Abstract
Increased androgen receptor (AR) levels are associated with prostate cancer progression to androgen independence and therapy resistance. Evidence has suggested that chronic inflammation is closely linked to various cancers including prostate cancer. Herein we show that the proinflammatory cytokine TNFα negatively regulates AR mRNA and protein expression and reduces androgen sensitivity in androgen-dependent LNCaP human prostate cancer cells. Decreased AR expression results from transcription repression involving essential in cis interaction of nuclear factor-κB (NF-κB) with the B-myb transcription factor at a composite genomic element in the 5′-untranslated region of AR. The negative regulation was abrogated when NF-κB activity was inhibited by a superrepressor of the inhibitory κB protein. In contrast, androgen-independent C4-2 (LNCaP-derived) cells fail to show AR down-regulation by TNFα, despite expression of B-myb and TNFα-induced NF-κB activity similar to that in LNCaP cells. The negatively regulated AR gene chromatin region showed TNFα-dependent enrichment of B-myb and the NF-κB proteins p65 and p50. In parallel, the histone deacetylase 1, corepressor silencing mediator of retinoid and thyroid hormone receptor and the corepressor-associated scaffold protein mSin3A were recruited to the inhibitory site. In C4-2 cells, neither NF-κB and B-myb, nor any of the corepressor components, were detected at the negative site in response to TNFα. Apoptosis was induced in TNFα-treated LNCaP cells, likely in part due to the down-regulation of AR. The androgen-independent, AR-expressing C4-2 and C4-2B (derived from C4-2) cells were resistant to TNFα-induced apoptosis. The results linking androgen dependence to the NF-κB and AR pathways may be insightful in identifying novel treatment targets for prostate cancer.
DIVERSE PHYSIOLOGY, MOST notably that related to male reproduction, muscle growth, and cognitive vigor, is dependent on the intracellular androgen signaling coordinated by the androgen receptor (AR), which is an inducible transcription factor of the nuclear hormone receptor family. The androgen-induced AR pathway plays a key role in the structural and functional integrity of the prostate, whereas misregulation of AR signaling leads to aberrant growth and malignant progression of prostate cells (1,2). In a wide variety of examples, the transcription regulatory function of AR is known to be modulated by multiple kinase-regulated signaling cascades such as those involving protein kinases A and C, MAPKs, and phosphatidylinositol 3-kinase/Akt-activated kinase pathways (3). Attenuation of AR activity due to transrepression by the nuclear factor-κB (NF-κB) has also been reported (4). Apart from its activity, the expression level of AR is another important determinant of androgen-regulated tissue functions. For example, moderate increases in the AR mRNA and protein levels were observed to associate with the transition of human prostate xenograft tumors from hormone dependence to hormone resistance (5). Auto-induced AR gene expression, observed in transfected cells (6), would conceivably amplify target gene responses to AR signaling and change AR-dependent cell/tissue functions. Furthermore, knockdown of AR using various approaches [antisense oligo; AR antibody; AR-specific hammerhead ribozyme; AR mRNA-targeting small interfering RNAs (siRNAs)] caused growth inhibition and apoptosis of both androgen-dependent and androgen-independent prostate cancer cells (7,8,9,10). In clinical samples, AR is expressed at nearly all stages of prostate cancer (2), and gene amplification along with elevated tissue AR levels are frequently observed in hormone-refractory cancers (11,12). Above experimental and clinical findings highlight the need to understand the molecular events that control prostate cell AR expression in the background of normal and pathogenic stimuli.
Accumulated evidence strongly implicates chronic inflammation as a key player in tumorigenesis (13). Malignant tissues, including prostate tumors, are normally under inflammatory stress rendered by the cytokines released from infiltrated inflammatory cells such as monocytes and macrophages (14). In an experimental model, prostate cancer cell interaction with monocytic cells converted the antiandrogen bicalutamide (casodex) to an androgen agonist (15). The functional impact of the antagonist to agonist conversion for bicalutamide was amplified when cancer cells were pretreated with the proinflammatory cytokine TNFα, indicating that the TNFα-elicited signaling profoundly impacts androgen-dependent prostate cell function. NF-κB is rapidly activated by TNFα in various cell types after a receptor-mediated transmembrane activation response that culminates in phosphorylation, ubiquitination, and proteasomal degradation of the NF-κB-associated inhibitory κ B (IκB) protein and release of the cytosol-retained NF-κB for nuclear translocation and transcription regulation (16). The mammalian NF-κB/Rel protein family has five members, of which p65/RelA, c-Rel and Rel B possess transactivation activity at the carboxyl terminus. The proteins p50 and p52 lack activation function, bearing only the sequence-specific DNA-binding activity. Using the common Rel homology domain, individual NF-κB members form homo- or heterodimers, generating functionally distinct NF-κB complexes. Upon inflammatory stimulation, the p65/p50 complex is the predominantly induced NF-κB in most cells. Diverse processes such as immune activation, cell growth, proliferation, differentiation, and apoptosis are impacted by target gene responses to the activated NF-κB.
In the majority of examples, NF-κB functions as a positive regulator of transcription. Nevertheless, we had previously shown that the rat AR gene activity in transfected HepG2 human hepatoma cells was inhibited by p65/p50-NFκB. The inhibition resulted from direct binding of NF-κB to a 21-bp DNA element at an upstream promoter location (17). Progressive loss of AR mRNA expression in the rat liver during aging in parallel to increased age-associated NF-κB activity led us to speculate that similar negative regulation of rat AR can be expected in vivo in the liver (17). Additional examples of NF-κB-repressible targets include the growth arrest- and DNA damage-inducible GADD 45 genes -α and -γ; glutamate transporter gene EAAT-2; and long-terminal repeat of the latent HIV virus (18,19,20). Inhibition of EAAT-2 expression in astroglioma cells by TNFα required juxtaposition of NF-κB and the oncoprotein N-Myc at the regulatory site.
Herein we present evidence that in androgen-dependent malignant (LNCaP) and nonmalignant (RWPE-1) human prostate cells, TNFα-activated NF-κB inhibits AR expression. In contrast, the inhibitory effect is absent in the LNCaP derived C4-2 and C4-2B prostate cancer cells that have progressed to androgen independence. TNFα caused apoptosis in LNCaP cells but not in the androgen-independent C4-2 and C4-2B cells. The reduced AR level in TNFα-treated LNCaP cells is due to transcription repression involving an essential interaction in cis between NF-κB and the transcription factor B-myb (a protooncoprotein) at a composite binding sequence in the genomic 5′-UTR (untranslated region) of AR. A silencing mediator of retinoid and thyroid hormone receptor (SMRT)/ histone deacetylase (HDAC)1/mSin3A-containing corepressor complex is involved in the negative regulation. In androgen-independent prostate cancer cells, TNFα did not induce recruitment of NF-κB, B-myb, and corepressors to the repressor-responsive site. Delineation of the factors regulating AR gene repression, or lack of it, in response to inflammation-induced NF-κB, may provide important insights on the search to identify new prostate cancer therapeutics.
RESULTS
TNFα-Controlled Inhibition of AR Expression in Human Prostate Cancer Cells and a Role of NF-κB in the Negative Regulation
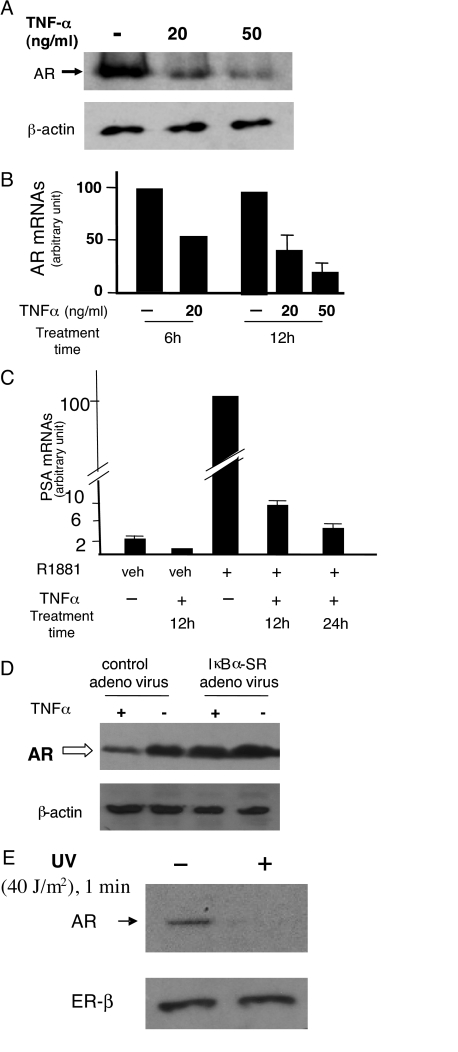
Because prostate tumor tissues are frequently infiltrated with inflammatory cells, and TNFα treatment of malignant or nonmalignant prostate epithelial cells can convert an antiandrogen to an AR agonist, we sought detailed insights into the effect of TNFα on AR expression in prostatic cells. Consistent with an earlier report (21), TNFα caused a dose-dependent reduction of the AR mRNA and protein levels in LNCaP prostate cancer cells (Fig. 1, A and B). The TNFα effect is rapid, because AR mRNAs declined markedly within 30 min of the cytokine treatment (data not shown). As expected, the reduced AR level was associated with attenuation of AR-mediated transactivation, because androgen-induced prostate-specific antigen (PSA) mRNA expression declined markedly in TNFα-treated cells (Fig. 1C).
Figure 1.
TNFα Suppressed AR Expression and Activity in LNCaP Human Prostate Cancer Cells
A, Reduced AR protein level. Cells were treated with TNFα at indicated doses for 12 h, and AR and β-actin proteins were assayed by Western blot. B, Reduced AR mRNAs in cells treated with TNFα for 6 h (at 20 ng/ml) or 12 h (at 20 ng/ml and 50 ng/ml). AR mRNAs were assayed by real-time RT-qPCR, normalized to β-actin mRNAs. Minus TNFα control cells were treated with vehicle (PBS). C, Reduced androgen sensitivity indicated by decreased androgen-induced PSA mRNA expression. Cells were incubated with 10 nm R1881 (or 0.001% ethanol as vehicle) for 24 h. Thereafter, TNFα (20 ng/ml) was added to the culture medium. Control cells (minus TNFα) received PBS. Incubation continued for 12 additional hours, after which cells were harvested for total RNA preparation. In one indicated case, TNFα incubation continued for additional 12 h (total TNFα treatment time: 24 h), thus extending R1881 incubation period to 48 h in this case. PSA mRNAs relative to the invariant β-actin mRNAs were assayed by real-time RT-qPCR. Error bars are averages of three experiments ± sd. PCR primers for AR, PSA, and β-actin mRNAs are described in Materials and Methods. Error bars are not shown in cases where assays were repeated twice, each in duplicate. Data in these cases are presented as average values. D, IκB-SR blocked TNFα-induced AR down-regulation. LNCaP cells were infected with IκB-SR-expressing adenovirus (at 20 moi) or the control GFP-expressing adenovirus (at 20 moi), and 48 h later cells were treated with TNFα (20 ng/ml) or PBS (vehicle) for an additional 24 h. Cell lysates were immunoblotted with anti-AR or anti-β-actin. E, Reduced AR expression in UV-C-irradiated LNCaP cells. UV-C (<290 nm) exposure was at 40 J/m2 (1 min). AR and ER-β protein levels were analyzed by Western blot. ER-β, Estrogen receptor-β; veh, vehicle.
NF-κB activity has an essential role in the TNFα-regulated inhibition, because adenovirus-mediated expression of the IκB-α superrepressor (IκB-SR), which specifically blocks NF-κB activity, prevented reduction of the AR level in TNFα-treated LNCaP cells (Fig. 1D). The SR with Ser→ Ala mutational changes (amino acids 32 and 36) is resistant to TNFα-induced phosphorylation and proteolysis (22), thus preventing the nuclear translocation and activity of NF-κB. The short-wavelength UV radiation UV-C (<290 nm), another activator of NF-κB, also reduced the immunoreactive AR level in LNCaP cells, whereas estrogen receptor-β (ER-β) expression was unaffected (Fig. 1E). These results support the conclusion that NF-κB is a negative regulator of AR gene expression in LNCaP cells.
Lack of TNFα Response in Androgen-Independent Prostate Cancer Cells
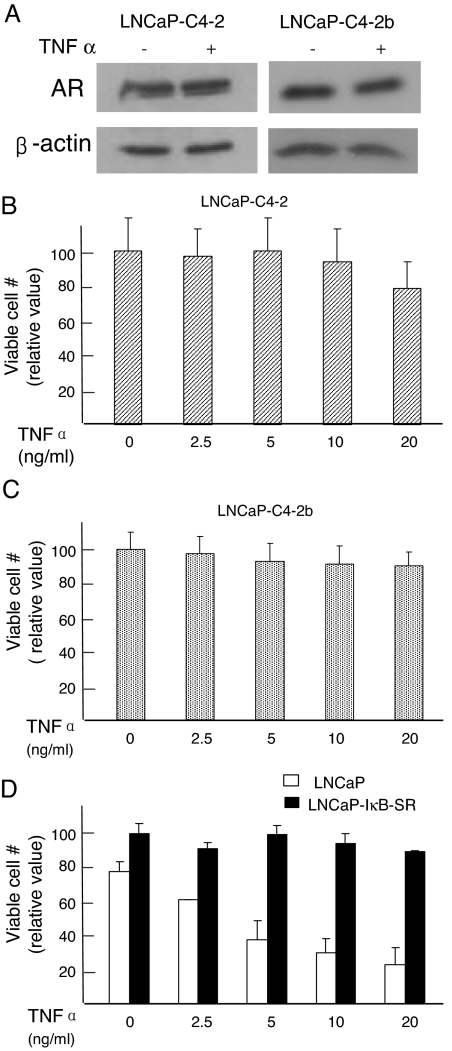
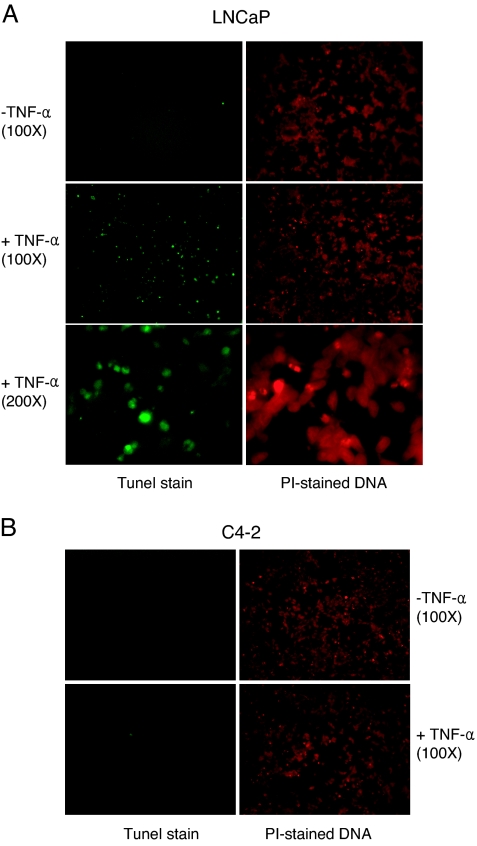
Given that the AR is expressed at all stages of prostate malignancy and the majority of clinical prostate tumor specimens are AR positive, we examined the impact of TNFα on AR expression and cell viability in androgen-independent, AR-positive human prostate cancer cells (Fig. 2). TNFα did not reduce AR levels in C4-2 and C4-2B androgen-independent prostate cancer cells (Fig. 2A). The lack of AR down-regulation correlated with almost a complete protection of C4-2 and C4-2B cells against loss of cell viability (Fig. 2, B and C). C4-2 cells originated from tumor xenografts in castrated mice produced by a mixture of LNCaP cells and bone stromal cells (23). The androgen-independent C4-2B cells were isolated from the bone metastasis of C4-2-derived xenograft tumors (24). In contrast to C4-2 and C4-2B cells, the viability of parental LNCaP cells decreased steadily with increasing doses of TNFα (Fig. 2D, open bars), consistent with a previous report (25). The essential role of NF-κB in the TNFα-induced cell death is evident from the result that stable expression of IκBα-SR protected LNCaP cells from the loss of viability at all tested doses of TNFα (Fig. 2D, solid bars). Resistance of C4-2 cells to TNFα-induced apoptosis is also evident from in situ terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL) assay (Fig. 3). TNFα-treated parental LNCaP cells (Fig. 3A), but not the C4-2 cells (Fig. 3B) showed TUNEL staining. Taken together, these results suggest that the prostate cancer cells that proliferate independently of androgens are unresponsive to TNFα-induced apoptosis, and this failure may be due, in part, to the absence of the TNFα-regulated reduction of AR expression in androgen-independent cells.
Figure 2.
Resistance of Androgen-Independent Prostate Cancer Cells to TNFα-Induced AR Down-Regulation and to Apoptosis
A, Unaltered AR protein levels (analyzed by Western blot) in LNCaP-derived androgen-independent C4-2 and C4-2B cells treated with TNFα (20 ng/ml). B and C, Sustained viability of C4-2 and C4-2B cells against TNFα. The cells were treated with increasing doses of TNFα for 5 d, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay determined the number of viable cells. D, Reduction in the number of viable cells for the parental LNCaP (open bars). Absent loss of viability for LNCaP-IκB-SR cells that stably express the IκB-SR (dark-filled bar). Viable cell numbers are averages of three experiments ± sd.
Figure 3.
Apoptosis of Androgen-Dependent (LNCaP) But Not Androgen-Independent (C4-2) Prostate Cancer Cells after TNFα Treatment
Cells were incubated with TNFα (or PBS for minus TNFα) for 5 d, and TUNEL staining was used to examine apoptosis. Representative cells within a microscopic field (at ×100 or ×200 magnification, as indicated) displaying TUNEL staining (left) and PI staining (right) are shown. TUNEL-stained LNCaP cells emitting green fluorescence are visible at ×100 and ×200. No TUNEL staining for C4-2 cells was detectable. Data are representative of green fluorescence in five different fields.
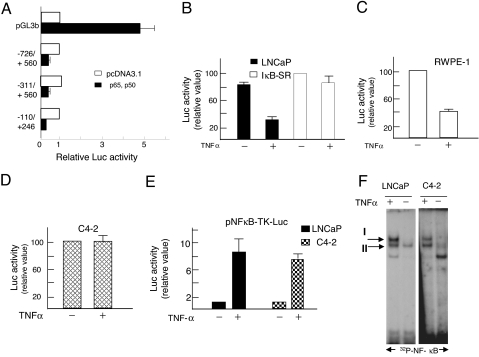
Transcriptional repression accounted for the TNFα/NF-κB-mediated reduction of AR expression in LNCaP cells, revealed from reporter assay in transfected cells (Fig. 4, A and B). Genomic fragments of the human AR cloned into the promoter-less pGL3b luciferase vector were examined for activity to direct reporter expression in p65- and p50-cotransfected LNCaP cells (Fig. 4A). The pGL3b vector itself was strongly stimulated upon overexpression of NF-κB/p65 and NF-κB/p50, due to the presence of an NF-κB-inducible sequence in the firefly luciferase cDNA. However, for the reporter constructs containing genomic AR fragments up to −726, −311, or −110 nucleotide of the upstream sequences and intragenic sequences to various lengths (shown in Fig. 4A), cotransfection of p65 and p50 reduced luciferase expression by 50% or more relative to the pcDNA3.1-transfected control cells (Fig. 4A). Therefore, an NF-κB-responsive negative regulatory region maps to a location delimited by −110 and +246 positions. Indeed, TNFα treatment itself also inhibited the activity of the −110 to +246 AR genome fragment in LNCaP cells, and the inhibition was completely blocked in the presence of IκB-SR (Fig. 4B). Of note is the finding that TNFα did not induce the pGL3b vector in LNCaP or RWPE-1 or C4-2 cells (data not shown). Therefore, in Fig. 4A, pGL3b stimulation by NF-κB is a result of p65/p50 overexpression.
Figure 4.
AR Promoter Repression by NF-κB-p65/p50 in Androgen-Dependent LNCaP and RWPE-1 Prostate Cells and Lack of the Repression in Androgen-Independent C4-2 Cells
A, Luciferase reporter activity is shown for each construct in p65 and p50 cotransfected LNCaP cells relative to pcDNA3.1-transfected cells. Reporter expression was directed by the human AR promoters (−110/+246, −311/+560, −726/+560) or from the pGL3b vector itself. Data are averages with sd from three independent transfections. B, Repression of the −110 to +246 AR promoter by TNFα in LNCaP cells, and lack of the negative regulation in the presence of IκB-SR. The AR promoter-reporter was transfected into LNCaP (solid bar) or LNCaP-SR-IκB cells (open bars), and 24 h later the cells were treated with vehicle or TNFα (20 ng/ml) for an additional 24 h before the cells were analyzed for luciferase. Luciferase values are presented relative to the 100% value set for LNCaP-IκB-SR cells in the absence of TNFα. C, Repression of the AR promoter (−110 to +246) in RWPE-1 (AR-positive, nontumorigenic) cells treated with TNFα. Transfection conditions were same as in panel B. D, Absence of the TNFα effect on the AR promoter (−110 to +246) in C4-2 cells. It should be noted that the luciferase values were comparable in LNCaP and C4-2 cells in the absence of TNFα treatment. In contrast to LNCaP cells, C4-2 cells showed no reduction of luciferase activity after TNFα treatment. E, Comparable NF-κB activation by TNFα in LNCaP and C4-2 cells. Transactivation of a canonical NF-κB element was assayed in transfected LNCaP and C4-2 cells with or without TNFα treatment. F, NF-κB DNA-binding activity in TNFα-treated LNCaP and C4-2 cells. The NF-κB element from the IL-6 gene was used as the EMSA probe. TNFα treatments in panels B–F were for 24 h at 20 ng/ml.
Similar to LNCaP cells, RWPE-1 nontumorigenic prostate epithelial cells were inhibited for AR promoter function by about 60% upon treatment with TNFα (Fig. 4C), indicating that the molecular machinery coordinating NF-κB-mediated repression of AR is not limited to LNCaP cells but is present also in nonmalignant, normal type prostate cells. In contrast, androgen-independent C4-2 cells were refractory to the TNFα-mediated inhibition of the AR promoter activity (Fig. 4D). The luciferase activity driven by the AR promoter in the absence of TNFα was comparable in C4-2 vs. LNCaP cells, indicating that the sensitivity of the repression assay is not limiting in C4-2 cells. Thus, we conclude that the lack of AR down-regulation in C4-2 cells reflects a block at the level of transcription.
Interestingly, NF-κB activation in LNCaP and C4-2 cells is similar, evident from: 1) comparable TNFα-induced luciferase expression from the NF-κB-responsive reporter construct in the two cell lines (Fig. 4E); 2) comparable NF-κB DNA-binding activity in the two cell lines (note EMSA complex I and II) in response to TNFα treatment (Fig. 4F). Thus, the lack of TNFα-induced apoptosis in androgen-independent prostate cancer cells correlated with the lack of repression of the AR gene by NF-κB. To elucidate the molecular basis for the differential NF-κB regulation of the AR gene in the androgen-dependent vs. androgen-independent prostate cells, we characterized the TNFα-responsive negative element in the human AR gene and explored the coregulator dynamics at the regulatory site.
TNFα-Responsive, NF-κB-Regulated Negative Element in the Human AR Gene
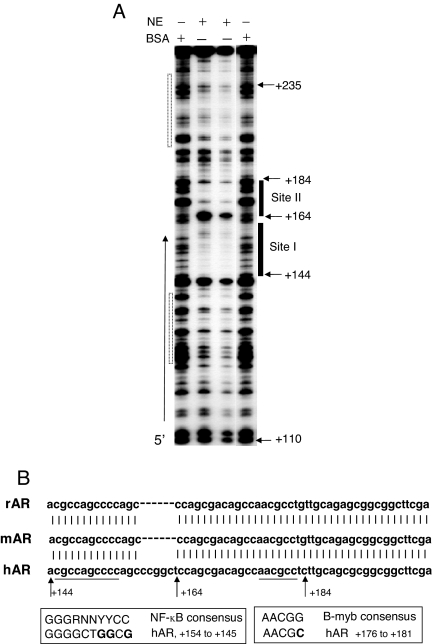
To pinpoint the TNFα/NF-κB-responsive negatively acting element, deoxyribonuclease I (DNase I) footprinting was conducted. The footprint within a segment of the 5′-UTR (from +110 to just beyond + 235) produced by the rat liver nuclear extract revealed four protein-binding sites (Fig. 5A). Site I is especially noteworthy because it includes an NF-κB-like element (GGGGCTGGCGT) at +154 to +144, in the opposite strand. The adjacent site II includes the consensus binding sequence (AACGCC) for the Myb family of transcription factors. The mouse and rat AR genes show 100% (excluding the 6-base deleted gap area) sequence homology with the human gene at site I, and the entire site II is 100% conserved with corresponding sequences in the rodent genes (Fig. 5B). Given the high across-the-species sequence conservation at sites I and II in the 5′-UTR, it is highly likely that this region is involved in regulatory events with important physiological implications.
Figure 5.
Protein-Binding Sites at the 5′-UTR of the Human AR Promoter
A, DNase I footprinting of human AR gene from −110 to +246 with rat liver nuclear extract. B, Sequence conservation of rat and mouse AR promoters with the human AR promoter at +144 to +204. An NF-κB-like element in site I and myb consensus sequence in site II are shown. hAR, human AR; mAR, mouse AR; rAR, rat AR; NE, nuclear extract.
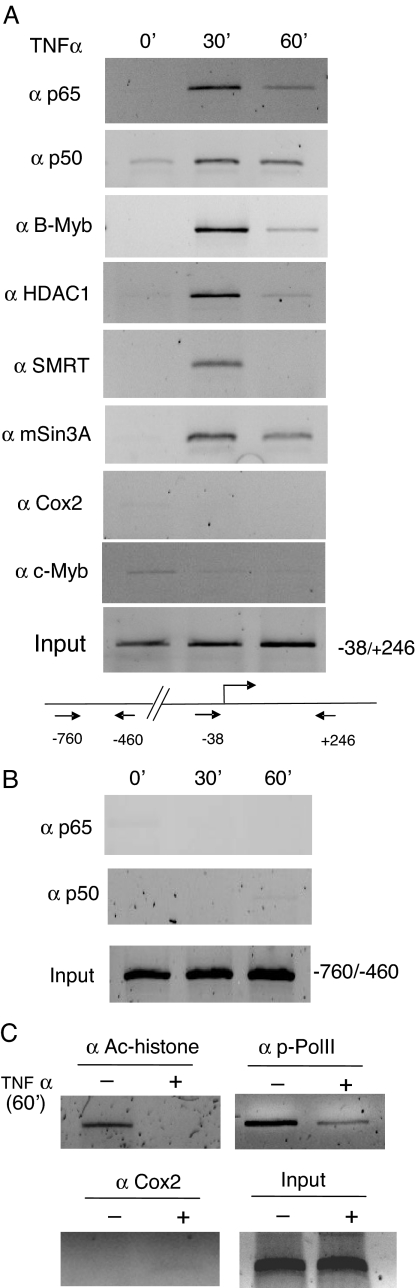
The chromatin area harboring site I and site II was explored for TNFα-regulated specific enrichment of NF-κB and possibly the proteins of the myb family, using chromatin immunoprecipitation (ChIP) (Fig. 6A). Within 30 min of exposing LNCaP cells to TNFα, NF-κB/p65 and NF-κB/p50 had associated with chromatin fragments containing site I and site II, because the immunoprecipitated DNA could be PCR amplified with a primer set specifying −38 to +246 positions. B-myb was also recruited to this region within 30 min. The closely related c-myb did not associate with this region, although c-myb and B-myb can bind to the same DNA elements in vitro. Recruitment of several known components of corepressor complexes, such as the histone deacetylase HDAC1, the transcription silencer SMRT, and the corepressor complex scaffold protein mSin3A, was also observed within 30 min of TNFα treatment. ChIP with unrelated anti-Cox-2 antibody (Fig. 6A), and with a primer set at −760 (forward), −460 (reverse) (Fig. 6B) did not generate any PCR signal. Association of acetylated histones and phospho-RNA polymerase II (two markers of transcription-active chromatin) with the negatively acting chromatin region was also markedly diminished in the presence of TNFα (Fig. 6C). Therefore, we conclude that B-myb and NF-κB p65, p50 are involved in the TNFα-regulated AR gene repression.
Figure 6.
TNFα-Responsive Enrichment of NF-κB, B-myb, HDAC1, SMRT, and mSin3A at the Chromatin Region Containing Site I and Site II of the AR Promoter in LNCaP Cells
Soluble chromatin fragments (average, ∼500 bp) from LNCaP cells treated with TNFα (for 30 min or 60 min), or with vehicle (PBS), were analyzed by ChIP. A, The immunoprecipitated DNAs were amplified with AR-specific PCR primers (forward at −38; reverse at +246). These primers are described in Materials and Methods. B, PCR of the immunoprecipitated DNAs using upstream primers corresponding to the AR promoter from −760 to −460 (negative control). C, ChIP using antibodies to acetylated histones and to phospho-RNA polymerase II. AR-specific primers as in panel A were used in PCR amplification. Anti-Cox2 served as a negative control antibody. Amplified PCR products of 1% input DNAs from cells with or without TNFα treatment are shown. Ac-histone, Acetylated histone; Pol II, polymerase II.
A Stabilizing Role of B-myb for NF-κB Binding to the Negative Element
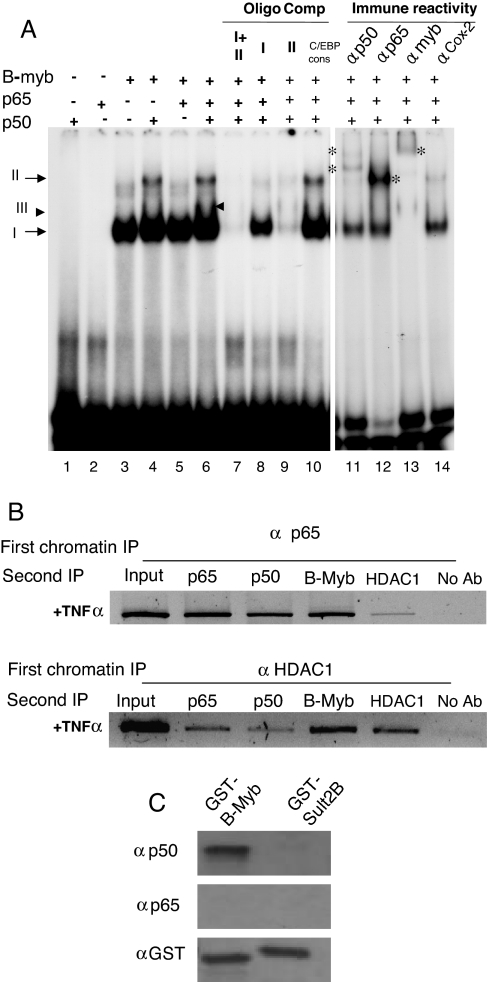
Whether recruitment of NF-κB and B-myb to the AR chromatin reflected direct binding of these transcription factors to 5′-UTR-located site I and site II, respectively, was investigated in EMSA using recombinant p65, p50, and B-myb and the DNA probe spanning both site I and site II (Fig. 7A). Recombinant p65 or p50 by themselves (lanes 1 and 2) or together (data not shown) did not produce any EMSA complex. In DNase I footprinting assay also, the combined presence of p65 and p50 did not reveal any protected region within the site I- and site II-included 5′-UTR (data not shown). B-myb (amino-terminal fragment containing the entire DNA-binding domain) produced a strong gel-shifted band (complex I, lane 3). B-myb together with p50 produced complex I and a second gel-shifted band (complex II, lane 4), indicating that complex II likely contains both p50 and B-myb. In the presence of p65 and B-myb, only complex I was detected (lane 5), indicating that B-myb did not produce a new complex in association with p65. When all three transcription factors were included in EMSA, it appears that a new poorly resolved complex III (in addition to I and II) might have appeared just above complex I (lane 6, arrowhead). Antibody supershift (lanes 11–14) shows that B-myb is part of both complex I and II (lane 13); complex II additionally contained p50 (lane 11; two supershifted bands, noted with asterisks). In the presence of the anti-p65 antibody, a strong band appeared at the complex II position, indicating the recognition of complex II by the p65 antibody (lane 12). Oligonucleotide competition showed specificity of individual EMSA complexes. Unlabeled oligo sequence covering sites I + II (lane 7) competed out all EMSA complexes, whereas only B-myb-containing complex I survived (lane 8) in the presence of unlabeled site I (+144 to 158). The unlabeled site II (+164 to 184) abolished all complexes (lane 9), indicating that p50 and p65 binding to site I is stabilized by the site II-bound B-myb.
Figure 7.
Stabilization of NF-κB Binding to the Negatively Acting Composite Site by the Adjacently Bound B-myb, and Concurrent Occupancy of the Regulatory Factors at the Negative Site in LNCaP Cells
A, Recombinant p65, p50, and B-myb singly, or in combination, were used in EMSA. Binding specificity was verified by competition with unlabeled double-stranded oligos. Identity of individual EMSA complexes was shown by antibody supershifts. Supershifted bands (lanes 11–13) are designated using asterisks. B, Two-step ChIP showing concurrent occupancy of p65, p50, B-Myb, and HDAC1 at the same genomic AR chromatin fragments harboring the negative site. LNCaP cells were treated with TNFα (20 ng/ml) for 30 min and then processed for two-step ChIP as described in Materials and Methods. C, GST-B-myb (NTD) bound to glutathione sepharose beads was mixed with recombinant p50 or p65, and retained proteins were identified (Western blot). GST-Sult2B (a GST fusion of the mouse dehydroepiandrosterone-sulfotransferase isoform Sult2B) is a negative control. Ab, Antibody; IP, immunoprecipitation; Comp, competitor; cons, consensus.
Evidence for the concurrent occupancy of p65, p50, B-myb, and HDAC1 in the same chromatin fragments of the genomic AR was provided by two-step ChIP on TNFα-treated LNCaP cells (Fig. 7B). Only TNFα-treated cells were analyzed, because in the absence of TNFα none of the regulatory factors were present at the negative site. The chromatin fragments immunoprecipitatable by anti-p65 were reimmunoprecipitated by anti-p65 (positive control), as well as by anti-p50 and anti-B-myb (upper panel). Re-ChIP with anti-HDAC1 also pulled down the same fragments, indicating association of HDAC1 with the same region of AR that was enriched for p65, p50, and B-myb. Co-occupancy of HDAC1 with p65, p50, and B-myb is further shown from another series of two-step ChIPs, in which anti-HDAC1 was used as the first antibody for the immunoprecipitation (lower panel). Absent PCR signals in no-antibody lanes confirmed specificity of the results.
B-myb is likely to directly associate with p50 at the TNFα-responsive negative regulatory site, whereas p65 appears to be part of this complex through its interaction with p50, because p50 and B-myb together formed a protein-DNA complex (i.e. complex II; Fig. 7A, lane 4), but no EMSA complex was detected with p65 and B-myb (Fig. 7A, lane 5). Interaction in vitro between B-myb and p50 and lack of an interaction between B-myb and p65 were observed in glutathione-S-transferase (GST) pull-down assay (Fig. 7C). Recombinant p50 bound to GST-B-myb, because immunoblotting detected p50 after its release from the glutathione beads. Absence of B-myb interaction with p65 is shown by the lack of Western blot signal for p65, indicating that p65 was not retained by the glutathione bead-immobilized GST-B-myb.
Interdependence of NF-κB and B-myb in Mediating TNFα-Directed Negative Regulation
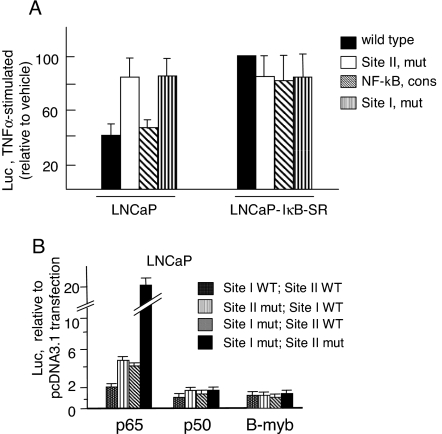
ChIP and EMSA (Figs. 6 and 7) indicated that functional interaction in-cis between NF-κB and B-myb at the negative response region may be a prerequisite for the inhibition of AR expression. Functional assay with the wild-type and mutant AR promoters showed that the requirement is indeed an important controlling feature (Fig. 8). In the natural −110 to +246 AR gene background, point mutations at a single site (site I or site II) led to higher luciferase expression compared with the wild-type promoter in TNFα-treated LNCaP cells. Thus, the repressive effect of activated NF-κB on the wild-type promoter is released upon mutational inactivation of either site I or site II (Fig. 8A). Notably, changing site I to an NF-κB consensus sequence (third bar graph from the left) retained TNFα-induced repression similar to that with the wild-type promoter. In the presence of IκB-SR (LNCaP-IκB-SR cells), TNFα did not repress the AR promoter function and, furthermore, the wild-type and mutant promoters were similarly active (Fig. 8A). Taken together, Fig. 8A shows that site I and site II act cooperatively to negatively regulate the AR gene activity.
Figure 8.
Essential Involvement of NF-κB (Site I) and B-myb (Site II) in the Repression of the AR Promoter
A, AR promoter (−110 to +246)-driven luciferase expression in TNFα-treated parental LNCaP or LNCaP-IκB-SR stable cells, relative to the corresponding cells treated with vehicle (PBS). All values are presented as percent of the luciferase expression from the wild-type promoter in LNCaP-IκB-SR cells, for which the value was set at 100%. The NF-κB consensus indicates replacement of site I with a consensus NF-κB element. Bar graphs indicate averages from three experiments (±sd). B, Negative regulation of the TNFα/NF-κB-responsive region in a heterologous promoter context. Averages of triplicate independent experiments, each performed in duplicate (±sd). Luciferase activity in vector (pcDNA3.1)-transfected cells was set to a value of unity. Mut, Mutated; cons, consensus; WT, wild type.
The combined site I and site II activity also conferred NF-κB-mediated negative regulation to a heterologous promoter (Fig. 8B). The reporter constructs contained two copies each of site I and site II sequences (combined as either both sites wild type or mutated, or mutated at only site I or site II). The heterologous sequences were cloned at the 5′-end of the minimal promoter vector (MCS-TATA-Luc). Upon p65 cotransfection, each single mutant construct caused derepression of luciferase activity by approximately 2-fold relative to the wild-type construct. Mutations at both sites (double mutant construct) led to about 10-fold higher luciferase expression compared with the wild-type construct (Fig. 8B). The results are presented as relative values and compared the luciferase activity of individual constructs in p65-cotransfected vs. pcDNA3.1-cotransfected cells. Because p65 cotransfection induced the vector itself (due to the NF-κB-inducible site in the luciferase cDNA), the construct with the wild-type site I plus site II was also induced (∼2-fold) by p65 compared with pcDNA3.1. Nevertheless, sequence mutation at site I and/or site II was associated with much higher luciferase induction, indicating robust derepression of the negative activity manifested in the wild-type sequence (site I + site II). The mutant constructs did not derepress AR gene activity after cotransfection of p50 and B-myb, either individually (Fig. 8B) or in combination (data not shown). This is expected, because the stimulatory activity of NF-κB requires the p65 transactivation domain.
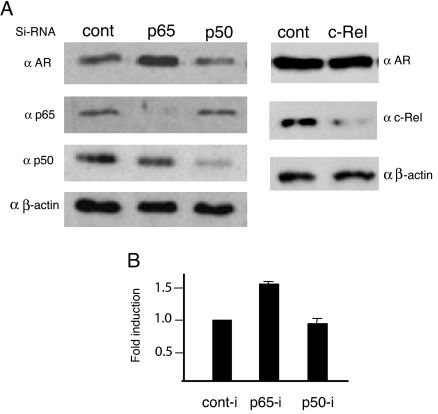
Knock down of p65/NF-κB in LNCaP cells caused approximately 1.5-fold increase in the endogenous AR level, further indicating that AR expression is under negative regulation by the NF-κB/p65 (Fig. 9). The endogenous p65 level in p65-siRNA-treated cells was reduced by 90% or more (Fig. 9A). Knock down of c-Rel did not alter the endogenous AR level, suggesting that c-Rel/NF-κB is not involved in the negative regulation. The endogenous AR level was also not altered by p50 knock down (Fig. 9, A and B), most likely due to the sustained NF-κB activity of the p65/p52 heterodimer complex. Even in unstimulated LNCaP cells, AR expression appears to be under NF-κB-directed repression because 1) activated NF-κB is detectable at a low level in untreated LNCaP cells (complex II, Fig. 4F); and 2) autocrine production of TNFα has been reported in unstimulated LNCaP cells (21).
Figure 9.
Released Repression of Endogenous AR in p65 Knocked Down LNCaP Cells
A, Western blot of AR, p65 and p50 (left), and AR, c-Rel (right). B, Quantification of AR levels in the immunoblots using quanti-software. Bar graphs are averages ± sd from triplicate experiments. cont-i, Control RNA interference.
Due to the presence of multiple positively acting myb-binding sites in the human AR gene, knock down of B-myb led to decreased expression of endogenous AR in LNCaP cells (data not shown). Nevertheless, functional assays in Fig. 8, A and B, clearly show that B-myb is an essential partner of NF-κB in the TNFα-regulated transcription repression of AR. Furthermore, ChIP results (Figs. 6A and 7B) show that B-myb is part of the corepressor complex assembled at the negative site in response to TNFα.
Altered Coregulator Dynamics at the Negative Site in Androgen-Independent C4-2 Cells
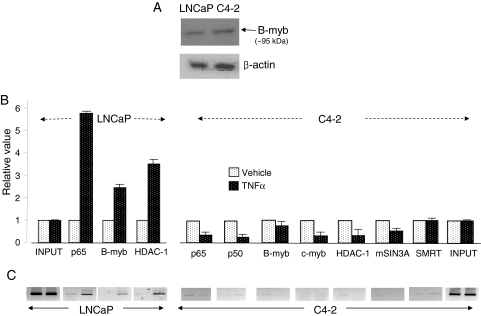
Given that NF-κB interaction with B-myb is an essential aspect for the TNFα-controlled down-regulation of AR, and that NF-κB activity is induced equally well by TNFα in C4-2 and LNCaP cells, we determined how B-myb expression levels and coregulator dynamics at the negative site in the genomic AR would compare between the two cell lines (Fig. 10). Probing of LNCaP and C4-2 cells using Western blot assay shows similar B-myb expression levels (Fig. 10A). B-myb levels also remained the same in the two cell lines after treatment with TNFα (data not shown). On the other hand, real-time quantitative PCR (qPCR) assay of DNAs from ChIP shows that upon TNFα treatment, the negative regulatory site in the C4-2 cells was not enriched for p65, p50, B-myb, and the components of the corepressor complex that were tested (HDAC1, SMRT, mSin3A). The signal for c-myb also remained at the background level (Fig. 10B). The results in C4-2 cells are in contrast to those in LNCaP cells in which TNFα induced robust recruitment of p65, B-myb, and HDAC1 (Fig. 10B, left panel). Similar results are also seen with the semiquantitative analysis of the PCR-amplified DNAs from immunoprecipitated chromatin fragments (Fig. 10C). Therefore, the absent negative regulation of AR in C4-2 cells is due to the inability of the required regulatory factors to occupy the negative regulatory site. Studies are under way to identify the cellular changes that prevent TNFα-induced engagement of relevant regulators at the negative site.
Figure 10.
B-myb Expression and TNFα-Induced Coregulator Dynamics at the Negative Regulatory Site in the AR Gene in LNCaP and C4-2 Cells
A, Western blot of B-myb, using β-actin as the normalization control. B, ChIP in LNCaP and C4-2 cells treated with TNFα (20 ng/ml for 30 min) or vehicle. Real-time qPCR quantified PCR signals. In each case, the value from vehicle-treated cells was set to unity. C, PCR products (285 bp) from the same ChIP analysis as in panel B, visualized as ethidium bromide-stained bands, on agarose gel.
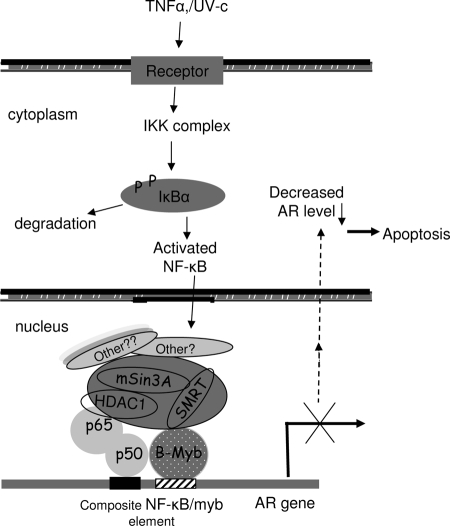
Figure 11 presents a model consistent with the notion that TNFα-induced interaction between NF-κB and B-myb would facilitate corepressor assembly at the negatively responsive composite NF-κB/B-myb element and initiate molecular events leading to AR gene repression.
Figure 11.
Model for the Role of NF-κB and B-myb in the TNFα-Controlled Transcription Repression of AR
Based on our results we propose: 1) NF-κB and B-myb are recruited to the composite element at the AR promoter in response to TNFα; 2) NF-κB binding to the composite site is dependent upon B-myb binding to this site; and 3) B-myb physically associates with p50, not p65; the trimeric B-myb/p50/p65 complex further recruits components of corepressor complex (SMRT, HDAC1, mSin3A, and others) to orchestrate AR gene repression.
DISCUSSION
TNFα is an autocrine and paracrine stimulator of prostate cancer cells in vivo, because prostate tumors, not the surrounding normal tissues, express TNFα (21), and local enrichment of this proinflammatory cytokine at tumor sites is brought about by infiltrating monocytes and macrophages (14,15). The present study shows that the endogenous AR in androgen-dependent LNCaP prostate cancer cells is reduced by TNFα. Additionally, TNFα-regulated repression of AR was observed in nonmalignant RWPE-1 androgen-dependent prostate cells. In contrast, the negative regulation is absent in the LNCaP descendant C4-2 and C4-2B cells, which are androgen independent. Importantly, TNFα inhibited proliferation and induced apoptosis of LNCaP cells but not C4-2 and C4-2B cells. The absent AR down-regulation appears to explain, at least in part, the resistance of androgen-independent prostate cancer cells to the cytokine-induced apoptosis, because AR knock down can trigger apoptosis of both androgen-dependent and androgen-independent prostate cancer cells (7,8,9,10).
Reduced AR expression in TNFα-treated LNCaP cells is due to transcription repression that requires a combined action of activated NF-κB and the B-myb transcription factor at a composite regulatory site in the 5′-UTR of the AR gene. Reduction of AR leads to decreased androgen sensitivity, evident from the inhibition of the androgen-induced PSA expression in LNCaP cells after TNFα treatment. Previously, a posttranscriptional mechanism for the TNFα action to suppress AR was suggested, based on the result that AR mRNA levels in TNFα-treated LNCaP cells declined even after actinomycin D inhibition of transcription (21). Our results unambiguously establish a transcription mechanism for the negative regulation of AR, because 1) TNFα inhibited activity of the transfected AR promoter in reporter assay; 2) TNFα induced recruitment of NF-κB and B-myb to the chromatin region in the genomic AR harboring the negative regulatory site; 3) NF-κB and B-myb specifically bound to cognate DNA sequences within the composite site; and 4) TNFα induced corepressor occupancy at the negative site, evident from the enriched presence of HDAC1, SMRT, and mSin3A in parallel to the recruitment of NFκB and B-myb.
Analysis of the activity of the mutagenized AR promoter showed that a functional interplay between NF-κB and B-myb at the composite regulatory site is essential for AR gene repression. Negative regulation by the composite site was also observed in a heterologous promoter background. EMSA results suggest that B-myb bound to the target regulatory site can stabilize NF-κB binding to the adjacent imperfect NF-κB-binding sequence. Two-step ChIP showed that the same population of genomic AR fragments are enriched for NF-κB, B-myb, and the corepressor components HDAC1, SMRT, and mSin3A in response to TNFα signaling. It appears from the GST pull-down result that B-myb engages in protein-protein interaction with p50 but not p65. Nevertheless, p65 should be an essential component of the negative regulation because two-step ChIP showed concurrent occupancy of p65, p50, and B-myb at the regulated site. Additionally, the endogenous AR level in LNCaP cells was repressed by NF-κB/p65, because the AR level increased after p65 knock down.
Despite the absent AR down-regulation, NF-κB was activated in androgen-independent C4-2 cells by TNFα similarly to LNCaP cells. B-myb expression levels were also similar in these two cell lines. Resistance of androgen-independent prostate cancer cells to the negative regulation of AR manifests a block in transcriptional regulation, because TNFα failed to inhibit the AR promoter activity in C4-2 cells (Fig. 4D). ChIP showed that neither the NF-κB subunits (p65, p50) and B-myb nor the corepressor components HDAC1, SMRT, and mSin3A were detected at the target-responsive region in C4-2 cells after TNFα stimulation. The failure to assemble coregulatory factors along with NF-κB and B-myb in C4-2 cells at the negative site may be due to one or more of the following possibilities: 1) the composite site is altered in C4-2 cells through mutation or altered DNA methylation; 2) posttranslational modification of a critical regulatory factor is altered in C4-2 cells due to changes in a specific intracellular signaling pathway (such as a specific kinase pathway), preventing assembly of required regulatory factors at the negative site; and 3) the expression of one or more yet-to-be identified factor(s) essential for transcription repression is lacking in C4-2 cells. These possibilities are currently under investigation.
The protooncogene product B-myb is an important regulator of G1→ S progression of cell cycle (26). A role for B-myb in prostate cancer progression is likely, because it can transactivate the gene for the antiapoptotic protein clusterin, which confers resistance of prostate cancer cells to apoptosis (27,28). The essential role of B-myb in the NF-κB-directed AR gene repression is reminiscent of the interdependence of N-myc and NF-κB in the TNFα-controlled negative regulation of the glutamate transporter gene (19).
NF-κB targeting of HDAC1, as seen here for the negative regulation of AR, has also been described in several other settings. For example, HDAC1 plays a role in the NF-κB-mediated transcriptional repression of the long-terminal repeat of the HIV genome, causing viral latency (20). HDAC1 appears to interact with NF-κB/p50 on the latent promoter. In another example, negative gene regulation by NF-κB involves direct association of HDAC1 with p65 via the Rel homology domain (29). The corepressor SMRT and the corepressor complex-associated scaffold protein mSin3A were recruited to the negative regulatory region in the AR gene under TNFα stimulation. SMRT (silencing mediator of retinoid and thyroid receptor) was originally identified as a coregulator involved in the transcription repression mediated by unliganded retinoic acid and thyroid hormone receptors (30). The role of SMRT and the related corepressor N-CoR (nuclear receptor corepressor) in transcription repression extends beyond nuclear receptors to other transcription factors (31). Sin3A is a large multidomain protein, which acts as a scaffold upon which other components of the corepressor assembly congregate (32). Transcription repression mediated by the unliganded thyroid hormone receptor as well as by the MAD family of transcription factors was shown to require interaction of these transcription factors with corepressor assembly containing mSin3, N-CoR, and HDAC1 (33,34). We did not detect enrichment of N-CoR at the genomic AR, indicating its absence in the corepressor assembly that plays a role in suppressing the AR gene in prostate cancer cells in response to TNFα-induced inflammation.
It has been reported that reduced AR expression through antisense oligos caused overexpression of the cell cycle-regulatory cyclin-dependent kinase 2 inhibitor p21 (Waf1/Cip1) in prostate cancer cells, and increased p21 expression partially restored androgen dependence of cell growth in androgen-independent prostate cancer cells (35). The androgen-independent cells used in this study were derived from androgen-dependent LNCaP cells that were passaged under chronically androgen-deprived culture conditions. Whether p21 expression would increase in LNCaP cells in association with TNFα-regulated AR repression needs to be assessed.
We may speculate that suppression of AR in androgen-dependent prostate tumors by inflammatory agents would lead to enhanced sensitivity of the cancer cells to apoptotic stimuli. An intriguing recent finding in this regard is that down-regulation of AR expression by the antitumor antibiotic Mithramycin, which inhibits binding of the Sp1 transcription factor to the 5′-UTR in the human AR promoter, reversed the resistance of AR-positive androgen-independent cells to the antiandrogen bicalutamide and increased sensitivity of these cells to the chemotherapeutic drug paclitaxel (36). Detailed knowledge of the role of specific factors and/or converged signal transduction pathways in AR gene repression by TNFα may reveal novel approaches to knock down AR in prostate tumors and increase tumor cell sensitivity to apoptotic stimuli.
MATERIALS AND METHODS
Cells, Culture Conditions, and UV Irradiation
The sources for different cell lines are as follows: LNCaP, RWPE-1 (American Type Culture Collection, Manassas, VA); C4-2, C4-2B (ViroMed Laboratories, Inc., Minnetonka, MN); LNCaP-SR-IκB (37), a gift from S. Maheswaran at Harvard University, Cambridge, MA). LNCaP and LNCaP-SR-IκB were cultured in RPMI; C4-2 and C4-2B were cultured in DMEM, and RWPE-1 were cultured in keratinocyte serum free medium. Media contained penicillin, streptomycin, and 10% fetal bovine serum. For androgen induction of PSA, LNCaP cells were cultured in RPMI containing 5% charcoal-stripped serum for 3 d, and changed to a fresh medium before hormone treatment. LNCaP cells (80% confluence) were irradiated with UV at 40 J/m2 for 1 min. After irradiation, medium was replaced, cells were further incubated (12 h, 37 C, 5% CO2), and cell lysate was analyzed.
Plasmids, Cell Transfections with Plasmids and siRNAs, and Adenovirus Infection
A human AR promoter (−1040 to +560), generated by PCR amplification of the HepG2 cell genomic DNA was sequence confirmed and used to prepare AR reporter constructs by inserting restriction enzyme-digested AR promoter fragments into KpnI/HindIII sites in pGL3b (Promega Corp., Madison, WI). Heterologous constructs were generated by cloning two copies of an oligonucleotide (separated by TTT) covering site I and site II (from +144 to +184) into the MCS-TATA-Luc vector (at the HindIII/EcoRI site). Wild-type or mutant oligos are as follows: 1) wild-type: 5′-ACGCCAGCCCCAGCCCGGCTCCAGCGACAGCCAACGCCTCT; 2) site I (NF-κB) mutant: 5′-ACGCCATCTCCAGTCTGGCTCCAGCGACAGCCAACGCCTCT; 3) site II (Myb) mutant: 5′-ACGCCAGCCCCAGCCCGGCTCCAGCGACAGCCATCGTCTCT; and 4) double mutant (site I + site II): 5′-ACGCCATCTCCAGTCTGGC-TCCAGCGACAGCCATCGTCTCT. The base mutations can inactivate binding of NF-κB or Myb to the cognate site.
Cells were seeded in 24-well plates, cultured overnight, and then transfected with plasmids for 48 h using Fugene6 liposome (Roche Molecular Biochemicals, Indianapolis, IN). Lysates from washed cells were assayed for luciferase activity and protein. Transfection of siRNAs was performed as per vendor recommendation (Invitrogen, Carlsbad, CA). Cells at 105 per well (six-well plate, medium, no antibiotics) were transfected with 20 μm siRNA oligo, using oligofectamine. Culture medium was changed to a fresh medium without serum, oligofectamine-siRNA complexes were added onto cells, and serum was added after 4 h. At 48 h after transfection, harvested cells were processed for Western.
For IκB-SR expression, cells were infected with the adenovirus at 20 multiplicity of infection (moi). After 48 h, cells were treated with TNFα for 12 h. Total cell lysates were used for Western blot. Viral amplification and titer determination were done as per vendor recommendation (Qbiogene, Irvine, CA). Other reagents are as follows: B-myb cDNA (full-length; ATCC); p65 and p50 cDNA plasmids (from Dr. Steve Harris, UTHSCSA, San Antonio, TX; originally from Dr. Albert Baldwin, Chapel Hill, NC). The p65 and p50 cDNAs were cloned in our laboratory into pcDNA 3.1. Adenovirus IκB-SR (and control virus) were gifts from Dr. Santanu Bose.
Chromatin Immunoprecipitation (ChIP), Re-ChIP
ChIP was performed as described elsewhere (38). Cells were seeded onto 10-cm dishes at 1 × 107 cells and incubated in medium with or without TNFα (20 ng/ml) for 30 min or 60 min. Control cells were treated with vehicle for 30 min. After formaldehyde-mediated cross-linking of the cells, chromatin fragments were sheared by sonication to approximately 500-bp size, and solubilized chromatins were incubated at 4 C with approximately 2–5 μl of antibody, followed by incubation with 40 μl protein-A sepharose (Upstate Biotechnology, Inc., Lake Placid, NY). Protein complexes were washed (twice) with low-salt, high-salt, and LiCl solution, and Tris-EDTA buffer (twice). Precipitated complexes were eluted (0.1 m sodium bicarbonate, 1% sodium dodecyl sulfate). A portion of the eluate (90 of 100 μl) was saved for re-ChIP. Eluate (10 μl) was brought to 0.2 m sodium chloride and incubated at 65 C (≤4 h). DNA was extracted by a DNA clean kit (Zymo Research, Inc., Orange, CA). For re-ChIP, 90 μl eluate was diluted 40-fold with ChIP dilution buffer and then incubated first with 2–5 μl of the second-step antibody and then with 40 μl protein-A sepharose. The washed precipitated complex was digested with proteinase K, and DNA was purified as above. DNA (3 μl of 100 μl total) was used for PCR amplification using primers corresponding to 5′-GACCCGACTCGCAAACTGTT at −38 (forward) and 5′-CCTCCGAGTCTTTAGC-AGCT at +246 (reverse).
GST-Pull Down
The N-terminal domain (NTD) of B-myb was fused in frame to the 3′-end of GST cDNA (pGEX-4T1), and GST-B-myb NTD expressed in Escherichia coli (BL21) were purified by glutathione-sepharose affinity chromatography. Recombinant p65 and p50 were commercially obtained [Active Motif (Carlsbad, CA) and Promega Corp., respectively]. GST-B-Myb NTD was incubated first with either p65 or p50 at 4 C for 4 h, and protein complexes were pelleted out after incubation with glutathione-Sepharose beads. Washed beads were boiled with Laemmli’s buffer and pelleted, and the supernatant was analyzed by Western blot using antibodies to p50 or p65 or GST.
DNase I Footprinting, EMSA, and Quantification of mRNAs
A radiolabeled coding strand probe for DNase I footprinting was generated by PCR of the human AR promoter using a 32P-end-labeled forward primer (at +50) and an unlabeled reverse primer from +240 site. Conditions for DNase I footprinting, EMSA, and nuclear extract preparation from rat liver were described elsewhere (39). LNCaP cell nuclear extract was prepared as described (40). Total RNAs (TRIzol extraction) were used to quantify AR and PSA mRNAs by RT-qPCR using SYBR Green PCR reagents kit (Bio-Rad Laboratories, Hercules, CA). Primer pairs are as follows. AR: 5′-TTCACCAATGTCAACTCCAGGA (+2262, forward); 5′-CTTGCACAGAGATGATCTCTG (+2689, reverse). PSA: 5′-TATTTCCAATG-ACGTGTG (+533, forward), 5′TGCACCA-CCTTGGTGTACAGG (+722, reverse). β-actin-sense: 5′-CGTACCACTGGCATCGTGAT-3′; antisense: 5′-GTGTTGGCGTACAGGTCTTT-3′. A single melting curve peak in qPCR assay ensured specificity of the PCR.
MTT Assay and TUNEL Staining
Cells (5000/well) were seeded in 96-well plates and treated with different amounts of TNFα for 5 d. Cells were incubated with Cell Titer96 AQueous One solution reagent (Promega) for 2 h at 37 C, 5% CO2. The blue color was quantified from absorbance at 490 nm using a 96-well plate reader (Victor3, PerkinElmer, Norwalk, CT).
For TUNEL staining, LNCaP and C4-2 cells were seeded onto 60-mm plate (coated with poly-l-lysine, glass-bottom culture dish) at 50% confluence, incubated with vehicle or TNFα for 5 d and stained using vendor-recommended protocol (Dead End Fluorometric TUNEL System protocol; Promega). Briefly, after TNFα (or vehicle) treatment, the cells were washed (PBS) and fixed with 4% methanol-free formaldehyde solution. Cells were then permeabilized by 0.2% Triton X-100 solution. Cells were incubated with recombinant TdT and nucleotide-mixed reagents (Promega) for 1 h at 37 C, after which the cells were washed with PBS and 2× SSC. Cells were then incubated with propidium iodide (PI) solution. Signals from green fluorescent protein (GFP) fluorescence and red fluorescence (PI-emitted) were analyzed under a fluorescence microscope.
Acknowledgments
We thank Dr. Shyamala Maheswaran (Harvard Medical School) for SR-IκB-expressing LNCaP cells; Dr. Steve Harris (UTHSCSA) for p65 and p50 cDNAs; and Dr. Santanu Bose (UTHSCSA) for IκB-SR and GFP control adenovirus. Isidro John De La Cruz provided technical support.
Footnotes
The study was supported by National Institutes of Health Grants AG-19660 and AG-10486 and a Veterans Affairs Merit-Review grant. B.C. is a Veterans Affairs Senior Research Career Scientist.
Present address for L.S.: Department of Veterinary Integrative Biosciences, College of Veterinary Medicine & Biomedical Science, Texas A&M University, College Station, Texas 77843.
Disclosure Statement: B.C. received research support from National Institutes of Health and Department of Veterans Affairs. Other authors have nothing to disclose.
First Published Online November 1, 2007
Abbreviations: AR, Androgen receptor; ChIP, chromatin immunoprecipitation; DNase I, deoxyribonuclease I; GFP, green fluorescent protein; GST, glutathione-S-transferase; HDAC, histone deacetylase; IκB, inhibitory κB; moi, multiplicity of infection; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; N-Cor, nuclear receptor corepressor; NTD, N-terminal domain; PI, propidium iodide; PSA, prostate-specific antigen; qPCR, quantitative PCR; siRNA, small interfering RNA; SMRT, silencing mediator of retinoid and thyroid hormone receptor; SR, superrepressor; TUNEL, terminal deoxynucleotide transferase-mediated dUTP nick end labeling; UTR, untranslated region.
References
- Tomlins SA, Rhodes DR, Perner D, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM 2005 Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648 [DOI] [PubMed] [Google Scholar]
- Litvinov IV, Griend DJ, Antony L, Dalrymple S, Demarzo AM, Drake CG, Isaacs JT 2006 Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci USA 103:15085–15090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioeli D 2005 Signal transduction in prostate cancer progression. Clin Sci 108:293–308 [DOI] [PubMed] [Google Scholar]
- Palvimo JJ, Reinikainen P, Ikonen T, Kallio PJ, Moilanen A, Janne OA 1996 Mutual transcriptional interference between RelA and androgen receptor. J Biol Chem 271:24151–24156 [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL 2004 Molecular determinants of resistance to antiandrogen therapy. Nat Med 10:33–39 [DOI] [PubMed] [Google Scholar]
- Grad JM, Dai JL, Wu S, Burnstein KL 1999 Multiple androgen response elements and a Myc consensus site in the androgen receptor (AR) coding region are involved in androgen-mediated up-regulation of AR messenger RNA. Mol Endocrinol 13:1896–1911 [DOI] [PubMed] [Google Scholar]
- Eder IE, Culig Z, Ramoner R, Thurnher M, Putz T, Messler-Menardi C, Tiefenthaler M, Barsch G, Klocker H 2000 Inhibition of LNCaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Ther 7:997–1007 [DOI] [PubMed] [Google Scholar]
- Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ 2002 Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res 62:1008–1013 [PubMed] [Google Scholar]
- Liao X, Tang S, Thrasher JB, Griebling TL, Li B 2005 Small interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther 4:505–515 [DOI] [PubMed] [Google Scholar]
- Zou JX, Zhong Z, Shi XB, Tepper CG, DeVere White RW, Kung HJ, Chen H 2006 ACTR/AIBI/SRC-3 and androgen receptor control prostate cancer cell proliferation and tumor growth through direct control of cell cycle genes. Prostate 66:1474–1486 [DOI] [PubMed] [Google Scholar]
- Linja MJ, Savinainen KJ, Saramäki OR, Tammela TLJ, Vessella RL, Visakorpi T 2001 Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 61:3550–3555 [PubMed] [Google Scholar]
- Ford III O, Gregory CW, Kim D, Smitherman AB, Mohler JL 2003 Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J Urol 170:1817–1821 [DOI] [PubMed] [Google Scholar]
- Mantovani A 2005 Inflammation by remote control. Nature 435:752–753 [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG 2007 Inflammation in prostate carcinogenesis. Nat Rev Cancer 7:256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Baek SH, Bourk EM, Ohgi KA, G-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW 2006 Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124:615–629 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S 2004 Signaling to NF-κB. Genes Dev 18:2195–2224 [DOI] [PubMed] [Google Scholar]
- Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK 1995 Nuclear factor κ B functions as a negative regulator for the rat androgen receptor gene and NF-κB activity increases during the age-dependent desensitization of the liver. J Biol Chem 270:837–842 [DOI] [PubMed] [Google Scholar]
- Zerbini LF, Wang Y, Czibere A, Correa RG, Cho J, Ijiri K, Wei W, Joseph M, Gu X, Grall F, Goldring MB, Zhou, J-R, Libermann TA 2004 NF-κB mediated repression of growth arrest- and DNA-damage-inducible proteins 45α and γ is essential for cancer cell survival. Proc Natl Acad Sci USA 101:13618–13623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS 2005 Positive and negative regulation of EAAT2 by NF-κB: a role for N-myc in TNF-α controlled repression. EMBO J 24:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC 2006 NF-κB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J 25:139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A, Gotoh A, Yamada H, Keller ET, Matsumoto T 2000 Tumor necrosis factor-α represses androgen sensitivity in the LNCaP prostate cancer cell line. J Urol 164:800–805 [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Mayo MW, Baldwin AS 1996 TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 274:784–787 [DOI] [PubMed] [Google Scholar]
- Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW 1994 Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer 57:406–412 [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang S, Ozen M, Pathak S, Chung LWK 2000 LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate 44:91–103 [DOI] [PubMed] [Google Scholar]
- Chopra DP, Menard RE, Januszewski J, Mattingly RR 2004 TNF-α-mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett 203:145–154 [DOI] [PubMed] [Google Scholar]
- Lin D, Fiscella M, O’Connor PM, Jackman J, Chen M, Luo LL, Sala A, Travali S, Appella E, Mercer WE 1994 Constitutive expression of B-myb can bypass p53-induced Waf1/Cip1-mediated G1 arrest. Proc Natl Acad Sci USA 91:10079–10083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellera M, Raschella G, Santilli G, Tanno B, Ventura A, Mancini, C, Sevignani C, Calabretta B, Sala A 2000 Direct transactivation of the anti-apoptotic gene apolipoprotein J (clusterin) by B-MYB. J Biol Chem 275:21055–21060 [DOI] [PubMed] [Google Scholar]
- Miyake H, Hara I, Kamidono S, Gleave ME, Ito H 2003 Resistance to cytotoxic chemotherapy-induced apoptosis in human prostate cancer cells is associated with intracellular clusterin expression. Oncol Rep 10:469–473 [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS 2001 The p65 (RelA) subunit of NF-κB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol 21:7065–7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Evans RM 1995 A transcriptional corepressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lozach J, Jepsen K, Sawka-Verhelle D, Perissi V, Sasik R, Rose, DW, Johnson RS, Rosenfeld MG, Glass CK 2004 A nuclear receptor corepressor transcriptional checkpoint controlling activator protein 1-dependent gene networks required for macrophage activation. Proc Natl Acad Sci USA 101:14461–14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Eisenman RN 1999 Sin meets NuRD and other tails of repression. Cell 9:447–450 [DOI] [PubMed] [Google Scholar]
- Alland L, Muhle R, Hou H, Potes J, Chin L, Schreiber-Agus N, Depinho RA 1997 Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen T-M, Söderström M, Laherty CD, Torchia J, Yang W-M, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG 1997 A complex containing NCoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43–48 [DOI] [PubMed] [Google Scholar]
- Wang LG, Ossowski L, Ferrari AC 2001 Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res 61:7544–7551 [PubMed] [Google Scholar]
- Wang LG, Ferrari AC 2006 Mithramycin targets Sp1 and the androgen receptor transcription level—potential therapeutic role in advanced prostate cancer. Translational Oncogenomics 2:19–31 [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Segev DL, Gupta V, Kawakubo H, Yeo G, Donahoe PK, Maheswaran S 2006 Mullerian inhibiting substance regulates androgen-induced gene expression and growth in prostate cancer cells through a nuclear factor-κB-dependent Smad-independent mechanism. Mol Endocrinol 20:2382–2391 [DOI] [PubMed] [Google Scholar]
- Song CS, Echchgadda I, Seo Y-K, Kim S, Kim S-A, Cho S, Shi L, Chatterjee B 2006 An essential role of the CAAT/enhancer binding protein-α in the vitamin D-induced expression of the human steroid/bile acid-sulfotransferase (SULT2A1). Mol Endocrinol 20:795–808 [DOI] [PubMed] [Google Scholar]
- Chatterjee B, Echchgadda I, Song S 2005 Vitamin D receptor regulation of the steroid/bile acid sulfotransferase SULT2A1. “Phage II: conjugation enzymes, glutathione transferases and transportsystems.” Methods Enzymol 400:165–191 [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebowitz RM, Roeder RG 1983 Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]