Abstract
Carney complex (CNC) is an autosomal dominant neoplasia syndrome caused by inactivating mutations in PRKAR1A, the gene encoding the type 1A regulatory subunit of protein kinase A (PKA). This genetic defect induces skin pigmentation, endocrine tumors, myxomas, and schwannomas. Some patients with the complex also develop myxoid bone tumors termed osteochondromyxomas. To study the link between the PRKAR1A mutations and tumor formation, we generated a mouse model of this condition. Prkar1a+/− mice develop bone tumors with high frequency, although these lesions have not yet been characterized, either from human patients or from mice. Bone tumors from Prkar1a+/− mice were heterogeneous, including elements of myxomatous, cartilaginous, and bony differentiation that effaced the normal bone architecture. Immunohistochemical analysis identified an osteoblastic origin for the abnormal cells associated with islands of bone. To better understand these cells at the biochemical level, we isolated primary cultures of tumoral bone and compared them with cultures of bone from wild-type animals. The tumor cells exhibited the expected decrease in Prkar1a protein and exhibited increased PKA activity. At the phenotypic level, we observed that tumor cells behaved as incompletely differentiated osteoblasts and were able to form tumors in immunocompromised mice. Examination of gene expression revealed down-regulation of markers of bone differentiation and increased expression of locally acting growth factors, including members of the Wnt signaling pathway. Tumor cells exhibited enhanced growth in response to PKA-stimulating agents, suggesting that tumorigenesis in osteoblast precursor cells is driven by effects directly mediated by the dysregulation of PKA.
CARNEY COMPLEX (CNC) is an autosomal dominant familial multiple neoplasia syndrome characterized by spotty skin pigmentation, cardiac and extracardiac myxomatosis, schwannomas, and endocrine tumors (1,2,3). In addition to these manifestations, patients with CNC may develop bone tumors, often arising in younger patients (4). These tumors are characterized by sheets of cells growing in a macro- or microlobular pattern in the setting of a loose myxoid matrix consisting of mesenchymal, cartilaginous, bony, and fibrous elements. These tumors appear to represent a distinct pathological entity and have been designated as osteochondromyxomas (4).
Genetic analysis of CNC patients has shown that the disease may be caused by inactivating mutations in PRKAR1A, the gene encoding the type 1A regulatory subunit of protein kinase A (PKA) (5,6,7). Loss of this regulatory subunit causes increased PKA activity in CNC tumors (5). Recent data indicate that mutations in the phosphodiesterase gene PDE11A can cause the adrenal tumors characteristic of CNC (8), but it is unknown yet whether other manifestations (including bone tumors) can be caused by mutations in this gene.
To model the tumor phenotype of CNC, mice carrying a null allele of Prkar1a have been generated (9,10). Although homozygous null mice die early in embryogenesis (9,10), heterozygous mice, expected to mimic the human phenotype, exhibit enhanced tumorigenesis (10,11). An attenuated but similar phenotype was also observed in a mouse line carrying an antisense construct as a means to reduce Prkar1a levels (12,13).
Although results from different models have produced different spectra of tumors, our studies have indicated that Prkar1a+/− mice develop tumors arising from cAMP-responsive cells such as Schwann cells and thyrocytes (10). Lesions on the tail vertebrae were observed in over 80% of heterozygous mice by 1 yr of age, and preliminary analysis suggested that these tumors arose from the osteoblast lineage (10). Although bone tumors may be seen as a background phenotype in certain strains of mice (14), the tumors in our mice were genotype specific, because they were never observed in control littermates.
To better understand the molecular mechanisms leading to the formation of these bone tumors, we have undertaken a more thorough analysis of these lesions. We present in this manuscript a detailed characterization of the histology of these bone tumors. Furthermore, we have established primary cultures of tumor cells and present our initial studies describing the molecular alterations associated with the tumor phenotype.
RESULTS
Structural Aspects of Bone Tumors in Prkar1a+/− Mice
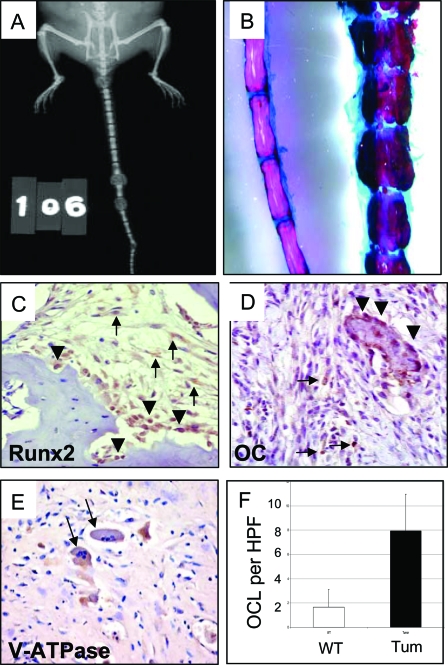
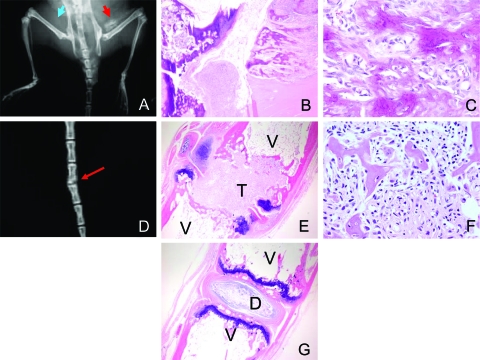
Prkar1a+/− mice, generated as a genetic model for CNC, frequently develop tumors in tail vertebrae and in the sacroiliac region (10). Mice begin to develop tumors at the age of 6 months; by 1 yr of age, approximately 80% of Prkar1a heterozygotes have developed these lesions. In tail tumors, the normal bone of individual vertebrae had been almost completely replaced by tumor (Fig. 1A), and tumors involving the sacroiliac region frequently showed erosion of local tissue structures (data not shown). To date, distant spread of tumors has not been observed. Staining of bone tumors with Alcian blue/Alizarin red showed that the tumors are composed of both bone and cartilage but that mineralized bone, although abnormal, is prevalent (Fig. 1B). This observation suggested that there might be increased bone turnover in the tumors, resulting in replacement of normal structures with abnormal bone.
Figure 1.
Gross and Immunohistochemical Characterization of Bone Tumors in Prkar1a+/− Mice
A, X-ray of a mouse with early tail tumors showing effacement of the normal vertebral bone; B, tail of WT (left) and Prkar1a+/− (right) mice stained for bone and cartilage with Alizarin red (bone) and Alcian blue (cartilage); C–E, immunohistochemical analysis of bone tumors for Runx2 (C), a marker of early osteoblast differentiation; osteocalcin (D), a marker of late osteoblast maturation; and V-ATPase (E), a marker for osteoclasts (black arrows); F, quantitation of osteoclast numbers per HPF in the bone tumors. The graph shows the average counts from 15 HPF from two WT and two tumor tails. In C and D, tumor cells are shown with black arrows, whereas normal staining osteoblasts are shown with black arrowheads.
To better understand the cell composition of the tumor, we performed immunohistochemistry for markers of the osteoblast lineage. Staining for Runx2 (Cbfa1), an early marker of osteoblast differentiation (15), revealed that the tumor cells frequently stained for this marker (Fig. 1C, arrows). Runx2 staining was also observed in the normal-appearing osteoblasts lining bony trabecular structures (Fig. 1C, arrowheads). As described previously (10), tumors also stained for osteocalcin, a late marker for osteoblast differentiation (Fig. 1D), although this was patchy. Consistent with enhanced bone remodeling, staining for the osteoclast marker V-ATPase demonstrated a significant (P < 0.0001) increase in the presence of these cells in the tumors (Fig. 1, E and F).
Establishment of Primary Cell Culture of Prkar1a+/− Tumor Cells
To better understand the origin and the function of the tumor cells, we established primary cell culture from bone tumor fragments, including tumors arising in tail vertebrae and in the sacroiliac region. Tumor cells grew readily under conditions used for the growth of osteoblasts (see Materials and Methods).
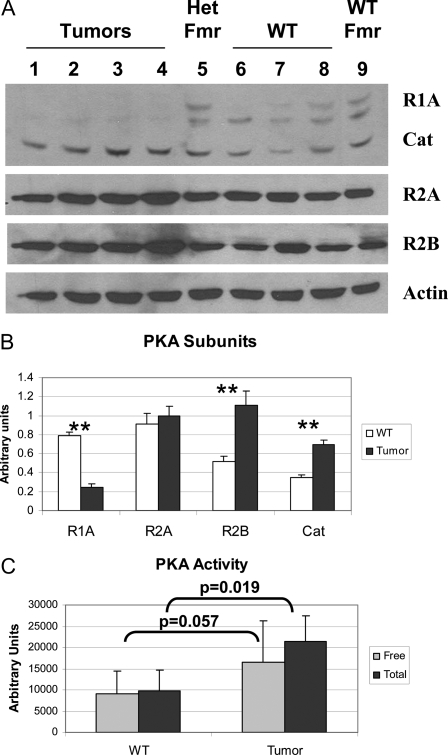
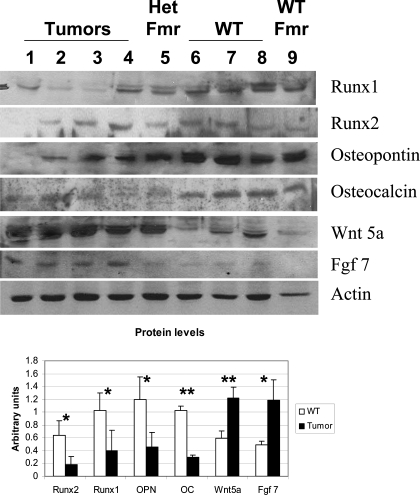
Tumors arising in human patients with mutations in PRKAR1A can exhibit variable expression of PKA subunits in the tumors (13,16) but typically are observed to have elevated PKA activity (5,16). To determine whether these mouse bone tumors exhibit similar findings, we first measured levels of the PKA subunits in the tumor cells by Western blotting of cultured tumor cell lysates (Fig. 2A). Although Prkar1a levels are decreased by 70% in the tumor cells, they remain detectable, suggesting that these cells retain heterozygosity for the Prkar1a locus. As has been observed in human tumors, levels of the Prkar2a regulatory subunit did not change significantly, whereas there is a 2-fold up-regulation of the Prkar2b subunit (13). Intriguingly, there was also up-regulation of the catalytic subunit of PKA, as has previously been described in mouse embryonic fibroblasts lacking Prkar1a (17). To determine the aggregate effects of these changes on PKA enzymatic activity, cell lysates from four independent preparations of control and tumor osteoblasts were assayed for PKA activity. These studies (Fig. 2C) confirmed that the level of total PKA was significantly elevated in the tumor cells (P = 0.019). Levels of the free enzyme also appeared higher in the tumor cells, although this comparison revealed significance only at P = 0.057. We believe this likely represents a significant result, because variability in the assay caused by the use of completely independent samples led to large confidence intervals for many of the aggregate statistics. However, in both wild-type (WT) and tumor osteoblasts, the majority of the PKA in the cells existed in the free form of the enzyme, because addition of cAMP to the reaction buffer produced only minimal (nonsignificant) augmentation of PKA activity under these conditions.
Figure 2.
Analysis of PKA in Primary Osteoblasts
Proteins were prepared from primary cultures of tumor or WT vertebral osteoblasts, as were single samples from primary cultures from heterozygous Prkar1a+/− mouse femurs (Het Fmr) or WT femurs (WT Fmr). A, Samples were Western blotted for PKA subunits as indicated at right. Actin was used as a loading control. B, Quantitation of the data for the four tumor and three WT vertebral samples. **, P < 0.01. This experiment was repeated three times, and a representative blot is shown. Note that all subunits tested demonstrated statistically significant changes with the exception of Prkar2a. C, PKA activity from WT or tumor cells was measured either in the absence of exogenous cAMP (free PKA activity) or in the presence of 5 μm cAMP (total PKA). P values for the comparisons are shown. In both cases, the difference between free and total levels of PKA activity were not significant.
Characterization of Tumor Osteoblasts
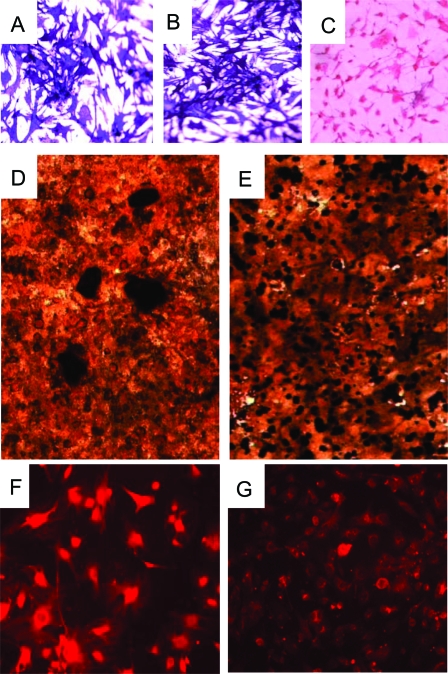
To determine the cellular origin of the tumor cells, we performed alkaline phosphatase staining (Fig. 3, A and B) of primary cells isolated from WT and tumor bone. This analysis confirmed the osteoblast lineage of these cells, because fibroblasts do not stain under these conditions (Fig. 3C). To further investigate the functional properties of the tumor osteoblasts, cells were cultured under mineralizing conditions. Although the tumor cells were able to form bone nodules, the nodules appeared immature, as evidenced by their smaller size and failure to coalesce into the large nodules formed by WT osteoblasts (Fig. 3, D and E). We also performed immunofluorescence of cultured cells for osteocalcin as a marker of complete osteoblast differentiation. As shown in Fig. 3G, tumor osteoblasts stained lightly for this marker, whereas WT cells (Fig. 3F) exhibited a much stronger signal.
Figure 3.
Morphological and Functional Analysis of Primary Tumor Cells from Prkar1a+/− Mouse Bone Tumors
A–C, Staining for alkaline phosphatase activity of primary cultures of cells isolated from control (WT) primary osteoblasts (A), primary tumor cells (B), and primary mouse embryonic fibroblasts (C); D and E, von Kossa staining of mineralization assay for WT osteoblasts (D) and tumor cells (E) (note that the mineralized nodules, black, fail to condense in the tumor cells); F and G, immunofluorescence for osteocalcin in WT osteoblasts (F) and tumor cells (G). Each of these assays was performed three to five times, with the exception of the immunofluorescence study, which was performed twice. Representative data from each assay are shown.
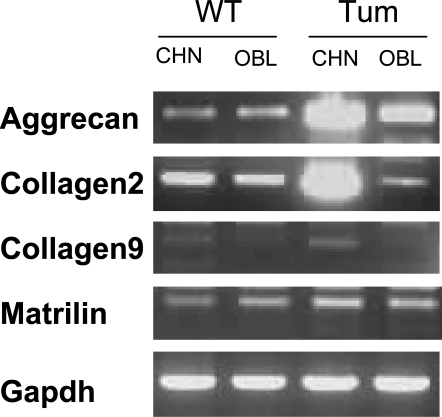
Because osteoblasts and chondrocytes both arise from the mesenchymal stem cell lineage, we next determined whether the cells exhibited chondrocyte markers as an indicator of incomplete differentiation. Cells were grown in either osteoblast medium or in chondrocyte differentiation medium for 3 d, and expression of the chondrocyte markers aggrecan, matrilin, and collagens 2 and 9 were assessed by semiquantitative real-time PCR (Fig. 4). In osteoblast medium, both WT and tumor cells expressed low levels of aggrecan and collagen 2, whereas collagen 9 was essentially undetectable. When the cells were grown in chondrocyte medium, both WT and tumor cells expressed low levels of collagen 9. However, whereas normal osteoblasts did not change levels of aggrecan or Col2, tumor cells demonstrated marked up-regulation of these markers, suggesting that the cells were arrested at a partially differentiated state.
Figure 4.
Analysis of Chondrocyte Markers in Primary Tumor Cells
WT or Prkar1a+/− tumor osteoblasts were isolated from mouse tails and grown in chondrocyte (CHN) or osteoblast (OBL) medium and analyzed by semiquantitative RT-PCR for the mRNAs shown at left. Gapdh was used as a normalization control. Note the marked increase in the chondrocyte markers aggrecan and collagen2 in the tumor osteoblasts. Shown are representative data from replicate experiments.
To rule out abnormalities of the osteoclast lineage, primary osteoclasts were prepared from the bone marrow of long bones from heterozygote mice and compared with similar preparations from WT mice. The osteoclasts did not show morphologic changes, and functional characterization by acid phosphatase staining or a bone pitting assay did not reveal differences between the groups (data not shown).
Although distant metastases were not observed in mice, the tumors were locally aggressive, causing distortion of the local tissue architecture. Because this property can also be observed in malignant tumors, we sought to determine whether the tumor cells had the ability to grow as xenotransplants. For this purpose, we injected WT or tumor osteoblasts into the flank (orthotopic) or into the vertebral space (eutopic) of immunocompromised mice (Fig. 5). None of the four mice (0%) injected with WT cells exhibited tumor formation. However, in two of four mice (50%) injected with tumor cells, calcification was radiologically detectable within 8 wk (Fig. 5A). Analysis of these lesions revealed the presence of tumor nests similar to the original bone tumors observed in Prkar1a+/− mice (Fig. 5, B and C). Additionally, one of four mice (25%) exhibited a kinking of the tail at a location of the eutopic injection of tumor cells. This also turned out to represent a tumor focus (Fig. 5, D–F). No such lesions were observed after eutopic injection of WT osteoblasts.
Figure 5.
Injection of Tumor Cells Can Recapitulate Tumor Formation in Immunocompromised Mice
A, WT osteoblasts injected sc over right hip (blue arrow) of NOD-SCID mice fail to produce tumors, whereas tumor osteoblasts injected over the left hip (red arrow) produce calcifying tumors; B and C hematoxylin- and eosin-stained sections of the tumors are shown at ×40 (B) and ×400 (C) magnification and demonstrate marked similarity to the initial tumor (e.g. Fig. 1); D–F, eutopic injection of tumor cells into the tail can also produce tumors in mice, shown by x-ray (D, red arrow), or by hematoxylin and eosin at ×40 (E) or ×400 (F); G, normal mouse tail for comparison. D, Intervertebral disk; T, tumor; V, vertebrae.
Analysis of Gene Expression in Tumor Osteoblasts
To better understand signaling pathways that might be altered in the tumor osteoblasts, we isolated mRNA from three independent control and tumor cell preparations, and analyzed mRNA expression patterns by cDNA microarray. All samples used in this portion of the study were derived from tail vertebral bones. The microarray study was analyzed in an unsupervised fashion, and probe sets showing changes at a statistical significance level of P < 0.001 by the logit-t test (18) were considered as having altered expression. In this analysis, we found significant alterations in the expression of 250 genes, including 144 known genes and 106 expressed sequence tags (supplemental Tables 2 and 3, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Of these transcripts, 99 (39.6%) were up-regulated, whereas 151 (60.4%) were down-regulated. To confirm the validity of the analysis, we selected 10 genes of interest from this list and performed quantitative RT-PCR (qRT-PCR) to confirm the difference in expression found by microarray. Transcripts selected for this analysis included factors involved in growth signaling (Wnt5a, Frzb, Fgf7, and Igf2bp), transcription factors (Cebpd and Runx1/Cbfa2), differentiation markers (Il6st and Cilp), and proteins thought to be involved in bone tumor metastasis and remodeling (Vil2/ezrin and Mmp9). In general, microarray analysis provided qualitatively similar data to that obtained with qRT-PCR, although the magnitude of some of the changes was variable (Table 1).
Table 1.
Identification of mRNA Transcripts Altered in Tail and Long Bones from Prkar1a+/− Mice
| mRNA | Microarray (Fold Change)
|
qRT-PCR (Fold Change)
|
||
|---|---|---|---|---|
| Tail | Femur | Tail | Femur | |
| Up-regulated | ||||
| Wnt 5a | 9.5 | 2.8 | 13.0 | 0.1 |
| Fgf 7 | 9.4 | 2.5 | 5.3 | 1.4 |
| Mmp 9 | 7.6 | 2.5 | 23.7 | 79.4 |
| Cebp-d | 4.0 | 3.3 | 3.9 | 2.0 |
| Frzb | 3.8 | 2.1 | 13.3 | 25.4 |
| Villin 2 | 2.9 | 1.6 | 73.5 | 13.9 |
| Down-regulated | ||||
| Runx1 | −2.1 | −1.3 | −5.3 | −1.2 |
| Il6st | −2.6 | 1.4 | −5.5 | −1.5 |
| Igfbp 2 | −5.5 | −1.1 | −87.3 | −4.3 |
| Cilp | −49.7 | −8.0 | −93.8 | −25.0 |
Data are expressed as fold expression in heterozygous animals compared with WT animals, where negative numbers indicate higher expression in the WT animals.
To determine whether expression changes were correlated with alterations in protein levels, we performed Western blotting analysis of some of the proteins identified in the microarray analysis as well as other protein markers of osteoblast differentiation, such as the transcription factor Runx2 (15). We also studied the related factor Runx1, which is expressed in bone but whose role in osteoblast function is unclear (19) (Fig. 6).
Figure 6.
Analysis of Selected Proteins by Western Blot
Proteins were prepared from the same samples as in Fig. 2 and Table 1 and Western blotted for the proteins indicated. Quantitation is shown below as in Fig. 2. Note that all tested proteins showed statistically significant changes, matching the results of mRNA expression analysis. *, P < 0.05; **, P < 0.01. This experiment was repeated five times, and a representative blot is shown.
All of the proteins whose transcripts were altered in the microarray analysis exhibited changes that correlated with mRNA alterations. For example, we noted a marked increase in both Wnt5a and Fgf7 proteins in the tumor cells. Conversely, Runx1 and Runx2 were both down-regulated at the protein level. In confirmation of the immunofluorescence data (Fig. 3, F and G), we also observed a significant reduction on osteocalcin, as well as another marker of osteoblast differentiation, osteopontin.
Growth Characteristics of Tumor Osteoblasts
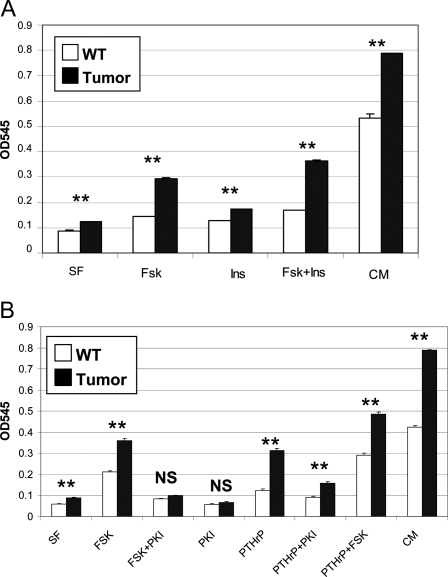
When tumor osteoblasts were placed in primary culture, it became evident that these cells proliferated more rapidly than their WT counterparts. After 5 d of growth in complete medium, tumor osteoblasts exhibited a 50% increased cell count compared with WT cells (Fig. 7, rightmost bars). Even under serum-free conditions, the tumor cells exhibited a slight but significant growth, whereas control cells did not proliferate in the absence of growth factors.
Figure 7.
Analysis of Growth Characteristics of Control and Tumor Osteoblasts
A, WT or tumor cells were plated in triplicate in 12-well dishes and allowed to grow in serum-free medium containing no additives (SF), FSK, insulin (Ins), or both (Fsk+Ins) or in complete medium containing 10% fetal calf serum (CM). After 5 d, cells were quantitated. B, Cells were grown in serum-free medium for 3 d with the addition of FSK, PTHrP, or PKI as indicated or in complete medium (CM). Doses of drugs are described in Materials and Methods. **, P < 0.001 for comparison of WT and tumor cells receiving the same treatment.
To examine factors that may promote growth of the cells, the samples were cultured in the presence of forskolin (FSK), an activator of the cAMP/PKA pathway, insulin, to stimulate the insulin receptor pathway, or both (Fig. 7A). Both agents caused enhanced proliferation of both WT and tumor cells. Insulin stimulated both cell types equally, leading to a 1.4-fold enhancement of growth compared with serum-free conditions. In contrast, FSK caused a 2.4-fold increase in the growth of the tumor cells, whereas control cells were stimulated only 1.6-fold. Similar effects were observed when cells were directly stimulated with 8-Br-cAMP (data not shown). When used together, FSK and insulin produced additive effects on growth, which might be expected if they promoted growth through different pathways; however, no synergy of signaling was observed, and cells did not proliferate to the same extent as observed in cells receiving complete growth medium.
To verify that the enhanced growth was mediated by stimulation of the PKA pathway, WT or tumor cells were treated with FSK in the presence of a cell-permeant derivative of the peptide PKA inhibitor, PKI. As shown in Fig. 7B, PKI completely reversed the stimulatory effects of FSK on tumor cells. Because normal osteoblasts activate the cAMP/PKA pathway by signaling downstream of the PTH/PTHrP receptor (PTHR1), we treated the cells with PTHrP to activate this pathway. PTHrP stimulated the growth of both normal and tumor osteoblasts, although tumor osteoblasts exhibited a markedly enhanced sensitivity to this treatment. Treatment with PKI reversed the majority of the effect of PTHrP, although a slight stimulatory effect was observed, suggesting that PTHrP may have modest effects on other growth-stimulatory pathways.
DISCUSSION
We present here a detailed characterization of bone tumors that arise in Prkar1a+/− mice, generated as a model of the CNC tumor syndrome. Bone tumors in CNC patients have a mixed histological appearance, with areas of abnormal bone and cartilage set in a loose myxoid matrix (4). These tumors are generally seen at young ages and may occur congenitally. In contrast, the analogous tumors are not observed in younger mice and develop with high penetrance as the mice approach 1 yr of age. Despite this difference, a comparison of photomicrographs of the tumors observed in mice (10) (Fig. 1) with those described in humans (4) reveals a striking similarity. The tumors in these mice, like those reported in CNC patients, are expansile and appear to be locally aggressive but do not exhibit features of malignancy. Specifically, mitotic figures are extremely rare in the tumors, and we have not yet observed an instance of a metastatic tumor, despite the fact that the primary tumors can grow to substantial size (10).
Somewhat surprisingly, two of four independent primary cultures of tumor cells were able to form tumors in NOD-SCID mice within 8 wk of injection. This observation confirms the locally invasive character of these lesions, which is a characteristic normally associated with a malignant phenotype (e.g. osteosarcomas). However, the lack of distant metastases, either in the primary tumors or in the xenografts suggests there may be some dissociation between the ability to form tumors and the malignant phenotype. This phenotype is also exhibited by normal parathyroid cells, which are able to establish themselves after orthotopic autotransplantation (20) and in xenotransplant models in mice (21). Thus, the connection between the ability to form tumors in a xenograft model and the malignant phenotype may not be so clear in this case. The rate of tumor formation after eutopic injection into the tail is probably lower because fewer cells can be injected into this limited space. It is possible that the 50% tumor formation rate may reflect individual variation of the primary cultures, although mRNA expression analysis by cDNA microarray indicated that various lines are quite similar (see supplemental data). Another possibility is that these tumors grow very slowly and that the incidence would have been higher if more time had been allowed for tumor growth.
At the cellular level, we have clearly demonstrated that the tumors arise from mesenchymal stem cells progressing along the osteoblast lineage, although they appear arrested in an incompletely differentiated state, as demonstrated by the retention of chondrocyte markers. These findings are similar in many ways to studies of bone tumors associated with the McCune-Albright syndrome (MAS), which is also thought to occur as a result of the dysfunction of stem cells directed at the osteoblast lineage (22). At the clinical level, MAS is the association of polyostotic fibrous dysplasia with café-au-lait spots and autonomous endocrine hyperactivity, classically manifest as precocious puberty in girls (23). The genetic defects responsible for MAS were identified in the early 1990s as activating mutations of the Gsα subunit, leading to enhanced activity of adenylyl cyclase, elevation of intracellular cAMP levels, and hyperstimulation of PKA (24). The fact that both mutations lead to dysregulation of PKA explains the clinical similarities of these two syndromes. In-depth characterization of the bone lesions in MAS was elegantly carried out in the lab of Robey and co-workers (25,26,27,28) and clearly demonstrated that the deposition of abnormal bone was a result of abnormal osteoblasts, in close analogy to the observations made here. Although the lesions appear similar at first blush, careful analysis by an experienced pathologist can differentiate the lesions of MAS from those of CNC (Carney, J. A., personal communication).
Like osteoblasts isolated from the fibrous dysplasia lesions of MAS patients, tumor osteoblasts observed in this model exhibit increased PKA activity (Fig. 2C). Similar observations have previously been made in adrenal tumors from humans (5) and in mouse embryonic fibroblasts (17). In accordance with the elevated PKA activity, tumor cells exhibit hyperresponsiveness to the growth-promoting effects of PKA-activating agents in this primary culture system. Although insulin signaling enhances growth, the effects are only modest when compared with those caused by activation of PKA. Elevation of cAMP, either directly (by the use of the adenylyl cyclase activator FSK) or indirectly by liganding of the PTHR1, leads to a specific and PKA-dependent stimulation of proliferation. Given that PTH is secreted as part of a constitutive feedback loop and that the PTHR1 signals (at least primarily) through the Gs/cAMP/PKA pathway (29), this may provide a chronic stimulus for cell proliferation, as has been previously reported in osteoblast cell line models (30). Because the tumor cells exhibit hyperresponsiveness to this signal, they are able to overcome normal controls on cell proliferation. Thus, both enhanced bone turnover and tumorigenesis as observed in this model are likely directly attributable to this hyperresponsiveness to cAMP.
Microarray analysis of tumor osteoblasts revealed approximately 250 distinct transcripts that were altered in these cells compared with their WT counterparts, including transcripts necessary for proper osteoblast function. One of the interesting findings was a significant reduction in Runx2, which has been proposed to be a major control gene for osteoblast differentiation (31). This was accompanied by a reduction of its downstream transcriptional targets, including osteocalcin and osteopontin. Not surprisingly, there was down-regulation of other markers of osteoblast function, including Il6st (IL-6 signal transducer), a gene that encodes the gp130 subunit of the IL-6 receptor (32) and is responsible for signal transduction in osteoblasts by IL-6 (33,34) and other cytokines (35). The transcript exhibiting the sharpest drop coded for Cilp, cartilage intermediate layer protein, a structural protein thought to play a role in matrix disposition during endochondral bone formation (36,37).
Genes that were up-regulated at the protein and mRNA level included the paracrine growth factors Wnt5a and Fgf7. Wnt5a is member of the secreted glycoprotein Wnt family, of which there are 18 members in mice and 19 members in humans (38). In addition to complexity in the Wnts themselves, the signaling pathway is similarly complicated, consisting of membrane-bound receptors (Frizzled family members) and co-receptors (Lrp5 and Lrp6) as well as a multiplicity of intracellular mediators including the canonical β-catenin-Lef/Tcf pathway as well as noncanonical pathways involving other G protein-coupled receptors (39). Wnt signaling is very well known to affect osteoblast function with resulting effects on bone mass, and mutations of the Lrp5 co-receptor can alter bone mass both in mice and humans (for reviews, see Refs. 31 and 40). Efforts to quantify β-catenin in tumor osteoblasts have not demonstrated an increase in levels (data not shown), but this does not rule out increased Tcf/Lef activity, because this may occur without gross changes in levels of β-catenin, as has previously been demonstrated in a PTH- and cAMP-dependent system (41). Also, a connection between CNC tumors and aberrant Wnt signaling was previously suggested in a serial analysis of gene expession analysis of adrenal tumors from CNC patients, although no evaluation of effects at the protein or biological level was performed (42). The possibility that alteration in Wnt signaling is playing a role in tumor cells, including the possibility of action through noncanonical Wnt signaling, is currently under active investigation. The role of Fgf7, also known as keratinocyte growth factor, is currently unknown, but this factor is thought to be secreted by osteoblasts as a means to affect the local environment (43).
In summary, we present in this paper a detailed characterization of bone lesions in Prkar1a+/− mice, initially generated to model the human tumor syndrome CNC. Bone lesions in the mice are closely analogous to those observed in human patients, and we have demonstrated that the lesions arise from incompletely differentiated cells of the osteoblast lineage. Tumorigenesis in this system appears to be driven predominantly by excess activation of PKA signaling, although other growth factors, including members of the Wnt pathway, may also play a role. These bone tumors, which provide the first example of an in vitro system, in either man or mouse, with which to study directly the biochemical alterations associated with tumorigenesis caused by PRKAR1A mutations, will provide insights not only into osteoblast biology but also into the general question of tumorigenesis associated with the CNC.
MATERIALS AND METHODS
Mouse Strains
Prkar1a+/− mice carrying a deletion of exon 2 have previously been described (10). Deletion of this exon removes the initiator ATG codon from the gene and results in an allele that does not produce any protein (10,17). For this study, the original mixed-background strain was backcrossed onto an FVB/N genetic background. All animal experiments in this study were conducted in accordance with the highest standards of animal care and were carried out under an Institutional Animal Care and Use Committee-approved animal protocol.
Staining for Bone and Cartilage
Tails were taken from Prkar1a +/− mice carrying tumors or from WT littermate mice. The skin was removed, and the tails were placed in 1% KOH solution at room temperature for 3 d. The tails were stained for cartilage and bone with Alcian blue 8GX and Alizarin red (Sigma Chemical Co., St. Louis, MO) using standard protocols (44).
Immunohistochemistry on Bone Tumor Sections and Wild-Type Bone
Tumor and WT tissues were removed from mice and subjected to a decalcification (TBD-2 decalcifier; Thermo Shandon, Pittsburgh, PA) before fixation in formalin. Sections were stained with hematoxylin and eosin or used for immunochemical analysis. The following antibodies were used in this study: osteocalcin (Biomedical Technologies, Inc., Palatine, IL), Runx2 and V-ATPase (Santa Cruz Biotechnology, Santa Cruz, CA). Samples were subject to antigen retrieval by steaming 20 min in Target Retrieval Solution (DAKO, Carpinteria, CA). Slides were developed with Vector Elite ABC reagents (Vector Laboratories, Burlingame, CA). Quantitation of osteoclasts was done by counting the number of multinucleated cells that have the shape of osteoclasts and are positive for V-ATPase per high-power field (HPF).
Primary Cultures of Mouse Osteoblasts
Primary cultures of mouse tumors and normal bones were established using a protocol based on the method of Bianco et al. (28). Briefly, tumors or vertebral bones were minced into small fragments of about 2–3 mm and incubated in collagenase A (Sigma) in DMEM at 37 C for 20 min. The remaining bone fragments were washed twice with Dulbecco’s PBS, and cultured in 60-mm culture dishes with DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 10,000 U/liter penicillin G, 10 mg/liter streptomycin, and 1% ascorbic acid (osteoblast medium). Media were refreshed at 3-d intervals. After 6 d, tumor cells that had attached to the culture dishes were harvested by trypsin treatment, plated into 100-mm dishes, and grown in osteoblast medium.
For preparation of cells from long bones, the epiphyses were excised and the bone marrow was flushed out with PBS. The diaphyses were then cut into small fragments of approximately 1–2 mm using sterile blades. The bone fragments were incubated with 5 ml collagenase per two femurs and two tibias at 37 C with shaking; after 60 min, the collagenase was discarded and the samples digested with 5 ml fresh trypsin for an additional 30 min. The remaining fragments were washed with DMEM supplemented with 10% fetal bovine serum and placed in 35-mm plates in osteoblast medium. After approximately 14 d, the bone cells attached to culture dishes were isolated by trypsin treatment and plated into 100-mm dishes in osteoblast medium. After four to six passages, cells were harvested for protein and mRNA extraction.
Alkaline Phosphatase Staining
To determine alkaline phosphatase activity, cells were plated on plastic coverslips placed into six-well plates (Corning) at a density of 15,000 cells per coverslip. Coverslips were washed with PBS and the cells fixed in 4% paraformaldehyde for 1 h at 4 C. After washing, cells were stained with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche, Indianapolis, IN) for 30 min at room temperature and counterstained with eosin before mounting and visualization.
Cell Growth Assay
WT osteoblasts from mouse tails and tumor cells extracted from tail tumors were plated in triplicate in 12-well dishes at 25,000 cells per well. Cells were incubated in serum-free DMEM with the following additives, as indicated in the text: 5 μm FSK, 2 μg/ml insulin (Sigma), 10 μm myristoylated PKI (Invitrogen, Carlsbad, CA) or 100 nm PTHrP (Bachem, King of Prussia, PA). For Fig. 7A, cells were allowed to proliferate for 5 d including one change of medium. For Fig. 7B, cells were cells were allowed to proliferate for 3 d. Cell quantitation was performed using crystal violet staining as described (45).
Mineralization and Differentiation Assay
For the mineralization assay, cells were plated at a density of 12,500 cells/ml on a bony substrate (Osteologic discs; BD Biosciences, San Diego, CA) in osteoblast medium. Media were refreshed at 3-d intervals, and the cells were allowed to mineralize for 21 d. To visualize bone nodules, the disks were subject to von Kossa staining and mounted in toto on glass slides. To determine the effects of medium on markers of differentiation, cells were grown in osteoblast medium (as above) or in chondrocyte differentiation medium (Cell Applications, Inc., San Diego, CA) for 3 d before analysis.
Protein Analyses by Western Blot
Cultured cells were lysed in M-PER protein extraction reagent containing HALT protease inhibitors (Pierce, Rockford, IL). Proteins were resolved by SDS-PAGE gels and transferred to nitrocellulose (Pall, East Hills, NY). Blots were developed with chemiluminescence solution (Western Lightning; PerkinElmer, Norwalk, CT). To detect the proteins of interest, we used the following antibodies: PKAR1A, PKAR2A, and PKAR2B were from BD Biosciences; osteocalcin was from Biomedical Technologies. CBFA2/AML-1 (Runx1) (sc-8563), CBFA1/PEBP2αA (Runx2) (sc-10758), osteopontin (sc-21742), Fgf-7 (sc-1365), and Wnt-5a (sc-23968) were from Santa Cruz Biotechnology, and actin (A5060) was from Sigma.
Immunofluorescence
Tumor cells and WT osteoblasts were analyzed by immunofluorescence for osteocalcin and actin as previously described (17). Briefly, cells were plated and grown on glass coverslips, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100 for 6 min, and slides were blocked with 2% BSA/PBS and then incubated overnight with the primary antibody. After washing, coverslips were then incubated with Alexa Fluor 594-conjugated antibodies (Invitrogen) for 1 h at room temperature. In the end, cells were mounted using Vectashield with 4′,6-diamidino-2-phenylindole (Vector Laboratories) and imaged using fluorescence and phase contrast microscopy.
Kinase Activity Assay
The activity of PKA was assessed as previously described (17). Briefly, cells were washed in ice-cold PBS and lysed in buffer solution containing 10 mmol/liter Tris-HCl (pH 7.1), 1 mmol/liter EDTA, 1 mmol/liter dithiothreitol, 0.5 mmol/liter phenylmethylsulfonyl fluoride, 0.1 mmol/liter vanadate, 1 mmol/liter NaF, and HALT protease inhibitors. Lysates were collected by centrifugation at 10,000 × g for 20 min at 4 C; supernatants were collected for measurement of free (−cAMP) and total (+cAMP) PKA activity. The total volume of the reaction was 30 μl and contained 30 μg protein lysate, 5 μg 6× His-tagged cAMP response element-binding protein (CREB), 50 mmol/liter ATP, 50 mmol/liter Tris, 10 mmol/liter MgCl2, and 1 mmol/liter dithiothreitol with and without 5 μmol/liter PKI and cAMP. The reaction was incubated 30 min at 30 C and terminated by incubating 5 min at 100 C with sample buffer. Phosphorylation of the CREB substrate was measured by Western blot using a phospho-CREB antibody (Cell Signaling Technology, Beverly, MA), and the bands were quantitated using Image J software. PKA activity was corrected for nonspecific phosphorylation by subtracting residual bands observed in the presence of PKI.
Microarray and qRT-PCR
mRNA was isolated from three independent primary cultures of WT vertebral osteoblasts and three independent cultures of tumor osteoblasts from tail tumors. Samples were isolated by Trizol extraction (Invitrogen) and repurified on RNeasy columns (QIAGEN, Valencia, CA). Labeled cRNA was hybridized to Affymetrix Mouse 430 v2.0 chips (Affymetrix, Santa Clara, CA), and the resulting data were analyzed by logit-t test, as described (18). For semiquantitative or quantitative RT-PCR, RNA (1 μg) was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative real-time PCR was done using an iCycler and iCycler SYBR Green reagents (Bio-Rad). The primers were designed using Primer3 web software (46) (http://frodo.wi.mit.edu/) and are included in supplemental Table 1. For quantitation of transcripts, all PCR were performed in triplicate, and the ΔΔCt method was used to calculate relative mRNA levels relative to a Gapdh standard.
Tumor Xenografts
Four NOD-SCID mice (Harlan) were injected in right flank with 106 WT osteoblasts and in the left flank with 106 tumor osteoblasts. At the same time, the mice received 105 WT and 105 tumor osteoblasts at distinct locations in the tail. The mice were monitored weekly and killed 8 wk later. X-rays were obtained by FAXITRON radiograph (Hewlett-Packard, Palo Alto, CA) before histological examination.
Statistical Analysis
All analyses in this manuscript were performed using a two-sided Student’s t test, as implemented by StatCrunch (http://www.statcrunch.com). Resulting P values are indicated in the text where appropriate. For analysis of PKA activity, a paired t test was used to minimize effects of independently run kinase assays.
Supplementary Material
Acknowledgments
We gratefully acknowledge Drs. J. Aidan Carney and Rebecca Jackson for their insights and helpful discussions of this project. We also thank Dr. Donna Kusewitt for technical guidance on this and other projects.
Footnotes
This work was funded in part by National Institutes of Health Grants HD01323 and CA112268-02 (to L.S.K.) and by National Cancer Institute Grant CA16058 to the Ohio State University Comprehensive Cancer Center.
Disclosure Statement: All authors declare no conflict of interest.
First Published Online October 11, 2007
Abbreviations: CNC, Carney complex; CREB, cAMP response element-binding protein; FSK, forskolin; HPF, high-power field; MAS, McCune-Albright syndrome; PKA, protein kinase A; PKI, PKA inhibitor; PTHR1, PTH/PTHrP receptor; qRT-PCR, quantitative RT-PCR; WT, wild type.
References
- Carney JA 1995 The Carney complex (myxomas, spotty pigmentation, endocrine overactivity, and schwannomas). Dermatol Clin 13:19–26 [PubMed] [Google Scholar]
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL 1985 The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64:270–283 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Stratakis CA 2002 Genetic analysis of Carney complex: current understanding and future questions. Curr Opin Endocrinol Diabetes 9:244–249 [Google Scholar]
- Carney JA, Boccon-Gibod L, Jarka DE, Tanaka Y, Swee RG, Unni KK, Stratakis CA 2001 Osteochondromyxoma of bone: a congenital tumor associated with lentigines and other unusual disorders. Am J Surg Pathol 25:164–176 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000 Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA 2000 Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum Mol Genet 9:3037–3046 [DOI] [PubMed] [Google Scholar]
- Casey M, Vaughan CJ, He J, Hatcher CJ, Winter JM, Weremowicz S, Montgomery K, Kucherlapati R, Morton CC, Basson CT 2000 Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest 106:R31–R38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA 2006 A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 [DOI] [PubMed] [Google Scholar]
- Amieux PS, Howe DG, Knickerbocker H, Lee DC, Su T, Laszlo GS, Idzerda RL, McKnight GS 2002 Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem 277:27294–27304 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns 2nd WH, Carney JA, Westphal H, Stratakis CA 2005 A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65:4506–4514 [DOI] [PubMed] [Google Scholar]
- Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, La Perle K, Vaughan CJ, O’Hagan A, Bennett KR, Meyer BJ, Legius E, Karttunen M, Norio R, Kaariainen H, Lavyne M, Neau JP, Richter G, Kirali K, Farnsworth A, Stapleton K, Morelli P, Takanashi Y, Bamforth JS, Eitelberger F, Noszian I, Manfroi W, Powers J, Mochizuki Y, Imai T, Ko GT, Driscoll DA, Goldmuntz E, Edelberg JM, Collins A, Eccles D, Irvine AD, McKnight GS, Basson CT 2004 Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci USA 101:14222–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos S, Robinson-White A, Lenherr S, Weinberg FD, Claflin E, Meoli E, Cho-Chung YS, Stratakis CA 2004 Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res 64:8811–8815 [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA, Stratakis CA 2004 A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet 41:923–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albassam MA, Wojcinski ZW, Barsoum NJ, Smith GS 1991 Spontaneous fibro-osseous proliferative lesions in the sternums and femurs of B6C3F1 mice. Vet Pathol 28:381–388 [DOI] [PubMed] [Google Scholar]
- Huang W, Yang S, Shao J, Li YP 2007 Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12:3068–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-White A, Meoli E, Stergiopoulos S, Horvath A, Boikos S, Bossis I, Stratakis CA 2006 PRKAR1A mutations and protein kinase A interactions with other signaling pathways in the adrenal cortex. J Clin Endocrinol Metab 91:2380–2388 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS 2005 Disruption of protein kinase A regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65:10307–10315 [DOI] [PubMed] [Google Scholar]
- Lemon WJ, Liyanarachchi S, You M 2003 A high performance test of differential gene expression for oligonucleotide arrays. Genome Biol 4:R67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS 2004 Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr 14:1–41 [PubMed] [Google Scholar]
- Baumann DS, Wells Jr SA 1993 Parathyroid autotransplantation. Surgery 113:130–133 [PubMed] [Google Scholar]
- Strieth S, von Johnston V, Eichhorn ME, Enders G, Krasnici S, Thein E, Hammer C, Dellian M 2005 A new animal model to assess angiogenesis and endocrine function of parathyroid heterografts in vivo. Transplantation 79:392–400 [DOI] [PubMed] [Google Scholar]
- Riminucci M, Saggio I, Robey PG, Bianco P 2006 Fibrous dysplasia as a stem cell disease. J Bone Miner Res 21(Suppl 2):P125–P131 [DOI] [PubMed] [Google Scholar]
- McKusick VA 2003 Online Mendelian inheritance in man. Baltimore: McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University; Bethesda, MD: National Center for Biotechnology Information, National Library of Medicine [Google Scholar]
- Shenker A, Weinstein LS, Sweet DE, Spiegel AM 1994 An activating Gsα mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab 79:750–755 [DOI] [PubMed] [Google Scholar]
- Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P 1997 Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol 151:1587–1600 [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Kuznetsov SA, Riminucci M, Fisher LW, Spiegel AM, Robey PG 1998 Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gsα-mutated skeletal progenitor cells. J Clin Invest 101:1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Robey PG, Bianco P 1999 The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gsα gene: site-specific patterns and recurrent histological hallmarks. J Pathol 187:249–258 [DOI] [PubMed] [Google Scholar]
- Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, Corsi A, Bone HG, Wientroub S, Spiegel AM, Fisher LW, Robey PG 2000 Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res 15:120–128 [DOI] [PubMed] [Google Scholar]
- Ahlstrom M, Lamberg-Allardt C 1997 Rapid protein kinase A-mediated activation of cyclic AMP-phosphodiesterase by parathyroid hormone in UMR-106 osteoblast-like cells. J Bone Miner Res 12:172–178 [DOI] [PubMed] [Google Scholar]
- Kano J, Sugimoto T, Fukase M, Fujita T 1991 The activation of cAMP-dependent protein kinase is directly linked to the regulation of osteoblast proliferation (UMR-106) by parathyroid hormone. Biochem Biophys Res Commun 177:365–369 [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM 2004 Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39 [DOI] [PubMed] [Google Scholar]
- Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T 1992 Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol 148:4066–4071 [PubMed] [Google Scholar]
- Dodds RA, Merry K, Littlewood A, Gowen M 1994 Expression of mRNA for IL1β, IL6 and TGFβ1 in developing human bone and cartilage. J Histochem Cytochem 42:733–744 [DOI] [PubMed] [Google Scholar]
- Ross FP, Christiano AM 2006 Nothing but skin and bone. J Clin Invest 116:1140–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing DP, Comeau MR, Friend DJ, Gimpel SD, Thut CJ, McGourty J, Brasher KK, King JA, Gillis S, Mosley B 1992 The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science 255:1434–1437 [DOI] [PubMed] [Google Scholar]
- Yao Z, Nakamura H, Masuko-Hongo K, Suzuki-Kurokawa M, Nishioka K, Kato T 2004 Characterisation of cartilage intermediate layer protein (CILP)-induced arthropathy in mice. Ann Rheum Dis 63:252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo P, Neame P, Sommarin Y, Heinegard D 1998 Cloning and deduced amino acid sequence of a novel cartilage protein (CILP) identifies a proform including a nucleotide pyrophosphohydrolase. J Biol Chem 273:23469–23475 [DOI] [PubMed] [Google Scholar]
- Miller JR 2002 The Wnts. Genome Biol 3:REVIEWS3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H 2005 Wnt signalling in stem cells and cancer. Nature 434:843–850 [DOI] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA 2006 Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni NH, Halladay DL, Miles RR, Gilbert LM, Frolik CA, Galvin RJ, Martin TJ, Gillespie MT, Onyia JE 2005 Effects of parathyroid hormone on Wnt signaling pathway in bone. J Cell Biochem 95:1178–1190 [DOI] [PubMed] [Google Scholar]
- Horvath A, Mathyakina L, Vong Q, Baxendale V, Pang AL, Chan WY, Stratakis CA 2006 Serial analysis of gene expression in adrenocortical hyperplasia caused by a germline PRKAR1A mutation. J Clin Endocrinol Metab 91:584–596 [DOI] [PubMed] [Google Scholar]
- Britto JA, Evans RD, Hayward RD, Jones BM 2001 From genotype to phenotype: the differential expression of FGF, FGFR, and TGFβ genes characterizes human cranioskeletal development and reflects clinical presentation in FGFR syndromes. Plast Reconstr Surg 108:2026–2039; discussion 2040–2026 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R 2003 Manipulating the mouse embryo: a laboratory manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, Loda M, Carrasco DR, DePinho RA 2002 Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419:162–167 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ 2000 Primer3 on the WWW for general users and for biologist programmers. In: Krawetz SA, Misener S, eds. Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press; 365–386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.