Abstract
Aquaporins facilitate the diffusion of water across cell membranes. We previously showed that acid pH or low Ca2+ increase the water permeability of bovine AQP0 expressed in Xenopus oocytes. We now show that external histidines in loops A and C mediate the pH dependence. Furthermore, the position of histidines in different members of the aquaporin family can “tune” the pH sensitivity toward alkaline or acid pH ranges. In bovine AQP0, replacement of His40 in loop A by Cys, while keeping His122 in loop C, shifted the pH sensitivity from acid to alkaline. In the killifish AQP0 homologue, MIPfun, with His at position 39 in loop A, alkaline rather than acid pH increased water permeability. Moving His39 to His40 in MIPfun, to mimic bovine AQP0 loop A, shifted the pH sensitivity back to the acid range. pH regulation was also found in two other members of the aquaporin family. Alkaline pH increased the water permeability of AQP4 that contains His at position 129 in loop C. Acid and alkaline pH sensitivity was induced in AQP1 by adding histidines 48 (in loop A) and 130 (in loop C). We conclude that external histidines in loops A and C that span the outer vestibule contribute to pH sensitivity. In addition, we show that when AQP0 (bovine or killifish) and a crippled calmodulin mutant were coexpressed, Ca2+ sensitivity was lost but pH sensitivity was maintained. These results demonstrate that Ca2+ and pH modulation are separable and arise from processes on opposite sides of the membrane.
Keywords: calmodulin, MIP, histidine, lens, killifish
INTRODUCTION
Aquaporins (AQPs) facilitate the rapid and selective movement of small neutral molecules, such as water and glycerol, across cell membranes. AQP1 from red blood cells was the first member of the family shown to be a functional water channel (Preston et al., 1992). AQP0, the major intrinsic protein of the lens, is expressed only in terminally differentiated fiber cells. The killifish (Fundulus heteroclitus) lens homologue of AQP0, designated MIPfun, was recently cloned (Virkki et al., 2001). MIPfun has 70% sequence identity with bAQP0 and 46% with human AQP1 (hAQP1) and exhibits a water permeability much higher than that of bAQP0 but close to that of hAQP1 in the oocyte expression system. Another member, AQP4, functions as a water-selective channel in epithelial cells in the kidney-collecting duct as well as in brain astrocytes (Shi and Verkman, 1996). Rat AQP4 (rAQP4) has 37% identity with bAQP0 and 41% identity with hAQP1 and also exhibits water permeability close to that of hAQP1 in the oocyte expression system. Some AQPs are sensitive to change in pH, including AQP0 (Németh-Cahalan and Hall, 2000), AQP3 (Zeuthen and Klaerke, 1999), and AQP6 (Yasui et al., 1999), but bovine AQP0 (bAQP0) is the only water channel shown to be modulated also by low Ca2+ (Németh-Cahalan and Hall, 2000).
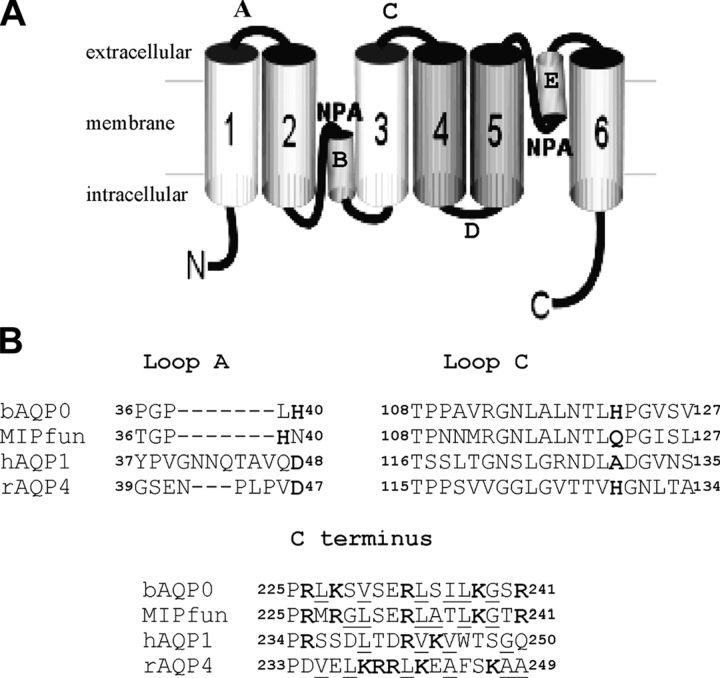
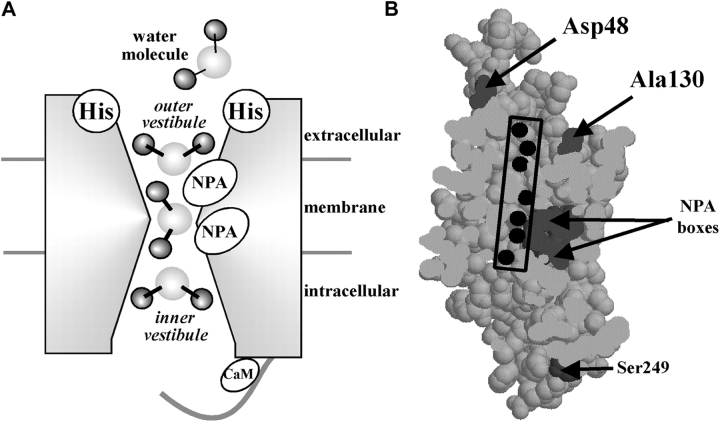
Fig. 1 A shows the predicted topology of aquaporins, confirmed by X-ray (Sui et al., 2001) and electron crystallography (Murata et al., 2000) with six transmembrane helices (1–6) and five connecting loops (A–E). Two highly conserved loops (B and E) each contain the signature motif present in every aquaporin, asparagine-proline-alanine (NPA), which forms one wall of the water pore. Fig. 1 B shows the sequence alignments of the critical regions that contribute to pH and Ca2+ sensitivity, loop A, loop C, and the COOH terminus tail. Loop A of bAQP0 contains the critical His40 residue, while two histidines are present in MIPfun at positions 39 and 43. In loop C, only bAQP0 and rAQP4 present an aligned His at positions 122 and 129, respectively. In both bAQP0 and MIPfun, the indicated sequence within the COOH terminus tail is atypical for a CaM-binding site, but fits the general requirement of an amphiphilic chain with alternation of positive charges (Peracchia and Girsch, 1989). Moreover, several reports demonstrate that calmodulin binds AQP0 (van den Eijnden-van Raaij et al., 1985, 1986; Peracchia and Girsch, 1989; Louis et al., 1990; Girsch and Peracchia, 1991; Swamy-Mruthinti, 2001).
Figure 1.
The predicted membrane topology of AQPs. (A) In the hourglass model, confirmed by high-resolution structures, loops B and E containing highly conserved NPA motifs fold into the bilayer from opposite sides of the membrane to form the water pore. (B) Sequence alignment of AQPs (using ClustalW at http://clustalw.genome.ad.jp). In loop A, bAQP0 contains His40, MIPfun contains His39 and His43, and hAQP1 and rAQP4 both contain Asp48 and Asp47, respectively. In loop C, both bAQP0 and rAQP4 present a His at the position 122 and 129, respectively. In both bAQP0 and MIPfun, the C-tail contains a consensus CaM binding site with alternating hydrophobic (underlined) and positive residues (in bold).
In this paper, we compare the effects of pH and Ca2+ on the water permeabilities of bAQP0, MIPfun, hAQP1, rAQP4. We demonstrate that the position of external histidines in loops A and C can tune the pH dependence. We suggest that histidines alter the structure of water in the outer vestibule of the channel and thus modulate the water permeability. We also show that Ca2+ modulation requires active CaM and that pH and Ca2+ act at two completely separate sites. Molecular mechanisms and physiological relevance are discussed.
MATERIALS AND METHODS
Preparation of Oocytes
Female Xenopus laevis were anesthetized, and stage V and VI oocytes removed and prepared as previously described (Németh-Cahalan and Hall, 2000). The day after isolation, oocytes were injected with 10 ng of each cRNA, and maintained in ND96 (in mM, 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 2.5 sodium pyruvate, 5 HEPES pH 7.5) supplemented with 10 μg/ml penicillin and 10 μg/ml streptomycin at 18°C. All chemicals were obtained from Sigma-Aldrich.
Expression Constructs and RNA Preparation
The expression constructs for bovine AQP0 (bAQP0) and human AQP1 (hAQP1) were gifts from Peter Agre and Greg Preston (Johns Hopkins University). The expression construct for MIPfun was a gift from Walter Boron (Yale University). The rat AQP4 (rAQP4) gene was purchased from American Type Culture Collection (#87184) and placed in the same expression vector. The gene for the crippled mutant CaM from Drosophila (B1234Q), a gift from Kathy Beckingham (Rice University), was placed in a PAGA expression vector (Mukherjea et al., 1996). RNA was transcribed in vitro using T3 or T7 RNA polymerase (mMESSAGE mMACHINE kit; Ambion).
Mutant Constructions
All the mutations were done using the QuikChange site-directed mutagenesis kit (Stratagene). The mutations were confirmed by sequencing (University of Chicago, DNA Sequencing Facility).
Swelling Assay and Measurement of Water Permeability
2 d postinjection, oocyte swelling assays were performed at room temperature (20–21°C) by transfer from 200 mosM (100% ND96) to 70 mosM (30% ND96) solution as previously described (Németh-Cahalan and Hall, 2000). Water permeability, Pf, was calculated from optical measurements of the increase in cross-sectional area of the oocyte with time in response to diluted ND96 using the formula:
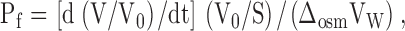 |
where V is the volume as a function of time, V0 is the initial volume, S is the geometric surface area, Δosm is the osmotic gradient, and Vw is the molar volume of water. Previously (Chandy et al., 1997; Németh Cahalan and Hall, 2000), we reported water permeabilities using the actual surface area of the oolemma, which is nine times the geometric surface area due to folds and microvilli as determined by morphological measurement and by oocyte membrane capacitance (Dick and Dick, 1970; Zampighi et al., 1995). To facilitate comparison with results in the bulk of the literature, the permeabilities reported here are calculated using the geometric surface area. The swelling assay is performed either under control conditions of pH 7.5 and 1.8 mM Ca2+, or experimental conditions of altered pH or Ca2+. Unless otherwise indicated in parentheses, each data point is the average of measurements from nine oocytes from three different batches.
Controls
Under standard conditions, uninjected oocytes had an average water permeability of 9.75 ± 0.7 × 10−4 cm/s (n = 52), and showed no change in water permeability under any of the experimental conditions (changes in pH or Ca2+ concentration).
pH Effect
For each experimental pH value, 100% and 30% ND96 solutions were made using HEPES for pH 8.5–7.0 and MES for pH 6.5–6.0. Before the swelling assay in 30% ND96 at the experimental pH, the oocytes were incubated in 100% ND96 at the experimental pH for 5 min. We define the factor of increase as the ratio of Pf at the experimental pH value (pH 6.5 or 8.5) and Pf at the standard pH value (7.5).
Calcium Effect
For the “no Ca2+” solution, 100% and 30% ND96 solutions were made omitting Ca2+. Before the swelling assay was performed in 30% ND96 without added Ca2+, the oocytes were incubated in 100% ND96 without added Ca2+ for 5 min.
Coinjection of Crippled Calmodulin and Aquaporin
In some experiments, 10 ng of aquaporin (AQP0, AQP0/H40C, or MIPfun) and 10 ng of B1234Q CaM mutant were coinjected. After 2 d, the swelling assay was performed.
RESULTS
Initially, we compared effects of pH and Ca2+ on bovine and killifish AQP0, MIPfun expressed in Xenopus oocytes. Fig. 2 A shows that bAQP0 water permeability was increased by acid pH or low Ca2+ in experiments performed at 21°C, consistent with our previous results at 15°C (Németh-Cahalan and Hall, 2000). MIPfun-injected oocytes have a water permeability 11 times higher than oocytes injected with the same quantity of AQP0 cRNA under control conditions. (Fig. 2 A, note different scales). And, although the water permeability of MIPfun did not increase at pH 6.5, either alkaline pH or low Ca2+ significantly increased its water permeability. These results demonstrate pH regulation in a water channel with much higher water permeability than bAQP0 and allow us to compare sequence motifs in killifish and bovine AQP0 critical for pH regulation of water permeability.
Figure 2.
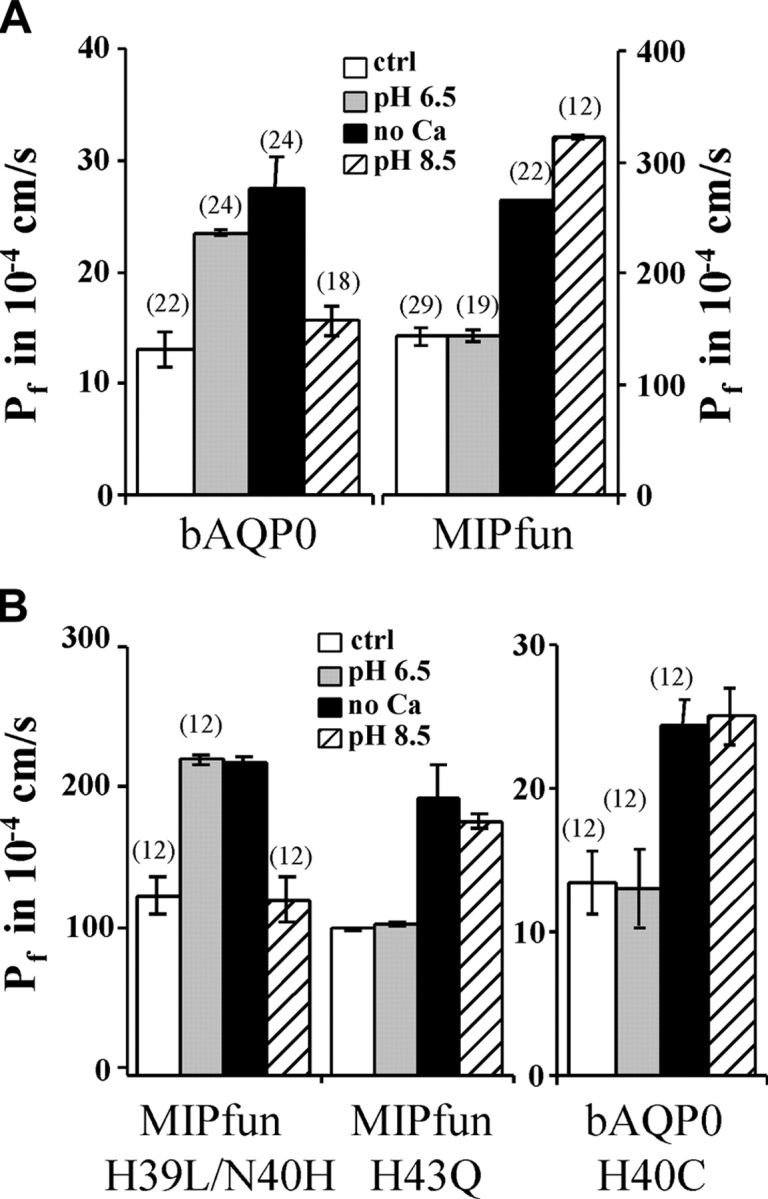
Role of histidines in loop A on bAQP0 and MIPfun water permeability. (A) The permeability of bAQP0-injected oocytes increases by a factor of 1.8 at pH 6.5 and by a factor of 2.1 with no added Ca2+. MIPfun-injected oocytes have a water permeability 11 times higher than oocytes injected with the same quantity of AQP0 cRNA under control conditions. The permeability of MIPfun-injected oocytes increases by a factor of 2.2 at pH 8.5 and by a factor of 1.9 with no added Ca2+. (B) Moving the His39 to the position 40 restores the acid pH sensitivity, but replacing His43 does not affect the pH sensitivity. Acid pH has no effect on the water permeability of bAQP0/H40C-injected oocytes, but no added Ca2+ or alkaline pH increases the permeability by a factor of 1.8 and 1.9, respectively. Note the different scales for bAQP0 and MIPfun.
Histidines in Loops A and C Regulate pH Sensitivity
In loop A, which contributes to the pH sensitivity of bAQP0, MIPfun lacks His40 but has instead two histidines at positions 39 and 43 (Fig. 1 B). We made mutations at corresponding positions in MIPfun to investigate the role of histidine position in pH sensitivity. To mimic the bAQP0 loop, we moved His39 to position 40, and found that this mutation (H39L/N40H) induced acid pH sensitivity, exactly like wild type bAQP0 (Fig. 2 B). In contrast, replacing His43 by a Gln, (MIPfun/H43Q, Fig. 2 B) had no effect, the mutant maintaining alkaline pH sensitivity just like wild-type MIPfun. Thus, His43 has no effect on water permeability. These results show that in MIPfun, His39 confers alkaline pH sensitivity, whereas His40 confers acid pH sensitivity. We also replaced His40 by a Cys in bAQP0 (Fig. 2 B, note different scales). The mutant bAQP0/H40C exhibited no sensitivity to acid pH but gained sensitivity to alkaline pH while retaining sensitivity to low Ca2+. These results show that His40 in bAQP0 is necessary to confer acid pH sensitivity. Furthermore, the effects of pH and Ca2+ are separable and act at different sites.
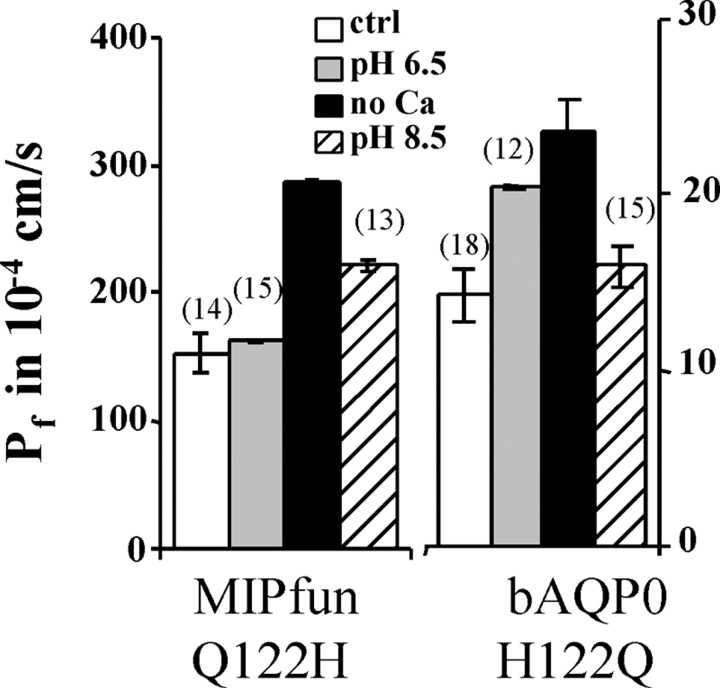
The results above demonstrated the role of histidines in loop A in the pH sensitivity of AQP0. We also investigated a possible role for histidine in the next external loop (loop C) by inserting His122 in MIPfun and removing it in bAQP0. Fig. 3 shows that MIPfun/Q122H retained alkaline pH sensitivity but with a reduction of 35% compared with the wild-type. Symmetrically, bAQP0/H122Q retained acid pH sensitivity but with a 17% reduction. We conclude that adding His122 in MIPfun does not change the alkaline pH sensitivity conferred by His39. And symmetrically, removing His122 in bAQP0 does not change the acid pH sensitivity conferred by His40. But because we observed a reduction of effect in both mutants, we can also conclude that there is a possible interaction between histidines in loop A and C.
Figure 3.
Role of histidines in loop C on bAQP0 and MIPfun water permeability. Adding a histidine at position 122 in MIPfun does not affect alkaline pH sensitivity, but the factor of increase is only 1.5 (32% less than wild-type increase). Removing the His122 in bAQP0 does not affect acid pH sensitivity but the factor of increase is only of 1.5 (17% less than wild-type increase). Note the different scales.
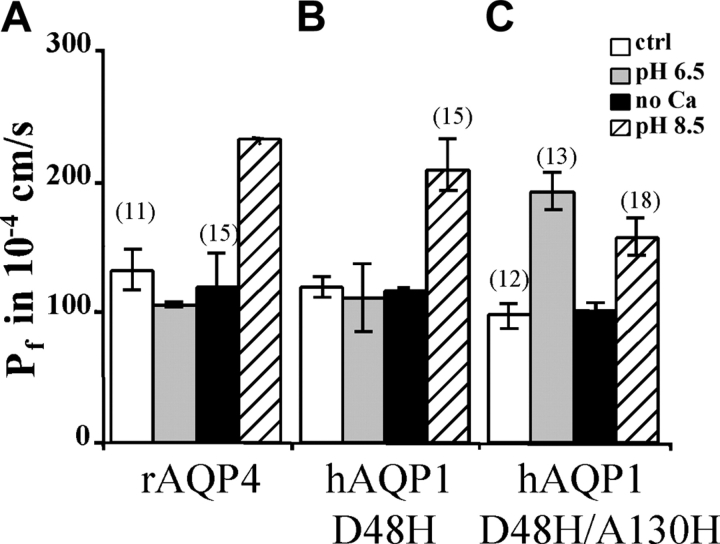
Rat AQP4 (rAQP4) is not acid pH sensitive (Németh-Cahalan and Hall, 2000); but because it has His129, the homologue of His122 in bAQP0, we decided to test for alkaline pH sensitivity (Fig. 4 A). Indeed, rAQP4 water permeability was markedly increased at alkaline pH. These results suggest that in rAQP4, His129 alone may confer alkaline pH sensitivity.
Figure 4.
Effect of pH and Ca2+ on AQP4 and mutants of AQP1. (A) The water permeability of rAQP4-injected oocytes increases at alkaline pH by a factor of 1.9, but acid pH and low Ca2+ have no effect. (B) In contrast to hAQP1 wild-type, the water permeability of hAQP1/D48H-injected oocytes increases with alkaline pH by a factor of 1.7. (C) The water permeability of hAQP1/D48H/A130H-injected oocytes increases with acid pH and alkaline pH by a factor of 1.9 and 1.6, respectively.
In our previous report, we demonstrated that human AQP1 (hAQP1) was not regulated by pH in the range from 6.5 to 8.5 (Németh-Cahalan and Hall, 2000). We sought to engineer pH sensitivity into hAQP1 by introducing a His40 and a His122 in the corresponding positions. By using sequence alignments (Fig. 1 B), we determined that the corresponding positions in AQP1 are Asp48 and Ala130. Fig. 4 B shows that hAQP1/D48H is not acid pH sensitive, but instead is alkaline pH sensitive. Fig. 4 C shows that the double mutant (hAQP1/D48H/A130H) is acid and alkaline pH sensitive. We conclude that His48 induces alkaline pH sensitivity in hAQP1 and that the combination of His48 and His130 can confer acid and alkaline pH sensitivity. Conferring pH sensitivity on AQP1 by introducing histidines did not induce Ca2+ sensitivity, offering further proof that pH and Ca2+ regulation are separable.
Calcium Regulation of Aquaporin Water Permeability
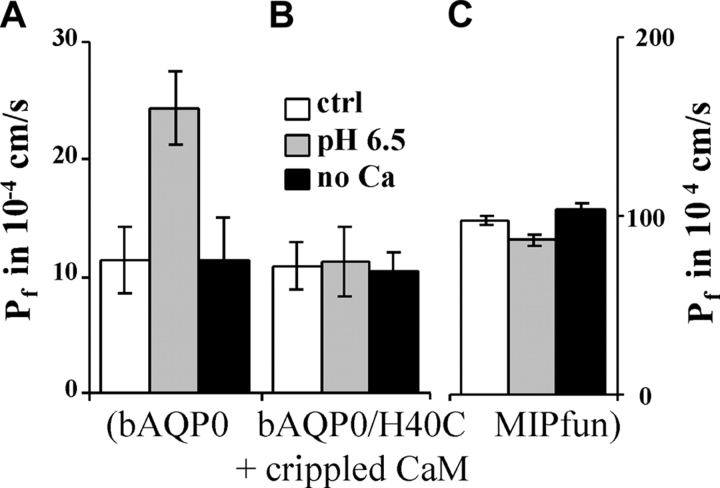
We showed above (Fig. 2, A and B) that bAQP0, bAQP0/H40C and MIPfun are Ca2+ sensitive and that their water permeabilities are increased upon Ca2+ removal by factors of 2.1, 1.8, and 1.9, respectively. The water permeabilities of rAQP4 and hAQP1 are not Ca2+ modulated (Fig. 4). In addition, we previously found that three different CaM inhibitors (TFP, CDZ, and W7) eliminated the Ca2+ sensitivity of bAQP0 water permeability (Németh-Cahalan and Hall, 2000), but these inhibitors are notoriously nonspecific. We now provide definitive evidence for the involvement of CaM by coinjecting B1234Q, a “crippled hands” CaM in which the four Ca2+-binding sites are mutated so as to prevent Ca2+ binding without changing its structure (Mukherjea et al., 1996). Coinjection of crippled CaM with bAQP0 abolished the increase in water permeability that would normally be produced by low Ca2+, but did not alter the increase produced by acid pH (Fig. 5 A). As a control for specificity, we also compared coinjection of crippled CaM with hAQP1, and found no effect on control Pf or Pf values at altered pH and low Ca2+ (unpublished data). These results prove that the Ca2+ effect is mediated by CaM, exactly as earlier results with CaM inhibitors suggested. That the increase in water permeability induced by pH is unaffected by the mutant CaM argues strongly that the sites at which pH and Ca2+ act are distinct and the effects of pH and Ca2+ completely separable.
Figure 5.
Elimination of Ca2+ sensitivity of bAQP0, bAQP0/H40C and MIPfun by crippled CaM. (A) The water permeability of bAQP0 and crippled CaM coinjected oocytes is still increased by low pH but not by low Ca2+. (B) The water permeability of bAQP0/H40C and crippled CaM coinjected oocytes is not increased by low pH or low Ca2+. (C) The water permeability of MIPfun and crippled CaM coinjected oocytes is not increased by low pH or low Ca2+. Note the different scale for MIPfun.
Fig. 5, B and C, shows the result of coinjection of crippled CaM with the mutant bAQP0/H40C and with MIPfun, respectively. The CaM mutant abolished the Ca2+ sensitivity of bAQP0/H40C and of MIPfun. Both bAQP0/H40C and MIPfun remain insensitive to acid pH. Thus, for both bAQP0/H40C and MIPfun, Ca2+ modulation requires active CaM, and the effects of pH and Ca2+ are entirely separable.
DISCUSSION
By creating conditions or mutations that modify either Ca2+ sensitivity or pH sensitivity while leaving sensitivity to the other ion intact, we have demonstrated that AQP0 is modulated by pH and Ca2+ at distinct sites.
Role of External Histidines in pH Sensitivity
Our results reveal the molecular basis for acid and alkaline pH sensitivity mediated by externally accessible histidines in the AQP family. Histidine position is critical, and histidines in different loops interact to produce results that are not additive combinations of the effects of the histidines individually (Table I) . In bAQP0, His40 in loop A conveys acid pH sensitivity, but if His40 is absent, we observed alkaline pH sensitivity. In rAQP4, we also observed alkaline pH sensitivity. In both cases, the His in loop C (His122 for bAQP0 and His129 for rAQP4) may be responsible for the alkaline pH sensitivity. In MIPfun, His39 confers alkaline pH sensitivity, whereas moving it to position 40 confers acid pH sensitivity, just like bAQP0. His43 is apparently not involved in pH sensitivity. In summary, a lone histidine in loop A can confer either acid or alkaline pH sensitivity according to its precise position within the loop. Shifting the histidine by only one amino-acid residue can tune pH sensitivity. A lone histidine in loop C may confer alkaline pH sensitivity. The crystal structure of AQP1 shows that the histidines in loops A and C are on opposite sides of the outer vestibule flanking the entrance to the water pore but close enough to interact with one another electrostatically (Fig. 6) . The possible significance of the flanking histidines for water permeability is discussed below.
TABLE I.
Summary of Modulation of pH Sensitivity with Histidine Position in Loops A and C
| Aquaporin | Loop A | Loop C | Maximum pH sensitivity | Factor of increase |
|---|---|---|---|---|
| bAQP0 | His40 | His122 | pH 6.5 | 1.8 |
| bAQP0/H40C | — | His122 | pH 8.5 | 1.9 |
| bAQP0/H122Q | His40 | — | pH 6.5 | 1.5 |
| MIPfun | His39 | — | pH 8.5 | 2.2 |
| MIPfun/H39L/N40H | His40 | — | pH 6.5 | 1.8 |
| MIPfun/H43Q | His39 | — | pH 8.5 | 2.3 |
| MIPfun/Q122H | His39 | His122 | pH 8.5 | 1.5 |
| hAQP1 | — | — | none | none |
| hAQP1/D48H | His48 | — | pH 8.5 | 1.7 |
| hAQP1/D48H/A130H | His48 | His130 | pH 6.5 and pH 8.5 | 1.9 and 1.6 |
| rAQP4 | — | His129 | pH 8.5 | 1.9 |
The factor of increase is calculated by dividing the Pf at the maximum pH sensitivity by the Pf at pH 7.5.
Figure 6.
The aqueous pore. (A) A cartoon shows the three different regions seen by a transiting water molecule: the outer vestibule, the maximum constriction near the NPA boxes, and the inner vestibule. Water molecules must change their orientations to pass from region to region. We propose that histidines (His) in loops A and C act on water in the outer vestibule under the influence of pH and that the COOH-terminal tail containing the CaM-binding site acts on water in the inner vestibule under the influence of Ca2+. (B) A section through the pore based on the crystal structure of AQP1 using the coordinates of Sui et al. (2001). The section shows the permeation pathway (with seven water molecules), the positions of Asp48 and Ala130 (homologues of His40 and His122 in bAQP0), and the NPA boxes. Ser249 is the last resolved residue visible in the crystal and is situated in the middle of the COOH terminus tail. This figure was prepared using Protein Explorer (http://proteinexplorer.org) (Martz, 2002).
Our results also demonstrate that the hAQP1 double histidine mutant is not the equivalent of bAQP0. Histidines in loop A and C can confer either acid or alkaline pH sensitivity, as in AQP0, MIPfun, or AQP4, or sensitivity to both acid and alkaline pH in the same molecule, as in the double histidine mutant of AQP1. The mutations in MIPfun are directly comparable with bAQP0, because of their 70% sequence identity and equal number of amino acids, but this is not the case for mutations in AQP1. But because the relevant His are close to the transmembrane helices, the comparison with His in bAQP0 is possible even if the extracellular loops of AQP1 are larger than those in bAQP0 and MIPfun, and the environment surrounding the His mutations in hAQP1 are entirely different.
Role of CaM in Ca2+ Sensitivity
In our previous study (Németh-Cahalan and Hall, 2000), we found that three bAQP0 mutants, H40A, H40K, and H40D, lost both pH sensitivity and Ca2+ sensitivity. All three mutants exhibited the same high water permeability that wild-type AQP0 showed at acid pH or low calcium. We also found that the effects of low calcium and acid pH were not additive. Furthermore, by increasing internal Ca2+ buffering and elevating internal Ca2+, we showed that Ca2+ regulation of water permeability in bAQP0 is mediated through an internal site (Németh-Cahalan and Hall, 2000). Therefore, we suspected that these three mutants were calcium insensitive not because they lack the ability to sense low calcium, but because they were already in a state of high water permeability. Results from our new H40C/bAQP0 mutant are consistent with this view; compared with wild-type, the H40C mutant loses pH sensitivity but retains the same calcium-dependent modulation in water permeability, indicating that effects of pH and Ca2+ are separable and mediated by different portions of the molecule. Since it was known that the COOH-terminal tail of bAQP0 contains a consensus CaM binding site with alternating hydrophobic and charged residues, and that a peptide with this sequence was able to bind CaM in vitro in a Ca2+-independent manner (Peracchia and Girsch, 1989), we tested the hypothesis that CaM mediates the calcium-sensitive modulation of water permeability. Though the sequence is somewhat atypical of consensus calmodulin binding sites, there are multiple reports in the literature of calmodulin binding to AQP0 (van den Eijnden-van Raaij et al., 1985, 1986; Peracchia and Girsch, 1989; Louis et al., 1990; Girsch and Peracchia, 1991; Swamy-Mruthinti, 2001), and our present results certainly strengthen the position that calmodulin binds AQP0 and MIPfun in a calcium-independent manner. Expression of a mutant CaM with “crippled” hands completely eliminated calcium sensitivity, but had no effect on normal water permeability or pH modulation. The crippled CaM mutant can presumably still bind AQP, but since it cannot bind Ca2+ it cannot convey Ca2+ sensitivity. These results reinforce the conclusion that effects of pH and Ca2+ on water permeability are completely separable and independent, and, because the CaM binding site is on the cytoplasmic side of the membrane, they suggest that calcium and calmodulin may alter the interactions of water with the cytoplasmic vestibule.
A Single-file Model
This paper strongly supports the conclusion that AQP0 has a high permeability mode and a low permeability mode. How do these two modes differ? Available structural data on the nature of the water-filled pore through AQP1 and the glycerol facilitator suggest a possible answer. For both, water and glycerol must move through the pore by single-file diffusion. The crystal structure and molecular dynamic simulations suggest that there are multiple water molecules in the pore. There are ∼6 waters seen in the narrow constriction of the pore in the X-ray structure (Sui et al., 2001), and molecular dynamic simulations suggest that there are ∼7 or 8 water molecules moving in concert in the single-file portion of the pore (Tajkhorshid et al., 2002; Zhu et al., 2004). The lack of passage of ionic current through AQP0 and AQP1 is explained by electrostatic considerations that strongly inhibit the movement of protons into the NPA region or hydroxyls into regions flanking either side of the NPA region (de Groot et al., 2003; Chakrabarti et al., 2004). Thus, movement of water through the channel is likely to be described, at least to a first approximation, in a manner analogous to ion movement through a single-file ion channels (Almers and McCleskey, 1984; Doyle et al., 1998; Morais-Cabral et al., 2001). A critical characteristic of single-file diffusion is that net movement of material from one side of the membrane to the other requires that each of the sites must be unoccupied by the permeating molecules for a significant fraction of the time in order for molecules from adjacent sites to move. Such a model is consistent with both the structure of AQP1 (Murata et al., 2000; Sui et al., 2001) and molecular dynamics simulations of water movement in the AQP1 pore (de Groot and Grubmuller, 2001; Tajkhorshid et al., 2002; de Groot et al., 2003; Chakrabarti et al., 2004).
We suggest that our results can be explained by a model in which pH, by titrating external histidines, modulates the orientation of water molecules near the outer vestibule and Ca2+, through the mediation of calmodulin, their orientation in the inner vestibular site (Fig. 6 A). Modulation of water orientation would in turn alter the orientation-dependent electrostatic interactions between water molecules, and thus their effective binding energies in the single-file pore. Molecular dynamic simulations show that water molecules must rotate their dipole moments to pass through the AQP1 channel and the glycerol facilitator (Jensen et al., 2001, 2002, 2003; Zhu et al., 2001). We speculate that, in the high permeability mode, water molecules in the vestibule are oriented, on average, so as to repel water molecules in the single-file pore, thus decreasing their binding energy to the pore and increasing the rate of water movement through the pore. In the low permeability state, water molecules in the vestibule are oriented on average in a less repulsive configuration, and thus the collective binding energy of water molecules in the single-file pore is higher and water permeability is reduced. In the high permeability mode, the properties of the single-file pore would be rate limiting, while in the low permeability mode, exit rate from the pore would be modulated to a degree by the hydration states of the vestibules. We emphasize the rather remarkable finding that pH and calcium tend to increase the water permeability by a factor of approximately two in aquaporins that have quite different single-molecule water permeabilities; this suggests a common mechanism likely to act by altering the kinetics of a particular rate constant by a similar amount in each of the different aquaporins.
Physiological Relevance
The mechanism that we describe here would provide a rapid and localized regulation of water permeability in response to ionic changes in the extracellular environment. It is of some importance to determine if the mechanisms regulating AQP0 water permeability in oocytes are physiological in the mammalian lens. Mutations in human and mouse AQP0 genes are associated with cataract (Berry et al., 1996, 2000; Shiels and Bassnett, 1996; Francis et al., 2000a,b). AQP0-dependent cataracts may be caused by a failure of the intrinsic microcirculation of the avascular lens (Mathias et al., 1997), and pH and Ca2+ are likely candidates for effecting regulatory control of this circulation. The lens interior is more acidic (Pasquale et al., 1990; Mathias et al., 1991), has higher Ca2+ than the surface (Baldo et al., 2002), and disturbances in Ca2+ concentration are associated with cataract (Takehana, 1990). Lens-fiber-cell vesicles isolated from an AQP0 knockout mouse have a water permeability reduced by 80% of wild-type mouse values (Varadaraj et al., 1999), thereby demonstrating in the native membrane that AQP0 is essential for water transport. Recently, these authors reported that water permeability of vesicles isolated from wild-type mouse or rabbit lenses was also modulated by Ca2+ and pH (Mathias et al., 2002). This modulation depended on the presence of AQP0 and was absent in vesicles from AQP0-knockout animals. These results strongly suggest that our findings in the oocyte are relevant to lens physiology. Finally, because His129 is conserved in bovine, rat, rabbit, and human AQP4, the alkaline pH sensitivity exhibited by rAQP4 could be physiologically relevant as a mechanism to modulate water permeability in the kidney, particularly in the cortical-collecting duct during alkaline load.
Acknowledgments
We would like to thank Mary Hawley for expert technical assistance including oocyte preparation, and Michael D. Cahalan for helpful discussions. We also would like to thank Peter Agre and Greg Preston for bovine AQP0 and human AQP1 constructs, Walter Boron for MIPfun constructs, and Kathy Beckingham for the crippled CaM mutant construct.
This work was supported by Grant EY5661 from the National Institutes of Health to J.E. Hall.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: AQP, aquaporin; bAQP0, bovine AQP-0; CaM, calmodulin; hAQP1, human AQP1; NPA, asparagine-proline-alanine; rAQP4, rat AQP4.
References
- Almers, W., and E.W. McCleskey. 1984. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J. Physiol. 353:585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo, G.J., J. Gao, X. Sun, and R. Mathias. 2002. Intracellular Ca2+ concentration gradient within the lens. Invest. Ophthalmol. Vis. Sci. 43:3539. [Google Scholar]
- Berry, V., P. Francis, S. Kaushal, A. Moore, and S. Bhattacharya. 2000. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat. Genet. 25:15–17. [DOI] [PubMed] [Google Scholar]
- Berry, V., A.C. Ionides, A.T. Moore, C. Plant, S.S. Bhattacharya, and A. Shiels. 1996. A locus for autosomal dominant anterior polar cataract on chromosome 17p. Hum. Mol. Genet. 5:415–419. [DOI] [PubMed] [Google Scholar]
- Chandy, G., G.A. Zampighi, M. Kreman, and J.E. Hall. 1997. Comparison of the water transporting properties of MIP and AQP1. J. Membr. Biol. 159:29–39. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, N., E. Tajkhorshid, B. Roux, and R. Pomes. 2004. Molecular basis of proton blockage in aquaporins. Structure (Camb.). 12:65–74. [DOI] [PubMed] [Google Scholar]
- de Groot, B.L., T. Frigato, V. Helms, and H. Grubmuller. 2003. The mechanism of proton exclusion in the aquaporin-1 water channel. J. Mol. Biol. 333:279–293. [DOI] [PubMed] [Google Scholar]
- de Groot, B.L., and H. Grubmuller. 2001. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 294:2353–2357. [DOI] [PubMed] [Google Scholar]
- Dick, E.G., and D.A.T. Dick. 1970. The effect of surface microvilli on the water permeability of single toad oocytes. J. Cell Sci. 6:451–476. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., C.J. Morais, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Francis, P., V. Berry, S. Bhattacharya, and A. Moore. 2000. a. Congenital progressive polymorphic cataract caused by a mutation in the major intrinsic protein of the lens, MIP (AQP0). Br. J. Ophthalmol. 84:1376–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, P., J.J. Chung, M. Yasui, V. Berry, A. Moore, M.K. Wyatt, G. Wistow, S.S. Bhattacharya, and P. Agre. 2000. b. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum. Mol. Genet. 9:2329–2334. [DOI] [PubMed] [Google Scholar]
- Girsch, S.J., and C. Peracchia. 1991. Calmodulin interacts with a C-terminus peptide from the lens membrane protein Mip26. Curr. Eye Res. 10:839–849. [DOI] [PubMed] [Google Scholar]
- Jensen, M.O., S. Park, E. Tajkhorshid, and K. Schulten. 2002. Energetics of glycerol conduction through aquaglyceroporin GlpF. Proc. Natl. Acad. Sci. USA. 99:6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, M.O., E. Tajkhorshid, and K. Schulten. 2001. The mechanism of glycerol conduction in aquaglyceroporins. Structure (Camb.). 9:1083–1093. [DOI] [PubMed] [Google Scholar]
- Jensen, M.O., E. Tajkhorshid, and K. Schulten. 2003. Electrostatic tuning of permeation and selectivity in aquaporin water channels. Biophys. J. 85:2884–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, C.F., P. Hogan, L. Visco, and G. Strasburg. 1990. Identity of the calmodulin-binding proteins in bovine lens plasma membranes. Exp. Eye Res. 50:495–503. [DOI] [PubMed] [Google Scholar]
- Martz, E. 2002. Protein Explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci. 27:107–109. [DOI] [PubMed] [Google Scholar]
- Mathias, R.T., J.L. Rae, and G.J. Baldo. 1997. Physiological properties of the normal lens. Physiol. Rev. 77:21–50. [DOI] [PubMed] [Google Scholar]
- Mathias, R.T., G. Riquelme, and J.L. Rae. 1991. Cell to cell communication and pH in the frog lens. J. Gen. Physiol. 98:1085–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias, R.T., K. Varadaraj, S. Kumari, G.J. Baldo, A. Shiels, and S. Bassnett. 2002. Water transport by Aquaporin 0 in the lens. Invest. Ophthalmol. Vis. Sci.. 43:4643. [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Mukherjea, P., J.F. Maune, and K. Beckingham. 1996. Interlobe communication in multiple calcium-binding site mutants of Drosophila calmodulin. Protein Sci. 5:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata, K., K. Mitsuoka, T. Hirai, T. Walz, P. Agre, J.B. Heymann, A. Engel, and Y. Fujiyoshi. 2000. Structural determinants of water permeation through aquaporin-1. Nature. 407:599–605. [DOI] [PubMed] [Google Scholar]
- Németh-Cahalan, K.L., and J.E. Hall. 2000. pH and calcium regulate the water permeability of aquaporin 0. J. Biol. Chem. 275:6777–6782. [DOI] [PubMed] [Google Scholar]
- Pasquale, L.R., R.T. Mathias, L.R. Austin, P.R. Brink, and M. Ciunga. 1990. Electrostatic properties of fiber cell membranes from the frog lens. Biophys. J. 58:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia, C., and S.J. Girsch. 1989. Calmodulin site at the C-terminus of the putative lens gap junction protein MIP26. Lens Eye Toxic. Res. 6:613–621. [PubMed] [Google Scholar]
- Preston, G.M., T.P. Carroll, W.B. Guggino, and P. Agre. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 256:385–387. [DOI] [PubMed] [Google Scholar]
- Shi, L.B., and A.S. Verkman. 1996. Selected cysteine point mutations confer mercurial sensitivity to the mercurial-insensitive water channel MIWC/AQP-4. Biochemistry. 35:538–544. [DOI] [PubMed] [Google Scholar]
- Shiels, A., and S. Bassnett. 1996. Mutations in the founder of the MIP gene family underlie cataract development in the mouse. Nat. Genet. 12:212–215. [DOI] [PubMed] [Google Scholar]
- Sui, H., B.G. Han, J.K. Lee, P. Walian, and B.K. Jap. 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature. 414:872–878. [DOI] [PubMed] [Google Scholar]
- Swamy-Mruthinti, S. 2001. Glycation decreases calmodulin binding to lens transmembrane protein, MIP. Biochim. Biophys. Acta. 1536:64–72. [DOI] [PubMed] [Google Scholar]
- Tajkhorshid, E., P. Nollert, M.O. Jensen, L.J. Miercke, J. O'Connell, R.M. Stroud, and K. Schulten. 2002. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science. 296:525–530. [DOI] [PubMed] [Google Scholar]
- Takehana, M. 1990. Hereditary cataract of the Nakano mouse. Exp. Eye Res. 50:671–676. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-van Raaij, A.J., A.L. de Leeuw, and R.M. Broekhuyse. 1985. Bovine lens calmodulin. Isolation, partial characterization and calcium-independent binding to lens membrane proteins. Curr. Eye Res. 4:905–912. [PubMed] [Google Scholar]
- van den Eijnden-van Raaij, A.J., A.L. de Leeuw, and R.M. Broekhuyse. 1986. Calcium-binding lens membrane proteins. Doc. Ophthalmol. 61:255–265. [DOI] [PubMed] [Google Scholar]
- Varadaraj, K., C. Kushmerick, G.J. Baldo, S. Bassnett, A. Shiels, and R.T. Mathias. 1999. The role of MIP in lens fiber cell membrane transport. J. Membr. Biol. 170:191–203. [DOI] [PubMed] [Google Scholar]
- Virkki, L.V., G.J. Cooper, and W.F. Boron. 2001. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281:R1994–R2003. [DOI] [PubMed] [Google Scholar]
- Yasui, M., A. Hazama, T.H. Kwon, S. Nielsen, W.B. Guggino, and P. Agre. 1999. Rapid gating and anion permeability of an intracellular aquaporin. Nature. 402:184–187. [DOI] [PubMed] [Google Scholar]
- Zampighi, G.A., M. Kreman, K.J. Boorer, D.D. Loo, F. Bezanilla, G. Chandy, J.E. Hall, and E.M. Wright. 1995. A method for determining the unitary functional capacity of cloned channels and transporters expressed in Xenopus laevis oocytes. J. Membr. Biol. 148:65–78. [DOI] [PubMed] [Google Scholar]
- Zeuthen, T., and D.A. Klaerke. 1999. Transport of water and glycerol in aquaporin 3 is gated by H+. J. Biol. Chem. 274:21631–21636. [DOI] [PubMed] [Google Scholar]
- Zhu, F., E. Tajkhorshid, and K. Schulten. 2001. Molecular dynamics study of aquaporin-1 water channel in a lipid bilayer. FEBS Lett. 504:212–218. [DOI] [PubMed] [Google Scholar]
- Zhu, F., E. Tajkhorshid, and K. Schulten. 2004. Theory and simulation of water permeation in aquaporin-1. Biophys. J. 86:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]