understood.
In experiments with a human carcinoma A431 cell line we previously described Imin plasma membrane Ca2+ channels that are activated by application of uridine triphosphate and bradykinin to cell-attached patches or by application of InsP3 to excised inside-out patches (Kiselyov et al., 1997, 1999b; Mozhayeva et al., 1990). We found that major functional properties of Imin channels, such as small conductance (1–1.5 pS for divalent cations) and sensitivity to block by SKF95365 are similar to ICRAC channels (Kiselyov et al., 1999b; Zubov et al., 1999). We further demonstrated that activation of Imin channels by InsP3 in inside-out patches is facilitated by anti-PIP2 antibodies and suggested a InsP3R–PIP2–Imin signaling complex in these cells (Kaznacheyeva et al., 2000). More recently we demonstrated activation of Imin channels in A431 cells by depletion of intracellular Ca2+ stores and renamed these channels ICRACL (ICRAC-like) (Kaznacheyeva et al., 2001).
Our previous characterization of Imin/ICRACL channels in A431 cells was performed at the single-channel level by patch-clamp technique (Mozhayeva et al., 1990; Kiselyov et al., 1997, 1999b; Kaznacheyeva et al., 2000, 2001; Zubov et al., 1999). In these experiments we determined that the monovalent single-channel conductance of Imin/ICRACL channels is in the range 8.5–10 pS (Kaznacheyeva et al., 2001). The ICRAC channels were described in Jurkat and RBL cells in whole-cell recordings (Hoth and Penner, 1992; Zweifach and Lewis, 1993; Premack et al., 1994) and the channels with 40 pS conductance for monovalent cations had been measured in Jurkat cells (Kerschbaum and Cahalan, 1999). Recently, Mg2+ and ATP-sensitive IMagNuM (Mg2+- and nucleotide-regulated metal) channels encoded by TRPM7 were described in RBL and Jurkat cells (Nadler et al., 2001; Runnels et al., 2001; Hermosura et al., 2002). Channels with identical properties were also uncovered in RBL and Jurkat cells by other groups and called IMIC (Mg2+-inhibited channels) (Kozak et al., 2002; Prakriya and Lewis, 2002). It appears that TRPM7 protein encodes both IMagNuM and IMIC channels, and in our paper we will use the originally proposed IMagNuM nomenclature (Nadler et al., 2001). IMagNuM channels display 40 pS conductance for monovalent cations (Prakriya and Lewis, 2002), and it appears that the previously measured 40 pS channels present in Jurkat cells (Kerschbaum and Cahalan, 1999) correspond to IMagNuM, not to ICRAC (Kozak et al., 2002). In fact, recent noise measurements suggested that the monovalent conductance of ICRAC channels in Jurkat cells is no more than 0.2 pS (Prakriya and Lewis, 2002).
These results pose a number of questions. What is a relationship between ICRAC, ISOC, IMagNuM, and Imin/ICRACL channels in A431 cells? What is a mechanism of Imin/ICRACL activation? To answer these questions, we analyzed store-operated Ca2+ influx pathways in A431 cells by whole-cell and single-channel recordings. We discovered that both Imin/ICRACL and ICRAC channels are activated by intracellular Ca2+ store-depletion, by cytosolic InsP3, and by PLC-linked agonists. However, ICRAC channels are activated by Ca2+ store-depletion faster than Imin/ICRACL channels and are more selective for divalent cations than Imin/ICRACL channels. In some experiments we also observed channels with the properties typical of IMagNuM (Clapham, 2002; Hermosura et al., 2002; Kozak et al., 2002; Prakriya and Lewis, 2002). These channels were clearly distinct from Imin/ICRACL channels. Thus, we concluded that Imin/ICRACL channels correspond to ISOC and not to ICRAC or IMagNuM channels in A431 cells. In additional experiments we demonstrated activation of Imin/ICRACL channels by the amino-terminal recombinant fragment of InsP3R1, in support of a conformational coupling activation model of ISOC in A431 cells.
MATERIALS AND METHODS
Cells
Human carcinoma A431 cells (Cell Culture Collection) were kept in culture as described elsewhere (Kiselyov et al., 1999b). For patch clamp experiments cells were seeded onto coverslips and maintained in culture for 1 to 3 d before use.
Electrophysiology
All electrophysiological experiments were performed using a PC-501A patch clamp amplifier (Warner Instruments) with a conventional 10 GΩ feed-back resistance in the head stage. Resistance of sylgard-coated, fire-polished glass microelectrodes varied from 3 to 5 MΩ. Series resistance was not compensated. During recording the currents were sampled at 2.5 kHz and filtered digitally at 500 Hz. In all whole-cell experiments the holding potential was 0 mV. Periodically (once every 4–30 s) the membrane potential was stepped to −100 mV (for 30 ms) and a 170 ms voltage ramp to 70 mV was applied. Traces recorded before ICRAC and ISOC current activation were used as a template for leak subtraction. The recorded currents were normalized to the cell capacitance. Mean value of cell capacitance was 25 ± 2 pF (n = 36). The current reversal potential (Erev) was corrected by a 5.9 mV liquid-junction potential (Barry, 1994) in calculations of PBa/PCs permeability ratio. To calculate PBa/PCs permeability ratio we used GHK equation (Hille, 2001):
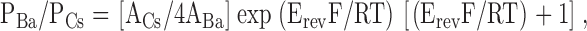 |
(1) |
where ACs = γCs[Cs]in and ABa = γBa[Ba]o; γCs = 0.82 and γBa = 0.46.
Single channel recordings data were collected from 10-s records at the given membrane potential (between −90 and −10 mV), digitized at 2.5 kHz and filtered at 100 Hz for analysis and presentation. Unitary current amplitude was determined from current records and all-point amplitude histograms. The experiments were performed at room temperature (22–24°C). Data are given as mean ± SE. Error bars denoting SE are shown where they exceed the symbol size.
Current Fluctuation Analysis
The whole-cell recordings in DFV media were performed as described above before and after application of UTP in the bath. The cells were maintained at 0 mV holding potential and currents were recorded by applying 700-ms voltage steps to −50- and −100-mV test potentials every 5 s. The current records at each test potential were sampled at 5 kHz and filtered at 1 kHz. The mean current and variance at each test potential were calculated from 200-ms segments of the digitized current using a software written on the basis of Microsoft Excel. The background noise and leak current at every potential were subtracted from mean current and variance.
The stationary noise analysis was performed as described in (Jackson and Strange, 1995; Prakriya and Lewis, 2002) with the following assumptions: (a) the ICRAC channels in the membrane are identical and independent; (b) the channels have two conductance states, open and closed; and (c) the single-channel conductance of channels in the membrane stays constant during current activation. With these assumptions, the current variance σ2 is related to the single-channel properties as
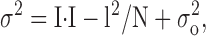 |
(2) |
where σ2 is the current variance, σo 2 is the variance of background noise, i is the single-channel current, N is a number of active channels in the membrane, and I is a whole cell current:
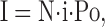 |
(3) |
where Po is the single channel open probability.
Combination of Eqs. 2 and 3 leads to:
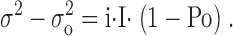 |
(4) |
In general case the Eq. 4 (and the equivalent Eq. 2) is parabolic. However, the Eq. 4 adapts linear form if open channel probability is low:
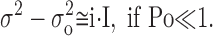 |
(5) |
Solutions
For whole-cell experiments, pipette solution contained (in mM) 145 NMDG aspartate, 10 Cs-EGTA (or 12 mM Cs-BAPTA), 10 Cs-HEPES, pH 7.3, 1.5 MgCl2, and either 4.5 CaCl2 (pCa 7.0) or no CaCl2 added (pCa > 9). 10 μM InsP3 was included in the pCa 7.0 pipette solution as indicated. Extracellular solution contained (in mM) 140 NMDG aspartate, 10 BaCl2, 10 Cs-HEPES, pH 7.3. The divalent-free (DVF) solution contained (in mM) 135 Na methanesulfonate, 5 NaCl, 10 HEDTA, 0.5 EGTA, and 10 HEPES pH 7.3. For single-channel experiments, the pipette solution contained (in mM): 105 BaCl2 or 140 NaCl as indicated and 10 Tris-HCl (pH 7.3). In inside-out experiments the intracellular solution contained (in mM): 140 K glutamate, 5 NaCl, 1 MgCl2, 10 K-HEPES pH 7.4, 2 EGTA and 1.13 CaCl2 (pCa 7.0), with or without InsP3 as indicated. In cell-attached experiments, the bath solution contained (in mM): 140 KCl, 5 NaCl, 10 K-HEPES, 1 MgCl2, and 2 CaCl2. In outside-out experiments the same solutions were used as in whole-cell experiments. The drugs and recombinant proteins were applied to the patches by bath perfusion. The time required for a complete change of solution around the patch was less than 1 s.
Expression and Purification of Recombinant CBD-InsP3R1N Proteins
CBD-InsP3R1N and CBD-InsP3R1N-K508R proteins were expressed in 0.5 liter of LB media for 14 h at 30°C in protease-deficient BL21 E. coli strain by 1 mM IPTG induction according to manufacturer's (Amersham Pharmacia Biotech) protocols. Cells were cooled on ice, collected by 20 min centrifugation at 4,500 rpm (Beckman JLA-10.5), and resuspended in 30 ml of wash buffer (20 mM TrisCl pH 8.0, 40 mM EDTA) with addition of 0.2 mg/ml lysozyme and protease inhibitors (1 mg/ml leupeptine, 200 μg/ml aprotinin, 1 mM PMSF). Cells were disrupted by repetitive 60 s sonication bursts on ice (Branson Ultrasonics). After centrifugation (4,500 g for 30 min; Beckman J-20 rotor) the pellet was twice washed with TED buffer (10 mM TrisCl pH 8.0, 1 mM EDTA, 1 mM DTT) and solubilized in 5 ml denaturing buffer (10 mM TrisCl pH 8.0, 8 M urea) for 3 h at RT. 5 ml of denatured protein was added to 250 ml of refolding buffer (50 mM TrisCl pH 8.0, 2 mM EDTA, 1.25 M NaCl, 0.5 M L-arginine) and incubated with stirring overnight in cold room. The resulting soluble fraction was concentrated to 5 ml on Amicon YM50 filters under nitrogen pressure and dialyzed against PBS.
[3H]InsP3 Binding Assay
Specific [3H]InsP3 binding was performed with minor modifications of a procedure described previously (Glouchankova et al., 2000). Briefly, 10–20 μg of purified CBD-InsP3R1N protein was incubated on ice with 10 nM [3H]InsP3 (Amersham Pharmacia Biotech) in the binding buffer (50 mM Tris-HCl, pH 8.3, 1 mM EDTA, 1 mM DTT, 100 mM NaCl) and precipitated with 12.5% PEG and 1.2 mg/ml γ-globulin at 14,000 g. Precipitates were quickly washed with the binding buffer, dissolved in Soluene (Packard Instrument Co.) and their [3H] content was determined by liquid scintillation counting. Nonspecific counts, determined in the presence of 25 μM nonlabeled InsP3, were subtracted from the total to yield specific binding.
Chemicals
HEPES, NMDG, and Na-methanesulfonate were from Sigma-Aldrich; EGTA and HEDTA were from Fluka Chemie AG; and UTP and InsP3 were from Calbiochem.
RESULTS
ICRAC and ISOC Divalent Whole-cell Currents in A431 Cells
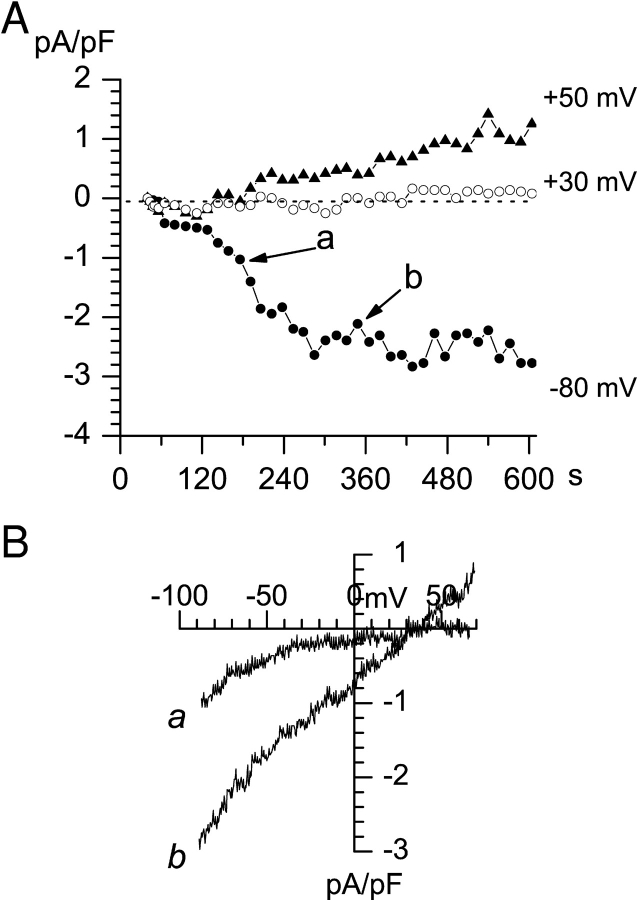
To investigate store depletion–activated divalent cation influx pathways in A431 cells, we performed a series of whole-cell current recordings using 10 mM Ba2+ as a current carrier. To stimulate store-depletion–activated currents, the pipette solution contained 10 mM EGTA or 12 mM BAPTA and 1.5 mM Mg2+ (pCa > 9.0). The A431 cells in these experiments were maintained at 0-mV holding potential and currents were recorded using 170-ms ramps from −100 to 70 mV. The inter-ramp interval was in the range from 4 to 30 s. The amplitudes of divalent currents recorded in a representative experiment at −80-, 30-, and 50-mV test potentials are plotted as a function of time on Fig. 1 A (time = 0 at the moment of break-in). The complete current-voltage curves measured at 159 s after break-in (curve a) and 275 s after break-in (curve b) in the same experiment are shown on Fig. 1 B. It is apparent that the current-voltage curves change not only in amplitude but also in shape. The curve a (measured at 159 s) displays strong inward rectification, with no detectable outward current with test potentials as positive as 70 mV. In contrast, the curve b (measured at 275 s) is practically linear and shows significant outward current at test potentials more positive than 30 mV. Curve a (Fig. 1 B) has the shape expected for a highly Ca2+–selective ICRAC current described in Jurkat and RBL cells (Hoth and Penner, 1992; Zweifach and Lewis, 1993; Premack et al., 1994). The shape of curve b (Fig. 1 B) corresponds to a less Ca2+–selective current.
Figure 1.
Activation of ICRAC and ISOC currents in A431 cells by intracellular store-depletion. Whole-cell recordings were performed at 0-mV holding potential using ramp protocol (test potentials from −100 to 70 mV; duration of the ramp, 200 ms; interramp interval is 10 s). Pipette solution contained (in mM) 10 Cs-HEPES pH 7.3, 145 NMDG aspartate, 10 Cs-EGTA (pCa > 9.0), 1.5 MgCl2. Extracellular solution contained (in mM) 10 Cs-HEPES pH 7.3, 140 NMDG aspartate, 10 BaCl2. (A) The amplitudes of peak currents recorded at each ramp at −80 mV (filled circles), 30 mV (open circles) and 50 mV (filled triangles) test potentials are plotted as a function of time after break-in. (B) Current-voltage relationships recorded at 159 s (curve a) and 275 s (curve b). Data from the same experiment are shown in A and B. Ramps corresponding to curves a and b in B are indicated by arrows in A. The data are representative of 19 experiments.
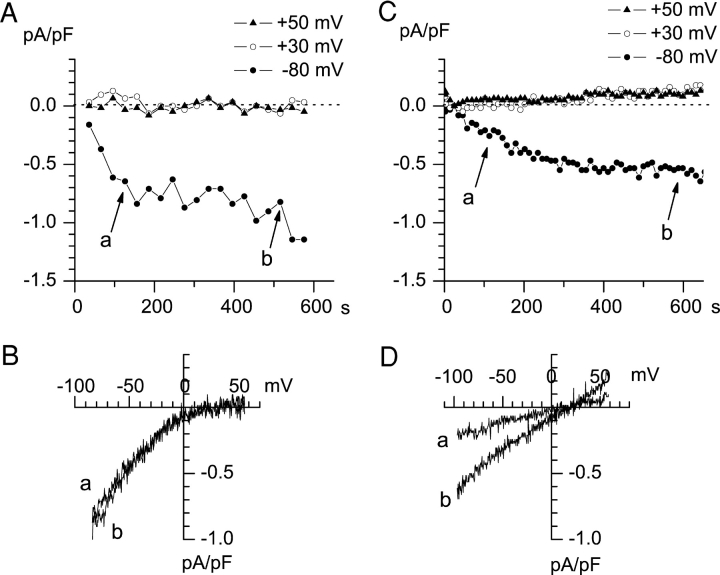
Two alternatives could account for the observed results. The first is that A431 cells express two types of Ca2+ influx channels: highly Ca2+–selective ICRAC and less Ca2+–selective store-operated channels (ISOC). The second possibility is that ICRAC channels activated by store depletion became less Ca2+ selective in the course of an experiment. Our data indicate that the coexistence of two different store-operated Ca2+ channel types in A431 cells is a more likely possibility. Indeed, in some cells (5/36 experiments, 14%) only highly Ca2+-selective ICRAC channels (Fig. 2, A and B) were observed. To facilitate Ca2+ store depletion, 10 μM InsP3 was included in the pipette solution in addition to 10 mM EGTA and 4.5 mM CaCl2 (pCa = 7.0) in the experiment shown on Fig. 2, A and B. In other cells (7/36 experiments, 19%) only moderately selective ISOC currents were observed using the same pipette solution (Fig. 2, C and D). The combination of ICRAC and ISOC currents (Fig. 1) was observed in 19/36 experiments (53%). We could not identify the reasons for variability in ICRAC and ISOC current expression in A431 cells. Nevertheless, we reasoned that the existence of “ICRAC only” (Fig. 2, A and B) and “ISOC only” (Fig. 2, C and D) A431 cells argues against the possibility of ICRAC to ISOC interconversion during our recordings.
Figure 2.
“ICRAC-only” and “ISOC-only” currents are activated by intracellular store-depletion. (A) Whole-cell recordings were performed as on Fig. 1 with the inter-ramp interval equal to 30 s. Pipette solution is the same as in Fig. 1 with addition of 4.5 CaCl2 (pCa 7.0) and 10 μM InsP3. Extracellular solution is the same as in Fig. 1. The amplitudes of the peak currents recorded at each ramp at −80 mV (filled circles), 30 mV (open circles), and 50 mV (filled triangles) test potentials are plotted as a function of time after break-in. (B) Current-voltage relationship recorded at 156 s (curve a) and 486 s (curve b). Ramps corresponding to curves a and b in B are indicated by arrows in A. The data are representative of 5 experiments. (C) Whole-cell recordings were performed as in Fig. 1 with the interramp interval equal to 10 s. The pipette and extracellular solutions are the same as on Fig. 2 A. The amplitudes of peak currents recorded at each ramp at −80 mV (filled circles), 30 mV (open circles), and 50 mV (filled triangles) test potentials are plotted as a function of time after break-in. (D) Current-voltage relationship recorded at 100 s (curve a) and 600 s (curve b) of the experiment. Ramps corresponding to curves a and b in D are indicated by arrows in C. The data are representative of seven experiments.
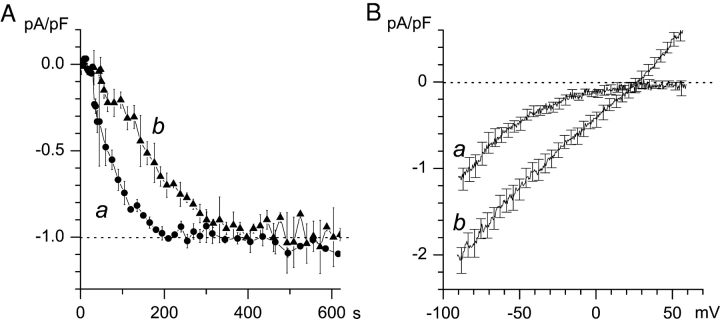
Both types of channels were activated by store depletion in our experiments, but with the different time course. Fig. 3 A illustrates an average time-course of ICRAC (filled circles, n = 4) and ISOC (filled triangles, n = 6) currents activation. It is apparent that ICRAC current reaches maximum value within 200 s after break-in (Fig. 3 A, curve a), whereas ISOC requires at least 300 s to reach its maximum value (Fig. 3 A, curve b). To further compare ICRAC and ISOC divalent currents in A431 cells, we calculated average current-voltage relationships measured in cells containing only ICRAC (Fig. 3 B, curve a, n = 4) or ISOC (Fig. 3 B, curve b, n = 6) currents. To generate curve a, the current voltage-relationship was determined when ICRAC channels were fully activated (Fig. 3 A, curve a). To generate curve b, ISOC currents were measured at least 340 s after break-in (Fig. 3 A, curve b). On average, the amplitude of ICRAC currents at −80 mV was equal to −0.96 ± 0.02 pA/pF (n = 4), and the amplitude of ISOC currents at −80 mV was equal to −2.1 ± 0.16 pA/pF (n = 6). Thus, an average ISOC current is twice the size of an average ICRAC current. The reversal potential of ICRAC currents could not be measured as we could not detect any significant outward current via ICRAC channels at test potentials as positive as 60 mV in our recording conditions (Fig. 3 B, curve a). For ISOC currents an average reversal potential (Erev) was equal to 30 ± 3 mV (n = 6) (Fig. 3 B, curve b). After correction for liquid junction potential we calculated PBa/PCs selectivity ratio of ISOC currents equal to 14.5 using GHK equation (Hille, 2001) as described in materials and methods. Thus, divalent selectivity of ISOC channels in A431 cells is similar to the divalent selectivity of ISOC channels described in other nonexcitable cells (Berridge, 1995; Parekh and Penner, 1997; Nilius and Droogmans, 2001; Putney et al., 2001; Venkatachalam et al., 2002).
Figure 3.
Time-course of current activation and current-voltage relationship of ICRAC and ISOC currents. (A) The average ICRAC (filled circles, n = 4, curve a) and ISOC (filled triangles, n = 6, curve b) currents are shown as a function of the time after break-in. The amplitude of the currents measured at −80 mV test potential in each experiment was normalized to the size of the currents recorded in the same experiment at 210 s for ICRAC and at 400 s for ISOC. (B) The average current-voltage relationship measured in cells exhibiting only ICRAC currents (n = 4, curve a) and only ISOC current (n = 6, curve b). To generate curve a, the current voltage-relationships were determined as shown on Fig. 2, A and B, when ICRAC channels were fully activated (at least 150–200 s after break-in). To generate curve b, the current voltage-relationships were determined as shown on Fig. 2, C and D, when ISOC channels were fully activated (at least 340 s after break-in). Data from the same experiments were used to generate A and B.
ICRAC and ISOC currents were observed in most (31/36) of the whole-cell recording experiments (86%). In a small fraction of cells (5/36, 14%), currents with very different properties were observed (unpublished data). In these cells, inward currents at −80-mV test potentials reached 10–20 pA/pF, 2–5-fold larger than typical ICRAC or ISOC currents (Fig. 3 B). At positive test potentials these cells displayed outward rectification leading to even larger currents at positive potentials (up to 30 pA/pF), in striking contrast with ICRAC or ISOC (Fig. 3 B). The reversal potential for these currents was close to 30 mV, similar to ISOC currents (Fig. 3 B). Based on the large size of inward currents, moderate selectivity for divalent cations and characteristic outward rectification, we reasoned that the currents observed in these experiments correspond to IMagNuM currents in A431 cells (Clapham, 2002; Hermosura et al., 2002; Kozak et al., 2002; Prakriya and Lewis, 2002). Detailed characterization of IMagNuM currents in A431 cells was precluded by their rare occurrence in our experiments, and in the remainder of this paper we focus on the analysis of more prevalent ICRAC and ISOC currents.
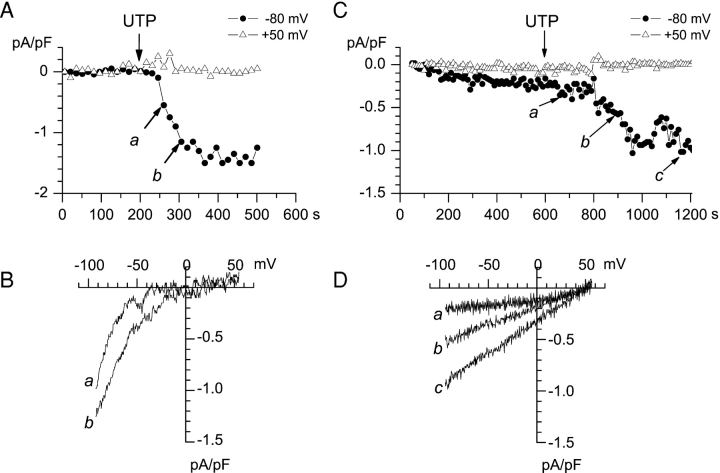
Activation of ICRAC and ISOC Currents by Extracellular UTP
In experiments shown on Figs. 1–2 ICRAC and ISOC currents were activated by depletion of intracellular Ca2+ stores. This was achieved by buffering cytosolic Ca2+ to pCa > 9.0 with 12 mM BAPTA or 10 mM EGTA (Fig. 1) or by addition of 10 μM InsP3 to cytosolic solution buffered to pCa = 7.0 (Fig. 2). In physiological situations, Ca2+ influx in nonexcitable cells is activated in response to activation of PLC-coupled receptors. Therefore, in the next series of experiments we used whole-cell recordings in A431 cells to measure Ba2+ currents activated by application of extracellular UTP. The intracellular Ca2+ concentration in these experiments was clamped to pCa 7.0 as in the experiments shown on Fig. 2, but no InsP3 was added. In these conditions passive store depletion is slow and ICRAC and ISOC currents were not activated within the first 200 s after break-in (Fig. 4 A) and within 600 s (Fig. 4 C). However, we found that 100 μM extracellular UTP efficiently activated both ICRAC and ISOC currents in A431 cells (Fig. 4). Similar to experiments with store-depletion (Figs. 1 and 2), “ICRAC-only” (Fig. 4, A and B) and “ISOC-only” (Fig. 4, C and D) currents were induced in some A431 cells in response to extracellular UTP. The current-voltage relationship of UTP-activated currents (Fig. 4, B and D) was identical to currents activated by Ca2+ store depletion (Fig. 3 B). Thus, we concluded that both ICRAC and ISOC currents could be activated in A431 cells by Ca2+ store depletion or in response to activation of PLC-coupled receptors.
Figure 4.
Activation of ICRAC and ISOC currents by extracellular UTP. Whole-cell recordings were performed with the pipette and extracellular solutions as in Fig. 2 without addition of InsP3. (A) The amplitudes of peak currents recorded at each ramp at −80 mV (filled circles) and 50 mV (open triangles) test potentials are plotted as a function of time after break-in. 100 μM UTP was applied to the cells at 208 s (arrow). (B) Current-voltage relationships recorded at 231 s (curve a) and 300 s (curve b) of the experiment. Ramps corresponding to curves a and b in B are indicated by the arrows in A. (C) The amplitudes of peak currents recorded at each ramp at −80 mV (filled circles) and 50 mV (open triangles) test potentials are plotted as a function of time after break-in. 100 μM UTP was applied to the cells at 630 s (arrow). (D) The current-voltage relationships recorded at 650 s (curve a), 850 s (curve b), and 1140 s (curve c) of the experiment. Ramps corresponding to curves a–c in D are indicated by the arrows in C.
ICRAC and ISOC Monovalent Whole-cell Currents in A431 Cells
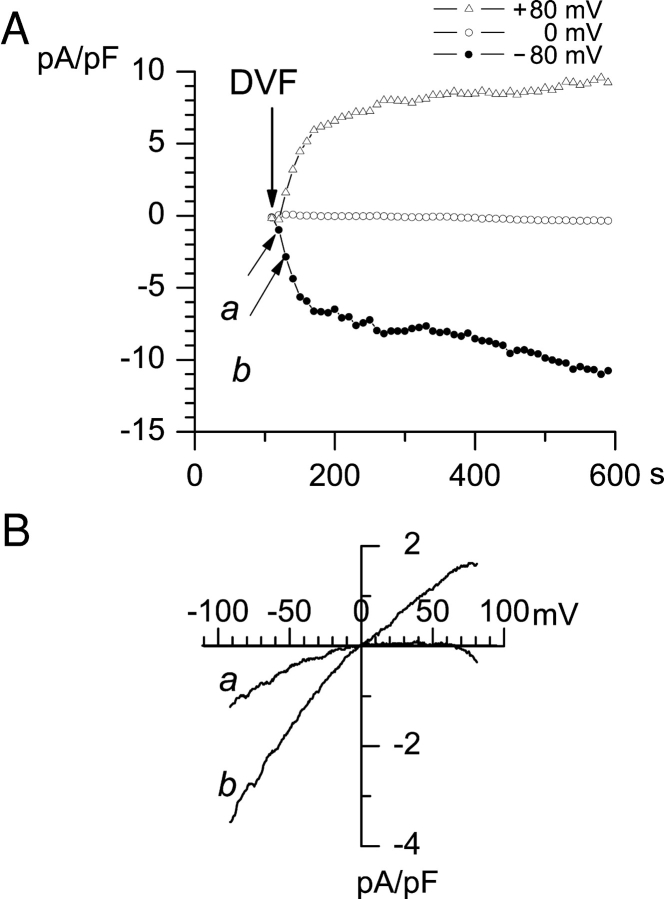
To further characterize ICRAC and ISOC currents in A431 cells, we performed a series of whole-cell recording experiments in DVF media containing 140 mM Na+. In the absence of divalent cations, Na+ and other monovalent cations carry a substantial current through voltage-gated Ca2+ channels (Almers et al., 1984; Hess and Tsien, 1984) and ICRAC (Hoth and Penner, 1993; Hermosura et al., 2002; Prakriya and Lewis, 2002). In most of the experiments performed in DVF media, large monovalent inward and outward currents were observed (Fig. 5 A). Similar to experiments with Ba2+ as a current carrier (Fig. 1), the shape of the current-voltage relationship recorded in DVF media was changing in a course of the experiments. Within the first 10 s after addition of DVF solution the current-voltage relationship displayed inward rectification, and outward currents were absent with test potentials as positive as 80 mV (Fig. 5 B, curve a). At later times, the current-voltage relationship became more linear, with a reversal potential at 0 mV and large outward currents (Fig. 5 B, curve b). The amplitude of Na+-currents continued to rise during first 10 min of recordings (Fig. 5 A). Thus, similar to recordings with Ba2+, recordings in DVF media revealed two types of channels in A431 cells activated in our recording conditions. The inwardly rectifying monovalent current observed in our experiments in DVF media (Fig. 5 B, curve a) is similar to Na-ICRAC current recorded in Jurkat and RBL cells (Hoth and Penner, 1993; Hermosura et al., 2002; Prakriya and Lewis, 2002). The large nonselective current observed in our experiments in DFV media (Fig. 5 B, curve b) is likely to correspond to Na-ISOC currents.
Figure 5.
ICRAC and ISOC currents in DVF medium. Whole-cell recordings were performed as described in Fig. 1 with the interramp interval of 11 s. Pipette solution contained (in mM) 120 Cs-Aspartate, 10 BAPTA (pCa > 9.0), 1.5 MgCl2, pH 7.3. Extracellular DVF solution contained (in mM): 135 Na-methansulfonate, 5 NaCl, 10 HEDTA, 0.5 EGTA, pH 7.3. (A) Amplitudes of peak currents recorded at each ramp at −80 mV (filled circles), 0 mV (open circles), and 80 mV (open triangles) test potentials are plotted as a function of time after break-in. Extracellular solution was replaced by DVF medium at 100 s after break-in (arrow). (B) Current-voltage relationship recorded at 120 s (curve a) and 130 s (curve b) of the experiment. Ramps corresponding to curves a and b in B are indicated by arrows in A. The data are representative of four experiments in DVF medium.
Imin/ICRACL Channels Support ISOC Currents in A431 Cells
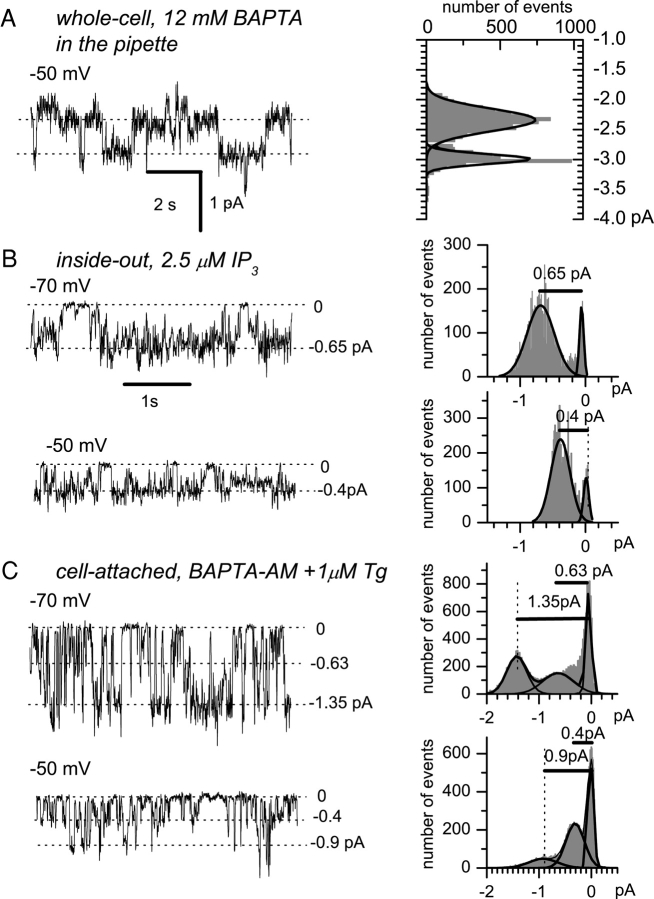
To gain insight into properties of channels supporting ICRAC and ISOC currents in A431 cells, we performed a series of single-channel recordings. In these experiments we took advantage of increased conductance of these channels in DVF media (Fig. 5). Because of different time course of ICRAC and ISOC activation (Fig. 3 A), we reasoned that the channels recorded within first 100 s after break-in are more likely to correspond to ICRAC, and the channels recorded between 300–600 s after break-in are more likely to correspond to ISOC. To measure single-channel currents in whole-cell experiments, we applied 10-s test pulses from −100 to 50 mV from the holding potential of 0 mV. In these experiments we failed to observe any channel activity within the first 100 s after break-in. However, with 12 mM BAPTA and 1.5 mM Mg2+ in the pipette (pCa > 9.0), we observed activity of multiple channels 200–600 s after break-in (Fig. 6 A). On average, the single-channel current through the channels was equal to 0.7 ± 0.05 pA at −70 mV test potential (Fig. 7 , triangles, n = 4).
Figure 6.
Patch-clamp recordings of single-channel activity. Single-channel activity recorded in whole-cell (A), inside-out (B), and cell-attached (C) patch-clamp mode. 140 mM Na+ is a current carrier in all experiments (see materials and methods for details). The single-channel activity was induced by: (A) store-depletion (12 mM BAPTA and 1.5 mM Mg2+ in the pipette, pCa > 9.0), representative of four whole-cell experiments; (B) 2.5 μM intracellular InsP3, representative of 8 inside-out experiments; (C) BAPTA-AM and 100 μM Tg in cell-attached mode, representative of four cell-attached experiments. The unitary current amplitudes were measured manually or determined from all-point amplitude histograms (shown on the right).
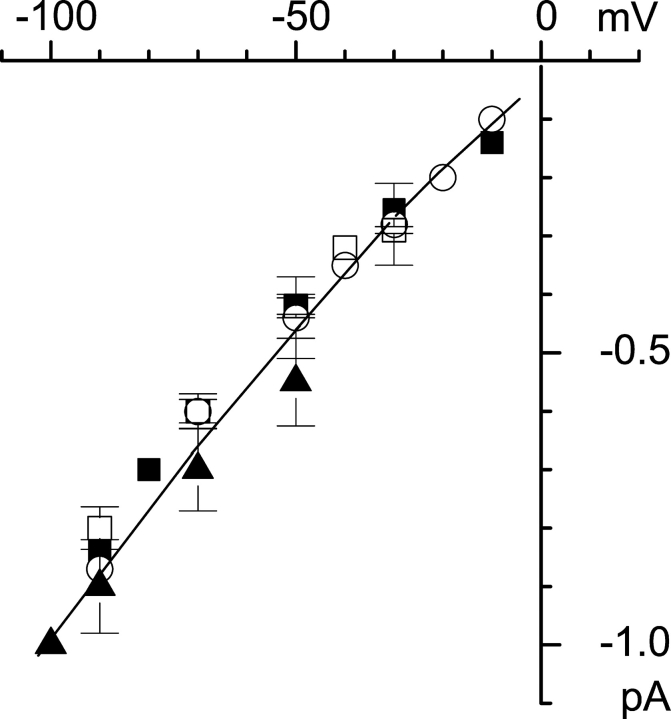
Figure 7.
Single-channel current-voltage relationships. The mean current-voltage relationship of single channels activated by store-depletion in whole cell mode (filled triangles, n = 4), by 2.5 μM intracellular InsP3 in inside-out mode (open circles, n = 8), by 100 μM Tg + BAPTA-AM in cell-attached mode (open squares, n = 4), or by 100 μM extracellular UTP in outside-out mode (filled squares, n = 4). The currents measured at several independent experiments from all-point amplitude histograms were averaged at the same test potential and shown as mean ± SEM for each recording condition. The single-channel slope conductance (line) at different potentials is 8.5–10 pS.
Channels with similar properties were recorded in excised patches after application of 2.5 μM InsP3 (inside-out, Fig. 6 B, n = 8), and after application of 1 μM thapsigargin and 1 mM BAPTA-AM (cell-attached, Fig. 6 C, n = 4; see also Kaznacheyeva et al., 2001) and in outside-out configuration in response to application of extracellular UTP (n = 4, current records not shown). The single-channel slope conductances for channels measured in all these experimental conditions were in the range 8.5–10 pS (Fig. 7). The properties of these channels are identical to the properties of Imin/ICRACL channels that we described previously (Kaznacheyeva et al., 2001). Importantly, Imin/ICRACL single-channels were observed in our whole-cell experiments from 100 s until 20 min after break-in, that is during the time period corresponding to ISOC activation (Fig. 3 A).
We did not observe Imin/ICRACL channel activity in whole-cell experiments within the first 100 s after break-in, that is in the time window of ICRAC current development (Fig. 3 A). From these experiments we concluded that the previously described Imin/ICRACL channels (Mozhayeva et al., 1990; Kiselyov et al., 1997, 1999b; Zubov et al., 1999; Kaznacheyeva et al., 2000, 2001) support ISOC currents in A431 cells.
Current Fluctuation Analysis of Na-ICRAC
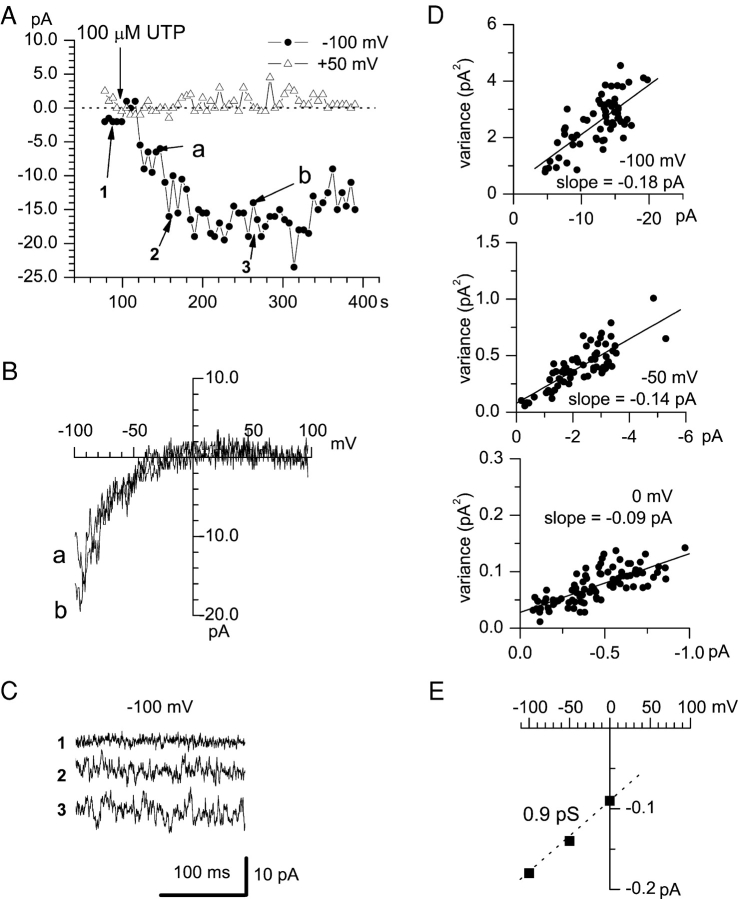
As described above, we were unable to measure single-channel activity of ICRAC channels using DVF media, most likely due to small conductance of these channels. Current fluctuation analysis has been used previously in studies of ICRAC channels in Jurkat cells (Zweifach and Lewis, 1993; Prakriya and Lewis, 2002). Here we applied the stationary current fluctuation analysis method (see materials and methods) to estimate the size of single-channel sodium currents via ICRAC channels activated by application of 100 μM UTP to A431 cells (Fig. 8 A). To perform fluctuation analysis, we selected experiments in DVF media with “ICRAC-only” cells as determined by the inward rectification shape of current-voltage relationship (Fig. 8, A and B). The intracellular Ca2+ concentration in these experiments was clamped to pCa 7.0 by 10 mM EGTA as in the experiments shown on Fig. 2. The mean macroscopic current (I) and corresponding variance (σ2) were measured at holding potential of 0 mV and at −50 and −100 mV test potentials as described in materials and methods. Application of UTP activated ICRAC (Fig. 8 A) and caused a significant increase in the noise of the current recorded at −100 mV test potential (Fig. 8 C).
Figure 8.
Fluctuation analysis of monovalent ICRAC currents. Whole-cell recordings in DVF media were performed as described in Fig. 4. The amplitudes of peak currents recorded at each ramp at −100 mV (filled circles) and 50 mV (open triangles) test potentials are plotted as a function of the time after break-in. 100 μM UTP was applied to the cells at 105 s (arrow). (B) Current-voltage relationships recorded at 158 s (curve a) and 278 s (curve b) of the experiment. Ramps corresponding to curves a and b in B are indicated by the arrows in A. The shapes of curves a and b are indicative of “ICRAC-only” cell. (C) Examples of 200-ms segments of currents recorded at −100 mV membrane potential before (1) and after (2 and 3) UTP application as indicated by the arrows in A. (D) Mean-variance analysis of Na-ICRAC currents. The plots show the mean value and variance of 200-ms current sweeps collected during development of agonist-induced Na+ current in DVF solution. The data points are fit by the straight lines with a slope of −0.18 pA (−100 mV), −0.14 pA (−50 mV), and −0.09 pA (0 mV). The data are representative of three independent experiments. (E) Current-voltage relationship of Na-ICRAC single channels obtained from current measurements as shown in D. The data points are fit by a line with a slope of 0.9 pS.
To determine the single-channel conductance of Na-ICRAC, we measured variance of the UTP-activated DVF current at 0, −50, and −100 mV membrane potentials. Plots of the current variance against mean current amplitude at 0, −50, and −100 mV could well be fitted by the straight lines (Fig. 8 D). Because of the observed linear relationship between σ2 and I we used linear approximation for relationship between σ2 and I (materials and methods, Eq. 5) in our calculations. The underlying assumption for linear approximation is that Na-ICRAC open channel probability Po is low (Jackson and Strange, 1995; Prakriya and Lewis, 2002). According to Eq. 5, the slope of σ2 versus I linear fit is equal to i, where i is the size of the Na-ICRAC single channel current. The fit to the data yielded the slope equal to −0.09 pA at 0 mV (Fig. 8 D, bottom), −0.14 pA at −50 mV (Fig. 8 D, middle), and −0.18 pA at −100 mV (Fig. 8 D, top). Obtained measurements are consistent with the slope single-channel conductance of Na-ICRAC channels in A431 cells equal to 0.9 pS (Fig. 8 E), which is 5 times greater than the chord conductance estimated for Na-ICRAC currents in Jurkat cells (Prakriya and Lewis, 2002), and 10 times smaller than the single-channel conductance of Na-Isoc in A431 cells (Fig. 7). The reasons for differences between our estimates and the results of Prakriya and Lewis (2002) may reflect the differences in ICRAC properties in A431 and Jurkat cells, or may be related to assumptions about channel open probability and gating properties inherent to the current fluctuation analysis approach.
Activation of Imin/ICRACL Channels by InsP3R1N
What mechanism is responsible for activation of Imin/ICRACL channels in A431 cells? Our previous results suggested that Imin/ICRACL channels may be activated via direct conformational coupling with the InsP3R (Zubov et al., 1999) or with the InsP3R–PIP2 complex (Kaznacheyeva et al., 2000). Which domain of the InsP3R is required for Imin/ICRACL activation? Based on its functional properties Imin/ICRACL is likely to be encoded by a member of the TRPC family (see discussion). Activation of TRPC3 channels by the amino-terminal region of InsP3R1 (InsP3R1N) has been reported (Kiselyov et al., 1999a). Can InsP3R1N gate Imin/ICRACL? To answer this question, we expressed the InsP3R1N fragment (aa 2–604) in bacteria as CBD-fusion protein (see materials and methods). In addition, we also generated and expressed a CBD-InsP3R1N-K508R mutant, which has been reported to lack specific InsP3 binding activity (Yoshikawa et al., 1996).
When expressed in E. coli BL21 cells, both CBD-InsP3R1N and CBD-InsP3R1N-K508R proteins were concentrated in inclusion bodies. In our experiments we purified the inclusion body fraction from bacteria, dissolved them in 8 M urea and refolded denatured proteins by rapid dilution. Refolded proteins were concentrated, dialyzed against PBS and used in functional experiments. Similar yields of CBD-InsP3R1N and CBD-InsP3R1N-K508R proteins were obtained in this purification scheme, but only CBD-InsP3R1N protein displayed specific [3H]-InsP3 binding activity (unpublished data). Thus, in agreement with the report published previously (Yoshikawa et al., 1996) and with the recently reported structure of the InsP3R1 ligand-binding core (Bosanac et al., 2002), the K508 residue plays a critical role in InsP3 binding.
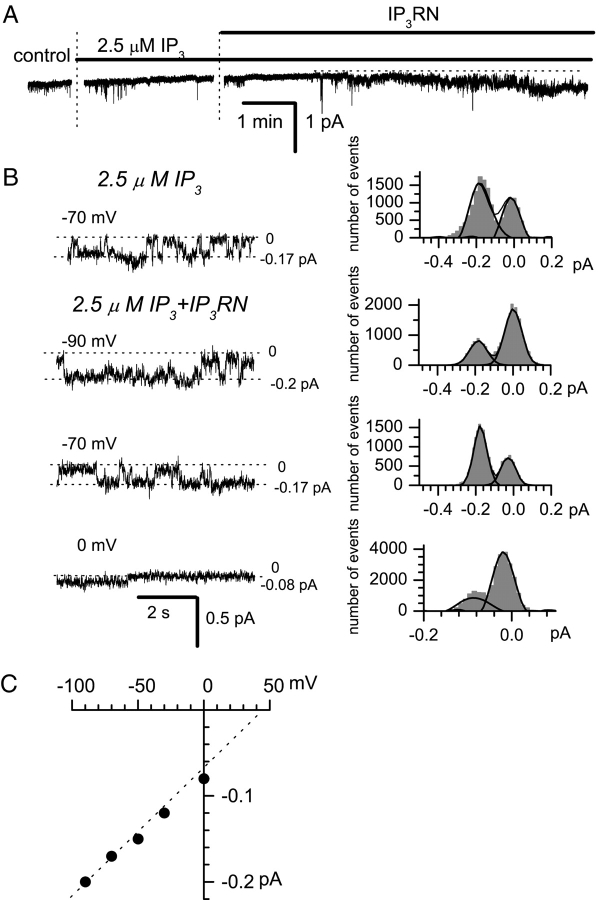
To test functional effects of InsP3R1N, we applied the CBD-InsP3R1N protein to inside-out patches excised from A431 cells using 105 mM Ba2+ in the pipette as a current carrier. As described previously (Mozhayeva et al., 1990; Kiselyov et al., 1997, 1999b; Zubov et al., 1999; Kaznacheyeva et al., 2000, 2001), a low activity of Imin/ICRACL channels was observed in excised patches in the presence of 2.5 μM of InsP3 (Figs. 9 A and 10 B). Addition of CBD-InsP3R1N protein to the cytosolic surface of the patch resulted in rapid facilitation of Imin/ICRACL channel activity (Figs. 9 A and 10 B). These channels displayed 1.5 pS conductance in 105 mM Ba2+ (Fig. 9 C), as described previously for Imin/ICRACL (Mozhayeva et al., 1990; Kiselyov et al., 1997, 1999b; Zubov et al., 1999; Kaznacheyeva et al., 2000, 2001).
Figure 9.
InsP3R1N activates Imin/ICRACL in inside-out patches. (A) Current traces in an inside-out patch recorded after application of InsP3 and CBD-InsP3R1N at −70 mV holding potential. (B) The current induced by InsP3 and CBD-InsP3R1N was recorded at the membrane potentials as indicated. Currents amplitudes were measured from all-point amplitude histograms (shown on the right). (C) The current-voltage relationship of Imin/ICRACL channels activated by InsP3 and CBD-InsP3R1N. The single-channel slope conductance (line) is 1.5 pS. Data are representative of 13 experiments.
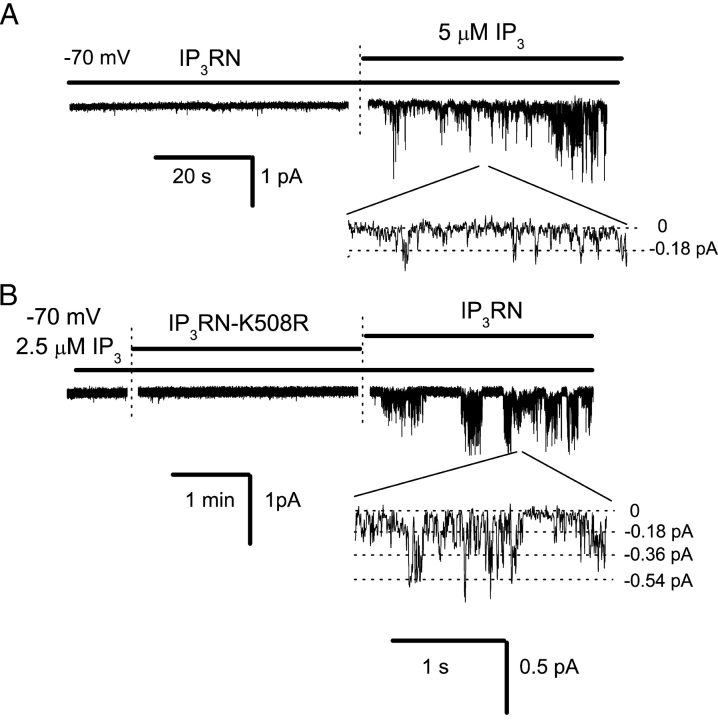
Can InsP3R1N activate Imin/ICRACL channels independently from InsP3? When CBD-InsP3R1N was added to excised patches in the absence of InsP3, no channel activity was observed (Fig. 10 A). However, addition of InsP3 to the same patch resulted in an immediate activation of Imin/ICRACL channels (Fig. 10 A). Thus, InsP3 is required for InsP3R1N activation of Imin/ICRACL channels. Is InsP3 binding to InsP3R1N important for the observed functional effects? When CBD-InsP3R1N-K508R protein was added to inside-out patches in the presence of 2.5 μM of InsP3, no channel activity was observed (Fig. 10 B). In contrast, addition of CBD-InsP3R1N to the same patch induced Imin/ICRACL activity (Fig. 10 B). These data suggest that the InsP3-liganded form of InsP3R1N is a true activator of Imin/ICRACL channels.
Figure 10.
InsP3 binding to InsP3R1N is required for Imin/ICRACL activation. Inside-out patch clamp recordings of Imin/ICRACL channel activity were performed as described in Fig. 9 at −70 mV. (A) InsP3 is required for Imin/ICRACL channel activation by CBD-InsP3R1N. Imin/ICRACL currents are shown on compressed (top) and expanded (bottom) time scales. Data are representative of eight experiments. (B) InsP3R1N, but not InsP3R1N-K508R, evokes ICRACL activity. Imin/ICRACL current activity in response to application of InsP3, CBD-InsP3R1N-K508R, and CBD-InsP3R1N to the patch is shown as indicated. Imin/ICRACL currents are shown on compressed (top) and expanded (bottom) time scales.
DISCUSSION
Store-operated Ca2+ influx pathways in A431 cells were analyzed in this paper by whole-cell and single-channel recordings. We discovered that ICRAC and ISOC currents are present in A431 cells. The main functional properties of ICRAC and ISOC (Imin/ICRACL) channels in A431 and other nonexcitable cells are briefly summarized in Table I . Implications of our findings are discussed below.
TABLE I.
Comparison of ICRAC and ISOC (Imin/ICRACL) Functional Properties
Imin/ICRACL and ICRAC
In the previous patch-clamp studies we described store-operated and InsP3-activated Imin/ICRACL channels in A431 cells (Mozhayeva et al., 1990; Kiselyov et al., 1997, 1999b; Zubov et al., 1999; Kaznacheyeva et al., 2000, 2001). Some of the properties of Imin/ICRACL channels, such as sensitivity to store-depletion, small conductance for divalent and monovalent cations, and sensitivity to block by SKF95365, indicated that Imin/ICRACL channels may correspond to ICRAC channels in A431 cells. However, a number of differences between Imin/ICRACL and ICRAC currents were uncovered in the present study. The main differences between Imin/ICRACL channels and ICRAC channels are (Table I): (a) Imin/ICRACL conductance for divalent cations is 1–1.5 pS, for ICRAC it is estimated at 10–25 fS; (b) Imin/ICRACL conductance for sodium is 8.5–10 pS, for ICRAC it is estimated at 0.2–0.9 pS; (c) Imin/ICRACL channels are moderately selective for divalent cations (PBa/PCs = 14.5), ICRAC channels are highly selective for divalent cations (PD/PM > 1,000); (d) Imin/ICRACL channels display linear current-voltage relationship, ICRAC currents display inward rectification; and (e) Imin/ICRACL currents require at least 300 s after initiation of Ca2+ store depletion for complete activation, ICRAC currents activate within 100–200 s. Thus, we concluded that Imin/ICRACL channels and ICRAC channels are different entities. In support of this conclusion, ICRAC-only cells were found in 5 of 36 whole-cell experiments (14%) and Imin/ICRACL-only currents were found in 7 of 36 experiments (19%). In 19 of 36 experiments (53%), both types of currents were present in the same cell. The different rates of ICRAC and Imin/ICRACL activation in response to store-depletion (Fig. 3 A) suggest that these two Ca2+ entry pathways could be gated by different mechanisms. Another possibility is that both ICRAC and Imin/ICRACL are activated by a similar “conformational coupling” mechanism (see below), but coupled to different Ca2+ pools, so that ICRAC is coupled to the pool that is depleted rapidly, and Imin/ICRACL is coupled to the pool that is depleted more slowly. The differences in the rates of pool depletion can be related to the differences in distribution of InsP3R and Ca2+ pumps, as well as to localized expression of different InsP3R isoforms. Future experiments will be needed to discriminate between these possibilities.
Imin/ICRACL and TRPC
Our results lead us to conclude that Imin/ICRACL channels support ISOC current in A431 cells. What is the molecular identity of Imin/ICRACL channels? Recent results support the hypothesis that members of the TRPC family are essential components of ISOC channels in nonexcitable cells. Indeed, genetic knockout of the TRPC4 subunit in mouse resulted in dramatic reduction of ISOC currents in endothelial cells (Freichel et al., 2001). Similar results were obtained when TRPC1 was genetically deleted in chicken B cell lymphocytes (Mori et al., 2002). Thus, it is likely that ISOC currents in other nonexcitable cells are also supported by the members of TRPC family. The main functional properties of Imin/ICRACL channels are consistent with the properties of channels formed by TRPC family members when expressed in heterologous expression system (for review see Birnbaumer et al., 1996; Clapham et al., 2001; Nilius and Droogmans, 2001; Montell et al., 2002; Venkatachalam et al., 2002; Zitt et al., 2002). Future molecular studies will be required to test this hypothesis and to identify the TRPC protein that encodes Imin/ICRACL channels in A431 cells.
Imin/ICRACL and InsP3R
What is a mechanism of Imin/ICRACL activation? Our previous results (Zubov et al., 1999; Kaznacheyeva et al., 2000) supported the conformational coupling model of Imin/ICRACL activation. Here we provide additional evidence in favor of the conformational coupling model. We established that the amino-terminal fragment of InsP3R1 (InsP3R1N) was able to activate Imin/ICRACL in excised inside-out patches (Fig. 9) and that InsP3R1N association with InsP3 is critical for Imin/ICRACL channel activation (Fig. 10). Importantly, the InsP3R1N fragment used in our experiments (amino acids 2–604) does not contain previously identified TRPC3- and TRPC4-binding sites, which are located within 669–821 region (Boulay et al., 1999; Tang et al., 2001). Thus, it is unlikely that the direct association of InsP3R1N with a member of TRPC family can account for an ability of InsP3R1N to activate Imin/ICRACL. Most likely, an additional adaptor protein is involved in the conformational coupling between InsP3R and Imin/ICRACL (TRPC). One potential candidate for this role is a Homer protein (Xiao et al., 2000) that binds to the residues 48–55 of the InsP3R1 amino-terminal region (Tu et al., 1998), which are present within InsP3R1N sequence. Identification of additional InsP3R1 amino-terminal binding partners may provide interesting insights into InsP3R1-Imin/ICRACL conformational coupling mechanism.
Acknowledgments
The authors are grateful to Dr. Bertil Hille for comments on the paper and to V. Alexeenko for technical support.
Supported by the Russian Basic Research Foundation 01-04-48809 (E. Kaznacheyeva), 01-04-48810 (G.N. Mozhayeva), SS-2178.2003.4 (G.N. Mozhayeva), the program of “Physical-Chemical Biology” Russian Academy of Sciences (G.N. Mozhayeva), the National Institutes of Health NS38082 (I. Bezprozvanny), the Robert J. Welch Foundation (I. Bezprozvanny), and the CRDF RB1-2018 (G.N. Mozhayeva and I. Bezprozvanny).
Olaf S. Andersen served as editor.
Footnotes
Abbreviations used in this paper: CCE, Ca2+ entry; DVF, divalent-free; SOC, store-operated Ca2+ entry.
References
- Almers, W., E.W. McCleskey, and P.T. Palade. 1984. A non-selective cation conductance in frog muscle membrane blocked by micromolar external calcium ions. J. Physiol. 353:565–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, P.H. 1994. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Methods. 51:107–116. [DOI] [PubMed] [Google Scholar]
- Berridge, M.J. 1995. Capacitative calcium entry. Biochem. J. 312:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer, L., X. Zhu, M. Jiang, G. Boulay, M. Peyton, B. Vannier, D. Brown, D. Platano, H. Sadeghi, E. Stefani, and M. Birnbaumer. 1996. On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc. Natl. Acad. Sci. USA. 93:15195–15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac, I., J.R. Alattia, T.K. Mal, J. Chan, S. Talarico, F.K. Tong, K.I. Tong, F. Yoshikawa, T. Furuichi, M. Iwai, et al. 2002. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 420:696–700. [DOI] [PubMed] [Google Scholar]
- Boulay, G., D.M. Brown, N. Qin, M. Jiang, A. Dietrich, M.X. Zhu, Z. Chen, M. Birnbaumer, K. Mikoshiba, and L. Birnbaumer. 1999. Modulation of Ca2+ entry by polypeptides of the inositol 1,4,5- trisphosphate receptor (IP3R) that bind transient receptor potential (TRP): evidence for roles of TRP and IP3R in store depletion-activated Ca2+ entry. Proc. Natl. Acad. Sci. USA. 96:14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham, D.E. 1996. TRP is cracked but is CRAC TRP? Neuron. 16:1069–1072. [DOI] [PubMed] [Google Scholar]
- Clapham, D.E. 2002. Sorting out MIC, TRP, and CRAC ion channels. J. Gen. Physiol. 120:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham, D.E., L.W. Runnels, and C. Strubing. 2001. The TRP ion channel family. Nat. Rev. Neurosci. 2:387–396. [DOI] [PubMed] [Google Scholar]
- Csutora, P., Z. Su, H.Y. Kim, A. Bugrim, K.W. Cunningham, R. Nuccitelli, J.E. Keizer, M.R. Hanley, J.E. Blalock, and R.B. Marchase. 1999. Calcium influx factor is synthesized by yeast and mammalian cells depleted of organellar calcium stores. Proc. Natl. Acad. Sci. USA. 96:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacion, M., W.G. Sinkins, and W.P. Schilling. 2001. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 530:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzius, D., M. Hoth, and R. Penner. 1994. Non-specific effects of calcium entry antagonists in mast cells. Pflugers Arch. 428:433–438. [DOI] [PubMed] [Google Scholar]
- Freichel, M., S.H. Suh, A. Pfeifer, U. Schweig, C. Trost, P. Weissgerber, M. Biel, S. Philipp, D. Freise, G. Droogmans, et al. 2001. Lack of an endothelial store-operated Ca2+ current impairs agonist- dependent vasorelaxation in TRP4−/− mice. Nat. Cell Biol. 3:121–127. [DOI] [PubMed] [Google Scholar]
- Glouchankova, L., U.M. Krishna, B.V.L. Potter, J.R. Falck, and I. Bezprozvanny. 2000. Association of the inositol (1,4,5)-trisphosphate receptor ligand binding site with phosphatidylinositol (4,5)-bisphosphate and adenophostin A. Mol. Cell Biol. Res. Commun. 3:153–158. [DOI] [PubMed] [Google Scholar]
- Hermosura, M.C., M.K. Monteilh-Zoller, A.M. Scharenberg, R. Penner, and A. Fleig. 2002. Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J. Physiol. 539:445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, P., and R.W. Tsien. 1984. Mechanism of ion permeation through calcium channels. Nature. 309:453–456. [DOI] [PubMed] [Google Scholar]
- Hille, B. 2001. Ionic Channels of Excitable Membranes. 3rd ed. Sinauer Associates, Sunderland, MA.
- Hoth, M., and R. Penner. 1992. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 355:353–356. [DOI] [PubMed] [Google Scholar]
- Hoth, M., and R. Penner. 1993. Calcium release-activated calcium current in rat mast cells. J. Physiol. 465:359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine, R.F. 1990. “Quantal” Ca release and the control of Ca entry by inositol phosphates - a possible mechanism. FEBS Lett. 263:5–9. [DOI] [PubMed] [Google Scholar]
- Jackson, P.S., and K. Strange. 1995. Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J. Gen. Physiol. 105:643–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva, E., A. Zubov, K. Gusev, I. Bezprozvanny, and G.N. Mozhayeva. 2001. Activation of calcium entry in human carcinoma A431 cells by store depletion and phospholipase C-dependent mechanisms converge on ICRAC-like calcium channels. Proc. Natl. Acad. Sci. USA. 98:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznacheyeva, E., A.N. Zubov, A. Nikolaev, V. Alexeenko, I. Bezprozvanny, and G.N. Mozhayeva. 2000. Plasma membrane calcium channels in human carcinoma A431 cells are functionally coupled to InsP3R-PIP2 complexes. J. Biol. Chem. 275:4561–4564. [DOI] [PubMed] [Google Scholar]
- Kerschbaum, H.H., and M.D. Cahalan. 1999. Single-channel recording of a store-operated Ca2+ channel in Jurkat T lymphocytes. Science. 283:836–839. [DOI] [PubMed] [Google Scholar]
- Kim, H.Y., D. Thomas, and M.R. Hanley. 1995. Chromatographic resolution of an intracellular calcium influx factor from thapsigargin-activated Jurkat cells. Evidence for multiple activities influencing calcium elevation in Xenopus oocytes. J. Biol. Chem. 270:9706–9708. [DOI] [PubMed] [Google Scholar]
- Kiselyov, K., G.A. Mignery, M.X. Zhu, and S. Muallem. 1999. a. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol. Cell. 4:423–429. [DOI] [PubMed] [Google Scholar]
- Kiselyov, K., X. Xu, G. Mozhayeva, T. Kuo, I. Pessah, G. Mignery, X. Zhu, L. Birnbaumer, and S. Muallem. 1998. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 396:478–482. [DOI] [PubMed] [Google Scholar]
- Kiselyov, K.I., A.G. Mamin, S.B. Semyonova, and G.N. Mozhayeva. 1997. Low-conductance high selective inositol (1,4,5)-trisphosphate activated Ca2+ channels in plasma membrane of A431 carcinoma cells. FEBS Lett. 407:309–312. [DOI] [PubMed] [Google Scholar]
- Kiselyov, K.I., S.B. Semyonova, A.G. Mamin, and G.N. Mozhayeva. 1999. b. Miniature Ca2+ channels in excised plasma-membrane patches: activation by IP3. Pflugers Arch. 437:305–314. [DOI] [PubMed] [Google Scholar]
- Kozak, J.A., H.H. Kerschbaum, and M.D. Cahalan. 2002. Distinct properties of CRAC and MIC channels in RBL cells. J. Gen. Physiol. 120:221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, C., L. Birnbaumer, and V. Flockerzi. 2002. The TRP channels, a remarkably functional family. Cell. 108:595–598. [DOI] [PubMed] [Google Scholar]
- Mori, Y., M. Wakamori, T. Miyakawa, M. Hermosura, Y. Hara, M. Nishida, K. Hirose, A. Mizushima, M. Kurosaki, E. Mori, et al. 2002. Transient receptor potential 1 regulates capacitative Ca2+ entry and Ca2+ release from endoplasmic reticulum in B lymphocytes. J. Exp. Med. 195:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhayeva, G.N., A.P. Naumov, and Y.A. Kuryshev. 1990. Inositol 1,4,5-trisphosphate activates two types of Ca2+-permeable channels in human carcinoma cells. FEBS Lett. 277:233–234. [DOI] [PubMed] [Google Scholar]
- Nadler, M.J., M.C. Hermosura, K. Inabe, A.L. Perraud, Q. Zhu, A.J. Stokes, T. Kurosaki, J.P. Kinet, R. Penner, A.M. Scharenberg, and A. Fleig. 2001. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 411:590–595. [DOI] [PubMed] [Google Scholar]
- Nilius, B., and G. Droogmans. 2001. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 81:1415–1459. [DOI] [PubMed] [Google Scholar]
- Parekh, A.B., and R. Penner. 1997. Store depletion and calcium influx. Physiol. Rev. 77:901–930. [DOI] [PubMed] [Google Scholar]
- Patterson, R.L., D.B. van Rossum, and D.L. Gill. 1999. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 98:487–499. [DOI] [PubMed] [Google Scholar]
- Prakriya, M., and R.S. Lewis. 2002. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 119:487–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack, B.A., T.V. McDonald, and P. Gardner. 1994. Activation of Ca2+ current in Jurkat T cells following the depletion of Ca2+ stores by microsomal Ca2+-ATPase inhibitors. J. Immunol. 152:5226–5240. [PubMed] [Google Scholar]
- Putney, J.W., Jr., and G.S. Bird. 1993. The signal for capacitative calcium entry. Cell. 75:199–201. [DOI] [PubMed] [Google Scholar]
- Putney, J.W., Jr., L.M. Broad, F.J. Braun, J.P. Lievremont, and G.S. Bird. 2001. Mechanisms of capacitative calcium entry. J. Cell Sci. 114:2223–2229. [DOI] [PubMed] [Google Scholar]
- Randriamampita, C., and R.Y. Tsien. 1993. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 364:809–814. [DOI] [PubMed] [Google Scholar]
- Runnels, L.W., L. Yue, and D.E. Clapham. 2001. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 291:1043–1047. [DOI] [PubMed] [Google Scholar]
- Tang, J., Y. Lin, Z. Zhang, S. Tikunova, L. Birnbaumer, and M.X. Zhu. 2001. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 276:21303–21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepakova, E.S., P. Csutora, D.L. Hunton, R.B. Marchase, R.A. Cohen, and V.M. Bolotina. 2000. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J. Biol. Chem. 275:26158–26163. [DOI] [PubMed] [Google Scholar]
- Tu, J.C., B. Xiao, J.P. Yuan, A.A. Lanahan, K. Leoffert, M. Li, D.J. Linden, and P.F. Worley. 1998. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 21:717–726. [DOI] [PubMed] [Google Scholar]
- Venkatachalam, K., D.B. van Rossum, R.L. Patterson, H.T. Ma, and D.L. Gill. 2002. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 4:E263–E272. [DOI] [PubMed] [Google Scholar]
- Xiao, B., J.C. Tu, and P.F. Worley. 2000. Homer: a link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 10:370–374. [DOI] [PubMed] [Google Scholar]
- Yao, Y., A.V. Ferrer-Montiel, M. Montal, and R.Y. Tsien. 1999. Activation of store-operated Ca2+ current in Xenopus oocytes requires SNAP-25 but not a diffusible messenger. Cell. 98:475–485. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, F., M. Morita, T. Monkawa, T. Michikawa, T. Furuichi, and K. Mikoshiba. 1996. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 271:18277–18284. [DOI] [PubMed] [Google Scholar]
- Zitt, C., C.R. Halaszovich, and A. Luckhoff. 2002. The TRP family of cation channels: probing and advancing the concepts on receptor-activated calcium entry. Prog. Neurobiol. 66:243–264. [DOI] [PubMed] [Google Scholar]
- Zubov, A.I., E.V. Kaznacheyeva, A.V. Nikolaev, V.A. Alexeenko, K. Kiselyov, S. Muallem, and G.N. Mozhayeva. 1999. Regulation of the miniature plasma membrane Ca2+ channel Imin by inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 274:25983–25985. [DOI] [PubMed] [Google Scholar]
- Zweifach, A., and R.S. Lewis. 1993. The mitogen-regulated calcium current of T lymphocytes is activated by depletion of intracellular calcium stores. Proc. Natl. Acad. Sci. USA. 90:6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]