Abstract
It has long been believed that vertebrate olfactory signal transduction is mediated by independent multiple pathways (using cAMP and InsP3 as second messengers). However, the dual presence of parallel pathways in the olfactory receptor cell is still controversial, mainly because of the lack of information regarding the single-cell response induced by odorants that have been shown to produce InsP3 exclusively (but not cAMP) in the olfactory cilia. In this study, we recorded activities of transduction channels of single olfactory receptor cells to InsP3-producing odorants. When the membrane potential was held at −54 mV, application of InsP3-producing odorants to the ciliary region caused an inward current. The reversal potential was 0 ± 7 mV (mean ± SD, n = 10). Actually, InsP3-producing odorants generated responses in a smaller fraction of cells (lilial, 3.4%; lyral, 1.7%) than the cAMP-producing odorant (cineole, 26%). But, fundamental properties of responses were surprisingly homologous; namely, spatial distribution of the sensitivity, waveforms, I-V relation, and reversal potential, dose dependence, time integration of stimulus period, adaptation, and recovery. By applying both types of odorants alternatively to the same cell, furthermore, we observed cells to exhibit symmetrical cross-adaptation. It seems likely that even with odorants with different modalities adaptation occurs completely depending on the amount of current flow. The data will also provide evidence showing that olfactory response generation and adaptation are regulated by a uniform mechanism for a wide variety of odorants.
Keywords: InsP3, cAMP, olfactory receptor cell, signal transduction, adaptation
INTRODUCTION
Olfaction begins at the cilia of the receptor cell in the olfactory epithelium. Odorant binding to receptor proteins on the ciliary surface activates enzymatic cascades that lead to the opening of ionic channels, causing membrane depolarization. One well-established mechanism underlying this signal transduction involves cAMP (for reviews see Bakalyar and Reed, 1991; Breer and Boekhoff, 1992; Reed, 1992; Ronnett and Snyder, 1992; Kurahashi and Yau, 1994; Schild and Restrepo, 1998), which finally activates cyclic nucleotide–gated (CNG) cation channels and Ca2+-activated Cl− channels (for reviews see Gold et al., 1990; Kurahashi and Yau, 1994; Firestein and Shepherd, 1995; Schild and Restrepo, 1998; Gold, 1999; Frings et al., 2000; Firestein, 2001). Another mechanism employs InsP3 as a second messenger (Huque and Bruch 1986; Breer and Boekhoff, 1991; for review see Schild and Restrepo, 1998) that activates InsP3-gated cation channel.
In the research history, the presence of multiple pathways was initially proposed by the experiment done by Sklar et al. (1986), who actually measured cAMP production biochemically with 65 different odorants in the olfactory ciliary membrane of the frog. They found that cAMP production rate varied among odorants, and further showed that certain types of odorants failed to produce cAMP. Later, those odorants (including lilial and lyral) that did not produce cAMP were found to produce InsP3 instead (in rodent, Breer and Boekhoff, 1991). In addition, Kashiwayanagi et al. (1996) showed in the bullfrog that single-cell responses induced by odorants that were subgrouped into InsP3- and cAMP-producing odorants did not cross-adapt with each other, and concluded that those olfactory transductions were mediated by independent pathways. Because of the consistencies of data obtained from various animals used in the past, the concept of multiple pathways has been universally applied to wide varieties of species within vertebrate, at least for volatile perceptions. For a long period of time, two transduction systems have been thought to be completely independent. However, a recent study by Vogl et al. (2000) showed that both pathways might have a cross-talk via cytoplasmic elements.
Thus, it has long been believed that olfactory receptor cells utilize multiple pathways for their signal transductions. On the other hand, however, recent studies that employed a combination of electro-olfactogram recordings and gene-targeting methods have argued against the presence of multiple pathways (Brunet et al., 1996; Belluscio et al., 1998; Wong et al., 2000). They showed that electro-olfactogram responses were disappeared or reduced in animals that lacked elements needed for cAMP metabolisms, and the presence of InsP3 pathway is still controversial (Gold, 1999). Even after these findings, however, there are still several arguments for the possibility of the presence of the InsP3 pathway (see e.g., Vogl et al., 2000; Kaur et al., 2001). A main reason why this issue remains unclear is a lack of detailed information regarding the response of single olfactory receptor cells to odorants that produce InsP3 exclusively. So far, responses to odorants were mainly recorded in single olfactory receptor cells by using cAMP-producing odorants, because the responsiveness of cells has been shown to be much higher than using InsP3-producing odorants (Lowe and Gold, 1993a). And, therefore, very little is known about the fundamental properties of membrane responses induced by InsP3-producing odorants. In this study, we obtained membrane responses to InsP3-producing odorants (lilial and lyral) by screening a large number (>2,800) of isolated single olfactory receptor cells under the whole-cell voltage clamp condition. We confirmed that electrophysiological properties of the membrane conductance induced by the InsP3-producing odorants were very similar to those of the response induced by cAMP-producing odorants. Furthermore, it was found that there were cells sensitive to both InsP3- and cAMP-producing odorants, and that these cells showed cross-adaptation to both types of odorants. It thus seems likely that olfactory adaptation occurs independently of the odorant modalities in the single cell. Our data will also add evidence supporting the notion that olfactory signal transduction is mediated by a single cytoplasmic cascade for a wide variety of odorants.
MATERIALS AND METHODS
Preparation
Olfactory receptor cells were dissociated enzymatically from the olfactory epithelium of the newt, Cynops pyrrhogasster. The experiments were performed under the latest ethical guidelines for animal experimentation at Osaka University, based on international experimental regulations. Dissociation protocols have been described elsewhere (Kurahashi, 1989; Hirono et al., 1992). In short, the animal was chilled on ice and double pithed. The mucosae excised from the olfactory cavity were incubated for 5 min at 37°C in a solution which contained enzymes (0.1% collagenase or 0.3% papain and 0.3% collagenase) with no added Ca2+ and Mg2+ (in mM): 110 NaCl, 3.7 KCl, 10 HEPES, 15 glucose, 1 pyruvate. In some experiments the tissue was further treated by a solution containing 0.1% DNase for 1.5 min to remove DNA filaments that interfere with the formation of giga-ohm seal. We did not see any remarkable differences in data between preparations with and without DNase. All solutions were adjusted to pH 7.4 with NaOH. The tissue was rinsed three times with normal Ringer's solution (in mM): 110 NaCl, 3.7 KCl, 3 CaCl2, 1 MgCl2, 10 HEPES, 15 glucose, 1 pyruvate, and triturated. Isolated cells were plated on the concanavalin A–coated glass coverslip. Cells were maintained at 4°C until use. In this study, we selected olfactory receptor cells having more than five cilia.
Recording Procedures
Membrane currents were recorded with the whole-cell recording configuration (Hamill et al., 1981). Patch pipettes were made of borosilicate tubing with filament (outer diameter, 1.5 mm; World Precision Instruments) by using a two-stage vertical patch electrode puller (PP-830; Narishige Scientific Instruments). The recording pipette was filled with a solution containing: (in mM) 119 CsCl, 1 CaCl2, 5 EGTA, 10 HEPES (pH was adjusted for 7.4 by CsOH) to suppress K channels that cause large current fluctuations. Normal Ringer's solution (for composition, see above) was used as the external solution for all recordings. The pipette resistance was 10–15 MΩ. The membrane potentials were corrected for by the liquid junction potential at the pipette tip (4 mV, see Kurahashi, 1989).
The recording pipette was connected to a patch clamp amplifier (Axopatch 1B, 1D or 200B; Axon Instruments, Inc.). The signal was low-pass filtered at 0.5 kHz, digitized by an A/D converter (sampling frequency, 1 kHz) connected to a computer (PC9821; NEC or IBM compatible PC). Simultaneously, signals were monitored on an oscilloscope and recorded on a chart recorder. Odor stimuli and the data acquisition were regulated by the same computer using a combination of original program and pClamp (ver. 6.0, Axon Instruments, Inc.). The results were analyzed by an offline computer and plotted by using Microcal Origin 6.0 software (OriginLab Corporation). For curve drawings, data sampled at 1/16 kHz were used. Experiments were performed at room temperature (23–25°C).
Odorant Stimulation
Odorants (cineole; eukalyptos commerce by Katayama Chemical Co. lyral and lilial, donated by Takasago Co.) were dissolved in DMSO and then diluted in the bath solution at 1 mM concentration with final DMSO concentration 0.2%. The stimulus solution was applied to the cilia from two independent puffer pipettes having a tip diameter of 1 μm. The tips of the puffer pipettes were, unless otherwise indicated, situated ∼20 μm from the tip of the dendrite. The pressure (maximum 150 kPa) was controlled by a computer-regulated pressure ejection system developed in our laboratory (Ito et al., 1995). There was a time lag between the actual chemical stimulation of the cell and the application of TTL pulse to the magnetic valve. This time lag, being 60 ms on average, was estimated from the liquid junction current caused by the same application system.
RESULTS
The Response Induced by InsP3-producing Odorants
Under the voltage clamp condition, puff application of InsP3-producing odorants (either 1 mM lilial alone or 1 mM lyral alone, or a mixture of both) induced an inward current at −54 mV (Fig. 1) . The amplitude of the inward current was 100 ± 110 pA (maximum value obtained in individual cells, mean ± SD, n = 111, ranging from 2.5 to 415 pA). With a cAMP-producing odorant (1 mM cineole), we obtained inward current responses of 215 ± 163 pA (n = 329, ranging from 9 to 724 pA). Thus, on average, responses induced by InsP3-producing odorants were relatively smaller in amplitude than responses induced by the cAMP-producing odorant.
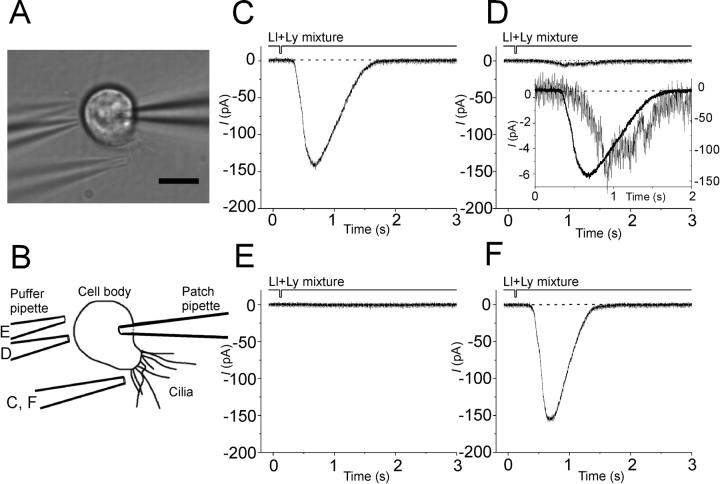
Figure 1.
Response induced by InsP3-producing odorants and spatial distribution of cell sensitivity. (A) Photo-micrograph of an olfactory receptor cell under the recording. Four photographs were superimposed so as to indicate the positions of a stimulus pipette. Bar, 10 μm. (B) Cell's shape and positions of stimulus pipette were traced from photograph presented in A. (C) Responses obtained from the olfactory receptor cell, when stimulated various parts. Data from the cell presented in A. Downward deflections of the top trace indicate the timing and duration of the odor stimulation. The alphabetical order (C–F) corresponds to the sequence of the experiments. Puffer pipette was filled with a solution containing a mixture of 1 mM lilial (abbreviated as Ll) and 1 mM lyral (Ly). In panel D inset, the current wave obtained in D (stimulated the cell body) was normalized and superimposed with the current from C (stimulated the cilia). Note that the rising phase of data presented in D is delayed from that of C. Duration, 25 ms. Holding voltage(Vh), −54 mV.
Heterogeneous Responsiveness of Cells to Odorants
As visualized in Fig. 2 , the probability that cells generate responses to InsP3-producing odorants was much smaller when compared with that obtained with a cAMP-producing odorant (cineole). This observation is actually consistent with a previous report that olfactory field potential (electro-olfactogram) responses are smaller with InsP3-producing odorants than with cAMP-producing odorants (Lowe et al., 1989). By applying both lilial and cineole to the same cell, we could observe cells that responded to both InsP3- and cAMP-producing odorants (Fig. 2 B). This observation was unpredictable, because it has been shown that both cAMP and InsP3 pathways use independent molecules (for review see Schild and Restrepo, 1998), and that individual receptor cells express only one type of receptor protein on their plasma membrane (Ngai et al., 1993; Ressler et al., 1993; Vassar et al., 1993; Chess et al., 1994). It seems likely that certain types of receptor protein can recognize both InsP3- and cAMP-producing odorants (see also Kajiya et al., 2001)
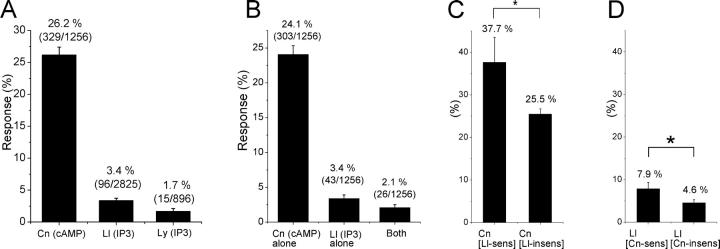
Figure 2.
Response-generative probabilities for three odorant stimuli, including a cAMP-producing odorant (cineole: abbreviated as Cn) and InsP3-producing odorants (abbreviated as IP3, lilial, and lyral). Numbers in parentheses plated on the bars indicate the number of sensitive cells and total number of cells examined. Error bars indicate SD (σ) that was obtained by σ = (pq/n)1/2, where p is the probability, q = 1 − p, and n is the number of cells tested. Response generation was defined as an induction of inward current under whole-cell voltage-clamp condition (Vh, −54 mV), regardless of the absolute amplitude of the inward current (nor cells' sensitivity). Concentration of odorants included in the pipette was 1 mM for all tested odorants. (A) Response probabilities for cineole, lilial, and lyral. (B) Heterogeneous responsiveness of cells when stimulated by both cAMP- (cineole) and InsP3-producing odorant (lilial). (C) Cross-correlation between cineole and lilial perception of single receptor cells. Data obtained from B. Probability of cineole-response occurrence in lilial-sensitive (Ll-sens) and lilial-insensitive cells (Ll-insens). Note that population of cells responding to cineole is larger in lilial-sensitive group than in lilial-insensitive group. In this kind of plot, it is assumed that there is a positive correlation, as the left bar is bigger than the right one. In an opposite case (the left was smaller than the right), there is a negative correlation. The asterisk indicates the presence of statistical significances between conditional probabilities with P < 0.05. (D) Response probability to lilial in cineole-sensitive and cineole-insensitive cells. The asterisk indicates the presence of statistical significances between conditional probabilities with P < 0.05.
It was interesting to note that cineole responses were observed more frequently within lilial-sensitive cells than in lilial-insensitive cells (Fig. 2 C), and vice versa that lilial responses were recognized more frequently in cineole-sensitive cells than in cineole-insensitive cells (Fig. 2 D). These results suggest that there is a positive cross-correlation between lilial and cineole sensitivities among cells.
Response Shape
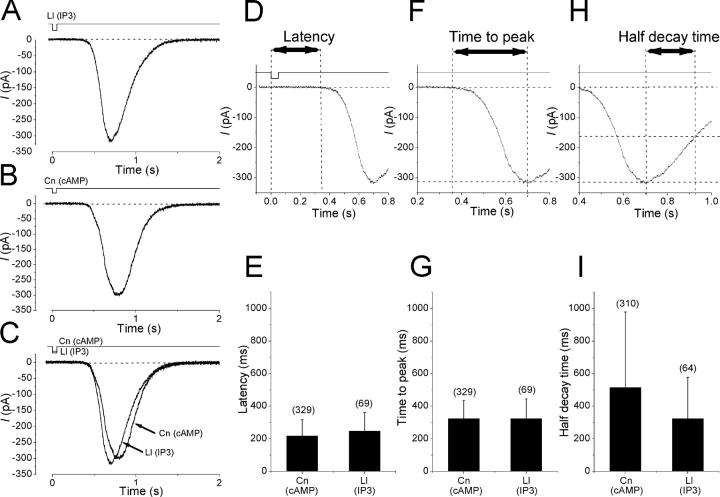
The shape of responses induced by InsP3-producing odorants was very similar to that of responses induced by cAMP-producing odorants (Fig. 3) . The inward current induced by InsP3-producing odorants started to develop 247 ± 115 ms (n = 69) after the onset of brief odorant pulse (50–100-ms duration). This value was almost comparable to that observed for the cAMP metabolism in the olfactory transduction in which the response latency attributes to the time needed for cytoplasmic cAMP concentration to increase high enough to drive CNG channels (Takeuchi and Kurahashi, 2002). The time to peak was ∼0.3 s (Fig. 3 F). After the peak, the inward current in turn gradually returned to zero level within a couple of seconds (Fig. 3 H). It was noticeable to see that the response lasted much longer than the period that the cell was exposed to the stimulant. In other words, the machinery activated by InsP3-producing odorants had ability for time amplification of the olfactory signal. In the cAMP pathway, time amplification of signal has been shown to be achieved by the prolonged activation of adenylyl cyclase and a prolonged cAMP lifetime (Takeuchi and Kurahashi, 2002). Similarity of response shapes between InsP3- and cAMP-producing odorants is also reported for olfactory cells from the tiger salamander (Chen et al., 2000).
Figure 3.
Membrane response induced by InsP3- (lilial) and cAMP-producing odorants (cineole). (A) Whole-cell current recorded from the olfactory receptor cell. Vh, −54 mV. Downward deflections of top traces indicate the timing and duration of the odor stimulation (pressure: lilial, 40 kPa; cineole, 30 kPa; duration, 100 ms). Response induced by lilial. (B) Response induced by cineole. Same cell as in A. (C) Superimposition of data from A and B. (D) Comparison of response latency. Bars indicate means of data and the vertical lines indicate the standard deviation. Numbers appeared in parentheses indicate the number of cells analyzed. Cells that expressed responses >50 pA were used for analyses. Responses obtained in cells showing smaller responses would not have fully driven the transduction machinery (and this situation is equivalent to a partial activation of enzymes), which actually affects the parameters exhibiting waveforms. (F) Comparison of time to peak. (H) Comparison of half-decay times. Cells which did not recovered to zero level for >10 s were excluded from the analysis. No statistical significance was recognized with t test (E, G, and I, P < 0.05).
Spatial Distribution of the Sensitivity
Cells' sensitivity to InsP3-producing odorants was the maximum at the apical dendritic part (Fig. 1) where sensory cilia were elongated. When the tip of the puffer pipette (∼1 μm opening diameter) was positioned close to the apical dendrite, the amplitude of the inward current was ∼140 pA. As the tip was shifted to proximal part, the amplitude of the inward current reduced, and became almost undetectable when the tip was positioned at proximal part of the cell body. Furthermore, the response latency was shortest when the pipette tip was positioned near apical dendrite (Fig. 1 D, inset). Probably, prolonged latency reflects the delayed arrival of stimulant to the receptive area. Thus, it appears likely that the cell sensitivity to InsP3-producing odorants is strongly localized to the apical dendritic part. Such strong polarization of the sensitivity has been observed for responses induced by cAMP-producing odorants (Kurahashi, 1989; Lowe and Gold, 1991) that activate CNG channels distributing densely at the ciliary membrane (Kurahashi and Kaneko, 1991, 1993; Larsson et al., 1997).
Voltage and Dose Dependence of Odorant-induced Current
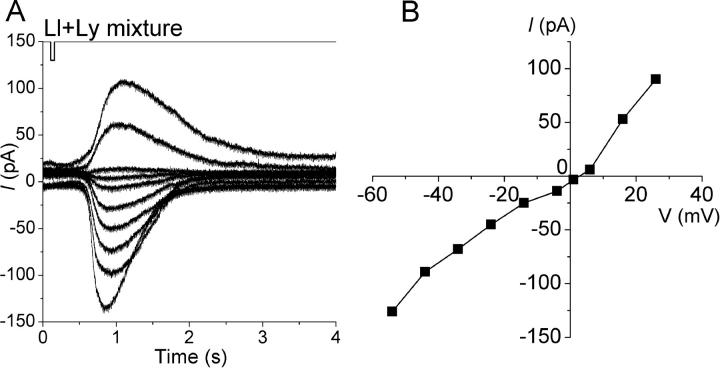
The amplitude and polarity of the current induced by InsP3-producing odorants were dependent on the holding voltage (Vh) (Fig. 4) . At negative potentials, the current polarity was inward. As the membrane potential was shifted to positive, the amplitude of the inward current reduced. Current reversal occurred at 0 ± 7 mV (n = 10). The I-V relation showed slight outward rectification (Fig. 4 B). The reversal potential and the shape of the I-V relation were all very similar to those reported previously for the current activated by cAMP-producing odorants (Kurahashi, 1989; Lowe and Gold, 1993a).
Figure 4.
Voltage dependence of the current response induced by the InsP3-producing odorant (a mixture of 1 mM lilial and 1 mM lyral). (A) Current responses under various holding potentials. Downward deflections of the upper trace indicate the timing and duration of the odor stimulation. Vh= −54 to +26 mV, pressure= 50 kPa, duration= 50 ms. (B) I-V relation of response induced by InsP3-producing odorant. Data from A.
Furthermore, it was frequently observed that the current falling phase became more gradual at positive potentials. This phenomenon has been also reported for responses induced by cAMP-producing odorants (Kurahashi and Shibuya, 1990; Lowe and Gold, 1993a). In the cAMP system the voltage dependence of the current shape has been explained by the contribution of Ca2+-feedback system on the current falling phase; the driving force for Ca2+ entry through the ion channels becomes bigger in proportion to the membrane hyperpolarization.
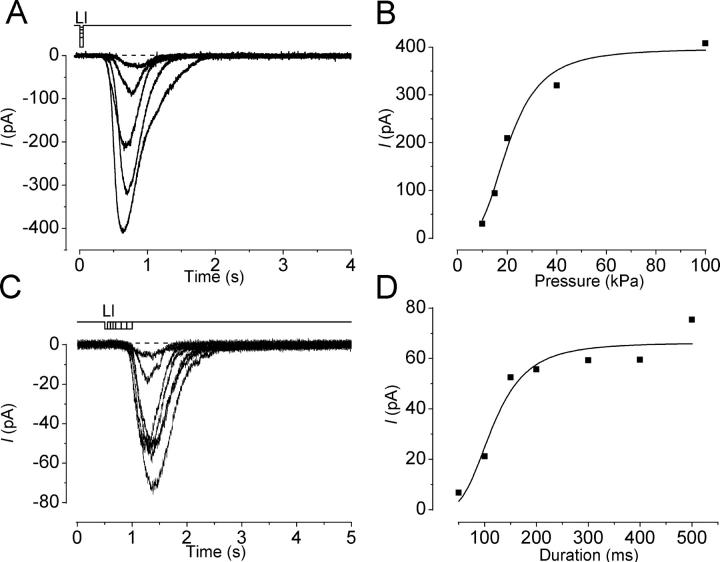
The response amplitude was dependent on the dose of odorant stimulation (Fig. 5) . With a fixed duration of 50 ms, an increase in the pressure applied to puffer pipette increased the current amplitude monotonically. One noticeable feature was that the slope of rising phase became steeper as the odorant pressure was increased (Fig. 5 A). The relation between response amplitude and pressure could be fitted by the Hill equation with high cooperativity (Hill coefficient, n H = 5.4 ± 4.3, n = 10), as has been reported for the olfactory receptor cell responding to cAMP-producing odorants (Lowe and Gold, 1993b).
Figure 5.
Dose dependence of the odorant-induced response. (A) Dependence of the response on the pressure applied to the puffer pipette. Downward deflections of the top trace indicate the amount of pressure, timing, and duration of the odor stimulation. Vh, −54 mV. (B) Pressure-response relation of the odorant-induced current. Peak amplitudes of responses obtained in A were plotted against the pressure. The smooth line was drawn by a least square fitting of the data points by the Hill equation, I = I max × P^n H /(P^nH+K 1/2^nH), where I is the current, P is the pressure of odorants, K 1/2 is the half maximum intensity, and n H is Hill coefficient. I max = 32.7 pA, K 1/2 = 15.3 kPa, and n H = 9.6. (C) Stimulus duration was changed, while the pressure applied to the puffer pipette was kept constant (20 kPa). Different cell as in A. Vh, −54 mV. (D) Duration-response relation of the odorant-induced current. Peak amplitudes of the responses obtained in C were plotted against the duration of stimuli. The smooth line was drawn by a least square fitting of the data points by the Hill equation with I max = 66.3 pA, K 1/2 = 115.8 ms and n H = 3.5.
The amplitude of current responses was dependent also on the duration of the odorant stimuli at fixed pressure (Fig. 5 C). The relation between current amplitude and stimulus duration was also fitted by the Hill equation (Fig. 5 B), with a Hill coefficient of 2.4 ± 0.8 (n = 5). It is thus likely that the response generation system has an ability to integrate the stimulus period, as has been reported for the cAMP cascades (Firestein et al., 1993; Takeuchi and Kurahashi, 2002).
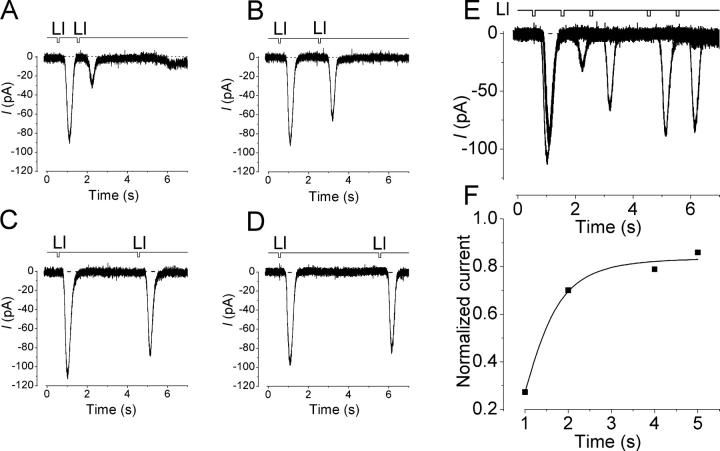
Self Adaptation to InsP3-producing Odorants
When cells were stimulated by double pulse protocols, the response induced by the second stimulus showed remarkable reduction in amplitude in comparison with the first response induced by a conditioning pulse (Fig. 6) . This phenomenon is very similar to that reported for olfactory adaptation analyzed with cAMP-producing odorants (Kurahashi and Menini, 1997).
Figure 6.
Self adaptation of response induced by lilial (1mM in the pipette). (A) Reduction of the current amplitude by a conditioning pulse. Reduction rate was 33.3% at 1-s interval. Pulse duration, 100 ms. The interstimulus interval was prolonged to 2 s (B), 4 s (C), and 5 s (D). Vh, −54 mV. (E) Superimposition of data appeared in A. (F) Recovery from adaptation. Peaks of the inward currents were plotted as a function of inter-stimulus intervals. The amplitude of the second response was normalized in reference to the amplitude of the first response. The solid line was drawn by a least square fitting of the data points by a sigmoid function.
With InsP3-producing odorants, reduction rate was 37 ± 14% (n = 8) when the second pulse was applied immediately after the first response was terminated. As the interstimulus interval was prolonged, the current amplitude gradually recovered. Full recovery was established ∼10–20 s after the onset of the conditioning pulse. These parameters relating adaptation were almost the same as those observed in responses induced by cAMP-producing odorants (see Kurahashi and Shibuya, 1990; Kurahashi and Menini, 1997).
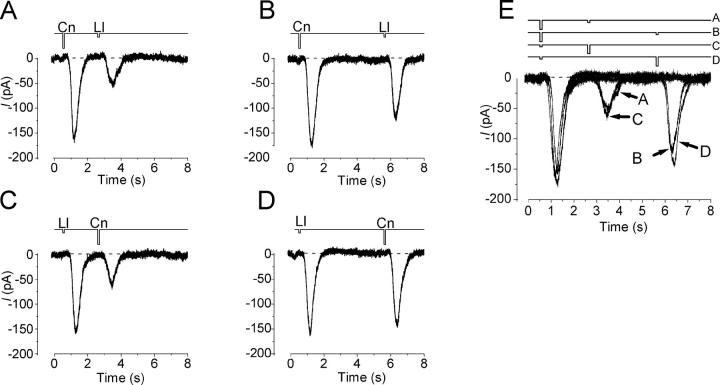
Cross-adaptation between InsP3- and cAMP-producing Odorants
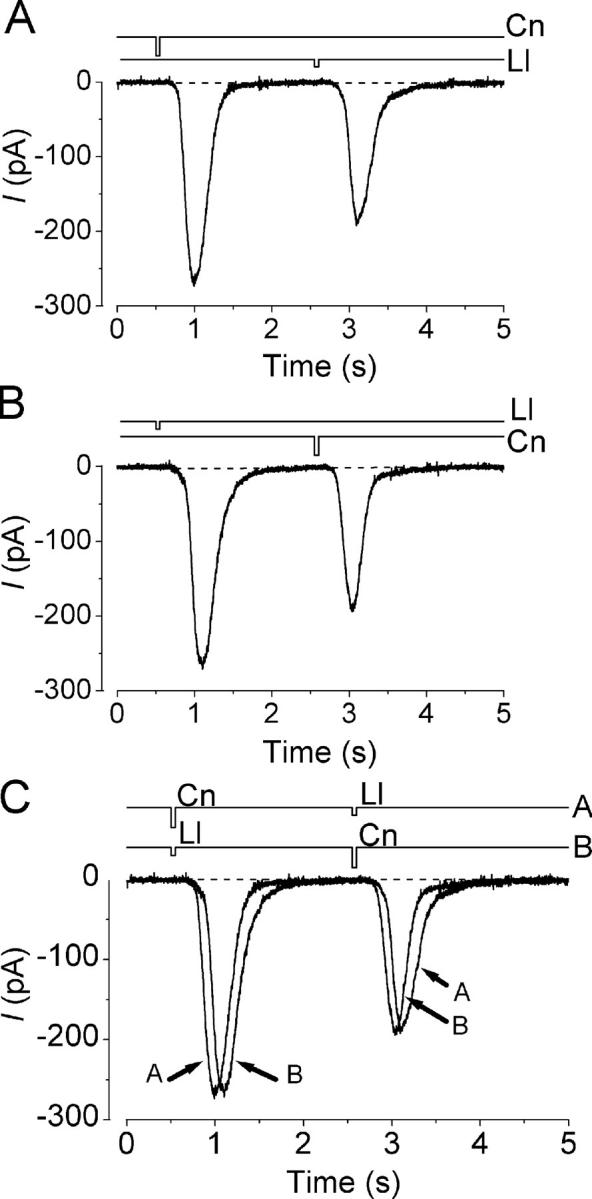
As mentioned before, we could find cells that responded to both InsP3- and cAMP-producing odorants. When both types of odorants were alternatively applied to the same cell as a double pulse protocol, cells expressed “cross-adaptation” (Fig. 7) ; the response induced by the InsP3-producing odorant was reduced by the conditioning pulse using cAMP-producing odorant, and the response induced by the cAMP-producing odorant was reduced by the InsP3-producing odorant. To analyze the degree of reduction more quantitatively, we first adjusted the amplitudes of responses induced by both InsP3- and cAMP-producing odorants to the same levels, and then those odorants were applied alternatively to the same cell. Interestingly, the second responses reduced to the same level, independent of the odorant species applied as conditioning pulse. Furthermore, the recovery phase was again the same (Fig. 8) . The most likely explanation for these results would be that both types of odorants activate the same transduction machinery. Further discussion on this matter will be made later.
Figure 7.
Cross-adaptation between InsP3- and cAMP-producing odorants. (A) Lilial stimulation was applied after the stimulation by cineole. Interval of stimuli were 2 s. Prior to the present experiment, maximum peaks of responses induced by lilial and cineole were adjusted to the same by controlling pressures applied to puffer pipettes. Cineole stimulus; pressure, 100 kPa, duration 100 ms. Lilial; pressure, 20 kPa, duration 100 ms. Vh = −54 mV. (B) Lilial stimulation was applied after the cineole pulse. (C) Superimpose of data from A and B. Note that the reduction in the amplitude is identical in these conditions.
Figure 8.
Recovery from cross-adaptation. (A) Lilial stimulation was applied after the stimulation by cineole with two different inter-stimulus intervals (A, 2 s; B, 5 s). Prior to the present experiment, maximum peaks of responses induced by lilial and cineole were adjusted to the same level by controlling the pressure applied to the puffer pipette. Pressure, 7 kPa (for lilial), 30 kPa (for cineole); duration, 50 ms. Vh = −54 mV. (C) Cineole stimulation was applied after the lilial pulse with two different inter-stimulus intervals. The same cell as in A. (E) Superimposition of data appeared in A–D. Note that the recovery processes show very similar time courses.
DISCUSSION
In this study, we obtained responses induced by InsP3-producing odorants (lilial and lyral) in single olfactory receptor cells. Fundamental properties, which include spatial distribution of the sensitivity, waveforms, I-V relation and reversal potential, dose dependence, time integration of stimulus period, adaptation, and recovery, were almost identical to those of responses induced by cAMP-producing odorants. A remarkable difference observed between InsP3- and cAMP-producing odorants was the probability of response generation; cineole responses were observed in 26% of cells, while lilial and lyral induced responses in 3.4% and 1.7% of cells, respectively. By applying both types of odorants alternatively to the same cell, furthermore, we observed cells to exhibit symmetrical cross-adaptation. Thus, it seems likely that, even with odorants with different modalities, adaptation occurs completely depending on the amount of current flow. This could be actually predicted by previous observation that CNG channel is the target of Ca2+-feedback expressing adaptation (Kurahashi and Menini, 1997).
Heterogeneous Sensitivities to Odorants in Relation to the Expression of Receptor Proteins
In the present study, we saw that the responsiveness of cells to odorants were heterogeneous. Looking at selectivity between lilial (InsP3-producing odorant) and cineole (cAMP-producing odorant), all four possible combinations were observed; namely, cells responding to lilial alone, to cineole alone, cells responding to both, and to neither. However, it is notable to see that the cineole response was observed more frequently in cells responding to lilial (38%) than in cells not responding to lilial (26%). And, vice versa, lilial response was observed more frequently in cells responding to cineole. This may suggest that, although cineole and lilial are subgrouped into cAMP- and InsP3-producing odorants, respectively, there are some positive correlation in cells' sensitivities. Since a cell expresses only one type of the receptor protein as predicted from molecular biological investigations (Ngai et al., 1993; Ressler et al., 1993; Vassar et al., 1993; Chess et al., 1994), these observations suggest that the receptor protein that accepts lilial is much more sensitive to cineole than the receptor protein insensitive to lilial.
The fraction of cells responding to InsP3-producing odorants was very small in comparison with the occurrence of cineole responses. This is consistent with a previous observation that the electrical field potential (electro-olfactogram) is smaller in amplitude for InsP3-producing odorants (Lowe et al., 1989). Amplitude of electro-olfactogram reflects a combination of response amplitude in individual cells and the number of cells responding. The result obtained in this study clearly shows that the number of cells responding to InsP3-producing odorants is smaller than the number of cells responding to cAMP-producing odorants. Obviously, cells expressing odorant receptors that accept InsP3-producing odorants are the minority.
Reversal Potential and Ionic Selectivity
The reversal potential is an index to estimate ionic selectivity of ionic channels generating currents. The InsP3-producing odorant response showed the reversal potential near 0 mV. This indicates that the underlying ionic channel is either cation and/or anion permeable. Although our present experiments do not address the ionic species directly, the reversal potential value is consistent with previous observations for the InsP3-activated channels to be a cation selective (Schild et al., 1995; Schild and Restrepo, 1998; Kaur et al., 2001). Furthermore, CNG channels are also cation selective (Kurahashi and Shibuya, 1990).
It is also reported that Ca2+ entry through the InsP3-activated cation channels can activate K-selective channels (Morales et al., 1997). In the present study, however, we used Cs pipette solution, and therefore cannot answer to the question if K(Ca) component was included in responses induced by InsP3-producing odorants.
Response Waveforms and Enzymatic Cascades
The response induced by InsP3-producing odorants showed a very similar waveform to the response induced by cAMP-producing odorants. This result actually argues against the notion that responses induced by InsP3- and cAMP-producing odorants are mediated by multiple pathways. It is generally figured out that cAMP and InsP3 pathways utilizes completely independent molecular components; namely, the cAMP pathway uses receptors, Golf, type III adenylyl cyclase, cAMP, and CNG channels; the InsP3 pathway uses receptors, Gq, phospholipase C, InsP3, and InsP3-gated cation channels. Any dynamic parameters of these elemental molecules could not be identical. Reactions and activated time courses of the enzymes would be different. Temporal patterns of cAMP and InsP3 formations, movements, and their hydrolyses cannot be identical. In the present study, however, the final output (channel activity) showed the same waveform in terms of the response latency, time to peak, and response shutdown. In general, these results would suggest that responses induced by two groups of odorants are mediated by the same second messenger system. Further quantitative information for the molecular elements underlying both systems would be indispensable to completely understand if responses are mediated by two independent pathways.
Ca Dynamics
In the olfactory receptor cell it has been well known that cytoplasmic Ca2+ plays key roles regulating adaptation (Kurahashi and Menini, 1997) and signal amplification via an activation of Ca2+-activated Cl− channel (Lowe and Gold, 1993b). Because of its importance, Ca2+ homeostasis is thought to be balanced in olfactory cilia by very critical regulatory systems; namely, a complex of molecular networks involving the Ca2+ influx through the transduction channels, the Na+-dependent Ca2+ extrusion system (Reisert and Matthews, 2001), and, presumably, the Ca2+-buffering system. Therefore, it is expected that the waveform of the odorant-induced current is not only regulated by the enzymes involving transduction, but also by the Ca2+ metabolisms. In the present study, we observed that responses induced by both categories of odorants expressed similar waveforms of responses. To express the same waveforms of responses with different transduction channels (InsP3-gated channels and CNG channels), both channels must be identical critically in terms of the Ca2+ permeability, activation, and shut-off kinetics. This would be very hard to imagine with a simple coincidence.
Self -adaptation and Cross-adaptation with cAMP-producing Odorants
Responses induced by InsP3-producing odorants expressed adaptation when examined by double pulse protocols. Adaptation of responses induced by cAMP-producing odorants has been shown to be regulated by the Ca2+-feedback to CNG channels (Kurahashi and Menini, 1997). Recovery from adaptation is regulated by the Na+-dependent Ca2+ extrusion system. As described above, since the InsP3 pathway has been thought to utilize independent molecules from the cAMP pathway, adaptation must be regulated by an independent mechanism. It is unlikely that, in addition to discussion made in previous sections, different systems can express the same adaptational time courses (reduction rate, time course for recovery).
Recently, Vogl et al. (2000) showed that InsP3 and cAMP pathways could suppress each other. Qualitatively, this finding seems likely to explain our observation of cross-adaptation. But, in our experiments, the reduction rate matched perfectly whether we used InsP3- or cAMP-producing odorant as a conditioning pulse. Coupled with step-by-step considerations described above, present observations would satisfactory lead us to conclude that responses induced by both InsP3- and cAMP-producing odorants in the same cell were mediated by the same system. Furthermore, it is highly likely that the mechanism underlying adaptation is uniform. However, our results do not exclude the possibility that InsP3-producing odorants produce InsP3. And, therefore, the findings by Vogl et al. (2000) may raise a new possibility that InsP3 is actually used for modulating the olfactory signal transduction.
This study showed that fundamental properties of single-cell responses induced by InsP3-producing odorants were very similar to those observed for responses induced by cAMP-producing odorants. As mentioned at the beginning of results, on average, amplitude of responses induced by InsP3-producing odorants was about half of that of cineole response. This statistical difference may be explained by assuming that odorants that have been thought to stimulate the InsP3 pathway indeed activate the cAMP metabolism with lower efficiency than the other odorants. The data obtained here would provide evidence, in addition to experiments done by Gold and his collaborators (Brunet et al., 1996; Belluscio et al., 1998; Wong et al., 2000) and Zufall's group (Chen, et al., 2000), showing that the response induced by InsP3-producing odorants is mediated by the cAMP pathway.
Acknowledgments
This work was supported by grants from HFSPO (to T. Kurahashi), MESSC (to H. Takeuchi and T. Kurahashi), ME (to T. Kurahashi), METI (to J. Hirono) and by a COE research project in Osaka University (to H. Takeuchi and T. Kurahashi). Part of the experiments done by Y. Imanaka was financially supported by a grant from METI to Dr. T. Sato.
Olaf S. Andersen served as editor.
Abbreviation used in this paper: CNG, cyclic nucleotide–gated.
References
- Bakalyar, H.A., and R.R. Reed. 1991. The second messenger cascade in olfactory receptor neurons. Curr. Opin. Neurobiol. 1:204–208. [DOI] [PubMed] [Google Scholar]
- Belluscio, L., G.H. Gold, A. Nemes, and R. Axel. 1998. Mice deficient in G(olf) are anosmic. Neuron. 20:69–81. [DOI] [PubMed] [Google Scholar]
- Breer, H., and I. Boekhoff. 1991. Odorants of the same odor class activate different second messenger pathways. Chem. Senses. 16:19–29. [Google Scholar]
- Breer, H., and I. Boekhoff. 1992. Second messenger signaling in olfaction. Curr. Opin. Neurobiol. 2:439–443. [DOI] [PubMed] [Google Scholar]
- Brunet, L.J., G.H. Gold, and J. Ngai. 1996. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 17:681–693. [DOI] [PubMed] [Google Scholar]
- Chen, S., A.P. Lane, R. Bock, T. Leinders-Zufall, and F. Zufall. 2000. Blocking adenylyl cyclase inhibits olfactory generator currents induced by IP(3)-odors. J. Neurophysiol. 84:575–580. [DOI] [PubMed] [Google Scholar]
- Chess, A., I. Simon, H. Cedar, and R. Axel. 1994. Allelic inactivation regulates olfactory receptor gene expression. Cell. 78:823–834. [DOI] [PubMed] [Google Scholar]
- Firestein, S. 2001. How the olfactory system makes senses of scents. Nature. 413:211–218. [DOI] [PubMed] [Google Scholar]
- Firestein, S., C. Picco, and A. Menini. 1993. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. J. Physiol. 468:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein, S., and G.M. Shepherd. 1995. Interaction of anionic and cationic currents leads to a voltage dependence in the odor response of olfactory receptor neurons. J. Neurophysiol. 73:562–567. [DOI] [PubMed] [Google Scholar]
- Frings, S., D. Reuter, and S.J. Kleene. 2000. Neuronal Ca2+ -activated Cl- channels–homing in on an elusive channel species. Prog. Neurobiol. 60:247–289. [DOI] [PubMed] [Google Scholar]
- Gold, G.H. 1999. Controversial issues in vertebrate olfactory transduction. Annu. Rev. Physiol. 61:857–871. [DOI] [PubMed] [Google Scholar]
- Gold, G.H., T. Nakamura, and G. Lowe. 1990. Studies on the mechanism of olfactory transduction in vertebrates. Neurosci. Res. Suppl. 12:S127–S134. [DOI] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Hirono, J., T. Sato, M. Tonoike, and M. Takebayashi. 1992. Simultaneous recording of [Ca2+]i increases in isolated olfactory receptor neurons retaining their original spatial relationship in intact tissue. J. Neurosci. Methods. 42:185–194. [DOI] [PubMed] [Google Scholar]
- Huque, T., and R.C. Bruch. 1986. Odorant- and guanine nucleotide-stimulated phosphoinositide turnover in olfactory cilia. Biochem. Biophys. Res. Commun. 137:36–42. [DOI] [PubMed] [Google Scholar]
- Ito, Y., T. Kurahashi, and A. Kaneko. 1995. Pressure control instrumentation for drug stimulation. Nippon Seirigaku Zasshi. 57:127–133. [PubMed] [Google Scholar]
- Kajiya, K., K. Inaki, M. Tanaka, T. Haga, H. Kataoka, and K. Touhara. 2001. Molecular bases of odor discrimination: Reconstitution of olfactory receptors that recognize overlapping sets of odorants. J. Neurosci. 21:6018–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwayanagi, M., K. Shimano, and K. Kurihara. 1996. Existence of multiple receptors in single neurons: responses of single bullfrog olfactory neurons to many cAMP-dependent and independent odorants. Brain Res. 738:222–228. [DOI] [PubMed] [Google Scholar]
- Kaur, R., X.O. Zhu, A.J. Moorhouse, and P.H. Barry. 2001. IP3-gated channels and their occurrence relative to CNG channels in the soma and dendritic knob of rat olfactory receptor neurons. J. Membr. Biol. 181: 91–105. [PubMed] [Google Scholar]
- Kurahashi, T. 1989. Activation by odorants of cation-selective conductance in the olfactory receptor cell isolated from the newt. J. Physiol. 419:177–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi, T., and A. Kaneko. 1991. High density cAMP-gated channels at the ciliary membrane in the olfactory receptor cell. Neuroreport. 2:5–8. [DOI] [PubMed] [Google Scholar]
- Kurahashi, T., and A. Kaneko. 1993. Gating properties of the cAMP-gated channel in toad olfactory receptor cells. J. Physiol. 466:287–302. [PMC free article] [PubMed] [Google Scholar]
- Kurahashi, T., and A. Menini. 1997. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 385:725–729. [DOI] [PubMed] [Google Scholar]
- Kurahashi, T., and T. Shibuya. 1990. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 515:261–268. [DOI] [PubMed] [Google Scholar]
- Kurahashi, T., and K.-W. Yau. 1994. Olfactory transduction. Tales of an unusual chloride current. Curr. Biol. 4:256–258. [DOI] [PubMed] [Google Scholar]
- Larsson, H.P., S.J. Kleene, and H. Lecar. 1997. Noise analysis of ion channels in non-space-clamped cables: estimates of channel parameters in olfactory cilia. Biophys. J. 72:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, G., and G.H. Gold. 1991. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J. Physiol. 442:147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, G., and G.H. Gold. 1993. a. Contribution of the ciliary cyclic nucleotide-gated conductance to olfactory transduction in the salamander. J. Physiol. 462:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, G., and G.H. Gold. 1993. b. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 366:283–286. [DOI] [PubMed] [Google Scholar]
- Lowe, G., T. Nakamura, and G.H. Gold. 1989. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc. Natl. Acad. Sci. USA. 86:5641–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, B., R. Madrid, and J. Bacigalupo. 1997. Calcium mediates the activation of the inhibitory current induced by odorants in toad olfactory receptor neurons. FEBS Lett. 402:259–264. [DOI] [PubMed] [Google Scholar]
- Ngai, J., M.M. Dowling, L. Buck, R. Axel, and A. Chess. 1993. The family of genes encoding odorant receptors in the channel catfish. Cell. 72:657–666. [DOI] [PubMed] [Google Scholar]
- Reed, R.R. 1992. Signaling pathways in odorant detection. Neuron. 8:205–209. [DOI] [PubMed] [Google Scholar]
- Reisert, J., and H.R. Matthews. 2001. Response properties of isolated mouse olfactory receptor cells. J. Physiol. 530:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler, K.J., S.L. Sullivan, and L.B. Buck. 1993. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 73:597–609. [DOI] [PubMed] [Google Scholar]
- Ronnett, G.V., and S.H. Snyder. 1992. Molecular messengers of olfaction. Trends Neurosci. 15:508–513. [DOI] [PubMed] [Google Scholar]
- Schild, D., F.W. Lischka, and D. Restrepo. 1995. InsP3 causes an increase in apical [Ca2+]i by activating two distinct components in vertebrate olfactory receptor cells. J. Neurophysiol. 73:862–866. [DOI] [PubMed] [Google Scholar]
- Schild, D., and D. Restrepo. 1998. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 78:429–466 (Review. [DOI] [PubMed] [Google Scholar]
- Sklar, P.B., R.R. Anholt, and S.H. Snyder. 1986. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J. Biol. Chem. 261:15538–15543. [PubMed] [Google Scholar]
- Takeuchi, H., and T. Kurahashi. 2002. Photolysis of caged cyclic AMP in the ciliary cytoplasm of the newt olfactory receptor cell. J. Physiol. 541:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar, R., J. Ngai, and R. Axel. 1993. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 74:309–318. [DOI] [PubMed] [Google Scholar]
- Vogl, A., J. Noe, H. Breer, and I. Boekhoff. 2000. Cross-talk between olfactory second messenger pathways. Eur. J. Biochem. 267:4529–4535. [DOI] [PubMed] [Google Scholar]
- Wong, S.T., K. Trinh, B. Hacker, G.C. Chan, G. Lowe, A. Gaggar, Z. Xia, G.H. Gold, and D.R. Storm. 2000. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 27:487–497. [DOI] [PubMed] [Google Scholar]