Abstract
The mode of action of extracellular protons on ATP-gated P2X2 receptors remains controversial as either enhancement or depression of ATP-mediated currents has been reported. By investigating, at different pH, the electrophysiological effect of ATP on P2X2 receptors and complementing it with receptor modelling, the present study suggests a unified mechanism for both potentiation and inactivation of ATP receptors by protons. Our experiments on patch-clamped PC12 cells showed that, on the same cell, mild acidification potentiated currents induced by low ATP concentrations (<0.1 mM) and attenuated responses to high ATP concentrations (>1 mM) with emergence of current fading and rebound. To clarify the nature of the ATP/H+ interaction, we used the Ding and Sachs's “loop” receptor model which best describes the behavior of such receptors with two open states linked via one inactivated state. No effects by protons could be ascribed to H+-mediated open channel block. However, by assuming that protons facilitated binding of ATP to resting as well as open receptors, the model could closely replicate H+-induced potentiation of currents evoked by low ATP doses plus fading and rebound induced by high ATP doses. The latter phenomenon was due to receptor transition to the inactive state. The present data suggest that the high concentration of protons released with ATP (and catecholamines) from secretory vesicles may allow a dual action of H+ on P2X2 receptors. This condition might also occur on P2X2 receptors of central neurons exposed to low pH during ischemia.
Keywords: pH, potentiation, fading and rebound, modelling, purinergic
INTRODUCTION
Membrane currents induced by extracellular ATP are due to activation of distinct subclasses of ionotropic receptors (P2X1–7; for review see North, 2002) that display differential sensitivity to changes in external pH (Stoop et al., 1997). As far as P2X2 receptors are concerned, acid pH strongly potentiate them when studied in expression systems (King et al., 1997) or in native tissue (Li et al., 1997). Another distinctive feature of P2X2 receptors is their slow inactivation that can be dramatically accelerated after PKC-dependent phosphorylation of their COOH-terminal (Boue-Grabot et al., 2000), high extracellular Ca2+ (Ding and Sachs, 2000), or excising the cell membrane (Nakazawa and Hess, 1993; Ding and Sachs, 2000). However, on P2X2 receptors of PC12 cells large ATP concentrations were reported to induce rapid decay of currents with subsequent recovery as soon as agonist application was terminated, a phenomenon termed “fading and rebound” (Giniatullin et al., 1996). Investigations with recombinant receptors attributed such a phenomenon to proton block of P2X2 receptors rather than agonist-dependent inactivation (Stoop and Quayle, 1998). Subsequent studies performed with single channels have not, however, revealed any blocking action by protons (Ding and Sachs, 1999), making unclear the nature of the P2X2 response fade. Nevertheless, it is difficult to resolve single channel kinetics when ATP is applied at concentrations ≥50 μM, a condition which would not enable detailed studies of channel inactivation behavior after the high doses (mM) of agonist required for fading and rebound.
A study by Clyne et al. (2002) has shown that a single histidine mutation (H319A) selectively removed proton-dependent potentiation of P2X2 receptors and disclosed an inhibitory effect of protons on currents induced by large ATP concentrations. Therefore, it seems likely that a discrete region located on the receptor extracellular loop can mediate current potentiation as well as inhibition by acid pH.
The action of ATP on PC12 cells or the closely related chromaffin cells remains of considerable physiological interest. In fact, ATP is stored together with catecholamines inside the highly acidic (pH 5.4) environment of secretory vesicles (Johnson, 1987; Hollins and Ikeda, 1997). This fact implies that, upon vesicular release, a sudden surge of high extracellular proton concentration could shape responses induced by ATP. Furthermore, the widespread distribution of P2X2 receptors in peripheral and central nervous systems (Dunn et al., 2001; Khakh et al., 2001; Pankratov et al., 2002) suggests that interplay between ATP and protons could occur extracellularly whenever ischemia, hypoxia, or stroke significantly lowers pH (Lutz, 1992; Lipton, 1999).
The present study analyzed the proton action on native P2X2 receptors of PC12 cells and examined the cause for current fading and rebound. In particular, we addressed the following issues: (a) Can protons rapidly up and down-regulate wild-type P2X2 receptors? (b) What conditions determine the balance between pH-evoked potentiation and inhibition? (c) What are the mechanisms responsible for the action of protons and can they be explained by a unitary kinetic scheme? For these goals we used patch-clamp recording as well as modeling of membrane ATP-induced currents, building on a recently reported model of ATP P2X2 receptors (Ding and Sachs, 1999). Our data are consistent with a bimodal modulation of ATP-elicited currents by protons whereby potentiation of ATP responses was based on increased affinity for agonist binding to P2X2 receptors, whereas inhibitory action was due to promotion of receptor inactivation by high concentration of ATP.
MATERIALS AND METHODS
Patch Clamp Recording
Rat PC12 cells were prepared as described previously (Giniatullin et al., 1996; Khiroug et al., 1997). Briefly, defrosted cells were resuspended with an adequate amount of DMEM or NB (plus 10% FBS). Finally, PC12 cells were plated on poly-l-lysine (5 mg·ml−1) coated Petri dishes and cultured for 3–6 d under an atmosphere containing 5% CO2. Whole-cell currents were recorded from cells of 15–20 μm diameter continuously superfused with control solution containing (in mM): NaCl 132, KCl 5, MgCl2 1, CaCl2 2, glucose 10, HEPES 10; pH was adjusted with HCl or NaOH. Patch pipettes had a resistance of 3–4 MΩ and were filled (in mM) with CsCl 130; HEPES 20; MgCl2 1, magnesium ATP 3; EGTA 5; pH was adjusted to 7.2 with CsOH. Cells were voltage-clamped at −70 mV with series resistance usually compensated by 80%. Currents were filtered at 1 kHz and acquired on IBM PC by means of pCLAMP 7.0 software (Axon Instruments, Inc.).
Modified solutions and drugs were applied by a rapid superfusion system (Rapid Solution Changer RSC-200; BioLogic Science Instruments) placed 100–150 μm away from the recorded cell. ATP applications were 2-s long. Time for solution exchange was ∼30 ms. According to Hollins and Ikeda (1997), single, P2X2 receptor-mediated events of apparently synaptic origin have a rise time of ∼30 ms and a decay lasting 120 ms, suggesting receptor kinetics generally slower than those of fast synaptic receptors. We have simulated the effect of applying 1 mM ATP with faster delivery on the rise and decay times of P2X2 receptor–mediated currents at standard pH using a theoretical model (model 2; see results) that best describes our experimental data. Reducing the solution exchange time from 30 to 3 ms decreased the current rise-time (10–90%) by 14% and the current decay time constant by 13% (unpublished data). Further diminution in delivery time had no effect on activation or deactivation of simulated currents, suggesting that at ∼3 ms agonist–receptor interaction rather than agonist delivery was apparently the rate-limiting factor. Decreasing the simulated solution exchange time from 30 to 3 ms had no effect on the current peak amplitude (unpublished data). These observations indicate that, in view of the seemingly slow receptor kinetics, the distortion in receptor activation and deactivation found experimentally with our application method was modest.
A number of cells displayed inward currents directly generated by switching from control to acid solution. The threshold for this phenomenon was pH 6.5; solutions with pH = 6 or lower induced currents which, at −70 mV holding potential, peaked at −91 ± 15 pA and were rapidly reversible on washout. Their reversal potential was ∼60 mV. Such responses have been described recently in PC12 cells (Chu et al., 2002) as due to activation of proton-sensitive channels, analogous to those found on mammalian brain neurons (Bolshakov et al., 2002). Whenever acid pH solutions evoked such responses, they were subtracted from those observed in the presence of ATP at the same pH value. The presence of pH-induced membrane currents was, however, not the cause for the observed changes in ATP responses as analogous data were obtained with PC12 cells essentially devoid of pH-elicited currents.
All chemicals, including enzymes for cell culture, were from Sigma-Aldrich; culture mediums were obtained from GIBCO BRL (Life Technologies).
Data Analysis
All data are presented as mean ± SEM mean (n = number of cells) with statistical significance assessed with paired t test (for parametric data) or Wilcoxon test (for nonparametric data). Statistical significance of data was assessed using SigmaStat (Jandel Scientific; version 2.0). A value of P < 0.05 was accepted as indicative of statistically significant difference.
The EC50 (ATP concentration producing 50% of maximal current amplitude) and IC50 (ATP concentration producing 50% decrease in maximal current amplitude) values were calculated with Eq. 1 from Papke and Porter Papke (2002):
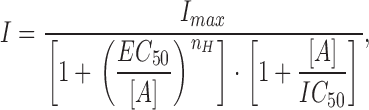 |
(1) |
where I is the peak current amplitude, I max is the peak current amplitude by maximal ATP concentration (1 mM), [A] is the ATP concentration, and n H is the Hill coefficient.
The extent of current fading was measured at the end of ATP application and calculated as a percentage of the first current peak amplitude. The extent of rebound was measured as a percentage increment over faded current (immediately before agonist washout).
Computer Simulation Method
In general, based on the mass action law, we formulated a set of differential equations for each kinetic state in analogy with the approach used by Chretien and Chauvet (1998), whereby:
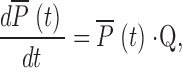 |
(2) |
where 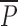 is a vector composed of probabilities of the receptor occupying each kinetic state at time t and Q is the matrix of transitions between the states. Our in-house–developed program was written in Pascal and used on an IBM-compatible PC to solve numerically this set of differential equations using the eight-order Runge-Kutta method (Baker et al., 1996).
is a vector composed of probabilities of the receptor occupying each kinetic state at time t and Q is the matrix of transitions between the states. Our in-house–developed program was written in Pascal and used on an IBM-compatible PC to solve numerically this set of differential equations using the eight-order Runge-Kutta method (Baker et al., 1996).
The P2X2 receptor currents were calculated according to
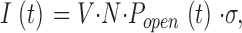 |
(3) |
where V is the holding voltage (mV), N is number of channels, P open is the probability of open channel state, and σ is the channel conductance. We assumed σ to be constant. The adequacy of the simulated responses to reproduce experimental records was judged by eye.
Online Supplemental Material
The values of the rate constants influenced lay protons and ATP in Models 1 and 2 are provided as supplemental material, available at http://www.jgp.org/cgi/content/full/jgp.200308825/DC1.
RESULTS
Potentiation and Inhibition by Protons of ATP Currents
Recordings were obtained from cells tested in standard pH (7.4) solution and subsequently studied after exposure to acid solution containing various concentrations of ATP.
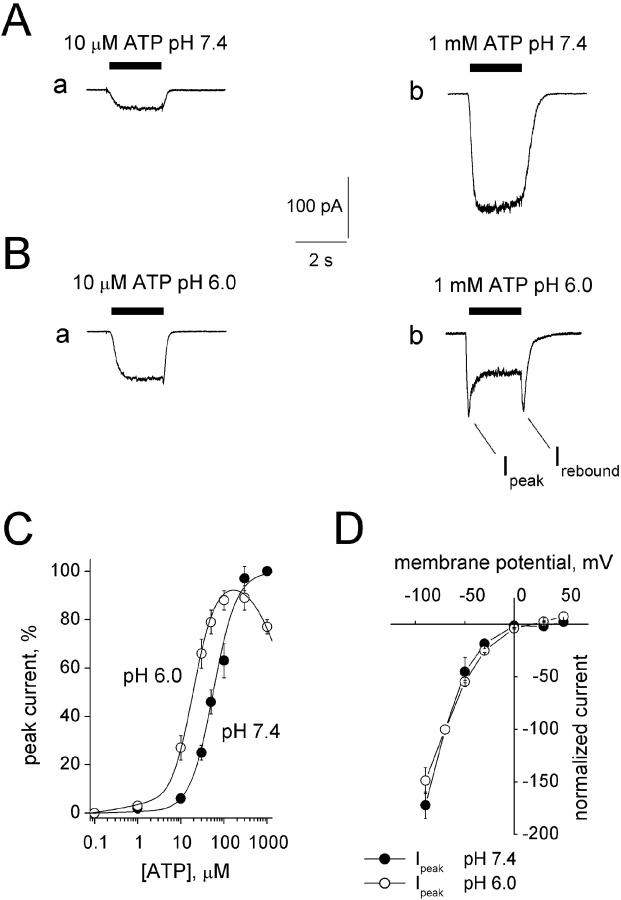
Fig. 1 A shows, for the same cell, how pH could differentially modulate inward currents induced by 10 μM or 1 mM ATP (2-s application). At physiological pH (7.4) the inward current evoked by 10 μM ATP had no fading, whereas the larger current observed with 1 mM ATP displayed slight fading from its peak (Fig. 1 A, a and b). When 1 mM ATP was applied for 8 s (unpublished data), a slow current fade developed with monoexponential time constant (τ) of 7,500 ± 1,500 ms (n = 9). This slow process of desensitization was not further investigated in the present study.
Figure 1.
Modulation of ATP currents by extracellular pH. A shows, at pH 7.4, inward currents induced by rapid superfusion of 10 μM (a) or 1 mM (b) ATP onto the same PC12 cell clamped at −70 mV. B shows responses (a and b) evoked by the same concentrations of ATP applied via acid solution (pH 6). Note enhancement of current amplitude in response to 10 μM ATP and reduction in peak amplitude in response to 1 mM ATP. In the latter case the response shows rapid fading to a steady-state level maintained during ATP application. After ATP washout and return to standard pH 7.4 solution, there is transient current rebound. (C) Plot of log ATP concentration versus peak current amplitude (as a percentage of the response to 1 mM ATP at pH 7.4; see materials and methods for fitting function). At pH 6.0 the curve is shifted to the left and shows reduced maximum response with amplitude decline at higher agonist concentration. Data are from 11 cells. (D) I/V curves for responses induced by 1 mM ATP tested at variable holding potentials. Current amplitude is normalized with respect to the one recorded at −70 mV, taken as 100% and assigned negative value. Note that the I/V is virtually the same for peak current amplitude at pH 7.4 or 6. No alteration in reversal potential is apparent. Data are from six cells.
When ATP was retested for 2 s at pH 6, the 10 μM ATP response was strongly enhanced (251%; Fig. 1 B, a). Conversely, the 1 mM ATP response had smaller peak amplitude (71%) than its control and was followed by fading to 50% with subsequent rebound (84%) on washout (Fig. 1 B, b). On average such a strong current fading, characterized by τ = 740 ± 50 ms (n = 9), led to an apparently steady-state current corresponding to 54 ± 2% of the peak amplitude. To minimize nonspecific effects of large extracellular proton concentrations, most experiments were restricted to testing responses at pH 6, although, for the purpose of comparison with other studies, responses were occasionally tested at pH 5.4 as well.
Fig. 1 C shows the log concentration-response curve for ATP at pH 7.4 or 6. Responses were measured in terms of their peak amplitude and normalized with respect to 1 mM response to ATP in standard solution. As shown by Fig. 1 C, acid pH enhanced responses elicited by concentrations up to 100 μM and depressed the 1 mM response. In our conditions, the calculated EC50 value for ATP shifted from 56 to 19 μM for 25 times change in proton concentration (from pH 7.4 to 6). The Hill coefficient remained similar (1.6) in control and at pH 6.
The rapid fading and rebound of currents induced by high concentrations of ATP at acid pH suggested quick inactivation and reactivation of open P2X2 receptors. If the mode of action by protons was to block a site within the channel (Stoop and Quayle, 1998), this effect should be modified by the electric field imposed to the membrane. Fig. 1 D indicates that, on average (n = 7), changing external pH from 7.4 to 6.0 did not alter the reversal potential of the ATP peak current. In addition, Fig. 2 shows experimental data concerning the voltage dependence of 1 mM ATP currents tested at pH 6. While there was a slight reduction in the degree of fading (measured as steady-state current normalized with respect to the response peak; Fig. 2 B) when cells were depolarized, neither the fading time constant (Fig. 2 A) nor the rebound current (Fig. 2 C) were related to the holding potential, indicating that proton-evoked inhibition of ATP currents was essentially voltage independent.
Figure 2.
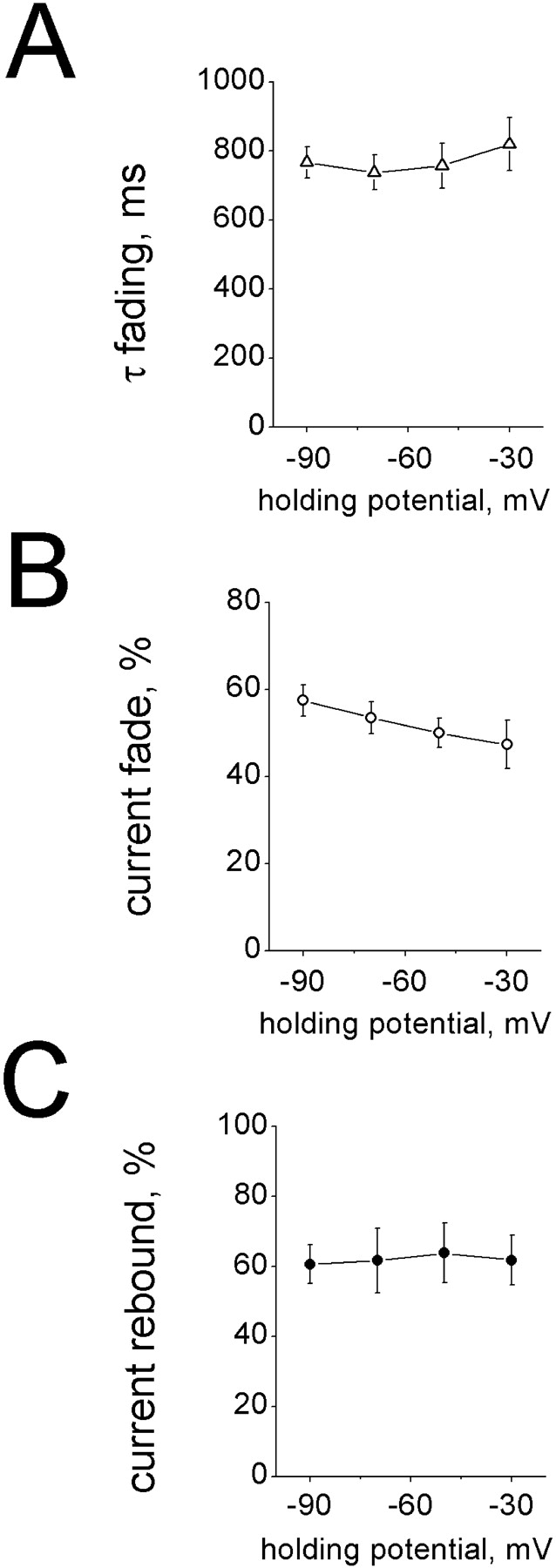
Voltage dependence of fading and rebound processes of currents induced by 1 mM ATP at pH 6. (A) Plot of changes in time constant of current fading (τfading; obtained by monoexponential fitting) versus holding membrane potential. (B) Degree of current fade (measured at the end of ATP application and expressed as a percentage of ATP peak current) at various holding potential values. (C) Rebound current (expressed as a percentage rise over the inactivated current) at various holding potentials. Data are from eight cells.
Fig. 3, A–C , shows the pH dependence for fading and rebound of ATP currents. In particular, acidification brought out fading (Fig. 3 B) and rebound (Fig. 3 C) only when the ATP concentration was ≥100 μM; both phenomena intensified with increasing proton concentrations and were associated with faster onset of fading, as demonstrated by the decrease in fading τ values (Fig. 3 A). These observations suggest that the main determinant for fading and rebound was the ATP concentration rather than the absolute value of pH. In accordance with this suggestion, there was a tight, negative correlation between the rebound amplitude and the extent of current inactivation with r = −0.93 (P < 0.001; n = 8; unpublished data).
Figure 3.
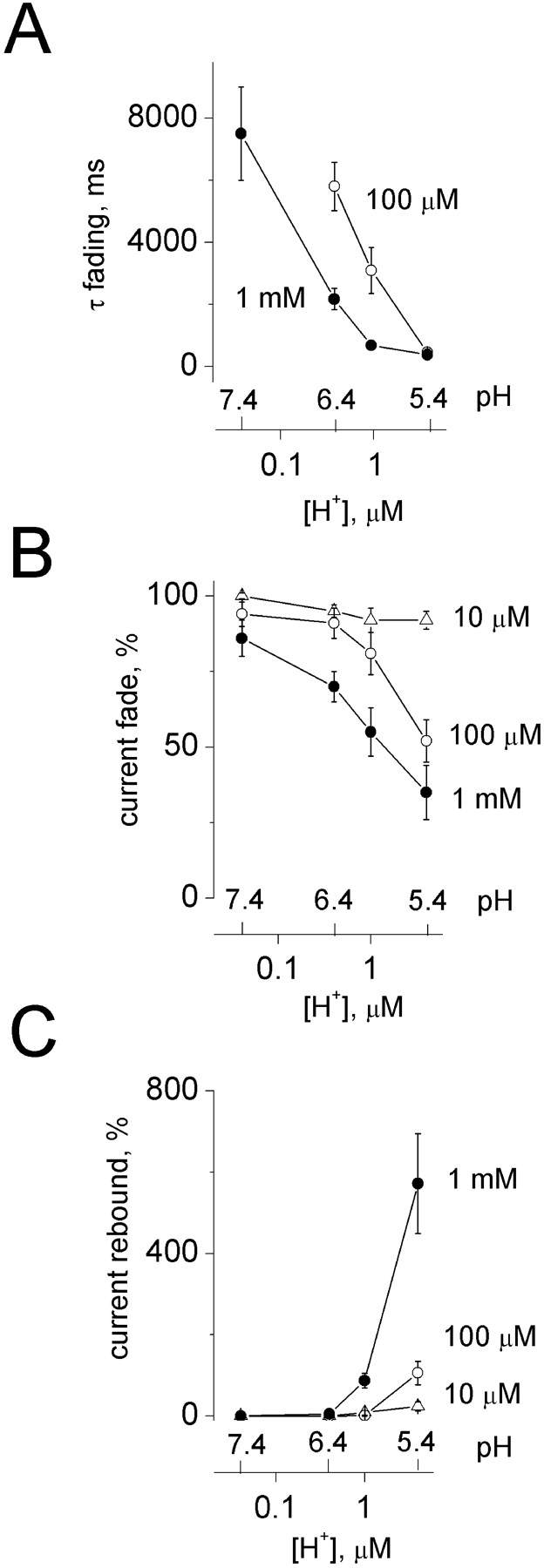
ATP current fading and rebound are dependent on ATP and proton concentration. (A) Plot of time constant of current fading (τfading) versus proton concentrations (log scale, μM; corresponding pH values are shown above) at two ATP concentrations. (B) Degree of current fade (measured at the end of ATP application and expressed as a percentage of ATP peak current) for various pH values. Note that 10 μM ATP cannot elicit current fading even at very acid pH. Fading becomes, however, apparent with 100 μM ATP applied at pH 6.0 and is very strong (∼50% current decay) with 1 mM ATP at pH 6.C, rebound current (expressed as a percentage rise over the inactivated current) at various pH values. Like current fade in B, rebound is absent with 10 μM ATP and manifested for higher ATP concentrations when pH is 6. Data are from nine cells.
The data in Figs. 1–3 show that protons produced two modulatory effects on P2X2 receptors. One consisted of facilitation of responses induced by small to moderate agonist concentrations. The other one was inhibition of ATP responses with corresponding onset of fading and rebound. We next used kinetic modeling of receptor activity to gain insight into the mechanisms underlying these phenomena.
Modeling Proton Effects on P2X2 Receptor Function
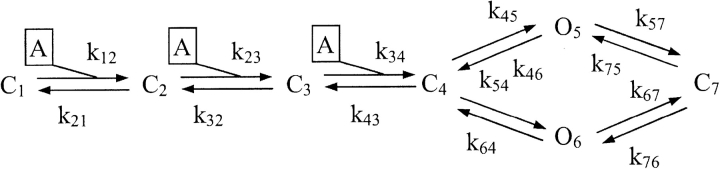
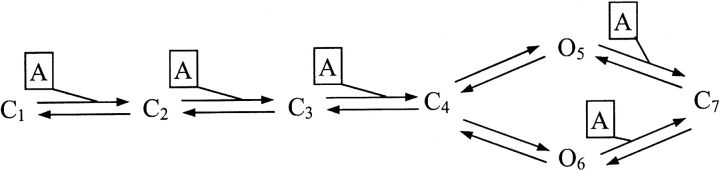
We considered that minimal requirement for the usefulness of any model was its ability to reproduce current time-course close to the observed one, to generate the phenomenon of fading and rebound with large ATP doses as well as to manifest potentiation of responses with low doses of ATP. Previous investigations by Ding and Sachs (1999) based on recording from outside out membrane patches have extensively analyzed the kinetics of P2X2 receptors expressed in HEK cells and provided an advantageous model to describe their behavior. The model that Ding and Sachs (1999) described as the most appropriate for P2X2 receptors was therefore applied to our present results, using their rate constant values based on single channel and modeling data. Such a model is termed 1–4 (see Fig. 13 in Ding and Sachs, 1999) and is represented as
 |
1 |
MODEL 1
where C1,2,3,4,7 represent closed states of the receptor, O5,6 are open states of the receptor, A is the ATP molecule, and knn are rate constants for the corresponding forward and backward reactions. Thus, this is a “loop” scheme that relies on two open receptor states (O5 is the most frequently detected one; Ding and Sachs 1999) reached via C4 (closed, and binding three ATP molecules), and four closed states, one of which (C7) we assumed to be the one responsible for ATP receptor inactivation. Fig. 4 A shows that the model adequately described the nondesensitizing nature of the responses produced by 1 mM or 10 μM ATP, although our experimental currents had slower onset and offset rates at the standard pH 7.4 of the control buffer solution (BS; compare with Fig. 1 A).
Figure 4.
Modeling the role of protons on fading and rebound of ATP currents. (A) Simulated inward current generated with Model 1 (Ding and Sachs, 1999) assuming 1 mM (solid line) or 10 μM (dotted line) ATP application in standard buffer solution (BS). (B) Simulation of fading and rebound of inward currents (elicited by 1 mM ATP applied at pH 6) generated with Model 1 assuming various degrees (a, b, and c; see Table I) of dependence of the forward rate constants k57 and k67 on proton concentration. (C) Simulated response evoked by 1 mM ATP transiently applied at pH 6.0 with washout back to standard BS. Note that rebound is always lost when washout is performed with BS at pH 6. Vertical calibration refers to probability of observing the receptor open state.
We first examined the possibility that this model could represent the generation of current fading and rebound observed when large doses of ATP were applied at acid pH. The first step was to consider in Model 1 whether by making the rate constants for transitions from open states to the closed one dependent on pH could generate fading and rebound. To simulate this process a constant (kn) became
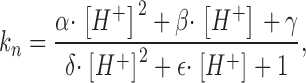 |
(4) |
which corresponds to Eq. 9 by Liu et al. (1996) to describe pH dependence of substrate binding to a protein. This simulation (applied to k57 and k67 for the O5 and O6 to C7 transition) assumed that 1 mM ATP was applied at pH 6. Fig. 4 B shows this model could generate fading and rebound as indicated by the three simulated responses (a, b, c), on the assumption of different degrees of pH dependence of the forward rate constants (Eq. 4). The actual values used for those simulations are listed in Table I . Assigning strong (trace a) or weak (trace c) reaction dependence on protons could affect the extent of fading (residual current at the end of ATP application), but it could not modify the very fast time constant of fading. This condition contrasts with the experimental observation of the dependence of fading on extracellular protons (see Fig. 3 A). Fig. 4 C shows that, regardless of the strength of pH dependence of such forward reactions (traces a, b, and c), rebound was lost if washout of ATP was performed with acid solution (pH 6).
TABLE I.
Coefficient Values Applied to Certain Rate Constants, k57 and k67, to Simulate pH Dependence in Model 1
| Constant | Versions | α | β | γ | δ | ε |
|---|---|---|---|---|---|---|
| k57 | a | 375 | 25 | 58 | 0.175 | 0.0025 |
| b | 156 | 12.5 | 59 | 0.175 | 0.0025 | |
| c | 37.5 | 2.5 | 59.5 | 0.175 | 0.0025 | |
| k67 | a | 5,756 | 384 | 890 | 0.175 | 0.0025 |
| b | 2,395 | 192 | 906 | 0.175 | 0.0025 | |
| c | 576 | 38 | 913 | 0.175 | 0.0025 |
The values of k57 and k67 are expressed as (mM s)−1 for proton concentration expressed as μM.
A distinct case for pH-induced inhibition of ATP currents would be direct block of ATP-gated channels by protons. This possibility draws analogies from the fact that fading and rebound are often caused by drug-induced block of open channels and subsequent prompt relief like, for instance, in the case of mecamylamine on nicotinic receptors (Giniatullin et al., 2000) or Mg2+ (or amantadine) on NMDA receptors (Sobolevski and Yelshansky, 2000). Despite the experimentally observed lack of voltage dependence for fading and rebound (Figs. 1 D and 2) that makes channel block by protons unlikely, we tested if fading and rebound in the presence of acid pH might have been due to simple proton block of the open states O5 and O6. The simulation yielded records (unpublished data) indistinguishable from those obtained for the proton dependence of the reactions leading the inactive state C7 (see Fig. 4, B and C), whenever we set the model to give similar level of steady-state current achieved under conditions of proton block.
Note that tests based on simplified receptor models comprising one open state only failed to yield adequate simulations because the rebound response was excessively large and long-lasting, and were not further used (unpublished data).
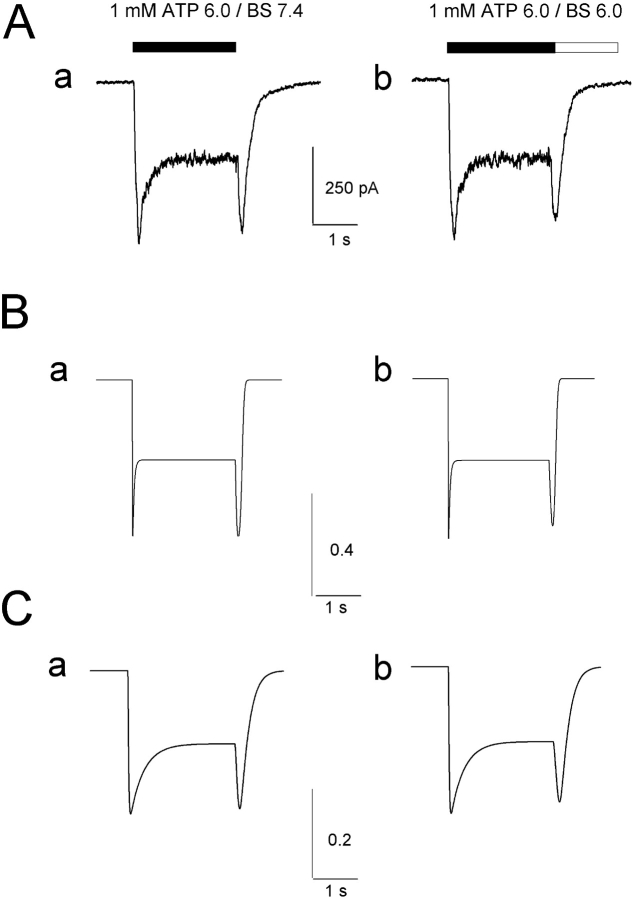
Proton dependence of the transitions to C7 and proton-induced channel block predicted lack of rebound on washout with acid pH medium (Fig. 4 C). Such a prediction could be readily verified with experimental tests. Data depicted in Fig. 5 A show that 1 mM ATP applied at pH 6.0 and then washed out with a solution at either pH 7.4 (Fig. 5 A, a) or pH 6.0 (Fig. 5 A, b) generated fading and rebound in both cases (same cell). On average for a sample of five cells all tested at two pH values, the rebound amplitude (as a percentage of steady-state current) was 87 ± 18% with pH 7.4 washout, and 54 ± 9% with pH 6.0 washout. These observations thus suggest that a model assuming either pH dependence for the P2X2 receptor transition to inactive state, or simple channel block induced by protons could not explain our electrophysiological data.
Figure 5.
Experimental and modeling data concur to show pH dependent fading and rebound. (A) Electrophysiological records of inward currents induced by 1 mM ATP applied at pH 6.0 and followed by washout with standard BS (a) or pH 6.0 solution (b). In either case fading and rebound are present. (B) Simulated currents evoked by 1 mM ATP assuming washout with standard solution (a) or acid solution (b). Traces are obtained with Model 2, which is based on pH and ATP dependence of the transition from open states to inactivated state. (C) Simulated trace reproducing responses to 1 mM ATP for the same conditions as in A and B and optimization of rate constants (Tables II and III). Vertical calibration for B and C refers to the probability of observing the open states O5 and O6 (see Model 2).
Protons may Promote P2X2 Receptor Inactivation by ATP
As neither protons nor ATP alone could induce fading, their concomitant action was an example of synergy at receptor level to obtain rapid onset of current inactivation. We therefore considered the possibility that protons could act as a cofactor to facilitate receptor inactivation by high doses of ATP via modification of channel conformation (Ding and Sachs, 1999). For the present simulation, we thus implied that with acid pH, additional ATP molecules could bind to the O5 and O6 states to promote their conversion to C7 (Model 2).
 |
2 |
MODEL 2
Of course, in this simplified scheme there is no identification of the additional ATP binding sites, which might be the agonist recognition sites or an allosteric site, but not the ion channel itself as ATP is strongly negatively charged and receptor inactivation is voltage independent. By assuming that the k57 and k67 rate constants for the interaction of free ATP molecules with the O5 and O6 state were also pH dependent (see Eq. 4), we obtained simulated traces that generated rebound even with acid pH washout (Fig. 5 B, a and b, with pH dependence similar to the one depicted for trace b in Fig. 4 B). Because rebound was generated from a quasi steady-state condition of agonist application and current level, it seemed likely that it represented a sudden surge of current due to the reaction from C7 to O5 and O6. The rate of rise of the rebound current would then reflect the receptor reactivation process from inactive state. Experimentally, we found that the risetime of the rebound current was virtually the same (13 and 14 ms, respectively; Fig. 5 A, a and b) regardless of the pH value of the washout solution, suggesting that protons were not presumably changing k75 and k76. This observation was consistent with Model 2 in which pH dependence was assigned only to the forward reaction from open states to inactive state. Although Model 2 contained all the essential features to reproduce the properties of fading and rebound, it generated records too fast with respect to those observed experimentally. This condition prompted us to optimize the reaction rate constants (Tables II and III) to slow down the fading and rebound processes. In this way, it was possible to simulate current records analogous to the experimental ones (Fig. 5 C, a and b). A full list of the rate constants used for simulations is provided as Online supplemental material available at http://www.jgp.org/cgi/content/full/jgp.200308825/DC1.
TABLE II.
Values of Rate Constants Used to Simulate ATP Responses (Model 2)
| Constant | k21 | k 32 | k 43 | k 45 | k 54 | k 46 | k 64 | k 75 | k 76 |
|---|---|---|---|---|---|---|---|---|---|
| s−1 | s−1 | s−1 | s−1 | s−1 | s−1 | s−1 | s−1 | s−1 | |
| Value | 20 | 40 | 60 | 20 | 40 | 5 | 14 | 0.005 | 8.2 |
TABLE III.
Coefficient Values Applied to Certain Rate Constants to Simulate Their pH Dependence with Facilitated Binding of ATP (Model 2)
| Constant | α | β | γ | δ | ε |
|---|---|---|---|---|---|
| k12 | 28,437.5 | 3,250 | 1,497 | 7.5 | 2.575 |
| k23 | 16,562.5 | 2,500 | 876 | 7.5 | 2.575 |
| k34 | 237,500 | 12,500 | 260 | 62.5 | 20 |
| k57 | 0.00625 | 0.1 | 0.043 | 0.0625 | 1.5 |
| k67 | 79.375 | 25.5 | 3 | 0.00313 | 0.5 |
The values of k12, k23, k34, k57, and k67 are expressed as (mM s)−1 for proton concentration expressed as μM. For the meaning of α, β, γ, δ, and ε, see Eq. 4.
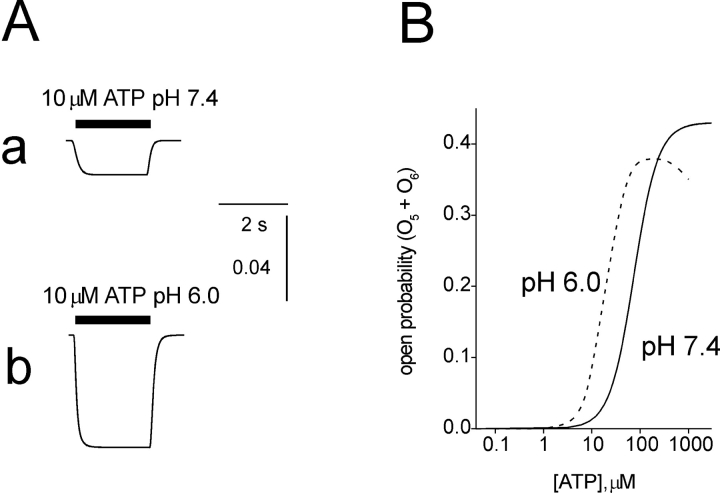
A further important requirement of the model was its ability to reproduce the enhancement of small responses to ATP by acid pH (King et al., 1997, and present Fig. 1). To include this response property in simulated data, we followed the suggestion that protons may increase the affinity of the binding site for ATP (Ding and Sachs, 1999). One way to simulate this condition was to make the ATP binding rate constants k12, k23, and k34 larger (see Model 2) and dependent on pH (see Table III) in analogy with the dependence of k57 and k67 on protons. Fig. 6 A, a and b, shows an example of the simulated enhancement by acid pH of responses to low concentrations of ATP with good correspondence with those found experimentally. The theoretical ATP dose response plots (Fig. 6 B) based on Model 2 are calculated assuming external pH 6.0 (dashed line) or pH 7.4 (solid line) and are similar to the experimental ones (Fig. 1 C). It is noteworthy that the present theoretical data closely accords with the experimental results by Ding and Sachs (1999) who report maximum open probability of 0.55 for 100 μM ATP.
Figure 6.
Modeling of pH induced potentiation of responses to small concentrations of ATP. (A) Model 2 is used to generate simulated traces to 10 μM ATP applied at standard pH (a) or pH 6.0 (b). Note potentiation of effect of ATP at acid pH. (B) Theoretical ATP dose response plots drawn on the basis of Model 2 for pH 6.0 (dashed line) or pH 7.4 (solid line). The ordinate shows open probability of ATP receptors. The Hill coefficients were 1.7 at 7.4 pH (EC50 value = 74 μM) and 1.9 at pH 6.0 (EC50 value = 19 μM).
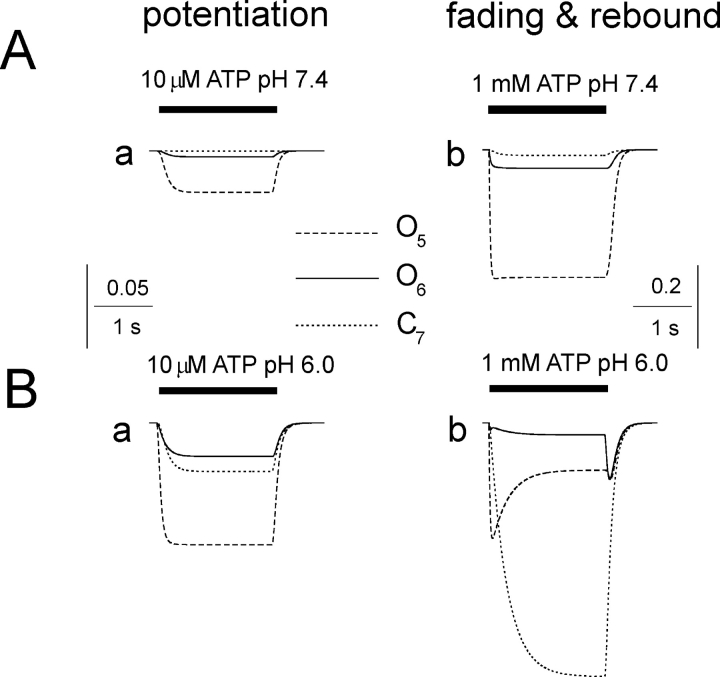
Unlike our experimental approach, modeling allowed exploring the relative role played by open states and the inactivated C7 state in the potentiation as well as fading plus rebound. Thus, we further examined the distinct contribution by O5, O6, and C7 to the simulated ATP currents at neutral or acid pH in analogy with the experimental data shown in Fig. 1, A and B. Each one of these three states was expressed in terms of calculated probability of occurrence prior, during, and after ATP application. The Fig. 7 A, a shows that at neutral pH both the O5 and, to lesser extent, the O6 states were responsible for the small current induced by 10 μM ATP while the C7 state had minimal probability of occurrence. Likewise, at neutral pH (Fig. 7 A, b), the application of 1 mM ATP was again associated with larger probability of O5 versus O6 because k45 was larger than k46 (see Model 2; Ding and Sachs, 1999). Fig. 7 B, a shows that, when 10 μM ATP was applied at pH 6, there was increased probability of detecting O5 and O6. There was also a measurable probability to detect the C7 state, although the latter was insufficiently common and too slow to generate obvious fading and rebound. Fig. 7 B, b (note different amplitude calibration from A, a) shows that, owing to the acid pH and the large dose (1 mM) of ATP, all three states now played a significant role in generating the complex waveform of the response. In fact, O5 appeared rapidly and decayed in coincidence with gradual increment in the O6 probability. However, the inactivated C7 state also developed strongly, so the decline (fading) of the overall current profile was probably determined by the emergence of C7, indicating conversion of receptors to inactive state. Upon termination of ATP application, the reaction from C7 to O6 was kinetically more favored owing to the larger value of k76 versus k75, and determined the appearance of the rebound component. Thus, simulated conditions suggest that fading was primarily due to rapid decrease in O5 states, whereas rebound was due to reemergence of O6 states. The model also demonstrates the need for two open states to simulate kinetically plausible peak current fade and rebound in accordance with the original scheme proposed by Ding and Sachs (1999).
Figure 7.
Contribution of ATP receptor states to the action of protons on current enhancement, fading, and rebound. (A) Simulated kinetic profile of probability for the receptor states, namely O5 and O6 (open receptor) and C7 (inactivated receptor) in the presence of 10 μM (a) or 1 mM (b) ATP transiently applied at standard pH. Note the larger contribution by O5 state to both responses. (B) Under analogous simulated conditions, transient application of ATP at pH 6.0 generates potentiation (a) or fading and rebound (b). Even at pH 6.0 with 10 μM ATP there is development of C7 state, although this is a slow process that cannot influence the macroscopic current primarily dependent on the strong enhancement of the O5 state probability. When 1 mM ATP application is simulated, O5 state largely contributes to the initial current peak while the C7 state probability is building up as a consequence of the transition of receptors from O5 to C7. This phenomenon is responsible for the large current fading. The O6 state is kinetically slower and mainly contributes to the rebound because the rate constant of C7 transition to O6 is larger than the one for C7 transition to O5.
DISCUSSION
The main result of the present study was the demonstration that a unitary mechanism could be responsible for the contrasting effects of protons on ATP receptors of PC12 cells. Thus, by assuming that agonist binding and receptor inactivation were similarly pH dependent, it was possible to account for fading and rebound of ATP currents generated by high agonist concentration, and enhanced current amplitude induced by low doses of ATP. These observations obtained with PC12 cells, which are of neural origin as they derive from adrenal chromaffin cells, can help to understand the complex interaction between protons and ATP receptor signaling that occurs on autonomic and sensory neurons.
Effects of pH on ATP Currents of PC12 Cells
Previous studies have shown that ATP currents recorded from PC12 cells are predominantly mediated by P2X2 receptors (Michel et al., 1996; Vulchanova et al., 1996; Khakh et al., 2001) with minimal propensity to inactivate despite sustained or closely repeated application of the agonist.
A former report described how rapid fade and rebound of ATP currents could be generated when large doses of ATP were applied (Giniatullin et al., 1996), a phenomenon subsequently attributed to rapid proton-induced block of ATP channels (Stoop and Quayle, 1998). This view is in contrast with a well-known feature of P2X2 receptors, namely that their responses are strongly enhanced by extracellular protons (King et al., 1997; Wildman et al., 1998; Ding and Sachs, 1999).
The present investigation tried to address these two apparent paradoxes concerning the action by ATP: is it facilitated or inhibited by protons, and does it inactivate quickly? A further goal was to understand the mechanism of action by protons using receptor modeling constrained by our electrophysiological data and to confirm model predictions with targeted experimental tests.
It should be noted that the present study relied on a relatively slow method of agonist application. For certain fast transmitters, like for example, GABA acting on GABAA receptors, the activation (and inactivation) kinetics are so fast that clear differences emerge between data obtained with fast superfusion of the agonist and ultra-fast agonist superfusion (Mozrzymas et al., 1999). In the case of P2X2 receptors, the kinetics of ATP–receptor interaction are comparatively slower as indicated by miniature events mediated by endogenous ATP with rise-time of ∼30 ms (Hollins and Ikeda, 1997) that is at least one order of magnitude slower than the one reported for GABAA receptors (0.7 ms, Mozrzymas et al., 1999; or even 0.2 ms, Williams et al., 1998). Simulation tests suggested that the present agonist delivery method introduced an error quantifiable in <15% in terms of receptor activation and deactivation, thus indicating a modest, yet detectable, distortion of the channel kinetics under investigation. The same simulations for 2-s long application of ATP, however, showed no changes in response amplitude.
At standard pH responses there was minimal fading (and no rebound) of responses induced by large or small doses of ATP. However, extracellular acidification largely potentiated small doses of ATP by shifting the bottom part of the log dose response curve to the left, whereas it inhibited currents induced by high doses. Hence, the dose response plot crossed the control one. These results confirmed that, under our conditions, both effects by protons, potentiation and inhibition, could be produced on the same cells.
Protons Do Not Block ATP Receptor Channels
An important aspect of responses to high doses of ATP in acid solution was the appearance of fading and rebound (threshold was pH 6.4 for 1 mM ATP), which displayed the same voltage dependence as the standard ATP current.
The voltage independence of proton inhibition makes unlikely proton-induced channel block as proposed by Stoop and Quayle (1998), although it might be conceivable that the proton site of action could be remote from the channel pore and little sensitive to membrane potential changes. This condition could be readily simulated by modeling pH induced channel block in the present study. In particular, when ATP was applied at acid solution and washed out with either the same agonist-free solution or neutral pH solution, in both cases fading and rebound were always found experimentally, unlike the prediction of channel block by protons. Furthermore, Ding and Sachs (1999), working on single channels, also failed to corroborate pH elicited channel block.
Unitary Mode of Action of Extracellular Protons
Previous studies of ATP receptors in expression systems have indicated that acidification increases the affinity of the P2X2 receptors for ATP (King et al., 1997; Ding and Sachs, 1999), a finding supported by the present study as long as low doses of ATP were used (Fig. 1 C) because the ATP EC50 value became significantly smaller (King et al., 1997; present data). We therefore wondered whether the most appropriate receptor model used by Ding and Sachs (1999) to account for the receptor operation might be extended to consider the contrasting action of protons on ATP currents.
The “loop” reaction model preferred by Ding and Sachs (1999) includes multiple closed or open states in which receptors can dwell. This provided us with a starting scheme that gave realistic simulations of ATP currents at standard pH and it enabled us to test the action of protons. The model most appropriate to simulate our data was based on pH dependence of the rate constants for most steps of the ATP–receptor binding reaction, regardless of their open or closed state. We postulated that, among the closed states, C7 represented the receptor inactive conformation bound by additional ATP. This condition is similar to the process whereby protons can modulate the cooperative binding of selective ligands (for instance, certain enzymatic substrates) to their multisubunit receptors or catalytic enzyme sites, and can thus impair enzymatic function by changing the substrate affinity (Sugasaki et al., 2001; Naught et al., 2002).
In our scheme, all main characteristics of ATP response modulation by protons could be reproduced, including potentiation as well as fading and rebound independent from the washout solution. On the basis of these data we suggest that there was a unitary explanation to interpret the diverse actions (enhancement and inhibition) of protons on ATP currents. In fact, assuming that the main effect of protons was to increase the affinity of ATP for binding the C1–3 (potentiation), and O5 and O6 (fading and rebound) states of P2X2 receptors, it was possible to reproduce all the properties of experimentally observed responses. It is noteworthy that, on AMPA (Ihle and Patneau, 2000; Lei et al., 2001), nicotinic (Abdrakhmanova et al., 2002), GABA (Huang and Dillon, 1999), or NMDA (Mott et al., 1998) receptors, the inhibitory action of protons is suggested to be due to facilitation of agonist-induced receptor inactivation, indicating analogies in the inhibitory action of protons on ionotropic receptors.
At neutral pH it was difficult to observe fading and rebound even with a large (1 mM) concentration of ATP. This result also indicates that, whenever ATP is applied to a PC12 cell, the interaction of ATP with its receptor does not necessarily follow all the steps of Model 2: in fact, reaching the C7 state (which is arrived at with a rate constant much smaller than those characterizing the transitions from C1 to C3) requires the facilitatory action of protons plus a large amount of ATP. A distinctive feature of the present model was the assumption of further binding of ATP to the open receptors to drive the reaction to the C7 state, a condition that therefore needs large agonist concentration. This suggestion is supported by our data demonstrating that fading and rebound strongly depended on ATP concentration at the same pH value.
Fading and Rebound
Fading and rebound of agonist-evoked membrane currents are not unique to ATP receptors. For example, neuronal nicotinic receptors of nerve cells (Maconochie and Knight, 1992) or muscle (Drapeau and Legendre, 2001) exposed to high agonist concentrations can generate currents that inactivate rapidly and bounce back upon termination of agonist application. In such cases fading and rebound were attributed to agonist-induced open channel block as the positively charged agonist may be attracted by the negatively charged cationic channel (Colquhoun and Sakmann, 1985). In the case of ATP receptors, which also comprise negatively charges cationic channels, such an explanation seems unlikely because ATP itself is negatively charged and neither fading nor rebound was voltage dependent. As in our model, fading and rebound required additional binding of ATP and the receptor open states were ATP occupied, it seems necessary to suggest that such additional binding of ATP took place at a site (not necessarily coincident with the agonist recognition site) which could promote receptor inactivation. Since the structure of P2X2 receptors is believed to be a tetra or pentameric assembly of homologous subunits (Kim et al., 1997; Khakh, 2001), it seems likely to contain the motifs for multiple agonist binding.
Functional Implications
The ability by protons to potentiate ATP-mediated currents is thought to be an important mechanism to modulate signals on sensory neurons. In fact, both ATP and acid pH are components released during the process of inflammation and their concurrent action may facilitate activation of P2X2 receptors on sensory neurons (Li et al., 1997; Stoop et al., 1997; Dunn et al., 2001; Khakh, 2001). If the extracellular level of ATP is very high, it is possible that pH-induced inhibition of ATP responses concurs to provide a self-limiting mechanism for sensory activation. In the central nervous system P2X2 receptors are widely distributed amongst neurons to mediate excitatory transmission (for review see Khakh, 2001; North, 2002). It is tempting to speculate that conditions like ischemia or anoxia that determine acid pH in brain tissue can significantly contribute to amplify and then limit the signals generated by ATP. Furthermore, even ATP receptors consisting of coassembled heterodimers of P2X6 and P2X2 subunits retain pH-sensitive potentiation and inhibition (King et al., 2000; Dunn et al., 2001), thus extending the targets for proton modulation of purinergic transmission.
Another interesting process for ATP–pH interaction is the vesicular release of these agents from catecholaminergic neurons, of which a well-tested example is provided by chromaffin cells, the progenitors of PC12 cells. In such vesicles ATP is highly concentrated (mM) in a very acid environment (5.4 pH, see Johnson, 1987) from which it is released together with catecholamines. The effective concentrations of ATP and protons reaching target cells remain unknown, but in the case of sufficient proximity of target cells, it is likely that ATP receptors may be modulated by protons. During this dynamic process of transient concentrations it may be hypothesized that pH favors potentiation rather than inhibition of ATP receptors, an issue which deserves future investigation.
Supplemental Material
Acknowledgments
This work was supported by grants from MIUR (co-fin), INFM, Research Fund from Friuli Venezia Giulia Region, RFBR, INTAS, and the Italian Ministry for Foreign Affairs through the agreement for cultural and scientific collaboration between Italy and Russia.
Angus C. Nairn served as editor.
References
- Abdrakhmanova, G., J. Dorfman, Y. Xiao, and M. Morad. 2002. Protons enhance the gating kinetics of the alpha3/beta4 neuronal nicotinic acetylcholine receptor by increasing its apparent affinity to agonists. Mol. Pharmacol. 61:369–378. [DOI] [PubMed] [Google Scholar]
- Baker, T.S., J.R. Dormand, J.P. Gilmore, and P.J. Prince. 1996. Continuous approximation with embedded Runge-Kutta methods. Appl. Numl. Math. 22:51–62. [Google Scholar]
- Bolshakov, K.V., K.V. Essin, S.L. Buldakova, N.A. Dorofeeva, S.N. Skatchkov, M.J. Eaton, D.B. Tikhonov, and L.G. Magazanik. 2002. Characterization of acid-sensitive ion channels in freshly isolated rat brain neurons. Neuroscience. 110:723–730. [DOI] [PubMed] [Google Scholar]
- Boue-Grabot, E., V. Archambault, and P. Seguela. 2000. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels. J. Biol. Chem. 275:10190–10195. [DOI] [PubMed] [Google Scholar]
- Chretien, J.M., and G.A. Chauvet. 1998. An algorithmic method for determining the kinetic system of receptor-channel complex. Math. Biosci. 147:227–257. [DOI] [PubMed] [Google Scholar]
- Chu, X.P., J. Miesch, M. Johnson, L. Root, X.M. Zhu, D. Chen, R.P. Simon, and Z.G. Xiong. 2002. Proton-gated channels in PC12 cells. J. Neurophysiol. 87:2555–2561. [DOI] [PubMed] [Google Scholar]
- Clyne, J.D., L.D. LaPointe, and R.I. Hume. 2002. The role of histidine residues in modulation of the rat P2X2 purinoceptor by zinc and pH. J. Physiol. 539:347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, D., and B. Sakmann. 1985. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J. Physiol. 369:501–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S., and F. Sachs. 2000. Inactivation of P2X2 purinoceptors by divalent cations. J. Physiol. 522:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, S., and F. Sachs. 1999. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 113:695–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau, P., and P. Legendre. 2001. Neuromuscular transmission on the rebound. Receptors Channels. 7:491–496. [PubMed] [Google Scholar]
- Dunn, P.M., Y. Zhong, and G. Burnstock. 2001. P2X receptors in peripheral neurons. Prog. Neurobiol. 65:107–134. [DOI] [PubMed] [Google Scholar]
- Giniatullin, R., L. Khiroug, M. Talantova, and A. Nistri. 1996. Fading and rebound of currents induced by ATP in PC12 cells. Br. J. Pharmacol. 119:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin, R.A., E.M. Sokolova, S. DiAngelantonio, A. Skorinkin, M.V. Talantova, and A. Nistri. 2000. Rapid relief of block by mecamylamine of neuronal nicotinic acetylcholine receptors of rat chromaffin cells in vitro: An electrophysiological and modeling study. Mol. Pharmacol. 58:778–787. [DOI] [PubMed] [Google Scholar]
- Hollins, B., and S.R. Ikeda. 1997. Heterologous expression of a P2X-purinoceptor in rat chromaffin cells detects vesicular ATP release. J. Neurophysiol. 78:3069–3076. [DOI] [PubMed] [Google Scholar]
- Huang, R.Q., and G.H. Dillon. 1999. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J. Neurophysiol. 82:1233–1243. [DOI] [PubMed] [Google Scholar]
- Ihle, E.C., and D.K. Patneau. 2000. Modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Mol. Pharmacol. 58:1204–1212. [DOI] [PubMed] [Google Scholar]
- Johnson, R.G. 1987. Proton pumps and chemiosmotic coupling as a generalized mechanism for neurotransmitter and hormone transport. Ann. NY Acad. Sci. 493:162–177. [DOI] [PubMed] [Google Scholar]
- Khakh, B.S. 2001. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat. Rev. Neurosci. 2:165–174. [DOI] [PubMed] [Google Scholar]
- Khakh, B.S., G. Burnstock, C. Kennedy, B.F. King, R.A. North, P. Séguéla, M. Voigt, and P.P.A. Humphrey. 2001. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 53:107–118. [PubMed] [Google Scholar]
- Khiroug, L., R. Giniatullin, M. Talantova, and A. Nistri. 1997. Role of intracellular calcium in fast and slow desensitization of P2-receptors in PC12 cells. Br. J. Pharmacol. 120:1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, M., O.J. Yoo, and S. Choe. 1997. Molecular assembly of the extracellular domain of P2X2, an ATP-gated ion channel. Biochem. Biophys. Res. Commun. 240:618–622. [DOI] [PubMed] [Google Scholar]
- King, B.F., S.S. Wildman, L.E. Ziganshina, J. Pintor, and G. Burnstock. 1997. Effects of extracellular pH on agonism and antagonism at a recombinant P2X2 receptor. Br. J. Pharmacol. 121:1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, B.F., A. Townsend-Nicholson, S.S. Wildman, T. Thomas, K.M. Spyer, and G. Burnstock. 2000. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 20:4871–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, S., B.A. Orser, G.R. Thatcher, J.N. Reynolds, and J.F. MacDonald. 2001. Positive allosteric modulators of AMPA receptors reduce proton-induced receptor desensitization in rat hippocampal neurons. J. Neurophysiol. 85:2030–2038. [DOI] [PubMed] [Google Scholar]
- Li, C., R.W. Peoples, and F.F. Weight. 1997. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflugers Arch. 433:446–454. [DOI] [PubMed] [Google Scholar]
- Lipton, P. 1999. Ischemic cell death in brain neurons. Physiol. Rev. 79:1431–1568. [DOI] [PubMed] [Google Scholar]
- Liu, S.-Q.J., F.-Y. Law, and P.A. Knauf. 1996. Effects of external pH substrate binding and on the inward chloride translocation rate constant of band 3. J. Gen. Physiol. 107:271–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz, P.L. 1992. Mechanisms for anoxic survival in the vertebrate brain. Annu. Rev. Physiol. 54:601–618. [DOI] [PubMed] [Google Scholar]
- Maconochie, D.J., and D.E. Knight. 1992. A study of the bovine adrenal chromaffin nicotinic receptor using patch clamp and concentration-jump techniques. J. Physiol. 454:129–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, A.D., C.B. Grahames, and P.P. Humphrey. 1996. Functional characterisation of P2 purinoceptors in PC12 cells by measurement of radiolabelled calcium influx. Naunyn-Schmiedebergs Arch. Pharmacol. 354:562–571. [DOI] [PubMed] [Google Scholar]
- Mott, D.D., JJ. Doherty, S. Zhang, M.S. Washburn, M.J. Fendley, P. Lyuboslavsky, S.F. Traynelis, and R. Dingledine. 1998. Phenylethanolamines inhibit NMDA receptors by enhancing proton inhibition. Nat. Neurosci 1:659–667. [DOI] [PubMed] [Google Scholar]
- Mozrzymas, J.W., A. Barberis, K. Michalak, and E. Cherubini. 1999. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J. Neurosci. 19:2474–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naught, L.E., S. Gilbert, R. Imhoff, C. Snook, L. Beamer, and P. Tipton. 2002. Allosterism and cooperativity in Pseudomonas aeruginosa GDP-mannose dehydrogenase. Biochemistry. 41:9637–9645. [DOI] [PubMed] [Google Scholar]
- Nakazawa, K., and P. Hess. 1993. Block by calcium of ATP-activated channels in pheochromocytoma cells. J. Gen. Physiol. 101:377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, R.A. 2002. Molecular physiology of P2X receptors. Physiol. Rev. 82:1013–1067. [DOI] [PubMed] [Google Scholar]
- Pankratov, Y., U. Lalo, O. Krishtal, and A. Verkhratsky. 2002. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J. Physiol. 542:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke, R.L., and J.K. Porter Papke. 2002. Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 137:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevski, A.I., and M.V. Yelshansky. 2000. The trapping block of NMDA receptor channels in acutely isolated rat hippocampal neurones. J. Physiol. 526:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop, R., A. Surprenant, and R.A. North. 1997. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 78:1837–1840. [DOI] [PubMed] [Google Scholar]
- Stoop, R., and J.M. Quayle. 1998. Fading and rebound of P2X2 currents at millimolar ATP concentrations caused by low pH. Br. J. Pharmacol. 125:235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasaki, A., K. Sugiyasu, M. Ikeda, M. Takeuchi, and S. Shinkai. 2001. First successful molecular design of an artificial Lewis oligosaccharide binding system utilizing positive homotropic allosterism. J. Am. Chem. Soc. 123:10239–10244. [DOI] [PubMed] [Google Scholar]
- Vulchanova, L., U. Arvidsson, M. Riedl, J. Wang, G. Buell, A. Surprenant, R.A. North, and R. Elde. 1996. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. USA. 93:8063–8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman, S.S., B.F. King, and G. Burnstock. 1998. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol. 123:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S.R., E.H. Buhl, and I. Mody. 1998. The dynamics of synchronised neurotransmitter release determined from compound spontaneous IPSCs in rat dentate granule neurones in vitro. J. Physiol. 510:477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.