Abstract
Estrogen receptors (ERs) regulate gene transcription by interacting with regulatory elements. Most information regarding how ER activates genes has come from studies using a small set of target genes or simple consensus sequences such as estrogen response element, activator protein 1, and Sp1 elements. However, these elements cannot explain the differences in gene regulation patterns and clinical effects observed with estradiol (E2) and selective estrogen receptor modulators. To obtain a greater understanding of how E2 and selective estrogen receptor modulators differentially regulate genes, it is necessary to investigate their action on a more comprehensive set of native regulatory elements derived from ER target genes. Here we used chromatin immunoprecipitation-cloning and sequencing to isolate 173 regulatory elements associated with ERα. Most elements were found in the introns (38%) and regions greater than 10 kb upstream of the transcription initiation site (38%); 24% of the elements were found in the proximal promoter region (<10 kb). Only 11% of the elements contained a classical estrogen response element; 23% of the elements did not have any known response elements, including one derived from the naked cuticle homolog gene, which was associated with the recruitment of p160 coactivators. Transfection studies found that 80% of the 173 elements were regulated by E2, raloxifene, or tamoxifen with ERα or ERβ. Tamoxifen was more effective than raloxifene at activating the elements with ERα, whereas raloxifene was superior with ERβ. Our findings demonstrate that E2, tamoxifen, and raloxifene differentially regulate native ER-regulatory elements isolated by chromatin immunoprecipitation with ERα and ERβ.
DRUGS THAT INTERACT with estrogen receptors (ERs) are commonly used to treat numerous reproductive conditions. Estrogens are mainly used for contraception and to treat a variety of menopausal symptoms, such as hot flashes and urogenital atrophy. However, the use of estrogens for menopausal symptoms has been severely curtailed after the results of the Women’s Health Initiative trial found that the risks of hormone therapy exceed the benefits (1). The selective estrogen receptor modulators (SERMs) differ from estrogens in that they exhibit both agonistic and antagonistic properties. Tamoxifen is the prototypic SERM that acts as an antagonist in the breast and an agonist in bone and uterus (2). Therefore, tamoxifen is used to treat and prevent ER-positive breast tumors (3). Raloxifene behaves similarly to tamoxifen in the breast and bone (4,5) but does not elicit an agonistic action in the uterus (6). Raloxifene is hence approved for the prevention and treatment of osteoporosis. Although the SERMs have some exciting properties, a major limitation is that they do not prevent hot flashes. Furthermore, the results of clinical trials with SERMs are showing that both drugs can produce serious adverse effects (7). These observations clearly indicate that it is essential to develop a new generation of safer drugs that target ERs. To achieve this goal, a greater understanding of how estrogens and SERMs regulate genes that mediate their beneficial and adverse effects is needed.
The execution of the biological effects of estrogens and SERMs is mediated by two ERs: ERα and ERβ. Once the ligand binds to these receptors, it induces a conformational change (8) that allows the receptor to interact with a regulatory element in target genes. Whereas the estrogen response element (ERE) is considered to be the major regulatory element in genes regulated by ERs (9), alternative elements, such as activator protein 1 (AP-1) (10) and Sp1 (11), are required for estradiol (E2) and SERMs to regulate the full spectrum of genes. When an estrogen-bound ER associates with a regulatory element, it recruits distinct classes of proteins, including p160s coactivators, mediator complex proteins, and cAMP response element-binding protein (CREB)-binding protein/p300, in a sequential and cyclical manner to activate gene transcription (12,13,14). In contrast, SERMs act as antagonists by recruiting corepressor proteins, such as nuclear receptor corepressor, that block the expression of the target gene (15). The mechanism of the agonist action that leads to transcriptional activation by SERMs is poorly understood but is thought to be mediated via the recruitment of coactivators to the activation function (AF)-1 domain of ERs (16,17).
Although the ERE and alternative elements are important mediators of ER regulation of genes, the pharmacology of E2 and SERMs on target genes cannot be understood solely by the known regulatory elements (18,19,20). For example, it is not yet known how E2 distinctly regulates genes with ERα and ERβ (18,21,22) and how some genes regulated by tamoxifen are different from those regulated by raloxifene (18,23). These observations indicate that in addition to the known regulatory elements, other types of elements exist in ER target genes. Identifying new regulatory elements in ER target genes is a critical step in the elucidation of how estrogens and SERMs induce the therapeutic and adverse effects observed in clinical trials. Previously, the standard way to identify ER-regulatory elements was to clone the promoter region and map the element with a series of deletion and point mutants upstream of a reporter gene. Recently, ER binding sites were identified by using a combination of chromatin immunoprecipitation (ChIP) and tiling arrays that contained chromosomes 21 and 22 (24) or the whole genome (25). ChIP also has been combined with sequencing to identify ER elements in MCF-7 cells (26). Here, we used a chromatin immunoprecipitation-cloning and sequencing (ChIP-CS) strategy to isolate genome-wide ER response elements from target genes in U2OS bone cells. Our goal was to assess the functional properties of a large set of ER-binding elements in response to different ligands. We isolated and tested 173 ER-regulatory elements for regulation by E2, raloxifene, and tamoxifen with both ERα and ERβ.
RESULTS
ChIP-Cloning and Sequencing Identifies ERα-Binding Elements
U2OS cells that express a stably transfected FLAG-ERα were treated for 2 h with E2 and then were cross-linked with formaldehyde. After shearing the chromatin, the ERα-bound DNA fragments were isolated using an antibody against the FLAG epitope. DNA fragments were then further purified with an antibody against ERα. Isolated DNA fragments were cloned into plasmids, and 192 were randomly selected and sequenced. Bioinformatics was used to match the sequences in the ChIP-CS library to genes by performing a BLAST search against the human genome. Of the clones sequenced, 173 contained inserts, which ranged in size from 200–500 bp. The chromosomal identifier and sequence of the 173 elements is shown in supplemental Table 1 published as supplemental data on The Endocrine Society’s Journals Online website at http://mend.endojournals.org. The location and name of each element relative to the nearest gene is shown in Tables 1 and 2.
Table 1.
ERα Binding Sites Located in Introns of Target Genes
| Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 | Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DnaJ (Hsp40) homolog, subfamily C, member 6 | 19284 | AB007942 | Adaptor-related protein complex 3 δ 1 subunit | 2933 | AC005545 | ||||||||||
| Protein tyrosine phosphatase, receptor type, Ma | 376229 | BC040543 | Astrotactin | 133783 | AB006627 | ||||||||||
| Cytoplasmic polyadenylation element bp 4 | 29026 | AB051460 | NY-REN-41 antigen | 1650 | AF155108 | + | |||||||||
| Similar to keratin, type I cytoskeletal 18 | 3550 | XM_497554 | + | Lipase, hepatica | 21702 | AF037404 | + | + | + | ||||||
| Protocadherin 11 X-linked | 348316 | AB026187 | + | + | + | + | + | FLJ42117 protein | 43282 | AK124111 | + | ||||
| Sema domain, seven thrombospondin repeats | 217329 | AC004615 | Fibroblast growth factor 12 | 11780 | AK125307 | + | |||||||||
| Solute carrier family 37 member 2 | 435 | AK074100 | + | Chromosome 10 open reading frame 112 | 226328 | AL590378 | |||||||||
| Phosphodiesterase 4Ba | 143115 | BC036108 | + | Putative nuclear protein ORF1-FL49 | 17774 | AJ245877 | + | + | + | ||||||
| CUB and Sushi multiple domains 1 | 779937 | AA889055 | + | Glutamate receptor, ionotropic, kainate 4 | 19998 | S67803 | |||||||||
| Calcium channel L type, α 1B subunit | 74357 | M94172 | + | Inhibitor of κ light polypeptide gene enhancer | 5571 | AF062089 | + | ||||||||
| RNA-binding motif single-stranded interacting protein | 3451 | AF023259 | + | + | + | + | Chromosome 14 open reading frame 171 | 31955 | AC007375 | ||||||
| Amyotrophic lateral sclerosis 2 | 346514 | AB053321 | + | Protein tyrosine phosphatase receptor type f | 43512 | BC034046 | + | ||||||||
| Cat eye syndrome chromosome region candidate 6 | 10 | AF307451 | + | + | + | Chromosome 20 open reading frame 44 | 12409 | AF173893 | |||||||
| Solute carrier family 19 member 1 | 9602 | AF004354 | + | + | Hypothetical protein LOC285692 | 586 | BC031253 | ||||||||
| TNF receptor member 19 | 34535 | BC047321 | Adenosine deaminase, RNA-specific, B1 | 29950 | AF001042 | + | |||||||||
| Ryanodine receptor 3 | 75898 | AB001025 | + | Plasminogen-like | 1645 | AK124365 | + | + | |||||||
| Hypothetical protein FLJ30707 | 8470 | AK055269 | + | Similar to ankyrin repeat domain 30A | 29389 | XM_291770 | + | + | |||||||
| l(3)mbt-like 2 | 3653 | AJ305226 | + | + | Rab6-interacting protein 2 | 68743 | BC037377 | ||||||||
| Glutamate receptor, ionotropic, kainate 2 | 142077 | AJ252246 | + | Guanine nucleotide- binding protein γ 7 | 51326 | AB010414 | + | ||||||||
| ATP-binding cassette, subfamily A member 8 | 78 | AB020629 | p21- Activated kinase 7 | 2122 | AB033090 | + | + | ||||||||
| Hemicentin | 217203 | AF156100 | + | Spectrin repeat containing, nuclear envelope 2 | 9450 | AB023228 | + | + | |||||||
| Family with sequence similarity 13 member C1 | 43164 | BC036453 | Lethal giant larve homolog 2 | 1991 | AK025401 | + | |||||||||
| EMI domain containing 2 | 6846 | AJ416091 | + | + | ATP synthase, H+ transporting | 1856 | AF088071 | + | |||||||
| Zinc and ring finger 3 | 20847 | AB051436 | + | Chromosome 2 open reading frame 13 | 28872 | BC030711 | + | + | |||||||
| Similar to CG7467-PA | 6095 | XR_000268 | Similar to hypothetical protein DKFZp586O0120.1 | 39692 | XM_496490 | + | + | + | |||||||
| Glycogen synthase kinase 3β | 71512 | BC000251 | + | START domain containing 9 | 68175 | AB037721 | + | + | + | ||||||
| γ-Aminobutyric acid receptor ρ 2 | 15479 | M86868 | + | KIAA1944 protein | 293645 | AB061814 | + | ||||||||
| Hypothetical protein FLJ22222 | 10514 | AK025875 | + | Spermatogenesis-associated 13 | 10701 | AK055770 | |||||||||
| Ankyrin repeat and SOCS box-containing 17 | 4339 | AF403035 | + | + | + | Hypothetical protein FLJ22301 | 1798 | AK025954 | + | + | |||||
| Naked cuticule homolog NKD | 7659 | AB062886 | + | Chromosome 10 open reading frame 11 | 364330 | AF267860 | + | ||||||||
| Similar to IFIT1; Interferon, α-inducible protein | 3368 | XM_497244 | + | Calcium channel voltage-dependent γ | 13500 | AF096322 | + | ||||||||
| RAB31, member RAS oncogene familya | 47283 | AF183421 | + | Similar to ribosome biogenesis protein BMS1 homolog | 14205 | XM_210011 | + | ||||||||
| Phosphodiesterase 8B | 434 | AB085824 | + | + | |||||||||||
| Total transcription factor sites | 9 | 24 | 13 | 19 | 12 |
The nearest gene associated with the ERα−binding element from the ChIP library was identified by the human genome. The presence of ERE, AP-1, Sp1, NFκB, and FOXA1-binding sites in clones were determined by bioinformatics using programs described in Materials and Methods. The accession numbers were obtained from the National Center for Biotechnology Information database.
Previously reported estrogen-regulated gene.
Table 2.
ERα Binding Sites Located in the Upstream Region of Target Genes
| Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 | Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tRNA alanine | 80 | Gene ID: 4553 | + | Receptor-interacting factor 1 | 23245 | AK001826 | + | ||||||||
| Single-stranded DNA binding protein 2a | 103 | AA013030 | + | + | Chemokine orphan receptor 1a | 26349 | AF030297 | ||||||||
| U2 small nuclear ribonucleoprotein A | 112 | AC002056 | + | + | Myeloid/lymphoid or mixed-lineage leukemia | 26492 | AK057058 | + | + | ||||||
| NADH dehydrogenase 6 | 154 and 884 | AY063322 | NYD-SP12 protein | 26758 | AF345909 | ||||||||||
| tRNA serine 1 | 266 | Gene ID:4574 | + | Family with sequence similarity 34, member A | 27290 | BC034621 | + | ||||||||
| Calbindin 2, 29 kDaa | 345 | BC015484 | + | Phosphodiesterase 4D interacting protein | 29581 | AB007923 | |||||||||
| Hypothetical protein FLJ35827 | 347 | AK093146 | + | + | + | Similar to Ig κ chain V region | 30659 and 49654 | XM_372941 | + | + | + | ||||
| Similar to olfactory receptor, family 7, subfamily A, 17 | 952 | XM_497067 | + | Similar to ADP,ATP carrier protein | 36373 | XM_498140 | + | + | |||||||
| THAP domain containing 8 | 965 | BC072416 | + | Hypothetical gene supported by BC040860 | 38885 | XM_498698 | + | + | |||||||
| Retinal outer segment membrane protein 1 | 1020 | BC008100 | + | + | + | Hypothetical protein LOC389072 | 40610 | BC020812 | |||||||
| Olfactory receptor, family 7 | 1121 | AC064843 | + | LOC441580 | 40920 | XM_499193 | + | + | + | + | + | ||||
| Killer cell lectin-like receptor subfamily C,3a | 1484 and 3100 | AF027164 | + | + | Cytidine monophosphate | 41193 | AF271388 | + | |||||||
| Neuronal PAS domain protein 2 | 1328 | AK026791 | + | Similar to ATP-dependent DNA helicase II | 41928 | XM_372262 | + | + | |||||||
| Snf2-related CBP activator protein | 1551 | AB002307 | + | SNAP25-interacting protein | 44729 | AB051471 | + | ||||||||
| Hypothetical LOC401038 | 1940 | XM_379163 | + | Similar to eukaryotic translation elongation factor 1 | 48771 | XM_497272 | + | ||||||||
| ATPase, Ca2+ transporting, ubiquitous | 2147 | AF458229 | Similar to putative | 50229 | XM_210400 | + | + | ||||||||
| FLJ46688 protein | 2759 | AK128530 | + | Tankyrase TRF1-interacting ankyrin | 51841 | AF082556 | + | + | + | ||||||
| KIAA0240 | 3021 | AL833540 | + | + | + | FAT tumor suppressor homolog 1a | 52081 | D88798 | |||||||
| Solute carrier family 4 | 3590 | AB018282 | Hypothetical protein MGC26143 | 52535 | BC014590 | ||||||||||
| Hypothetical gene supported by BC031617 | 3808 | BC031617 | + | Olfactory receptor family 4 subfamily C | 52688 | AC126345 | + | ||||||||
| fem-1 homolog b | 3952 | AB007856 | + | Similar to 33-kDa protein | 55690 | XM_498064 | + | ||||||||
| Oligodendrocyte lineage transcription factor 2 | 4109 | AF221520 | + | Likely ortholog of mouse limb-bud and heart gene | 56452 | AF110224 | |||||||||
| Polycystic kidney and hepatic disease 1 | 4442 | AF480064 | + | + | + | + | Ubiquinol-cytochrome c reductase | 57627 | AC007786 | + | + | + | |||
| Complement component 1 | 4508 | BC008983 | + | Glutamate receptor ionotropic, δ 1 | 61700 | AB033046 | |||||||||
| Similar to zinc finger protein 92 | 4551 | XM_066859 | + | + | Hypothetical gene supported by AK094765 | 63164 | XM_498861 | + | + | ||||||
| Similar to salivary proline-rich protein | 4645 | XM_497733 | + | + | + | Solute carrier family 8 member 1 | 65007 | AC007281 | + | + | |||||
| Similar to nudix type motif 4 isoform β | 4749 | BT020109 | + | Similar to FLJ10378 protein isoform 2 | 67385 | Gene ID:442109 | + | ||||||||
(Table continues)
Many Elements Do Not Contain an ERE or Other Known ER-Regulatory Elements
We found that 38% of the regulatory elements were located in introns (Table 1); 24% of the regulatory elements were found less than 10 kb upstream region of the transcription start site (TSS), whereas the remaining 38% of the elements were located beyond 10 kb of the TSS (Table 2). A histogram shows the distance of the elements from the TSS (Fig. 1). To explore whether our ChIP-CS library contains authentic regulatory elements, we searched ER-responsive gene databases as well as our microarray data (18) for known ER target genes. We found that 10% of the genes in our ChIP-CS library were reported to be activated by E2 or SERMs (Tables 1 and 2).
Table 2A.
Continued
| Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 | Gene Description | Location | Accession No. | ERE | AP-1 | SP1 | NFκB | FOXA1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Similar to seven transmembrane helix receptor | 5219 | XM_499490 | + | + | Inter-α inhibitor H5 | 70933 | AB075833 | + | + | + | |||||
| Similar to DEAD box polypeptide 10 | 5508 | GeneID:401533 | Similar to SPCPB16A4.07c | 81779 | XM_498163 | + | + | ||||||||
| cAMP responsive element binding protein 3-like 2 | 5741 | AJ549092 | + | + | Forkhead box L1 | 83326 | AF315075 | + | |||||||
| Sorting nexin family member 27 | 6195 | AB007957 | + | + | + | Hypothetical protein FLJ10305 | 89737 | AK001167 | + | ||||||
| H19, imprinted maternally untranslated mRNAa | 6488 | AC004556 | + | Ribosomal protein L18 pseudogene 1 | 90890 | AL512359 | + | ||||||||
| Ig superfamily receptor | 6490 | AF329490 | + | Similar to Ig heavy-chain-2 light-chain | 95986 | XM_372543 | + | ||||||||
| Hornerin | 7029 | BR000036 | + | + | SH2 domain containing 4B | 98724 | NM_207372 | + | |||||||
| Similar to TFIIH basal transcription factor | 7206 | GeneID:342784 | + | + | Hypothetical protein LOC283432 | 120703 | BC037211 | + | |||||||
| Transducin-like enhancer of split 2 | 7496 | BC017364 | + | Eukaryotic translation elongation factor 1 ε 1 | 122468 | AB011079 | + | ||||||||
| Progesterone receptora | 8931 | AF016381 | + | Hypothetical LOC285307 | 123535 | XM_211837 | + | ||||||||
| Hypothetical protein FLJ90586 | 9024 | AK075067 | + | Family with sequence similarity 10, member A3 | 149575 | NG_004762 | |||||||||
| Ubiquitin-conjugating enzyme E2N | 9084 | AK098233 | Peptidylglycine α-amidating monooxygenasea | 150061 | AB095007 | + | |||||||||
| Methylcrotonoyl-Coenzyme A carboxylase 1 | 9317 | BC004187 | + | + | + | Hypothetical protein FLJ13197 | 160477 | AK023259 | + | + | |||||
| Similar to ribosome biogenesis protein BMS1 homolog | 11530 | XM_372108 | + | Glycine receptor, α 1 | 187921 | BC074980 | + | + | |||||||
| Syntaxin-binding protein 6 | 12475 | AF161505 | + | + | + | 7SL cytoplasmic, pseudogene 2 | 196320 | NG_002426 | |||||||
| Discoidin, CUB and LCCL domain containing 2a | 12949 | AB073146 | + | + | Similar to Calcyclin binding protein | 204123 | AK093425 | ||||||||
| Adrenergic, β, receptor kinase 2 | 13589 | AK055687 | + | + | + | Similar to Rpl7a protein | 205123 | XM_498041 | + | ||||||
| Polymerase II polypeptide D | 14534 | BC002958 | Cadherin 18 type 2 | 246876 | BC031051 | + | + | ||||||||
| Platelet-activating factor receptora | 14597 | AY275466 | + | Similar to RIKEN cDNA 5730421E18 gene | 298531 | AK094007 | + | ||||||||
| Similar to galectin-related interfiber protein | 15092 | XM_499395 | + | + | Neuron navigator 3a | 307930 | AB023155 | ||||||||
| U56B small nuclear | 17308 | AC005994 | Roundabout, axon guidance receptor | 312713 | AB046788 | + | + | ||||||||
| Coiled-coil domain containing 5 | 18850 | AK097403 | + | + | CUB and Sushi multiple domains 3 | 357892 | AB067481 | ||||||||
| UDP glycosyltransferase 2 family polypeptide | 19256 | AF179879 | + | + | Ribosomal protein L10-like | 542861 | AB063608 | + | + | + | |||||
| FLJ45721 protein | 20310 | AK127623 | + | Similar to chromosome 9 ORF 140 | 579108 | BC048267 | + | + | |||||||
| ρ Guanine nucleotide exchange factor 3 | 21857 | AF249744 | + | + | Hypothetical LOC389202 | 606000 | XM_371690 | ||||||||
| COMM domain containing 3 | 22486 | AY542159 | |||||||||||||
| Total transcription factor sites | 10 | 47 | 18 | 38 | 29 | ||||||||||
The nearest gene associated with the ERα−binding element from the ChIP library was identified by the human genome. The presence of ERE, AP-1, Sp1, NFκB, and FOX1A binding sites in clones were determined by bioinformatics using programs described in Materials and Methods. The accession numbers were obtained from the National Center for Biotechnology Information database.
Previously reported estrogen-regulated gene.
Figure 1.
Distances of Fragments from the Nearest Gene in the ChIP-CS Library Are Widely Distributed
Distances of each fragment to the nearest TSS (Tables 1 and 2) were plotted on a histogram. The y-axis represents the number of the clones, whereas the x-axis represents the distance of the clone to the nearest TSS.
Many Elements Do Not Contain an ERE or other Known ER-Regulatory Elements
We searched multiple databases, including TRANSFAC and Dragon ERE finder, to identify potential regulatory elements in the ChIP-CS library. Only 11% of the inserts contained classical EREs (Tables 1 and 2). AP-1, Sp1, FOXA1, or nuclear factor-κB (NFκB) sites were present in 41%, 18%, 24%, and 33% of the inserts, respectively (Tables 1 and 2). Surprisingly, 23% of the inserts did not have an ERE or one of the other alternative elements. These data support our hypothesis that additional types of regulatory elements exist in ER target genes.
E2 Recruits ERα and p160 Coactivators to Elements Derived from the ChIP Library in U2OS-ERα Cells
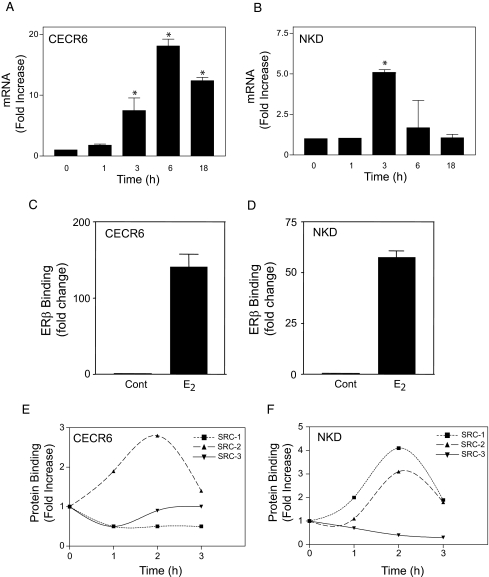
We selected elements from six genes to determine whether ERα binds to those elements in the native genes in U2OS-ERα cells. In these cells, the native ERα is driven by a tetracycline-inducible CMV promoter that allows for titration of the ERs to approximate physiological levels (18). ChIP shows that E2 recruited ERα to the cat eye syndrome chromosome region candidate 6 (CECR6), spermatogenesis associated 13 (SPATA13), naked cuticule homolog (NKD), lethal giant larvae homolog 2, (LLGL2), H19, imprinted maternally expressed untranslated mRNA (H19), and killer cell lectin-like receptor subfamily C (NKG2) genes (Fig. 2). E2 activates genes by recruiting p160 coactivators (14,27), steroid receptor coactivator (SRC)1, SRC2 (transcriptional intermediary factor 2, glucocorticoid receptor interacting protein 1, and nuclear receptor coactivator 2), and SRC3 (pCIP, receptor-associated coactivator 3, amplified in breast cancer 1, and activator of thyroid and retinoic acid receptor) to the AF-2 surface of ER (28,29,30). Using ChIP, we examined whether E2 recruits p160 coactivators to the six genes. E2 produced a time-dependent recruitment of p160 coactivators to the six genes with maximal recruitment occurring at 1–2 h (Fig. 3). However, distinct coactivators were recruited to the genes. All three coactivators were recruited to the CECR6 (Fig. 3A) and H19 (Fig. 3E) genes, whereas SRC-2 and SRC-3 were recruited to NKD (Fig. 3C) and NKG2 (Fig. 3F) genes. SRC-1 and SRC-2 were recruited to LLGL2 (Fig. 3D), whereas only SRC-3 was recruited to the SPATA13 (Fig. 3B) gene.
Figure 2.
ERα Interacts with Elements from the ChIP-CS Library
Tetracycline-inducible U2OS-ERα cells were treated with 10 nm E2 for 3 h, and ChIP was performed using antibodies to ERα. Immunoprecipitated chromatin fragments were PCR amplified and quantitated by real-time PCR using primers for the CECR6, SPATA13, NKD, LLGL2, H19, and NKG2 elements. Cont, Control.
Figure 3.
E2 Recruits p160 Coactivators to Genes from the ChIP-CS Library
Tetracycline-inducible U2OS-ERα cells were treated with 10 nm E2 for increasing times, and ChIP was performed using antibodies to the p160 coactivators, SRC-1, SRC-2, or SRC-3. Immunoprecipitated chromatin fragments were PCR amplified and quantitated by real-time PCR using primers for the CECR6, SPATA13, NKD, LLGL2, H19, and NKG2 elements (panels A–F, respectively).
E2 Activates and Recruits ERβ and p160 Coactivators to the CECR6 and NKD Genes in U2OS-ERβ Cells
The regulation of CECR6 and NKD genes by E2 was also examined in the U2OS-ERβ cells. U2OS-ERβ cells were treated for increasing time with E2, and the levels of CECR6 and NKD mRNA were measured by real-time PCR. E2 produced a maximal activation of the CECR6 gene in 6 h and at 3 h with the NKD gene (Fig. 4, A and B). The activation of the genes was associated with the recruitment of ERβ to the genes (Fig. 4, C and D). In contrast to the ERα cells, ChIP found that only SRC-2 was recruited by E2 to the CECR6 (Fig. 4E) gene, whereas SRC-1 and SRC-2 were recruited to the NKD (Fig. 4E) gene in the U2OS-ERβ cells. Maximal recruitment of the coactivators was observed after 2 h (Fig. 4, E and F). These findings demonstrate that both ERα and ERβ bind to the CECR6 and NKD elements, but they recruit different p160 coactivators with E2.
Figure 4.
ERβ Activates the CECR6 and NKD Genes and Recruits p160 Coactivators
Tetracycline-inducible U2OS-ERβ cells were treated with 10 nm E2 for increasing times. CECR6 (A) or NKD (B) mRNA was measured by qPCR. Each data point is the average of triplicate determinations ± sem. The asterisk represents a significant difference from control (P < 0.05). ChIP assays were performed using antibodies to ERβ (C and D) and the p160 coactivators, SRC-1, SRC-2, or SRC-3 (E and F). Immunoprecipitated chromatin fragments were PCR amplified and quantitated by real-time PCR using primers for the CECR6 (C and E) and NKD (D and F) elements. Cont, Control.
Tamoxifen Is More Effective at Regulating the Elements Than Raloxifene with ERα, Whereas Raloxifene Is Superior with ERβ
The ChIP library represents a powerful set of regulatory elements to study the differential regulation by E2, tamoxifen, and raloxifene. The 173 elements were cloned upstream of the minimal thymidine kinase (tk) promoter and then transfected into wild-type U2OS cells with an ERα or ERβ expression vector. Cells were then treated with E2, tamoxifen, or raloxifene, and luciferase activity was measured. We found that 80% of the elements were regulated by at least one of the ligands with ERα or ERβ (Table 3). Many elements that were located outside the promoter region were regulated by the ligands (Table 3). Several regulated elements were located more than 500 kb from the transcription initiation site of the nearest gene. We also found that different elements derived from the same gene were differentially regulated by E2, tamoxifen, and raloxifene (Table 3, boldface entries). Some elements were differentially regulated by E2, tamoxifen, or raloxifene. Surprisingly, however, we found that even though the library was derived from cells expressing ERα and treated with E2, more elements were regulated by SERMs than E2. Moreover, E2, tamoxifen, and raloxifene activated more elements in the presence of ERβ compared with ERα (Table 3). We also found that more elements were activated by tamoxifen, compared with raloxifene, in the presence of ERα, whereas raloxifene was more effective than tamoxifen at activating the elements in the presence of ERβ (Table 4).
Table 3.
Effects of E2 and SERMs on the Elements in the ChIP Library
| ERα | ERβ
|
ERα | ERβ
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | E2 | T | R | E2 | T | R | Gene Name | E2 | T | R | E2 | T | R |
| tRNA alanine | 0.9 | 1.5 | 1.3 | 0.5 | 2.2 | 5.3 | Similar to Ig heavy-chain-2 light-chain | 0.7 | 1.1 | 1.1 | 0.8 | 3.3 | 5.9 |
| Single-stranded DNA-binding protein 2 | 0.9 | 1.3 | 1.2 | 0.5 | 2.4 | 3.1 | SH2 domain containing 4B | 1.0 | 2.0 | 1.2 | 0.7 | 1.4 | 1.5 |
| u2 Small nuclear ribonucleoprotein A | 0.8 | 1.1 | 3.8 | 1.0 | 2.9 | 7.3 | Hypothetical protein LOC283432 | 0.9 | 1.9 | 1.6 | 0.9 | 1.8 | 2.5 |
| NADH dehydrogenase 6 | 0.9 | 2.0 | 1.5 | 0.6 | 3.4 | 7.2 | Eukaryotic translation elongation factor 1 ε 1 | 0.9 | 1.2 | 1.5 | 2.1 | 4.0 | 12.6 |
| NADH dehydrogenase 6 | 0.6 | 1.4 | 1.4 | 1.1 | 1.5 | 1.8 | Hypothetical LOC285307 | 0.8 | 2.0 | 1.7 | 1.3 | 2.1 | 2.7 |
| tRNA serine 1 | 1.1 | 1.3 | 1.1 | 0.8 | 1.8 | 8.0 | Hypothetical LOC285307 | 0.9 | 0.8 | 1.3 | 0.7 | 2.6 | 5.6 |
| Calbindin 2,29 kDa | 1.3 | 2.2 | 3.9 | 0.5 | 1.5 | 1.5 | Family with sequence similarity 10, member A3 | 0.9 | 2.0 | 1.5 | 1.5 | 1.4 | 1.3 |
| Hypothetical protein FLJ35827 | 1.0 | 1.5 | 1.2 | 0.6 | 2.6 | 4.1 | Peptidylglycine α-amidating monooxygenase | 0.5 | 1.2 | 1.6 | 1.2 | 4.5 | 7.0 |
| THAP domain containing 8 | 1.0 | 1.3 | 1.6 | 0.8 | 1.1 | 1.7 | Hypothetical protein FLJ13197 | 0.7 | 0.7 | 0.7 | 0.8 | 0.7 | 0.8 |
| Retinal outer segment membrane protein 1 | 0.7 | 1.1 | 1.2 | 1.0 | 2.0 | 3.2 | Glycine receptor, α 1 | 1.1 | 2.8 | 2.0 | 1.3 | 1.3 | 1.3 |
| Olfactory receptor, family 7 | 3.6 | 2.8 | 3.9 | 0.9 | 2.5 | 5.5 | 7SL, cytoplasmic, pseudogene 2 | 0.7 | 1.4 | 1.6 | 1.2 | 1.3 | 0.9 |
| Killer cell lectin-like receptor subfamily C | 32.0 | 4.5 | 3.0 | 10.0 | 0.1 | 0.1 | Similar to Calcyclin binding protein | 0.9 | 0.8 | 0.9 | 1.0 | 0.8 | 0.9 |
| Killer cell lectin-like receptor subfamily C | 1.1 | 1.2 | 1.1 | 0.6 | 3.9 | 5.5 | Similar to Rpl7a protein | 0.9 | 2.1 | 1.6 | 1.8 | 3.0 | 3.0 |
| Neuronal PAS domain protein 2 | 2.5 | 4.3 | 2.6 | 0.8 | 2.2 | 3.4 | Cadherin 18, type 2 | 0.8 | 1.2 | 1.4 | 0.7 | 2.3 | 3.4 |
| Snf2-related CBP activator protein | 0.8 | 1.6 | 1.4 | 0.1 | 1.2 | 1.9 | Similar to RIKEN cDNA 5730421E18 gene | 1.0 | 0.9 | 0.8 | 1.3 | 3.4 | 4.9 |
| Hypothetical LOC401038 | 0.5 | 1.5 | 1.3 | 0.3 | 6.4 | 9.0 | Neuron navigator 3 | 0.9 | 1.3 | 1.3 | 2.3 | 6.9 | 4.8 |
| ATPase, Ca2+ transporting, ubiquitous | 1.4 | 2.2 | 2.8 | 0.4 | 3.4 | 5.5 | Roundabout, axon guidance receptor | 0.9 | 1.9 | 2.2 | 0.4 | 2.9 | 2.8 |
| FLJ46688 protein | 0.6 | 1.3 | 1.5 | 0.7 | 2.6 | 3.3 | CUB and Sushi multiple domains 3 | 0.6 | 1.3 | 1.5 | 0.7 | 2.4 | 5.2 |
| KIAA0240 | 0.5 | 2.7 | 2.3 | 1.0 | 1.0 | 1.2 | Ribosomal protein L10-like | 0.5 | 2.6 | 1.6 | 2.7 | 11.8 | 47.0 |
| Solute carrier family 4 | 0.8 | 1.5 | 0.7 | 0.7 | 3.9 | 6.0 | Similar to chromosome 9 open reading frame 140 | 0.9 | 1.6 | 1.1 | 0.5 | 2.8 | 3.1 |
| Hypothetical gene supported by BC031617 | 0.8 | 2.0 | 1.4 | 0.4 | 3.3 | 4.4 | Hypothetical LOC389202 | 1.3 | 2.3 | 1.7 | 1.1 | 1.4 | 3.3 |
| fem-1 homolog b | 1.2 | 2.2 | 2.1 | 1.4 | 1.2 | 1.7 | DnaJ (Hsp40) homolog, subfamily C, member 6 | 0.7 | 1.1 | 1.2 | 0.6 | 1.3 | 5.9 |
| Oligodendrocyte lineage transcription factor 2 | 2.3 | 3.7 | 2.9 | 0.7 | 2.7 | 4.7 | Protein tyrosine phosphatase, receptor type, M | 1.5 | 2.1 | 3.2 | 2.2 | 6.0 | 4.5 |
| Polycystic kidney and hepatic disease 1 | 0.7 | 1.5 | 1.5 | 1.2 | 1.0 | 1.0 | Cytoplasmic polyadenylation element bp 4 | 0.4 | 0.4 | 0.5 | 0.3 | 3.3 | 4.4 |
| Complement component 1 | 1.1 | 1.7 | 1.3 | 0.8 | 0.7 | 0.8 | Similar to keratin, type I cytoskeletal 18 | 0.8 | 2.0 | 2.7 | 0.3 | 4.7 | 8.0 |
| Similar to zinc finger protein 92 | 0.6 | 1.2 | 1.3 | 1.2 | 2.5 | 4.0 | Protocadherin 11 X-linked | 1.0 | 1.7 | 1.3 | 1.1 | 1.1 | 1.2 |
| Similar to salivary proline-rich protein | 1.1 | 2.9 | 3.1 | 0.3 | 1.7 | 2.7 | Sema domain, seven trombospondin repeats | 0.8 | 0.8 | 1.0 | 1.2 | 1.2 | 1.1 |
| Similar to nudix type motif 4 isoform β | 1.1 | 2.0 | 1.6 | 0.8 | 2.9 | 5.9 | Solute carrier family 37 member 2 | 0.9 | 1.6 | 2.0 | 0.9 | 8.4 | 9.7 |
| Similar to seven- transmembrane helix receptor | 1.1 | 2.0 | 1.7 | 0.6 | 3.7 | 5.7 | Phosphodiesterase 4B | 1.0 | 2.0 | 1.4 | 1.2 | 2.1 | 2.6 |
| Similar to DEAD box polypeptide 10 | 1.5 | 0.9 | 1.1 | 0.7 | 2.4 | 6.3 | CUB and sushi multiple domains1 | 0.8 | 1.7 | 1.9 | 1.0 | 1.7 | 4.0 |
| cAMP-responsive element binding protein 3-like 2 | 0.8 | 1.3 | 1.5 | 0.7 | 2.9 | 6.2 | Calcium channel l-type α 1B subunit | 0.8 | 0.7 | 0.8 | 0.7 | 2.9 | 4.4 |
| Sorting nexin family member 27 | 1.2 | 3.1 | 3.6 | 0.9 | 2.0 | 5.6 | RNA binding motif single Strand interacting protein | 1.3 | 1.0 | 1.1 | 1.5 | 1.5 | 1.1 |
(Table continues)
Table 3A.
Continued
| ERα | ERβ
|
ERα | ERβ
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | E2 | T | R | E2 | T | R | Gene Name | E2 | T | R | E2 | T | R |
| H19, imprinted maternally expressed untranslated mRNA | 0.9 | 2.5 | 2.1 | 0.8 | 1.2 | 1.2 | Amyotrophic lateral sclerosis 2 | 0.8 | 1.5 | 1.5 | 0.8 | 3.4 | 7.3 |
| Ig superfamily receptor | 0.9 | 1.7 | 1.5 | 0.8 | 1.0 | 1.2 | Cat eye syndrome chromosome region candidate 6 | 70.2 | 18.9 | 8.1 | 15.8 | 1.4 | 2.4 |
| Hornerin | 1.0 | 1.5 | 1.5 | 1.7 | 2.0 | 8.4 | Solute carrier family 19 member 1 | 0.7 | 1.7 | 1.7 | 3.4 | 1.4 | 2.0 |
| Similar to TFIIH basal transcription factor | 7.1 | 2.2 | 2.0 | 2.9 | 1.5 | 2.5 | TNF receptor member 19 | 0.5 | 0.9 | 0.8 | 1.1 | 1.3 | 0.6 |
| Transducing like enhancer of split 2 | 0.8 | 2.1 | 2.0 | 0.9 | 3.2 | 12.1 | Ryanodine receptor 3 | 1.2 | 2.3 | 3.1 | 0.6 | 3.0 | 4.5 |
| Progesterone receptor | 1.1 | 1.0 | 1.3 | 0.8 | 2.0 | 6.5 | Hypothetical protein FLJ30707 | 1.1 | 0.9 | 0.7 | 0.8 | 0.9 | 0.8 |
| Hypothetical protein FLJ90586 | 0.9 | 1.4 | 1.4 | 1.2 | 4.1 | 9.4 | I (3) mbt like 2 | 0.9 | 1.3 | 1.0 | 1.0 | 2.1 | 3.3 |
| Ubiquitin- conjugated enzyme E2N | 0.9 | 1.4 | 1.3 | 0.8 | 1.6 | 2.1 | Glutamate receptor ionotrophic kainate 2 | 0.8 | 1.8 | 1.6 | 0.5 | 3.0 | 6.3 |
| Mtylcortonyl coenzyme A carboxylase 1 | 0.8 | 1.7 | 1.8 | 0.9 | 1.0 | 1.5 | ATP-binding cassette, sub-family A member 8 | 1.6 | 2.2 | 1.3 | 1.0 | 1.2 | 1.3 |
| Similar to ribosome biogenesis protein BMS1 homolog | 1.0 | 1.6 | 1.5 | 0.5 | 1.4 | 1.4 | Hemicentin | 1.0 | 2.0 | 1.5 | 0.8 | 2.8 | 5.1 |
| Syntaxin-binding protein 6 | 1.0 | 1.3 | 1.0 | 0.7 | 2.1 | 3.3 | Family with sequence similarity 13 member C1 | 0.8 | 0.6 | 0.8 | 0.9 | 1.1 | 1.4 |
| Discoidin, CUB and LCCL domain containing 2 | 0.9 | 3.8 | 2.6 | 1.1 | 1.4 | 1.1 | EMI domain containing 2 | 1.9 | 2.1 | 3.5 | 1.0 | 1.6 | 1.6 |
| Adrenergic β receptor kinase 2 | 0.9 | 1.3 | 1.3 | 0.5 | 0.9 | 1.0 | Zinc and ring finger 3 | 0.7 | 1.1 | 0.9 | 1.1 | 3.7 | 4.5 |
| Polymerase II polypeptide D | 0.8 | 1.9 | 1.7 | 1.2 | 2.5 | 3.2 | Similar to CG7467-PA | 1.0 | 2.5 | 2.1 | 0.4 | 2.6 | 3.6 |
| Platelet-activating factor receptor | 1.3 | 1.3 | 1.3 | 0.7 | 1.0 | 0.9 | Glycogen synthase kinase 3 β | 0.8 | 1.4 | 1.2 | 0.8 | 1.0 | 0.6 |
| Similar to galectin-related inter-fiber protein | 0.7 | 2.0 | 1.7 | 0.4 | 3.4 | 6.9 | γ−Αminobutyric acid receptor rho2 | 1.3 | 2.3 | 2.1 | 0.8 | 1.3 | 1.8 |
| U56B small nuclear | 0.9 | 1.8 | 1.6 | 0.8 | 1.3 | 1.3 | Hypothetical protein FLJ22222 | 0.8 | 1.6 | 1.3 | 0.6 | 4.0 | 5.5 |
| Coiled-coil domain containing 5 | 1.1 | 1.3 | 1.4 | 0.7 | 1.1 | 0.8 | Ankyrin repeat and SOCX box-containing 17 | 24.6 | 2.5 | 1.5 | 0.3 | 4.1 | 5.1 |
| UDP glycosyltransferase 2 family, polypeptide | 0.9 | 1.3 | 1.4 | 1.2 | 2.9 | 9.2 | Naked cuticule homolog | 3.5 | 2.5 | 1.5 | 2.4 | 2.3 | 2.1 |
| FLJ45721 protein | 0.3 | 0.7 | 0.7 | 0.6 | 3.4 | 4.0 | Similar to IFIT1; Interferon, α-inducible protein | 1.1 | 2.0 | 1.6 | 0.7 | 5.2 | 11.3 |
| ρ−Guanine nucleotide exchange factor 3 | 1.1 | 1.6 | 1.7 | 1.6 | 1.9 | 1.2 | RAB31, member RAS oncogene family | 1.0 | 2.2 | 1.6 | 1.1 | 2.2 | 2.9 |
| COMM domain containing 3 | 1.0 | 1.5 | 1.3 | 1.2 | 1.1 | 1.4 | Phosphodiesterase 8B | 0.8 | 1.4 | 1.8 | 2.5 | 4.1 | 4.1 |
| Receptor-interacting factor 1 | 1.4 | 2.4 | 1.8 | 1.2 | 4.0 | 8.9 | Adaptor-related protein complex 3 δ 1 subunit | 1.0 | 2.7 | 2.7 | 0.8 | 1.2 | 1.5 |
| Chemokine orphan receptor 1 | 1.1 | 7.4 | 2.9 | 0.9 | 4.3 | 7.2 | Astrotactin | 1.5 | 2.4 | 2.0 | 1.0 | 1.8 | 2.9 |
| Myeloid/lymphoid or mixed-lineage leukemia | 0.6 | 0.8 | 1.0 | 0.7 | 1.3 | 1.4 | NY-REN-41 antigen | 0.7 | 1.0 | 1.0 | 0.6 | 3.1 | 4.3 |
| NYD-SP-12 protein | 0.6 | 1.6 | 1.5 | 1.3 | 0.9 | 1.0 | Lipase hepatic | 0.5 | 1.3 | 1.7 | 0.4 | 5.6 | 11.6 |
| Family with sequence similarity 34, member A | 0.7 | 1.0 | 1.4 | 0.3 | 1.5 | 1.8 | FLJ42117 protein | 0.6 | 1.2 | 1.5 | 0.5 | 3.9 | 5.7 |
| Phosphodiesterase 4D-interacting protein | 0.9 | 0.9 | 1.0 | 0.6 | 4.5 | 6.2 | Fibroblast growth factor 12 | 0.6 | 1.2 | 1.3 | 0.4 | 1.8 | 2.5 |
| Similar to Ig κ chain V region | 0.6 | 1.0 | 0.9 | 0.5 | 3.1 | 4.5 | Chromosome 10 open reading frame 112 | 0.7 | 1.1 | 1.2 | 1.0 | 1.8 | 1.5 |
| Similar to Ig κ chain V region | 1.0 | 0.9 | 1.3 | 1.1 | 2.5 | 3.3 | Putative nuclear protein ORF1-FL49 | 1.0 | 2.1 | 1.4 | 0.5 | 3.6 | 5.6 |
| Similar to ADP,ATP carrier protein | 4.6 | 9.4 | 4.5 | 0.6 | 4.3 | 6.1 | Glutamate receptor ionotrophic kainate 4 | 0.7 | 1.4 | 1.4 | 1.2 | 1.1 | 1.1 |
| Hypothetical gene supported by BC040860 | 0.4 | 1.1 | 1.1 | 0.8 | 4.8 | 6.5 | Inhibitor of κ light polypeptide gene enhancer | 1.0 | 1.4 | 2.1 | 0.6 | 2.9 | 6.4 |
| Hypothetical protein LOC389072 | 0.5 | 1.4 | 1.1 | 1.0 | 1.7 | 2.9 | Chromosome 14 open reading frame 171 | 0.9 | 2.6 | 2.0 | 0.4 | 2.9 | 6.6 |
(Table continues)
Table 3B.
Continued
| ERα | ERβ
|
ERα | ERβ
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Name | E2 | T | R | E2 | T | R | Gene Name | E2 | T | R | E2 | T | R |
| LOC441580 | 1.0 | 2.6 | 2.0 | 0.4 | 2.4 | 3.9 | Protein tyrosine phosphatase, receptor type, F | 0.8 | 1.2 | 1.2 | 0.8 | 2.0 | 3.4 |
| Cytidine monophosphate | 0.8 | 1.1 | 0.9 | 0.5 | 3.0 | 3.5 | Chromosome 20 open reading frame 44 | 1.1 | 2.2 | 2.2 | 0.6 | 3.9 | 7.0 |
| Similar to ATP-dependent DNA helicase II | 0.9 | 0.9 | 0.7 | 1.2 | 3.2 | 7.0 | Hypothetical protein LOC285692 | 0.9 | 1.6 | 1.6 | 0.4 | 2.5 | 2.9 |
| SNAP25-interacting protein | 0.6 | 1.7 | 1.6 | 0.6 | 3.0 | 4.8 | Adenosine deaminase RNA- specific B1 | 0.8 | 0.9 | 0.6 | 0.6 | 3.8 | 4.7 |
| Similar to eukaryotic translation elongation factor 1 | 1.6 | 3.9 | 2.4 | 1.0 | 1.8 | 3.9 | Plasminogen like | 0.7 | 1.6 | 1.4 | 1.0 | 1.7 | 2.0 |
| Similar to putative | 0.8 | 1.2 | 1.1 | 0.7 | 2.3 | 5.0 | Similar to ankyrin repeat domain 30A | 1.0 | 2.0 | 1.7 | 1.1 | 1.3 | 2.4 |
| Tankyrase, TRF1-interacting ankyrin | 0.5 | 1.6 | 1.3 | 1.2 | 2.4 | 5.5 | Rab6-interacting protein 2 | 0.6 | 1.7 | 1.9 | 1.0 | 1.1 | 1.9 |
| FAT tumor suppressor homolog 1 | 0.8 | 1.4 | 1.2 | 0.6 | 1.1 | 1.8 | Guanine nucleotide- binding protein (G protein), γ 7 | 0.6 | 1.4 | 1.3 | 0.9 | 0.6 | 0.6 |
| Hypothetical protein MGC26143 | 0.5 | 0.8 | 1.1 | 0.6 | 3.5 | 5.1 | p21-Activated kinase 7 | 0.9 | 1.6 | 1.3 | 0.6 | 5.4 | 6.0 |
| Olfactory receptor, family 4, Subfamily C | 0.8 | 1.4 | 1.5 | 1.2 | 2.1 | 2.9 | Spectrin repeat containing, nuclear envelope 2 | 0.7 | 0.8 | 0.9 | 0.8 | 1.2 | 1.6 |
| Similar to 33 kDa protein | 1.1 | 1.1 | 1.0 | 0.7 | 1.0 | 1.7 | Lethal giant larve homolog 2 | 0.7 | 1.2 | 1.4 | 1.0 | 1.2 | 1.5 |
| Likely ortholog of mouse limb-bud and heart gene | 1.6 | 2.7 | 1.8 | 1.0 | 2.3 | 2.5 | ATP synthase, H+ transporting | 1.7 | 1.9 | 1.4 | 0.6 | 2.1 | 1.9 |
| Ubiquinol-cytochrome c reductase | 0.7 | 1.3 | 1.1 | 0.9 | 1.7 | 3.3 | Chromosome 2 open reading frame 13 | 0.7 | 1.2 | 1.6 | 0.6 | 1.9 | 2.7 |
| Glutamate receptor, ionotropic, δ 1 | 1.2 | 1.5 | 1.7 | 0.9 | 1.8 | 3.2 | Similar to hypothetical protein DKFZp586O0120.1 | 0.6 | 1.4 | 1.4 | 0.3 | 2.4 | 3.0 |
| Hypothetical gene supported by AK094765 | 0.6 | 1.1 | 1.4 | 0.6 | 2.1 | 2.4 | START domain containing 9 | 1.4 | 1.9 | 1.3 | 0.7 | 1.9 | 3.4 |
| Solute carrier family 8 member1 | 1.0 | 1.9 | 2.0 | 1.0 | 1.9 | 4.1 | KIAA1944 protein | 0.9 | 1.3 | 1.4 | 1.7 | 0.4 | 1.2 |
| Similar to FLJ10378 protein isoform 2 | 1.2 | 1.3 | 1.3 | 0.6 | 2.3 | 2.7 | Spermatogenesis associated 13 | 0.6 | 1.3 | 1.3 | 0.7 | 1.6 | 3.3 |
| Inter-α inhibitor H5 | 1.0 | 1.4 | 1.3 | 0.9 | 1.0 | 1.4 | Hypothetical protein FLJ22301 | 0.9 | 1.4 | 1.4 | 1.0 | 1.6 | 2.5 |
| Similar to SPCPB16A4.07c | 1.1 | 2.2 | 1.5 | 1.0 | 2.3 | 2.7 | Chromosome 10 open reading frame 11 | 0.6 | 0.9 | 1.1 | 0.5 | 4.1 | 6.4 |
| Forkhead box L1 | 0.9 | 1.6 | 1.5 | 0.7 | 1.7 | 1.3 | Calcium channel, voltage-dependent, γ | 1.2 | 2.1 | 1.7 | 1.0 | 2.5 | 7.7 |
| Hypothetical protein FLJ10305 | 0.9 | 1.7 | 1.4 | 0.4 | 3.5 | 5.8 | Similar to ribosome biogenesis protein BMS1 homolog | 0.7 | 1.4 | 1.5 | 0.6 | 0.8 | 1.8 |
| Ribosomal protein L18 pseudogene 1 | 0.9 | 1.4 | 1.2 | 0.9 | 1.1 | 1.3 | |||||||
Wild-type U2OS cells were transfected with the different elements from the ChIP library, located upstream of the minimal tk promoter, and an ERα or ERβ expression vector. Cells were then treated with 10 nm E2, 1 μm tamoxifen or 1 μm raloxifene for 18 h and luciferase assays were performed. Boldface entries indicate different elements located adjacent to one gene. Each number represents the mean of three measurements. The sem was less than 10%. T, Tamoxifen; R, raloxifene.
Table 4.
Summary of the Genes Activated by E2, Tamoxifen, and Raloxifene
| No. of Genes Regulated by 2-Fold or More | |
|---|---|
| ERα | |
| Estradiol | 19 |
| Tamoxifen | 51 |
| Raloxifene | 34 |
| Tamoxifen > raloxifene | 13 |
| Raloxifene > tamoxifen | 6 |
| ERβ | |
| Estradiol | 38 |
| Tamoxifen | 94 |
| Raloxifene | 116 |
| Tamoxifen > raloxifene | 3 |
| Raloxifene > tamoxifen | 75 |
The numbers listed are for genes activated with the drug by at least 2-fold. For the genes listed for tamoxifen vs. raloxifene the magnitude of activation by one of the drugs ERα and ERβ was at least 1.5-fold greater.
To compare the effects of the ligands on mRNA expression with the transfection results, we examined whether 35 genes randomly selected were regulated by E2 and SERMs in the previously characterized, tetracycline-inducible U2OS-ERα cells (18). Cells were treated for various times with E2, tamoxifen, or raloxifene, and mRNA levels were determined by quantitative real-time PCR (qPCR). Similar to the transfection assays, several genes were regulated only by tamoxifen or raloxifene, even though the library was derived from cells treated with E2. Of the 35 genes examined, several showed good correlation with transfection assays. A similar pattern of regulation with transfection assays and qPCR were found with the NKG2E, CECR6, and NKD genes (Table 5). The EMI domain containing 2 and calbindin 2,29 kDa genes were activated with E2 by qPCR, but E2 did not activate the elements in transfection studies. In contrast, elements from the oligodendrocyte lineage transcription factor 2, fem-1 homolog b, protein tyrosine phosphatase, receptor type, M, and astrotactin genes were activated by SERMs in transfection assays, but were not activated by qPCR.
Table 5.
Comparison of the Regulation of Selected Elements and Genes by E2 and SERMs
| Estradiol
|
Tamoxifen
|
Raloxifen
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luc. | PCR | Luc | PCR | Luc | PCR | ||||||||
| Killer cell lectin-like receptor subfamily C | 32.0 | 20.6 ± 9.7 | 43.5 ± 6.7 | 86.1 ± 11.2 | 4.5 | 5.6 ± 1.2 | 8.2 ± 7.8 | 17.3 ± 14.6 | 3.0 | 1.8 ± 0.7 | 4.1 ± 1.6 | 7.0 ± 3.0 | |
| Cat eye syndrome chromosome region candidate 6 | 70.2 | 29.9 ± 2.2 | 39.4 ± 2.4 | 13.0 ± 1.0 | 18.9 | 2.5 ± 0.4 | 2.6 ± 0.8 | 4.0 ± 0.4 | 8.1 | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.3 ± 1.0 | |
| H19, imprinted maternally expressed untranslated mRNA | 0.9 | 10.4 ± 4.6 | 11.2 ± 0.9 | 12.7 ± 2.2 | 2.5 | 1.7 ± 0.4 | 2.4 ± 0.4 | 1.9 ± 0.2 | 2.1 | 0.9 ± 0.2 | 1.1 ± 0.4 | 0.9 ± 0.2 | |
| EMI domain containing 2 | 1.9 | 4.2 ± 1.2 | 3.6 ± 1.6 | 3.4 ± 2.6 | 2.1 | 1.4 ± 0.3 | 1.8 ± 0.5 | 1.4 ± 0.1 | 3.5 | 1.6 ± 0.5 | 2.1 ± 1.4 | 1.8 ± 1.2 | |
| Retinal outer segment membrane protein 1 | 0.7 | 3.2 ± 2.2 | 2.4 ± 0.1 | 8.8 ± 7.1 | 1.1 | 1.9 ± 0.2 | 1.8 ± 0.4 | 1.7 ± 0.3 | 1.2 | 0.7 ± 0.4 | 4.4 ± 1.0 | 2.5 ± 1.8 | |
| Naked cuticule homolog | 3.5 | 2.5 ± 0.2 | 2.3 ± 0.3 | 2.1 ± 0.2 | 2.5 | 2.6 ± 0.9 | 2.2 ± 0.1 | 1.9 ± 0.3 | 1.5 | 1.1 ± 0.1 | 1.3 ± 0.3 | 1.1 ± 0.1 | |
| Solute carrier family 4 | 0.8 | 2.2 ± 0.0 | 3.2 ± 0.9 | 3.5 ± 0.8 | 1.5 | 0.7 ± 0.2 | 2.4 ± 0.1 | 2.7 ± 0.6 | 0.7 | 0.9 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Spermatogenesis associated 13 | 0.6 | 2.8 ± 0.5 | 2.5 ± 0.5 | 1.7 ± 0.4 | 1.3 | 2.5 ± 0.9 | 3.2 ± 0.7 | 3.5 ± 1.1 | 1.3 | 1.8 ± 0.7 | 2.2 ± 0.5 | 2.4 ± 0.6 | |
| Calcium channel l-type α 1B subunit | 0.8 | 1.4 ± 0.0 | 1.9 ± 0.3 | 3.7 ± 0.5 | 0.7 | 1.1 ± 0.0 | 1.3 ± 0.2 | 1.2 ± 0.1 | 0.8 | 0.8 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.2 | |
| Cytoplasmic polyadenylation element bp 4 | 0.4 | 1.9 ± 0.3 | 2.1 ± 0.0 | 2.1 ± 0.4 | 0.4 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.7 ± 1.0 | 0.5 | 1.0 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.1 | |
| Glutamate receptor ionotrophic kainate 4 | 0.7 | 1.0 ± 0.4 | 1.0 ± 0.1 | 2.8 ± 0.0 | 1.4 | 0.3 ± 0.0 | 0.1 ± 0.0 | 1.2 ± 0.6 | 1.4 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.7 ± 0.4 | |
| Calbindin 2,29 kDa | 1.3 | 1.8 ± 0.2 | 2.4 ± 0.1 | 2.7 ± 0.0 | 2.2 | 1.0 ± 0.0 | 1.6 ± 0.3 | 1.3 ± 0.2 | 3.9 | 1.0 ± 0.3 | 2.4 ± 0.1 | 2.3 ± 0.0 | |
| UDP glycosyltransferase 2 family, polypeptide | 0.9 | 1.0 ± 0.2 | 1.4 ± 0.4 | 2.9 ± 0.3 | 1.3 | 1.0 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.1 | 1.4 | 1.3 ± 0.5 | 0.9 ± 0.5 | 1.7 ± 0.7 | |
| Chromosome 10 open reading frame 11 | 0.6 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.9 ± 0.2 | 0.9 | 0.8 ± 0.1 | 0.8 ± 0.3 | 0.4 ± 0.1 | 1.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.1 | |
| Solute carrier family 19 member 1 | 0.7 | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.0 | 1.7 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.0 | 1.7 | 1.1 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.3 | |
| Eukaryotic translation elongation factor 1 ε 1 | 0.9 | 0.8 ± 0.1 | 0.6 ± 0.0 | 0.9 ± 0.3 | 1.2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.0 | 1.5 | 0.6 ± 0.3 | 0.7 ± 0.4 | 1.0 ± 0.1 | |
| Protocadherin 11 X-linked | 1.0 | 1.1 ± 0.1 | 0.9 ± 0.4 | 0.5 ± 0.0 | 1.7 | 1.1 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.3 | 1.3 | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.7 ± 0.1 | |
| START domain containing 9 | 1.4 | 0.5 ± 0.0 | 0.5 ± 0.2 | 0.5 ± 0.1 | 1.9 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.1 | 1.3 | 0.6 ± 0.2 | 0.9 ± 0.0 | 0.9 ± 0.1 | |
| Single-stranded DNA binding protein 2 | 0.9 | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.0 | 1.3 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 1.2 | 0.7 ± 0.0 | 1.2 ± 0.4 | 0.9 ± 0.2 | |
| Putative nuclear protein ORF1-FL49 | 1.0 | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.6 ± 0.0 | 2.1 | 0.9 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.1 | 1.4 | 0.7 ± 0.0 | 1.2 ± 0.6 | 1.1 ± 0.3 | |
| I (3) mbt like 2 | 0.9 | 0.9 ± 0.2 | 0.5 ± 0.1 | 0.5 ± 0.0 | 1.3 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.0 | 0.9 ± 0.1 | 1.3 ± 0.3 | 1.1 ± 0.3 | |
| Plasminogen like | 0.7 | 1.2 ± 0.2 | 0.6 ± 0.2 | 0.4 ± 0.1 | 1.6 | 1.2 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.2 | 1.4 | 0.8 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.4 | |
| RNA binding motif single strand interacting protein | 1.3 | 0.4 ± 0.0 | 0.3 ± 0.1 | 0.4 ± 0.1 | 1.0 | 0.6 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.1 | 1.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | |
| Glutamate receptor ionotrophic kainate 2 | 0.8 | 0.7 ± 0.5 | 0.1 ± 0.1 | 0.2 ± 0.0 | 1.8 | 1.0 ± 0.2 | 1.9 ± 0.5 | 1.6 ± 0.6 | 1.6 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.3 | |
| CUB and sushi multiple domains1 | 0.8 | 1.0 ± 0.1 | 1.0 ± 0.0 | 1.3 ± 0.0 | 1.7 | 0.8 ± 0.2 | 1.0 ± 0.3 | 1.0 ± 0.5 | 1.9 | 0.8 ± 0.1 | 0.9 ± 0.4 | 1.1 ± 0.1 | |
| Fibroblast growth factor 12 | 0.6 | 0.8 ± 0.0 | 0.9 ± 0.2 | 1.1 ± 0.0 | 1.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.1 | 1.3 | 0.9 ± 0.0 | 1.1 ± 0.4 | 0.9 ± 0.2 | |
| Adenosine deaminase RNA specific B1 | 0.8 | 1.6 ± 0.2 | 1.1 ± 0.4 | 1.3 ± 0.5 | 0.9 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 | 0.7 ± 0.3 | 1.1 ± 0.1 | 0.9 ± 0.2 | |
| Protein tyrosine phosphatase, receptor type, M | 1.5 | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.3 ± 0.5 | 2.1 | 1.2 ± 0.1 | 1.8 ± 0.1 | 1.7 ± 0.2 | 3.2 | 0.8 ± 0.1 | 1.2 ± 0.5 | 1.0 ± 0.2 | |
| COMM domain containing 3 | 1.0 | 1.3 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.5 | 1.0 ± 0.3 | 1.3 ± 0.4 | 1.6 ± 0.3 | 1.3 | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.2 | |
| Phosphodiesterase 8B | 0.8 | 1.4 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.4 | 0.8 ± 0.3 | 0.8 ± 0.3 | 1.1 ± 0.2 | 1.8 | 0.7 ± 0.1 | 1.4 ± 0.5 | 0.8 ± 0.2 | |
| Astrotactin | 1.5 | 0.7 ± 0.0 | 0.8 ± 0.0 | 1.1 ± 0.1 | 2.4 | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.1 | 2.0 | 0.9 ± 0.1 | 1.1 ± 0.4 | 1.0 ± 0.2 | |
| Fem-1 homolog b | 1.2 | 1.0 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1 | 2.2 | 0.8 ± 0.0 | 1.1 ± 0.0 | 0.7 ± 0.1 | 2.1 | 0.7 ± 0.1 | 1.2 ± 0.3 | 1.1 ± 0.1 | |
| Hypothetical protein FLJ30707 | 1.1 | 0.6 ± 0.2 | 0.6 ± 0.0 | 0.8 ± 0.2 | 0.9 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.2 | 0.7 | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.9 ± 0.1 | |
| cAMP responsive element binding protein 3-like 2 | 0.8 | 0.8 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.3 | 0.8 ± 0.2 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.5 | 1.1 ± 0.0 | 1.2 ± 0.3 | 1.3 ± 0.1 | |
| Oligodendrocyte lineage transcription factor 2 | 2.3 | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.4 | 3.7 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.0 | 2.9 | 1.0 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.0 | |
| Time of treatment (h) | 18 | 6 | 12 | 24 | 18 | 6 | 12 | 24 | 18 | 6 | 12 | 24 | |
Luciferase activity of the elements was measured by transfection studies. The expression of the corresponding gene was measured by real-time PCR after 6, 12 and 24 h of treatments with the ligands in U2OS-ERα cells. Each data point is the average of triplicate determinations ± sem.
DISCUSSION
Microarray data from our laboratory (18) showed that E2 and SERMs exhibit a complex pattern of gene regulation that cannot be explained by the pharmacology of known ER-responsive elements such as ERE, AP-1, and Sp1. These data raise the possibility that many ER target genes contain unknown regulatory elements. Whereas microarrays easily identify ER target genes (19,21,31) they do not provide any information on the location or nature of the regulatory elements in the target gene. Thus, new genome-wide approaches are essential to rapidly isolate regulatory elements in target genes to understand how E2 and SERMs exert tissue-specific clinical effects and produce the complex gene expression profiles observed with microarrays.
ChIP-chip is a powerful strategy that has been used to identify elements in target genes for ERα (25,35,36,37). This approach is valuable to identify and annotate different classes of ER elements and to identify transcription factors that interact with ER-binding sites (25). Here we used a ChIP-CS strategy to rapidly isolate and characterize ER-regulatory elements. We isolated 173 ER-binding elements that were tested for functional activity in response to E2 and SERMs with ERα and ERβ.
We found that most elements were derived from introns and regions beyond 10 kb of the TSS. Only 24% of the elements were found within 10 kb of the TSS. ChIP-chip studies also showed that many ER-regulatory elements are located at a distance far from the TSS (24,25). It also has been shown that 63% of the glucocorticoid response elements are found beyond 10 kb of the TSS (39). These results demonstrate that the proximal promoter region only contains a small subset of response elements for steroid receptors.
Only 11% of the genes derived from the ChIP-CS library had an ERE despite the use of a fairly low stringency for detection. Although the ERE is considered to be the major response element in target genes, these results suggest that most ER target genes are regulated by response elements other than the classical ERE. Of the elements in the ChIP-CS library, 41% contained an AP-1 site, whereas 18% of the genes contained Sp1 elements. The most surprising finding was that 23% of the clones from the ChIP library did not contain an ERE or any of the known alternative elements. However, our transfection studies showed that 70% of the elements without a classical ERE, AP-1, Sp1, FOXA1, or NFκB element were regulated by E2 or SERMs, demonstrating that many regulatory sequences from the ChIP library contain unknown elements. This notion was supported by the data showing that E2 activated and recruited p160s coactivators to the NKD gene, which did not have any known regulatory element.
A major advantage of having numerous elements from the ChIP library is that they can be examined for activity with different ligands and ERs. Most of the 173 elements were regulated by E2, tamoxifen, or raloxifene with ERα or ERβ using transfection assays, demonstrating that the library contained genuine regulatory elements. By testing a large number of elements, we were able to discover several surprising and interesting observations. We found that more elements were regulated by SERMs, even though ChIP was done with E2-treated cells. One explanation for this finding is that E2 regulation might require other factors that are lost during shearing of the chromatin. Genome-wide tiling arrays showed that many ER-binding sites are associated with transcription factor elements, such as FOX1A, AP-1, Oct, and CCAAT enhancer binding protein, which are important for regulation by E2 (25). Our findings suggest that the E2-ER complex binds to the elements and is recognized by the ChIP antibody. However, some elements are probably functionally inactive because the ER is unable to interact with factors that are absent from the ChIP element and required for transcriptional regulation by E2. Alternatively, it is conceivable that the antibody, which we used for ChIP, selectively recognizes an ER conformation that is inactive or inhibitory when bound to E2. Other antibodies, such as the one used in the tiling arrays (25), might recognize an ER conformation that is capable of detecting elements activated by E2. Finally, it is likely that some of the ER-binding sites might be silent in U2OS cells and do not function as regulatory elements in response to E2. Some of these inactive elements might be regulated by E2 in other cell types.
Another important finding from the functional studies of the ChIP library was that the magnitude of activation of most elements by tamoxifen was greater with ERα, whereas raloxifene was more effective at activating the elements in the presence of ERβ. The reason for the differences in activation with the two receptor subtypes is unclear. Tamoxifen binds equally to ERα and ERβ (40), whereas raloxifene binds with about 2-fold greater affinity to ERβ than ERα (41). These results demonstrate that differences in binding do not account for the difference in the activity of tamoxifen and raloxifene with ERs. Structural studies found that tamoxifen and raloxifene produce different conformations of ERα, with raloxifene causing a greater increase in the mobility of helix 12 of the ligand-binding domain (30). The different conformations elicited by tamoxifen and raloxifene might lead to a differential binding of ERs to the regulatory elements or the recruitment of different coregulators by ERα and ERβ at the elements. We previously showed that tamoxifen and raloxifene activate the NKG2E gene by recruiting different coactivators (42). We also found that tamoxifen activated the CECR6 and NKD genes from the ChIP library by recruiting coactivators (data not shown). In contrast, raloxifene did not activate these genes or recruit coactivators. These results indicate that the SERMs produce different conformations in ER, which leads to the differential recruitment of coactivators. The observation that the agonist activity of SERMs is mediated by coactivators is consistent with the findings that coactivators can interact with the AF-1 in the A/B domain (16,17).
One of the more surprising findings was the lack of correlation between the gene expression data and transfection studies. Previous studies selected a few elements derived from ChIP to correlate gene expression with function in response to E2 with transfection studies (25,26). To avoid any potential bias in selecting elements to examine, we cloned all 173 elements from the library upstream of tk-luciferase. Only a few of the 35 genes that were tested for expression data by PCR showed an exact correlation with transfection assays. There are several possible explanations for these findings. First, the element from the ChIP library was assigned to the nearest gene. However, it is possible that the element actually regulates one of the other three adjacent genes, rather than the nearest gene. Second, because of compact folding of chromatin, the regulatory element isolated from the ChIP library could actually be in close proximity to distant target genes. It is well recognized that enhancers can operate at far distances (43). For example, the E2 enhancer region interacts with distant Sp1 transcription factors by forming stable DNA loops that can be visualized by electron microscopy (44). These findings suggest that the elements from the ChIP library might bypass adjacent genes and cause regulation of distant ER target genes. Third, it is likely that some regulatory elements function differently in the context of native genes. Genes also contain silencers, which are elements that bind repressor proteins that repress the activation of the gene. Thus, some elements from the ChIP library that are functional when isolated and inserted upstream of the tk promoter might be silenced by other factors that interact with the native gene. This might be an important mechanism for tissue-specific gene expression if the factors are differentially expressed in different cell types. The results from our qPCR and luciferase data suggest that it is highly probable that the element actually regulates the assigned gene only when there is good correlation between ER binding by ChIP, mRNA expression, and transfection data.
Our results suggest that many ER target genes are regulated by elements other than ERE, AP-1, Sp1, FOXA1, and NFκB. We performed extensive bioinformatic analysis of the elements to identify other motifs that might be involved in regulation by ER. However, we were unable to discover any new motifs present in the majority of elements. This might be due to the fact that the ChIP library contained too few genes to detect new motifs. However, our detailed analysis of one element from the ChIP library (42) suggests it will be extremely difficult to detect single motifs even with many more elements. We found that the NKG2E gene contains a complex composite element (45), which comprises four elements (AP-1, heat shock factor-2, CCAAT enhancer binding protein-β, and a variant ERE) that cooperate to regulate the gene by E2 and SERMs (42). The results with the NKG2E-regulatory element and our ChIP library, showing that different elements, even within the same gene, are differentially regulated by E2, tamoxifen, and raloxifene, suggest that many ER targets are regulated by a combination of cooperative elements rather than a single element, such as ERE, AP-1, and Sp1. The tiling arrays suggest that ER cooperates with a preferred set of transcription factors (25). However, our data suggest that many different combinations of factors interact with ER, because we were unable detect any consistent combination of elements in the ChIP library. Furthermore, it is also likely that the factors with which ER interacts to regulate genes varies in different cell types. This notion is consistent with our observation that some of the ChIP library elements were regulated differently by E2 and SERMs in various cell types (data not shown).
The data from our ChIP library and tiling arrays clearly indicate that most ER target genes are regulated by ER interacting with a combination of multiple different transcription factors, rather than binding exclusively to an ERE. Identifying combinatorial regulatory elements will likely require detail mapping of many elements with functional studies. The isolation of regulatory elements from native genes with techniques such as ChIP-CS is a major step toward understanding how ERα and ERβ regulate different genes in response to diverse ligands, and how E2 and SERMs produce unique clinical effects. However, our findings also suggest that whereas ChIP-chip and ChIP-CS can identify ER-binding sites and regulatory elements, it can be difficult to identify the exact gene that is regulated by the element, even when the element is in close proximity or within a gene. A major challenge will be to develop strategies to accurately assign the proper gene that is regulated by the elements identified with tiling arrays or sequencing.
MATERIALS AND METHODS
Preparation of Stable Cell Lines
U2OS cells stably expressing Flag-tagged ERα were prepared by transfecting wild-type U2OS cells with pcDNA 6/V5-His vector containing Flag-ERα. Cells were selected and maintained using 10 μg/ml blasticidin. Tetracycline-inducible U2OS-ERα and U2OS-ERβ cells were prepared, characterized, and maintained as previously described (18).
Chromatin Immunoprecipitation Cloning and Sequencing (ChIP-CS)
U2OS-Flag-ERα cells were treated with 10 nm E2 for 2 h and then cross-linked, washed, collected, and lysed as previously described (18,46). Nuclei were then resuspended in FLAG protein immunoprecipitation buffer (50 mm Tris HCl, pH 7;4, 150 mm NaCl; 1 mm EDTA; 1% Triton X-100) and sonicated. The supernatant fraction was collected and incubated with anti-flag M2 agarose beads (Sigma-Aldrich, St. Louis, MO) for 2 h at 4 C. After the beads were washed (0.05 m Tris HCl, pH 7.4; 0.15 m NaCl), the proteins were eluted using 150 ng/μl 3X FLAG peptide (Sigma-Aldrich) in 0.05 m Tris HCl (pH 7.4)-0.15 m NaCl. Eluted proteins were then incubated with anti-ERα (DAKO Corp., Carpinteria, CA) coated magnetic beads (Dynabeads M-280) for 2 h. Beads were washed with PBS containing 1% BSA, and cross-linking of DNA/proteins was reversed with 1% sodium dodecyl sulfate-0.1 m NaHCO3 at 65 C. DNA fragments were purified, (QIAquick PCR Purification Kit; QIAGEN, Chatsworth, CA), blunt ended, and cloned, and plasmids were isolated and sequenced (Lark Technologies, Houston, TX).
Plasmids, Transfections, and Luciferase Assays
Inserts from the plasmids were cloned upstream of −32 to +45 tk-luciferase. Transfections of the various vectors into U2OS cells were carried out by electroporation (47). Cells were assayed for luciferase activity according to manufacturer’s protocol (Promega Corp., Madison, WI).
RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted and then treated with DNAse using the Aurum Total RNA Mini Kit (Bio-Rad Laboratories, Hercules, CA). Reverse transcription reactions were performed using the iScript cDNA Synthesis Kit with 1 μg of total RNA as previously described (48). qPCR was performed with a Bio-Rad iCycler Thermal Cycler System using iQ SYBR Green Supermix (Bio-Rad). Mean ± sem was calculated using Prism curve-fitting program (GraphPad Software, Inc., San Diego, CA). The primers used for qPCR are listed in supplemental Table 2.
Chromatin Immunoprecipitation (ChIP)
After treatments, cells were cross-linked, washed, collected, and lysed as described above. Immunoprecipitations were performed overnight at 4 C with anti-ERα (HC-20), (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-SRC-1 (1135) (Upstate Biotechnology, Inc., Lake Placid, NY), anti-SRC-2 (ab9261), and anti-SRC-3 (ab2782), (Abcam, Cambridge, MA). DNA fragments were purified (QIAquick PCR Purification Kit; QIAGEN) and PCR amplified. The primers used for ChIP are listed in supplemental Table 2.
Bioinformatics
To find putative ER target genes, the 192 sequences from the ChIP library were mapped to the human genome (HG 35.1 reference assembly) using BLAST with a threshold 1e-30. A set of 173 sequences that had significant hits was returned with four genes each: the nearest gene on each strand in each of the forward and reverse directions. Putative target genes were selected according to their positions relative to the hits. The computational programs MATCH (49) and Dragon ERE finder (50) were used to scan the 173 sequences for putative EREs, AP-1, SP1, foxA1, and NFκB. These two programs search for sites by scoring their similarity to experimentally verified EREs. When applying MATCH, two ERE position weight matrices, one from the TRANSFAC (51) database (matrix ID: V $ER_Q6), and the other one built from 25 EREs known in literature (52) were used. In these cases the threshold for declaring a putative ERE was set to minimize the sum of the false positives and false negatives. When applying the Dragon ERE finder, the default threshold for obtaining 0.83 sensitivity was used.
Supplementary Material
Acknowledgments
We thank Pierre Chambon and Jan-Åke Gustafsson for providing plasmids, and Paul Webb for valuable comments on the manuscript.
Footnotes
This work was supported by grants from the National Institutes of Health (DK061966) and the American Cancer Society.
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 25, 2007
Abbreviations: AF, Activation function; AP-1, activator protein 1; CECR6, Cat eye syndrome chromosome region candidate 6; ChIP, chromatin immunoprecipitation; ChIP-CS, ChIP-cloning and sequencing; E2, estradiol; ER, estrogen receptor; ERE, estrogen response element; H19, imprinted maternally expressed untranslated mRNA; NFκB, nuclear factor-κB; LLGL2, lethal giant larvae homolog 2; NKD, naked cuticule homolog; NKG2, killer cell lectin-like receptor subfamily C; NKG2E, killer cell lectin-like receptor; qPCR, quantitative real-time PCR; SERM, selective estrogen receptor modulator; SPAT13, spermatogenesis associated 13; SRC, steroid receptor coactivator; tk, thymidine kinase; TSS, transcription start site.
References
- Writing Group for the Women’s Health Initiative 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- MacGregor JI, Jordan VC 1998 Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev 50:151–196 [PubMed] [Google Scholar]
- Jordan VC 2004 Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5:207–213 [DOI] [PubMed] [Google Scholar]
- Cummings S, Eckert S, Krueger K, Grady D, Powles T, Cauley J, Norton L, Nickelsen T, Bjarnason N, Morrow M, Lippman ME, Black D, Glusman J, Costa A, Jordan V 1999 The effect of raloxifene on risk of breast cancer in postmenopausal women. JAMA [Erratum (1999) 282:2184] 281:2189–2197 [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black D, Mitlak B, Knickerbocker R, Nickelsen T, Genant H, Christiansen C, Delmas P 1999 Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA 282:637–644 [DOI] [PubMed] [Google Scholar]
- Christodoulakos GE, Botsis DS, Lambrinoudaki IV, Papagianni VD, Panoulis CP, Creatsa MG, Alexandrou AP, Augoulea AD, Dendrinos SG, Creatsas GC 2006 A 5-year study on the effect of hormone therapy, tibolone and raloxifene on vaginal bleeding and endometrial thickness. Maturitas 53:413–423 [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon Jr ER, Wade III JL, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N 2006 Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295:2727–2741 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW 1995 Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol 9:659–669 [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU 1986 An estrogen-responsive element derived from the 5′ flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell 46:1053–1061 [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ 1995 Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456 [DOI] [PubMed] [Google Scholar]
- Safe S 2001 Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 62:231–252 [DOI] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M 2000 Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Smith CL, O’Malley BW 2004 Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71 [DOI] [PubMed] [Google Scholar]
- Shang Y, Brown M 2002 Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ 1998 Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol 12:1605–1618 [DOI] [PubMed] [Google Scholar]
- Dutertre M, Smith CL 2003 Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol 17:1296–1314 [DOI] [PubMed] [Google Scholar]
- Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC 2004 Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges LC, Cook JD, Lobenhofer EK, Li L, Bennett L, Bushel PR, Aldaz CM, Afshari CA, Walker CL 2003 Tamoxifen functions as a molecular agonist inducing cell cycle-associated genes in breast cancer cells. Mol Cancer Res 1:300–311 [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145:3473–3486 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC 2003 Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERα or ERβ. J Cell Biochem 90:315–326 [DOI] [PubMed] [Google Scholar]
- Frasor J, Stossi F, Danes JM, Komm B, Lyttle CR, Katzenellenbogen BS 2004 Selective estrogen receptor modulators: discrimination of agonistic versus antagonistic activities by gene expression profiling in breast cancer cells. Cancer Res 64:1522–1533 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Lin Z, Reierstad S, Huang CC, Bulun SE 2007 Novel estrogen receptor-α binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res 67:5017–5024 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW 2005 Expanding functional diversity of the coactivators. Trends Biochem Sci 30:126–132 [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M 1997 Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758 [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL 1998 The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937 [DOI] [PubMed] [Google Scholar]
- Nettles KW, Greene GL 2005 Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 67:309–333 [DOI] [PubMed] [Google Scholar]
- Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC 2005 Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 19:1555–1568 [DOI] [PubMed] [Google Scholar]
- Choi I, Gudas LJ, Katzenellenbogen BS 2000 Regulation of keratin 19 gene expression by estrogen in human breast cancer cells and identification of the estrogen responsive gene region. Mol Cell Endocrinol 164:225–237 [DOI] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Giguere V 2005 Functional genomics identifies a mechanism for estrogen activation of the retinoic acid receptor α1 gene in breast cancer cells. Mol Endocrinol 19:1584–1592 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Inoue S, Hiroi H, Orimo A, Kawashima H, Muramatsu M 1998 Isolation of estrogen-responsive genes with a CpG island library. Mol Cell Biol 18:442–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V 2005 From the cover: location analysis of estrogen receptor α target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA 102:11651–11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Swarbrick A, Musgrove EA, Sutherland RL 2002 Mechanisms of growth arrest by c-myc antisense oligonucleotides in MCF-7 breast cancer cells: implications for the antiproliferative effects of antiestrogens. Cancer Res 62:3126–3131 [PubMed] [Google Scholar]
- Jin VX, Leu YW, Liyanarachchi S, Sun H, Fan M, Nephew KP, Huang TH, Davuluri RV 2004 Identifying estrogen receptor α target genes using integrated computational genomics and chromatin immunoprecipitation microarray. Nucleic Acids Res 32:6627–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S 2004 Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol 18:1411–1427 [DOI] [PubMed] [Google Scholar]
- So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR 2007 Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet 3:e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Weatherman RV, Clegg NJ, Scanlan TS 2001 Differential SERM activation of the estrogen receptors (ERα and ERβ) at AP-1 sites. Chem Biol 8:427–436 [DOI] [PubMed] [Google Scholar]
- Levy N, Zhao X, Tang H, Jaffe RB, Speed TP, Leitman DC 2007 Multiple transcription factor elements collaborate with ERα to activate an inducible estrogen response element in the NKG2E gene. Endocrinology 148:3449–3458 [DOI] [PubMed] [Google Scholar]
- Levine M, Tjian R 2003 Transcription regulation and animal diversity. Nature 424:147–151 [DOI] [PubMed] [Google Scholar]
- Li R, Knight JD, Jackson SP, Tjian R, Botchan MR 1991 Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell 65:493–505 [DOI] [PubMed] [Google Scholar]
- Diamond MI, Miner JN, Yoshinaga SK, Yamamoto KR 1990 Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science 249:1266–1272 [DOI] [PubMed] [Google Scholar]
- Burakov D, Crofts LA, Chang CP, Freedman LP 2002 Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J Biol Chem 277:14359–14362 [DOI] [PubMed] [Google Scholar]
- Tzagarakis-Foster C, Geleziunas R, Lomri A, An J, Leitman DC 2002 Estradiol represses human T-cell leukemia virus type 1 Tax activation of tumor necrosis factor-α gene transcription. J Biol Chem 277:44772–44777 [DOI] [PubMed] [Google Scholar]
- Paruthiyil S, Parmar H, Kerekatte V, Cunha GR, Firestone GL, Leitman DC 2004 Estrogen receptor β inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res 64:423–428 [DOI] [PubMed] [Google Scholar]
- Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E 2003 MATCH: a tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res 31:3576–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajic VB, Tan SL, Chong A, Tang S, Strom A, Gustafsson JA, Lin CY, Liu ET 2003 Dragon ERE Finder version 2: a tool for accurate detection and analysis of estrogen response elements in vertebrate genomes. Nucleic Acids Res 31:3605–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys V, Fricke E, Geffers R, Gossling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Munch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E 2003 TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31:374–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Lone R, Frith MC, Karlsson EK, Hansen U 2004 Genomic targets of nuclear estrogen receptors. Mol Endocrinol 18:1859–1875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.