Abstract
We show that cytochrome P450scc (CYP11A1) in either a reconstituted system or in isolated adrenal mitochondria can metabolize vitamin D3. The major products of the reaction with reconstituted enzyme were 20-hydroxycholecalciferol and 20,22-dihydroxycholecalciferol, with yields of 16 and 4%, respectively, of the original vitamin D3 substrate. Trihydroxycholecalciferol was a minor product, likely arising from further metabolism of dihydroxycholecalciferol. Based on NMR analysis and known properties of P450scc we propose that hydroxylation of vitamin D3 by P450scc occurs sequentially and stereospecifically with initial formation of 20(S)-hydroxyvitamin D3. P450scc did not metabolize 25-hydroxyvitamin D3, indicating that modification of C25 protected it against P450scc action. Adrenal mitochondria also metabolized vitamin D3 yielding 10 hydroxyderivatives, with UV spectra typical of vitamin D triene chromophores. Aminogluthimide inhibition showed that the three major metabolites, but not the others, resulted from P450scc action. It therefore appears that non-P450scc enzymes present in the adrenal cortex to some extent contribute to metabolism of vitamin D3. We conclude that purified P450scc in a reconstituted system or P450scc in adrenal mitochondria can add one hydroxyl group to vitamin D3 with subsequent hydroxylation being observed for reconstituted enzyme but not for adrenal mitochondria. Additional vitamin D3 metabolites arise from the action of other enzymes in adrenal mitochondria. These findings appear to define novel metabolic pathways involving vitamin D3 that remain to be characterized.
Keywords: cytochrome P450scc, mass spectrometry, mitochondria, NMR, vitamin D3
The predominant source of the main form of vitamin D in humans, vitamin D3 (cholecalciferol), is derived from its precursor 7-dehydrocholesterol (7-DHC). 7-DHC is localized to the plasma membrane of basal epidermal keratinocytes (80% of skin’s total 7-DHC content), where upon stimulation with photons of ultraviolet light B (UVB; wavelength 290–320 nm) it undergoes photolysis to previtamin D3 [1]. At normal skin temperature previtamin D3 undergoes internal rearrangement to form vitamin D3 [1]. Circulating vitamin D3 is successively hydroxylated in liver and kidney to 1,25-dihydroxyvitamin D3 (calcitriol), the active regulator of calcium metabolism [1]. Calcitriol and its precursors also have immune and neuroendocrine activities, and tumorostatic and anticarcinogenic properties, affecting proliferation, differentiation and apoptosis in cells of different lineages, and protecting DNA against oxidative damage [1–4]. Besides the liver and kidney, vitamin D3 hydroxylation at positions 25 and 1 can also occur in the epidermis [3,5,6]. The corresponding hydroxy-derivatives may have additional significant local actions on formation of the skin barrier, functional differentiation of adnexal structures, modulation of skin immune system and protection against UVB-induced DNA damage [1–3,5,6].
The crucial initial activation reaction of vitamin D, 25-hydroxylation, is performed by the microsomal enzymes CYP2R1, CYP2J3, and perhaps CYPC11 and/or CYP2D, as well as the mitochondrial enzyme CYP27A1 [1,7–13]. 25-Hydroxyvitamin D [25(OH)D] becomes fully active after its hydroxylation at position 1, carried out by mitochondrial CYP27B1 [1,8–10]. Both 1,25(OH)2D, and 25(OH)D are inactivated by the mitochondrial enzyme CYP24A, which introduces a hydroxyl group at position 24 [1,9,10]. There are also at least 30 additional derivatives, some of which are metabolically active with CYP24A being involved in production of some of these compounds [8,13–16].
P450scc (CYP11A1), the enzyme that performs cleavage of the cholesterol side chain, was recently discovered to also metabolize vitamin D3 and its precursor, 7-DHC [17,18]. Thus, purified bovine P450scc in a reconstituted system converts vitamin D3 into four compounds of which the two major products are 20(OH)- and 20,22(OH)2-vitamin D3 [18]. Unlike the action of P450scc on cholesterol [19,20] or 7-DHC [17], the side chain of vitamin D3 was not cleaved. These novel enzymatic activities of P450scc therefore give rise to a new family of products whose biological activity remains to be determined. We now further characterize this pathway using bovine cytochrome P450scc with vitamin D3 as a substrate. We used MS and NMR as tools for the characterization of secosteroid products. Furthermore, we tested vitamin D3 biotransformation by isolated mitrochondria from the adrenal gland, the tissue expressing the highest cytochrome P450scc activity.
Results and Discussion
Incubation of vesicle-reconstituted P450scc and its redox partners with vitamin D3 substrate generated three products that migrated on TLC plates at rates different from native vitamin D3, and that were not present in control incubations lacking an electron source (Fig. 1). From a 50 mL incubation of D3 with P450scc, 0.26 μg of TLC-purified P1 product was obtained, representing a yield of 16% from the original vitamin D3. The yield of TLC-purified products P2 and P3 were 1.7 and 4%, respectively. The proportion of product P2 varied between incubations, being barely detectable in some. Time courses for product formation based on the intensity of spots following TLC showed no further accumulation beyond 3 h of incubation indicating the reconstituted enzyme system had lost activity. The combined products from three 50 mL incubations were used for NMR analysis of P1 and P3.
Fig. 1.

Metabolism of vitamin D3 by purified bovine P450scc. Incubations were carried out in a reconstituted system comprising purified P450scc (3 μM), FDXR, FDX1 and phospholipid vesicles containing vitamin D3 at a molar ratio to phospholipid of 0.2. Reaction products were analyzed by TLC as described in Experimental Procedures. Control (incubation without NADPH (1); experimental incubation with NADPH (2); pregnenolone standard (3): products of vitamin D3 metabolism, P1, P2 and P3, are marked by arrows.
NMR analysis of compound P1 showed that it represents 20-hydroxyvitamin D3 (Fig. 2, Fig. S1). Compared with vitamin D3 and as an effect of quaternization of carbon atom C-20, the 1H NMR spectrum of compound P1 shows the singlet signal of methyl group CH3-21 instead of the doublet. Concomitant with this change there is also a significant downfield shift (Δ = 0.32 p.p.m) of the CH3-21 signal. The same shift had been reported for a preparation of 20-hydroxyvitamin D3 [18]. The difference between the chemical shifts of CH3-21 in cholesterol and in 20α(S)- and 20β(R)-hydroxycholesterol has been reported as 0.30 and 0.13 p.p.m., respectively [21]. The magnitude of the shift we observe suggests that hydroxylation of vitamin D3 by P450scc is stereospecific and produces exclusively 20S-hydroxyvitamin D3. Indeed, the presence of the hydroxyl group at C-20 in product P1 is further confirmed by 13C NMR spectrum, which shows C-20 as a quaternary signal at 75.4 p.p.m. This interpretation is further confirmed by HMBC correlation of this signal with proton resonance of methyl group CH3-21.
Fig. 2.
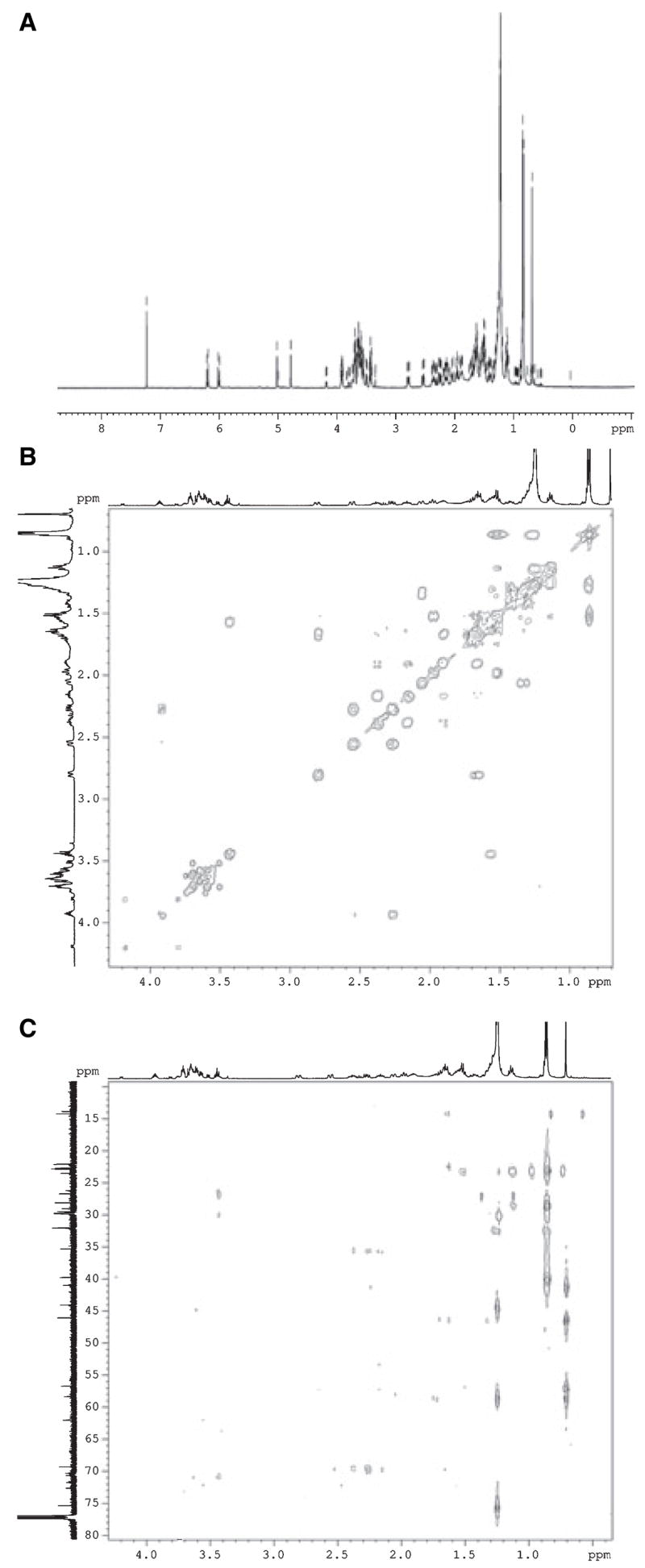
NMR spectra of the vitamin D3 metabolite (P1) identified as 20(S)-hydroxyvitamin D3. (A) 1H NMR; (B) COSY; (C) HMBC.
1H NMR of compound P3 has a broad peak at 4.10 p.p.m., which is not present in the NMR spectrum of vitamin D3 or in the NMR spectrum of 20(OH)D3 (Fig. 3). This chemical shift of this proton is similar to that of the proton at the 3-OH position, and it is a proton characteristic of a vicinal hydroxyl group. Compared with the results of Guryev et al. [18], this is the fingerprint of 20,22(OH)-vitamin D3, as in fact confirmed by COSY. Thus, the peak at 4.10 p.p.m., which is assigned to 22-OH, has three correlations in the COSY spectrum: they are 22(OH) to 22-CH, 22(OH) to 23-CH2 and 22(OH) to 24-CH2. Additional confirmation was provided by LS/MS/MS analysis (Fig. 4). Thus, the mass spectrum at a retention time of 3.6–3.8 min showed characteristics of the dihydroxyvitamin D3 fragment [22] with (M + 1)+ at m/z 399 (417 – H2O), m/z 381 (417 – 2 H2O) and m/z 363 (417 – 3H2O) (Fig. 4A). In addition, we also detected a mass spectra pattern consistent with trihydroxyvitamin D3 [22] with major fragments at m/z 415 (433 – H2O), m/z 397 (433 – 2H2O) and m/z 379 (433 – 3 H2O) (Fig. 4B). Fragmentation of the m/z 397 species demonstrated additional m/z 361 (433 – 4 H2O) and m/z 297 and 279 that are present on MS3 of vitamin D3.
Fig. 3.
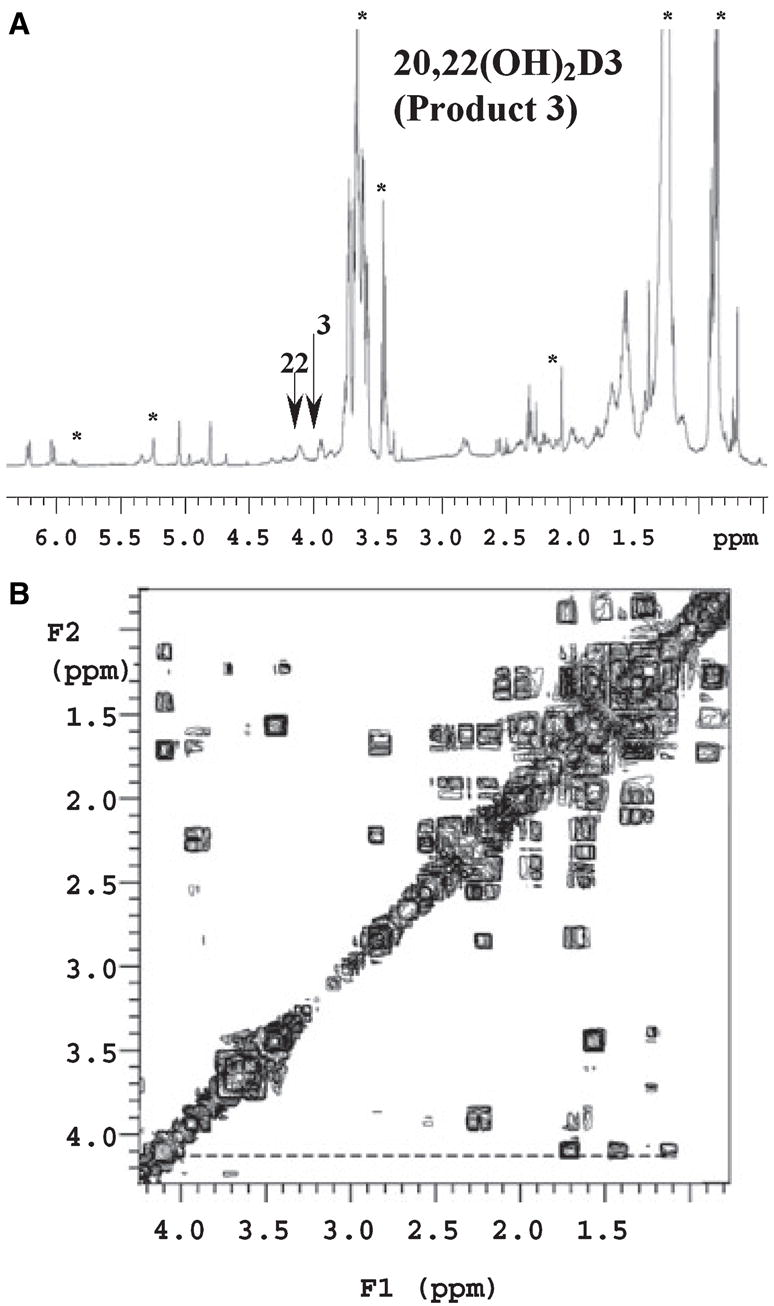
1H NMR spectra of metabolite P3, identified as 20(R),22-dihydroxyvitamin D3. (A) 1H NMR. The two hydroxyl groups (3.96 p.p.m. assigned to 3-OH and 4.10 p.p.m. assigned to 22-OH) are marked by arrows. Finger print peaks for D3 are labeled with * in the 1D proton spectrum. (B) 1H-1H COSY. The line indicates the correlations of 22-OH proton to protons at C22, C23 and C24.
Fig. 4.
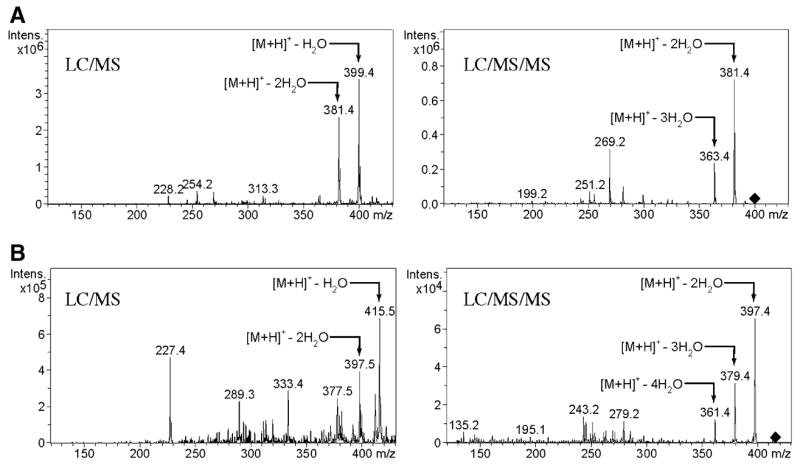
Mass spectrometry of product P3. (A) LC/MS/MS of fragment with (M + 1)+ at m/z 399. (B) LC/MS/MS of fragment with (M + 1)+ at m/z 415.
There was insufficient product P2 from the action of P450scc on vitamin D3 to perform NMR and therefore a full scan mass spectrum of the product was obtained from flow injection. This showed a mixture of fragment ions (M + 1)+ of which the most abundant were ions at m/z 299 and 383 (not shown). Further analyses by LC/MS, MS/MS and MS3 showed that in relation to a pregnenolone standard, m/z 299 represented the fragment ion of pregnenolone after loss of water (−18) that occurred during MS (m/z 317 was also present in the mixture at an identical retention time to the standard). This pregnenolone is derived from a small amount of cholesterol that copurifies with P450scc isolated from adrenal mitochondria [23]. Similarly, the compound at m/z 383 would appear to represent (M + 1)+ – H2O of hydroxycholestrol (m/z 401 was also present at the same retention time). This species, while having a different retention time (4.7 min) and MS/MS pattern from pregnenolone, nevertheless shared an identical ‘fingerprint-type’ pattern on MS3 indicating that it is structurally related to pregnenolone (not shown). We confirmed that P2 represents a product arising from contaminating cholesterol by showing that it is not generated when vitamin D3 is incubated with recombinant P450scc that does not contain bound cholesterol (not shown).
Thus, we confirmed that purified bovine P450scc does hydroxylate the side chain of vitamin D3, with 20-hydroxy and 20,22-dihydroxyvitamin D3 as the major reaction products [18] and provide complete NMR identification. We also identified two additional reaction products, pregnenolone and hydroxycholesterol that appear to arise from cholesterol copurifying with P450scc. Lastly, we detected as a novel fifth product of vitamin D3 metabolism by P450scc, trihydroxyvitamin D3. Our NMR analysis allowed tentative definition of the stereochemical structure of the main compound, 20-hydroxyvitamin D3, as 20(S)-hydroxycholecalciferol (5Z,7E-9,10-seco-5,7,10(19)-cholestatriene- 3,20S-diol). This serves as substrate for the second reaction and therefore by analogy with the generation of 20(R),22-dihydroxycholesterol from 20(S)-hydroxycholesterol, the second product should represent 20(R),22-dihydroxycholecalciferol (5Z,7E-9,10-seco- 5,7,10(19)-cholestatriene-3,20R,22-triol). Furthermore, the patterns of multiple hydroxylations of vitamin D3 by P450scc strongly suggest that these reactions occur sequentially and in a stereospecific manner, although based only on NMR data we were not able to establish configuration at C-22 (Fig. 5). The significant accumulation of 20(S)-hydroxyvitamin D3 (Fig. 1A) indicates easy release from the active site of the enzyme with only a minor portion remaining (or rebinding) for further hydroxylation. In fact, the yield of dihydroxy product was only 4% of the vitamin D3 load, compared with a 16% yield of 20-hydroxyvitamin D3. This is in contrast to the P450scc-mediated conversions of cholesterol into pregnenolone, or of 7-DHC (previtamin D3) into 7-dehydropregnenolone (7-DHP), where release of the intermediates hydroxycholesterol or hydroxy-7-dehydrocholeterol is undetectable while the reaction is proceeding [17,20].
Fig. 5.
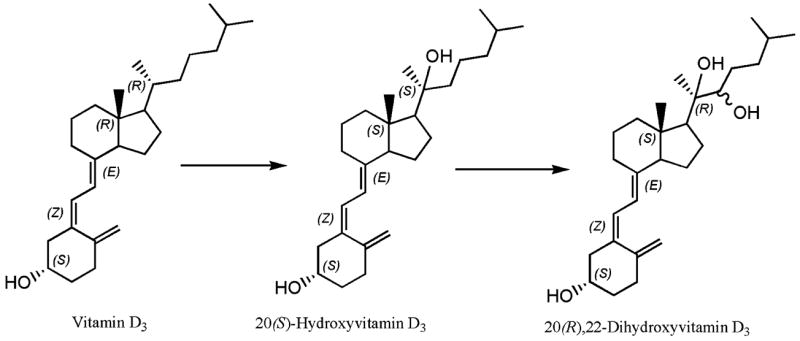
Proposed sequence of P450scc catalyzed transformation of vitamin D3 and structures of the first two reaction products.
We also specifically tested the capability of 25-hydroxyvitamin D3 to serve as substrate for P450scc. Our analysis of reaction mixtures supplemented with 25-hydroxyvitamin D3 failed to show any evidence for metabolism of 25-hydroxyvitamin D3 by P450scc (not shown). This finding is consistent with a previous study that showed reduction in the activity of human and bovine P450scc after introduction of a 25-hydroxyl group into the cholesterol side chain [20,24]. Since 25-hydroxylation of vitamin D3 is a limiting step in its activation, the latter finding has potential physiological implications. Once hydroxylated in 25 position, the hydroxyvitamin D3 would be protected from the P450scc-mediated pathway of metabolism thus permitting formation of fully active calcitriol by hydroxylation at C1, or inactivation of 25-hydroxycalciferol by hydroxylation at C-24. At the autocrine or paracrine levels the biological significance may extend to the skin, where both the synthesis and metabolism of vitamin D3 occur [1,3,5], physiological and clinical actions have been observed [1,3] and full expression of functional P450scc has been reported [17].
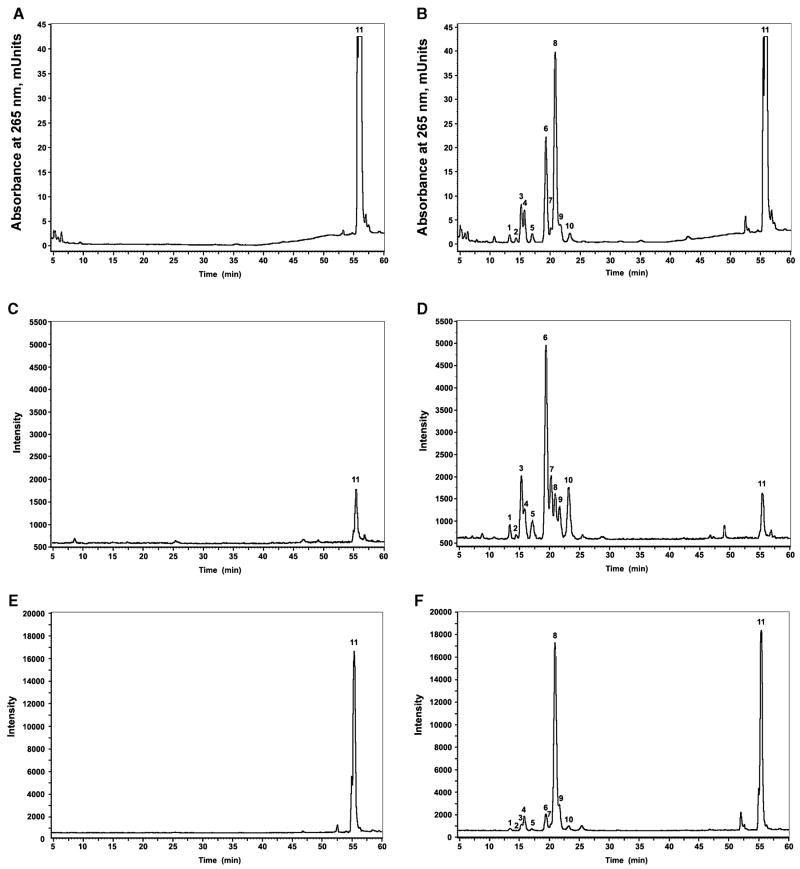
To further characterize biological production of vitamin D3 metabolites by reactions catalysed by cytochrome P450scc, we incubated mitochondria from the adrenal (which expresses the highest concentrations of P450scc of any tissue) with vitamin D3. Incubations were done in the presence (experimental) or absence (control) of NADPH and isocitrate and reaction products subjected to LC/MS or LC with UV spectral analyses (Fig. 6). Eleven main products of vitamin D3 metabolism (absent in controls) were identified by UV monitoring at 265 nm (Fig. 6A,B). The UV spectra of compounds 1–10 were typical of the vitamin D triene chromophore with λmax at 265 nm and λmin at 228 nm (not shown). LC/MS analyses of reaction products demonstrated that peaks 1–10 contained ions (M + 1)+ at m/z 401 and 383 at ratios that differed with the retention time of the product (Fig. 6C–F). This finding suggests differences in the capacity of the different products to lose water during ionization, likely related to each having a hydroxyl group at a different position. Therefore, we conclude that peaks 1–10 probably represent different isomers of hydroxyvitamin D3. The species with m/z at 401 corresponds to (M + 1)+ of hydroxyvitamin D3 (real mass 400 Da) (Fig. 6C,D), whereas that with m/z at 383 corresponds to hydroxyvitamin D3 minus water [(M + 1)+ – H2O; Fig. 6E,F]. Peak #4 contained 25-hydroxyvitamin D3 as it had a retention time and mass spectrum matching the corresponding standard. None of the major peaks showed detectable amounts of species with m/z at 417, 399 and 381 that represent dihydroxyvitamin D3 and products resulting from water removal from the molecule during chemical ionization.
Fig. 6.
LC/MS and UV spectra of products of vitamin D3 metabolism by adrenal mitochondria. (A, C, E) Control (incubation without NADPH and isocitrate); (B, D, F) experimental incubation (with NADPH and isocitrate). The HPLC elution profile was monitored by absorbance at 265 nm (A, B). The selected ion monitoring (SIM) mode was used to detect ions with m/z = 383 (E, F) and m/z 401 (C, D). The peaks designated as 1–10 correspond to vitamin D3 metabolites. The peak designated as 11 corresponds to vitamin D3. Product #4 has a retention time and mass spectrometric characteristics identical to 25OH-vitamin D3 standard. Elution was carried out as described in Experimental procedures.
To confirm that the vitamin D3 metabolites observed with adrenal mitochondria originated from P450scc action, 100 μM aminogluthetimide (a specific P450scc inhibitor) [25] was added to the reaction mixture and the reaction products analysed by RP-HPLC (Fig. 7). The resulting chromatogram showed that the major products (products 6, 8 and 3) largely disappeared in the presence of the inhibitor, with a slight decrease in products 2 and 4 also occurring (Fig. 7). From the above results we conclude that adrenal mitochondria do metabolize vitamin D3, that the ensuing reactions generate predominantly 10 hydroxyvitamin D3 products of which at least the three major ones are P450scc dependent. The identity of the enzymes involved in generation of the remaining hydroxyvitamin D3 products are yet to be defined. It should be noted that none of the mitochondrial enzymes known to hydroxylate vitamin D3 (CYP27A1, CYP27B1 and CYP24A) has been reported to be expressed in the adrenal gland. Thus, we provide the first evidence that adrenal mitochondria have the capability of hydroxylating vitamin D3 and we further show that P450scc is involved in the process. Although it seems likely that the major product of vitamin D3 metabolism by adrenal mitochondria is 20(S)-hydroxycalciferol, which is the major product produced by purified P450scc, this remains to be confirmed.
Fig. 7.
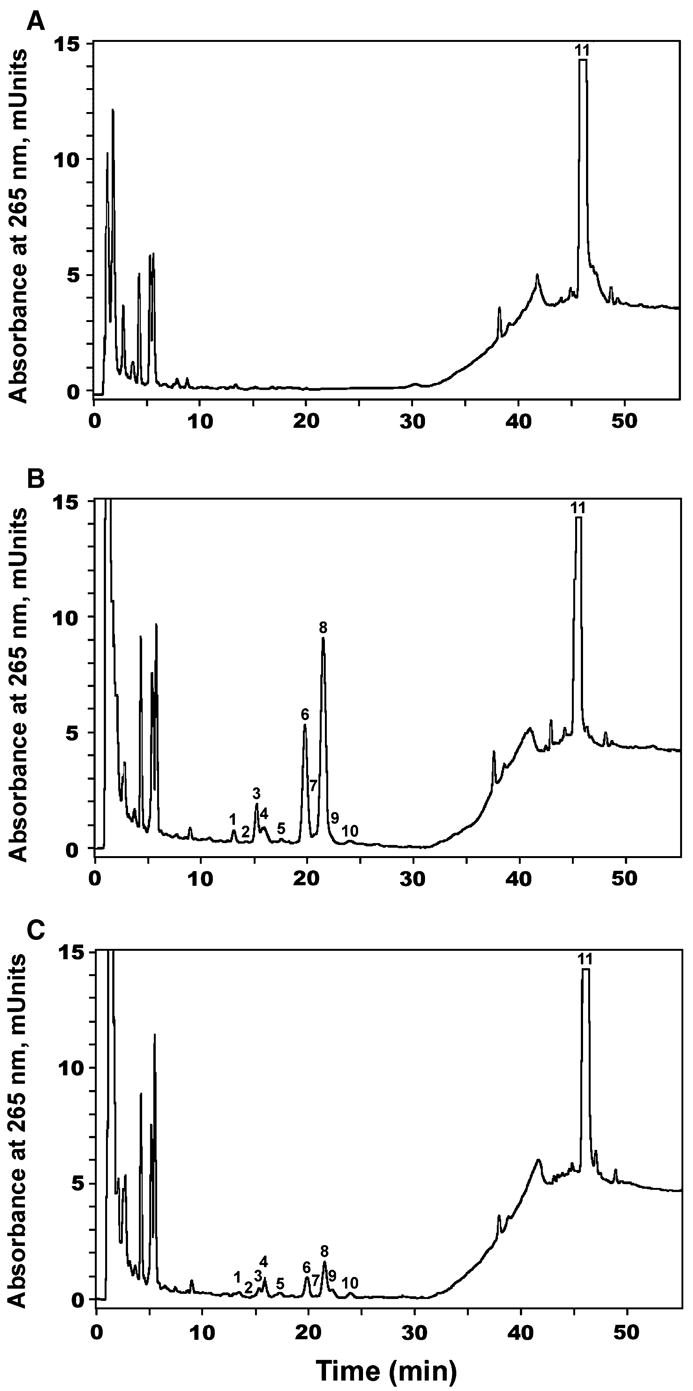
Inhibition of vitamin D3 metabolism by DL-aminoglutethimide. (A) Control (incubation without NADPH and isocitrate); (B) Experimental incubation (with NADPH and isocitrate); (C) Experimental incubation (with NADPH and isocitrate) in the presence of DL-aminoglutethimide (100 μM). The mobile phases were slightly modified in comparison with Fig. 6 and consisted of 85% methanol and 0.1% acetic acid from 0 to 25 min, followed by linear gradient to 100% methanol and 0.1% acetic acid from 25 to 35 min; and 100% methanol and 0.1% acetic acid from 35 to 55 min. Note the marked disappearance of products 6, 8 and 3.
It is also apparent from our results that P450scc opens a novel pathway for the metabolism of secosteroids. According to kinetic data generated by Guryev et al. [18] the rate (Vmax) for P450scc processing vitamin D3 is 43% of that for cholesterol, whereas the Km is approximately the same. From our own calculations this novel pathway (apparently expressed in an adrenal gland configuration) could accommodate up to 20% of the vitamin D3 load (100 μM). These rates of metabolism make a strong case for the pathway being significant under pathologic, and perhaps physiologic conditions, depending on the supply of secosteroids. Thus in organs expressing high levels of P450scc such as adrenals (bovine: 391 pmol·mg−1) [26], corpus luteum (bovine: 250 pmol·mg−1) [26], follicles and corpus luteum (porcine: 2–11 and 78 pmol·mg−1, respectively) [27], and placenta (human: 2.6 pmol·mg−1 protein) [28], production of vitamin D3 metabolites may possibly have systemic effects. In organs expressing low levels of P450scc, which include brain [29], gastrointestinal tract [30], kidney [31] and skin [17], the same metabolites could serve local para-, auto- or intracrine roles. This may be relevant to some of the pleiotropic activities of vitamin D3 that include immunomodulatory, neuroendocrine, anticarcinogenic and protective properties [1–3,32–34]. Regardless, the P450scc-initiated pathway would be clearly implicated in the Smith–Lemli–Optitz syndrome characterized by large excesses of 7-DHC [35–39]. In this condition, circulating vitamin D3 levels are not as high as would be expected [40], while concomitantly 7-DHP is increased [36,39]. Thus, whether P450scc provides an inactivation pathway and is actively involved in the pathogenesis of vitamin D deficiency syndrome or whether it generates novel bioactive molecules are some of the pressing issues that remain to be investigated.
In summary, we have characterized the transformation of vitamin D3 by P450scc. The main reaction product from the purified enzyme is 20S-hydroxycholecalciferol, which may be further metabolized to 20,22- dihydroxycholecalciferol and trihydroxycholecalciferol. In intact adrenal mitochondria a number of monohydroxy vitamin D3 metabolites were identified with the major ones requiring P450scc for their synthesis. This novel pathway of vitamin D3 metabolism may have wide biological and perhaps, clinical repercussions depending on the supply of cholecalciferol substrate, and the local level of P450scc activity.
Experimental procedures
Side-chain modification of vitamin D3 by reconstituted cytochrome P450scc
Bovine cytochrome P450scc and adrenododoxin reductase (FDXR) were isolated from adrenals [41,42]. Adrenodoxin (FDX1) was expressed in E. coli and purified as described previously [43]. Reactions to modify the side chain of vitamin D3 were performed with purified bovine P450scc and its electron transfer system in a manner similar to that described for 7-DHC [17]. The incubation mixture comprised 510 μM phospholipid vesicles (dioleoyl PC plus 15 mol% cardiolipin) with a vitamin D to phospholipid molar ratio of 0.2, 50 μM NADPH, 2 mM glucose 6-phosphate, 2 U·mL−1 glucose 6-phosphate dehydrogenase, 0.3 μM FDXR, 6.5 μM FDX1, 3.0 μM cytochrome P450scc and buffer pH 7.4 [43]. For TLC analysis incubations were 0.5 mL. To obtain products for NMR, incubations were scaled up to 50 mL. After incubation at 35 °C for 3 h the mixture was extracted with methylene chloride and dried under nitrogen. Products were analyzed and purified by preparative TLC on silica gel G with three developments in hexane/ethyl acetate (3:1, v/v) (representative visualization of charred vitamin D3 reaction products is shown in Fig. 1). Products were eluted from the silica gel with chloroform/methanol (1:1, v/v); dried separately under nitrogen and shipped for NMR and MS analyses on dry ice.
Side chain-modification of vitamin D3 by mitochondria isolated from the adrenal gland
Adrenals were obtained from male Wistar rats aged 3 months, killed under anesthesia. The animals were housed at the vivarium of the Department of Biotestings of Bioorganic Chemistry Institute, Minsk, Belarus. The experiments were approved by the Belarus University Animal Care and Use Committee. All animal experimentation described was conducted in accord with accepted standards of humane animal care, as outlined in the ethical guidelines.
A mitochondrial fraction was prepared from the adrenals by homogenizing the tissue in 5 vol. of ice-cold 0.25 M sucrose. The homogenate was centrifuged at 600 g for 10 min at 4 °C and the resulting supernatant was centrifuged at 9000 g for 20 min at 4 °C to sediment the mitochondrial fraction. The pellet was resuspended in 0.25 M sucrose and the mitochondrial fraction was again sedimented under the same conditions. The washed mitochondrial fraction was resuspended in 0.25 M sucrose and used for enzymatic reactions.
Isolated mitochondria were preincubated (10 min at 37 °C) with vitamin D3 (200 μM) in 0.5 mL medium comprising 0.25 M sucrose, 50 mM Hepes pH 7.4, 20 mM KCl, 5 mM MgSO4, 0.2 mM EDTA. Vitamin D3 (200 μM was dissolved in 45% 2-hydroxypropyl-cyclodextrin. The reactions were started by adding NADPH (0.5 mM) and isocitrate (5 mM) to the samples. After 120 min at 37 °C, the reactions were stopped by adding 1 mL ice-cold methylene chloride and the mixtures were re-extracted two more times with 1 mL methylene chloride. The methylene chloride layers were combined and dried using a rotational vacuum concentrator RVC 2–18 (Christ, Germany). The residues were dissolved in methanol and subjected to LC-MS analysis.
NMR
Samples were dissolved in CDCl3 (Cambridge Isotope Laboratories, Inc., Andover, MA) [17]. Analyses of 0.69 mg of product P1 (Fig. 1) included proton and proton 2D spectra (GCOSY, GHMQC and GHMBC) recorded on a Bruker DRX 500 MHz NMR spectrometer equipped with a Nalorac 3 mm inverse Z-axis gradient probe (MIDG- 500). Carbon and DEPT spectra were recorded on a Varian Unity Inova 600 MHz spectrometer equipped with a Nalorac 3 mm direct detect probe (MDBC600F). The NMR data was processed using xwinnMr 3.5 running on red hat linux 7.3.
For product P3 (150 μg), proton NMR spectra were acquired by using Varian Inova-500M NMR equipped with a 4 mm gHX Nanoprobe (Varian NMR Inc., Palo Alto, CA). The sample was spinning at 2000 Hz at a temperature of 21 °C. An interpulse delay of 5 s was used. 1H-1H COSY spectra were acquired by using a standard d1-90°–t1-90°-acquisition pulse sequence. The COSY spectrum consisted of 1024 (t2) by 512 (t1) data points covering 8000 Hz sweep width. Standard sine apodization function and zero filling were used in both dimensions before Fourier transformation.
MS analyses
LC/MS analysis
The products of mitochondrial activity (see above) were dissolved in methanol and analyzed on a HPLC mass spectrometer LCMS-QP8000α (Shimadzu, Japan) equipped with a Restec Allure C18 column (150 × 4.6 mm; 5 μm particle size; and 60 A pore size), UV/VIS photodiode array detector (SPD-M10Avp) and quadrupole mass spectrometer [17]. The LC-MS workstation Class-8000 software was used for system control and data acquisition (Shimadzu). Elution was carried out at flow rate of 0.75 mL·min−1 at 40 °C. The mobile phases consisted of 85% methanol and 0.1% acetic acid from 0 to 30 min, followed by linear gradient to 100% methanol and 0.1% acetic acid from 30 to 45 min; and 100% methanol and 0.1% acetic acid from 45 to 60 min. The MS operated in atmospheric pressure chemical ionization (APCI) positive ion mode and nitrogen was used as the nebulizing gas. The MS parameters were as follows: the nebulizer gas flow rate was 2.5 L·min−1; probe high voltage was 3.5 kV, probe temperature was 300 °C, the curved desolvation line heater temperature was 230 °C. Analyses were carried out in the scan mode from m/z 200 to 450 or in SIM mode.
MS/MS and LC/MS/MS
Products P2 and P3 (Fig. 1) were dissolved in methanol containing 0.1% acetic acids and analysed directly by MSn or LC/MS/MS using an Agilent 1100 LC/MSD-Trap-XCT system (Agilent technologies, Palo Alto, CA). The system was operated with the APCI source in the positive ion mode. For MSn the sample P1 (1 ng·μL−1) was infused using a syringe pump at a flow rate of 10 μL·min−1. The acquisition parameters were as follows. Tune source: trape drive (42.9), octopole RF amplitude (300.0 vpp), lens 2 (−69.0 V), capillary exit (152.5 V), skimmer (15.0 V), lens 1 (−4.7 V), oct 1 DC (9.1 V), oct 2 DC (2.39 V), dry temp and APCI temp (350 °C), nebulizer (15.00 p.s.i.), nitrogen gas (5 L·min−1), HV capillary (2680 V), HV end-plate offset (−500 V). The scan (average of three spectra) was between 100 and 400 m/z with maximal Accu time of 200000 μs and ICC target 150000. Fragmentation was set with SmartFrag Ampl between 30 and 200%, fragmentation width (10.00 m/z), fragmentation time (40 000 μs) and fragmentation delay (0 μs).
For LC/MS/MS the samples were separated on a 1100 LC capillary equipped with Zorbax SDC18 column (150 × 2.1 mm; 3.5 μm particle size) coupled with the 1100 LC/MSD-Trap-XCT system. Separation was performed at flow rate of 150 μL·min−1 with 70% acenitrile/30% methanol/0.1% acetic acid as a mobile phase. The MS operated in APCI positive ion mode with the scan from 120 to 410 m/z, capillary exit (111.0 V), skin 1 (15.0 V), trap drive (42.9), accumulation time (42216 μs) and with auto MS/MS on.
Supplementary Material
Fig. S1. NMR spectra of the vitamin D3 metabolite (P1) identified as 20S-hydroxyvitamin D3. (A–C) 1H NMR; (D, E) COSY; (F) 13C NMR; (G–I) HMBC; (J) HMQC.
Acknowledgments
The work was supported in part by NIH grant AR047079 to AS.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- 7-DHC
7-dehydrocholesterol
- 7-DHP
7-dehydropregnenolone
- FDX1
adrenodoxin
- FDXR
adrenodoxin reductase
- P450scc
cytochrome P450 side chain cleavage
- UV
ultraviolet light
- UVB
ultraviolet B
References
- 1.Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 4.Wiseman H. Vitamin D is a membrane antioxidant. Ability to inhibit iron-dependent lipid peroxidation in liposomes compared to cholesterol, ergosterol and tamoxifen and relevance to anticancer action. FEBS Lett. 1993;326:285–288. doi: 10.1016/0014-5793(93)81809-e. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161–171. doi: 10.1016/s0303-7207(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Calcium and vitamin D. Diagn Ther Clin Lab Med. 2000;20:569–590. [PubMed] [Google Scholar]
- 7.Holick MF, Garabedian M, Schnoes HK, DeLuca HF. Relationship of 25-hydroxyvitamin D3 side chain structure to biological activity. J Biol Chem. 1975;250:226–230. [PubMed] [Google Scholar]
- 8.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 9.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Bouillon R, Okamura WH, Norman AW. Structure–function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 11.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA. 2004;101:7711–7715. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamasaki T, Izumi S, Ide H, Ohyama Y. Identification of a novel rat microsomal vitamin D3 25-hydroxylase. J Biol Chem. 2004;279:22848–22856. doi: 10.1074/jbc.M311346200. [DOI] [PubMed] [Google Scholar]
- 13.Ohyama Y, Yamasaki T. Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci. 2004;9:3007–3018. doi: 10.2741/1455. [DOI] [PubMed] [Google Scholar]
- 14.Kamao M, Tatematsu S, Hatakeyama S, Sakaki T, Sawada N, Inouye K, Ozono K, Kubodera N, Reddy GS, Okano T. C-3 epimerization of vitamin D3 metabolites and further metabolism of C-3 epimers: 25-hydroxyvitamin D3 is metabolized to 3-epi-25-hydroxyvitamin D3 and subsequently metabolized through C-1alpha or C-24 hydroxylation. J Biol Chem. 2004;279:15897–15907. doi: 10.1074/jbc.M311473200. [DOI] [PubMed] [Google Scholar]
- 15.Sawada N, Kusudo T, Sakaki T, Hatakeyama S, Hanada M, Abe D, Kamao M, Okano T, Ohta M, Inouye K. Novel metabolism of 1 alpha,25- dihydroxyvitamin D3 with C24–C25 bond cleavage catalyzed by human Cyp24a1. Biochemistry. 2004;43:4530–4537. doi: 10.1021/bi030207f. [DOI] [PubMed] [Google Scholar]
- 16.Lohnes D, Jones G. Side chain metabolism of vitamin D3 in osteosarcoma cell line UMR-106 characterization products. J Biol Chem. 1987;262:14394–14401. [PubMed] [Google Scholar]
- 17.Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuckey RC, Cameron KJ. Side-chain specificities of human and bovine cytochromes P-450scc. Eur J Biochem. 1993;217:209–215. doi: 10.1111/j.1432-1033.1993.tb18235.x. [DOI] [PubMed] [Google Scholar]
- 20.Tuckey RC. Cholesterol side-chain cleavage by mitochondria from the human placenta. Studies using hydroxycholesterols as substrates. J Steroid Biochem Mol Biol. 1992;42:883–890. doi: 10.1016/0960-0760(92)90097-3. [DOI] [PubMed] [Google Scholar]
- 21.Mijares A, Cargill DI, Glasel JA, Lieberman S. Studies on the C-20 epimers of 20-hydroxycholesterol. J Org Chem. 1967;32:810–812. doi: 10.1021/jo01278a066. [DOI] [PubMed] [Google Scholar]
- 22.Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, Ohyama Y, Inouye K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem. 2000;267:6158–6165. doi: 10.1046/j.1432-1327.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- 23.Larroque C, Rousseau J, van Lier JE. Enzymebound sterols of bovine adrenocortical cytochrome P-450scc. Biochemistry. 1981;20:925–929. doi: 10.1021/bi00507a043. [DOI] [PubMed] [Google Scholar]
- 24.Tuckey RC, Cameron KJ. Catalytic properties of cytochrome P-450scc purified from the human placenta: comparison to bovine cytochrome P-450scc. Biochim Biophys Acta. 1993;1163:185–194. doi: 10.1016/0167-4838(93)90180-y. [DOI] [PubMed] [Google Scholar]
- 25.Toaff ME, Schleyer H, Strauss JF., 3rd Metabolism of 25-hydroxycholesterol by rat luteal mitochondria and dispersed cells. Endocrinology. 1982;111:1785–1790. doi: 10.1210/endo-111-6-1785. [DOI] [PubMed] [Google Scholar]
- 26.Hanukoglu I, Hanukoglu Z. Stoichiometry of mitochondrial cytochromes P-450, adrenodoxin and adrenodoxin reductase in adrenal cortex and corpus luteum. Implications for membrane organization and gene regulation. Eur J Biochem. 1986;157:27–31. doi: 10.1111/j.1432-1033.1986.tb09633.x. [DOI] [PubMed] [Google Scholar]
- 27.Tuckey RC, Kostadinovic Z, Stevenson PM. Ferredoxin and cytochrome P-450scc concentrations in granulosa cells of porcine ovaries during follicular cell growth and luteinization. J Steroid Biochem. 1988;31:201– 205. doi: 10.1016/0022-4731(88)90055-6. [DOI] [PubMed] [Google Scholar]
- 28.Tuckey RC, Kostadinovic Z, Cameron KJ. Cytochrome P-450scc activity and substrate supply in human placental trophoblasts. Mol Cell Endocrinol. 1994;105:103–109. doi: 10.1016/0303-7207(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 29.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395– 403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 30.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J Exp Med. 2004;200:1635–1646. doi: 10.1084/jem.20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalla Valle L, Toffolo V, Vianello S, Belvedere P, Colombo L. Expression of cytochrome P450scc mRNA and protein in the rat kidney from birth to adulthood. J Steroid Biochem Mol Biol. 2004;88:79–89. doi: 10.1016/j.jsbmb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 32.De Haes P, Garmyn M, Verstuyf A, De Clercq P, Vandewalle M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J Photochem Photobiol B. 2005;78:141–148. doi: 10.1016/j.jphotobiol.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Lin R, White JH. The pleiotropic actions of vitamin D. Bioessays. 2004;26:21–28. doi: 10.1002/bies.10368. [DOI] [PubMed] [Google Scholar]
- 34.Sutton AL, MacDonald PN. Vitamin D: more than a ‘bone-a-fide’ hormone. Mol Endocrinol. 2003;17:777– 791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 35.Fitzky BU, Moebius FF, Asaoka H, Waage-Baudet H, Xu L, Xu G, Maeda N, Kluckman K, Hiller S, Yu H, et al. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith–Lemli–Opitz/RSH syndrome. J Clin Invest. 2001;108:905–915. doi: 10.1172/JCI12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shackleton C, Roitman E, Guo LW, Wilson WK, Porter FD. Identification of 7 (8) and 8 (9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith– Lemli–Opitz syndrome) J Steroid Biochem Mol Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 37.Nowaczyk MJ, Whelan DT, Hill RE. Smith–Lemli–Opitz syndrome: phenotypic extreme with minimal clinical findings. Am J Med Genet. 1998;78:419–423. doi: 10.1002/(sici)1096-8628(19980806)78:5<419::aid-ajmg5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Tint GS, Irons M, Elias ER, Batta AK, Frieden R, Chen TS, Salen G. Defective cholesterol biosynthesis associated with the Smith–Lemli–Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- 39.Marcos J, Guo LW, Wilson WK, Porter FD, Shackleton C. The implications of 7-dehydrosterol- 7-reductase deficiency (Smith–Lemli–Opitz syndrome) to neurosteroid production. Steroids. 2004;69:51–60. doi: 10.1016/j.steroids.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Irons M, Elias ER, Abuelo D, Bull MJ, Greene CL, Johnson VP, Keppen L, Schanen C, Tint GS, Salen G. Treatment of Smith–Lemli–Opitz syndrome: results of a multicenter trial. Am J Med Genet. 1997;68:311– 314. [PubMed] [Google Scholar]
- 41.Tuckey RC, Stevenson PM. Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem. 1984;16:489–495. doi: 10.1016/0020-711x(84)90165-4. [DOI] [PubMed] [Google Scholar]
- 42.Tuckey RC, Stevenson PM. Properties of bovine luteal cytochrome P-450scc incorporated into artificial phospholipid vesicles. Int J Biochem. 1984;16:497– 503. doi: 10.1016/0020-711x(84)90166-6. [DOI] [PubMed] [Google Scholar]
- 43.Woods ST, Sadleir J, Downs T, Triantopoulos T, Headlam MJ, Tuckey RC. Expression of catalytically active human cytochrome p450scc in Escherichia coli and mutagenesis of isoleucine-462. Arch Biochem Biophys. 1998;353:109–115. doi: 10.1006/abbi.1998.0621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. NMR spectra of the vitamin D3 metabolite (P1) identified as 20S-hydroxyvitamin D3. (A–C) 1H NMR; (D, E) COSY; (F) 13C NMR; (G–I) HMBC; (J) HMQC.