Abstract
Carney complex (CNC) is an inherited neoplasia syndrome characterized by spotty skin pigmentation, myxomas, endocrine tumors, and schwannomas. Among the endocrine tumors that comprise the syndrome, GH-producing pituitary tumors are seen in approximately 10% of patients, although biochemical abnormalities of the GH axis are much more common. To explore the role of loss of the CNC gene PRKAR1A on pituitary tumorigenesis, we produced a tissue-specific knockout (KO) of this gene in the mouse. For these studies, we generated a mouse line expressing the cre recombinase in pituitary cells using the rat GHRH receptor promoter. These mice were then crossed with Prkar1a conditional null animals to produce tissue-specific KOs. Although prolactinomas were observed in KO and control mice, the KO mice exhibited a significantly increased frequency of pituitary tumors compared with wild-type or conventional Prkar1a+/− mice. Characterization of the tumors demonstrated they were composed of cells of the Pit1 lineage that stained for GH, prolactin, and TSH. At the biochemical level, levels of GH in the serum of KO animals were markedly elevated compared with controls, regardless of the presence of a frank tumor. These data indicate that complete loss of Prkar1a is sufficient to allow the formation of pituitary tumors and abnormalities of the GH axis, in close analogy to human patients with CNC.
PITUITARY TUMORS ARE common neoplasms in the population. A recent meta-analysis of the English-language literature on these tumors estimated that the overall prevalence of pituitary adenomas is 16.7% and suggested that a significant fraction of these tumors may be clinically relevant (1). Although the large majority of these are sporadic, pituitary tumors can also occur in the setting of the genetic syndromes multiple endocrine neoplasia type 1 (MEN1) and the Carney complex (CNC). In MEN1, pituitary tumors are observed in 10–60% of patients, and prolactinomas predominate (2). In CNC, GH-producing pituitary adenomas are observed in approximately 10% of patients (3), although biochemical abnormalities of the GH axis are much more common, likely due to somatotroph hyperplasia (4,5). Prolactinomas have also been reported in CNC kindreds, although these are infrequent. Familial pituitary tumors have also been described in isolation, occurring either as isolated familial somatotropinomas when GH-producing tumors are involved (6) or as familial isolated pituitary adenomas when other types of pituitary tumors are seen in a family (7). Although not occurring in an inherited fashion, pituitary adenomas are also associated with the McCune-Albright syndrome (8).
At the genetic level, CNC can be caused by null mutations in the PRKAR1A gene, encoding the type 1A regulatory subunit of protein kinase A (PKA) (9). Two independent mouse lines heterozygous for a null allele of the Prkar1a gene do not develop pituitary tumors up to 2 yr of age (10,11).
These observations suggested that haploinsufficiency for Prkar1a might not be sufficient for the development of pituitary tumors in the mouse. To address this question directly, we generated mice expressing the cre recombinase in pituitary cells of the Pit1 lineage, including GH-, prolactin (Prl)-, and TSH-producing cells. We used this line to produce tissue-specific knockout (KO) mice and report that these mice develop pituitary tumors with high frequency by 18 months. Furthermore, we find that the tumors are accompanied by abnormalities of the GH axis, analogous to observations made in human patients with CNC (5). These data indicate that complete loss of Prkar1a is required for CNC-associated pituitary tumorigenesis and that the secretory effects of the loss of Prkar1a are limited to GH-secreting cells.
RESULTS
Generation of rGHRHR-cre Mice
To generate the pituitary-specific KO of Prkar1a in our mice, it was first necessary to obtain a mouse line expressing the cre recombinase in the appropriate tissue. A mouse line that used the glycoprotein hormone α-subunit promoter to drive cre expression in the pituitary (αGSU-cre) had previously been described (12). This line was reported to express cre in all five pituitary cell subtypes, although there was significant expression in other tissues, most notably skeletal and cardiac muscle. For our studies, we elected to generate a new line of cre mice using the rat GHRH receptor (rGHRHR) promoter to drive cre expression. The promoter for this gene was chosen because GHRHR had been reported to be expressed exclusively in the pituitary and the kidney (13); furthermore, transgenic mice containing a rGHRHR-luciferase transgene expressed luciferase solely in the pituitary gland (14), suggesting that it could function in a highly tissue-specific fashion.
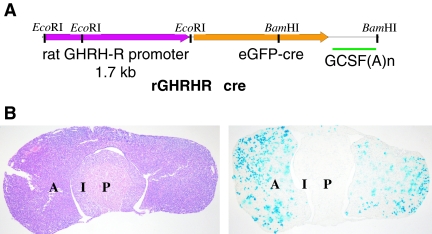
To generate the construct, the rat Ghrhr promoter was isolated and cloned in front of the EGFP-cre structural gene (15) followed by a granulocyte colony-stimulating factor (GCSF) polyadenylation signal (Fig. 1A). Multiple transgenic lines carrying the construct were identified and screened by crossing the offspring to the Z/AP (16) or Rosa26(lacZ) (17) reporter lines. One of the lines generated with the construct (3242) showed stable expression of cre activity (as detected by reporter enzyme staining, Fig. 1B) in a discrete subset of cells of the pituitary gland, and this line was chosen for further characterization.
Figure 1.
rGHRHR-cre Mice Express Cre Recombinase Activity in the Anterior Pituitary
A, Diagram of the rGHRHR-cre transgene. See Materials and Methods for details of the construction of the transgene; B, rGHRHR-cre mice express cre activity in the anterior pituitary. Left, H&E staining of a frozen section of the pituitary of an rGHRHR-cre(3242) mouse; right, alkaline phosphatase staining (purple) from a Z/AP cross reveals cre activity in isolated clusters of cells in the anterior pituitary. A, Anterior lobe; I, intermediate lobe; P, posterior lobe.
Screening of a panel of 16 mouse tissues, including major organs, endocrine glands, and other tissues, demonstrated that reporter activity was confined to the pituitary gland. A full description of the rGHRHR-cre line, including the embryonic pattern of expression of the transgene, will be reported elsewhere (40).
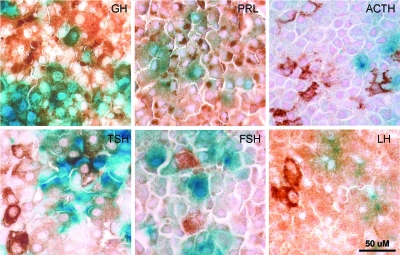
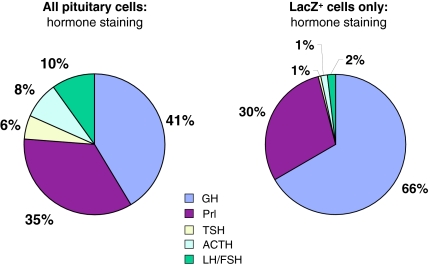
To determine which cells of the pituitary expressed cre activity, mice carrying the transgene were crossed with the Rosa26(lacZ) reporter strain, which expresses lacZ in the presence of cre activity (17). Pituitaries from these mice were isolated and stained in whole mount for β-galactosidase activity. The samples were then postfixed, sectioned, and subjected to immunohistochemistry using specific antisera for each of the pituitary hormones (Fig. 2). As shown in Fig. 3, quantitation of hormonal staining from the anterior pituitary showed a distribution of cell types similar to that observed in previous studies (18). However, when only lacZ-positive cells were counted, it became clear that the majority of cre activity is confined to cells producing GH and Prl. Because TSH-, ACTH, and LH/FSH-producing cells are present in significantly lower numbers in the pituitary, it was thought that the low rate of double staining (<2% of cells) of these cell types represented nonspecific background. However, as discussed below, it appears that the TSH staining may be a real phenomenon. Even for cells producing GH or Prl, the penetrance of cre expression in these cell types was less than 100% (Table 1), similar to what has been observed in the αGSU-cre line (12).
Figure 2.
Cre Activity Is Limited to Cells that Produce GH, Prl, and TSH
Pituitary glands from lacZ reporter crosses were stained in whole mount for β-galactosidase (blue), postfixed, and then analyzed by immunohistochemistry for hormone subunit production. Staining for each of the hormones is indicated by the brown diaminobenzidine color. Each panel shows sections of the same pituitary gland stained for the hormones indicated in the upper right of each picture. Note the colocalization of blue and brown colors in the GH, Prl, and TSH panels, whereas no colocalization is observed for ACTH, LH, or FSH. A scale bar, applicable to all panels in the figure, is shown at lower right.
Figure 3.
Distribution of Hormone Staining and Cre Activity in rGHRHRcre Mice
Hormone staining data from pituitaries (as shown in Fig. 2) were quantitated, and the summary data are shown to indicate the relative distribution of the individual hormone-producing pituitary cell types. A, The distribution of cell subtypes for the entire pituitary gland (note that gonadotroph cells producing LH and FSH are shown as a single cell type); B, distribution of cell subtypes for those cells expressing cre recombinase, as determined by lacZ staining. Percentages for each slice of the pie chart are indicated.
Table 1.
Frequency of Cre-Mediated Recombination in Pituitary Cell Types
| Cell Type | %cre Positive | %cre Negative |
|---|---|---|
| GH | 72.9 | 27.1 |
| Prl | 38.8 | 61.2 |
| TSH | 4.7 | 95.3 |
| ACTH | 7.1 | 92.9 |
| LH/FSH | 11.4 | 88.6 |
Tumorigenesis in PitKO Mice
To determine whether loss of Prkar1a was sufficient to cause pituitary tumorigenesis, mice carrying the rGHRHRcre transgene were crossed to mice carrying a conditional null allele of Prkar1a (10). These mice contain loxP sites surrounding exon 2 of the Prkar1a gene, and excision of this sequence results in a complete null allele (10). The mice were bred for two generations to produce mice of both the rGHRHRcre;Prkar1aloxP/+ and the rGHRHRcre;Prkar1aloxP/loxP genotype (henceforth referred to as pitHET and pitKO mice, respectively). The mice were born in expected Mendelian ratios, and no difference in viability or in weight of the mice was detectable up to 9 months of age (data not shown).
Small groups of mice were killed at 9, 12, and 18 months, and the pituitaries were analyzed for tumor formation. No tumors were observed at 9 or 12 months, but tumors were noted at histological examination from the first group of mice studied at 18 months. Based on these preliminary studies, we elected to study a large group of mice at 18 months of age to determine tumor incidence and subtype.
For this study, we analyzed 49 mice, including 21 pitKOs, 13 pitHETs, and 15 wild-type (WT) mice. The WT mice lacked a cre transgene and thus carried two normal alleles of Prkar1a in their pituitaries. Because we did not observe any difference in any facet of the study between pitHETs and WT mice, these groups were combined to form a single control group for comparison with the pitKO group. Of note, the control and KO mice were littermates all derived from three to four breeding pairs. One pitKO mouse had a visible tumor at the time of necropsy, whereas all others were grossly normal. For this reason, the pituitary gland from each mouse in the study was isolated and studied by histology (Fig. 4) and immunohistochemistry (Fig. 5), where appropriate.
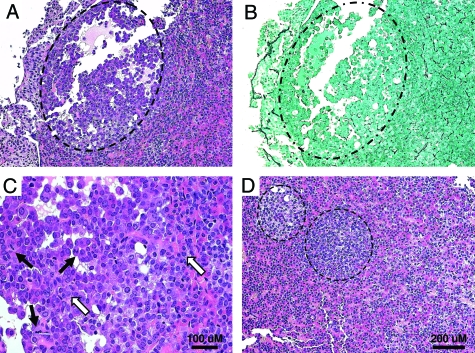
Figure 4.
PitKO Mice Exhibit Pituitary Tumorigenesis
A, H&E-stained section of a pitKO gland showing the presence of a small adenoma, indicated with the dashed line; B, section of the same tumor shows loss of the normal reticulin staining pattern in the region of the tumor (dashed circle); C, higher-power view of the same tumor showing the presence of multiple mitotic figures (black arrows) and dysplastic nuclei (white arrows); D, H&E-stained section of another pitKO pituitary showing the presence of two small adenomas (dashed lines) in the gland. A scale bar applicable to panels A, B, and D is shown at lower right. A separate scale bar for panel C is included in that panel.
Figure 5.
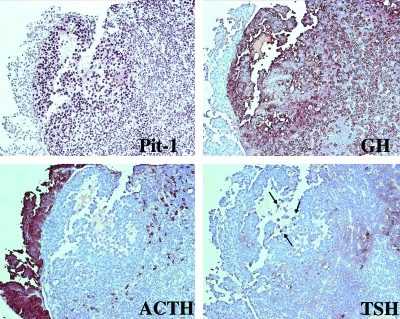
Immunohistochemical Characterization of PitKO Tumors Demonstrates Proliferation of Pit1-Derived Cell Types
The tumor from Fig. 3, A–C, was stained for Pit1 and pituitary hormones. Note that the tumor stains clearly for the Pit-1 transcription (upper left) and strongly for GH (upper right). There is also focal TSH staining, indicated by the arrows (lower right). Note the absence of ACTH staining in the tumor, although this marker strongly stains the intermediate lobe of the pituitary, as expected (lower left). This tumor also exhibited staining for Prl but not for FSH or LH.
Overall, as shown in Table 2, tumors were observed in 15 of the 49 mice (30.6%), but the frequency was significantly higher in the pitKO mice (10 of 21, 48%) than in the control animals (five of 28, 18%; P < 0.05). All tumors in the control group except one were prolactinomas (n = 4), providing a background rate of pituitary tumors in this group of 14% (four of 28), which was not different from the rate of prolactinomas in the pitKO group (four of 21, 19%; P nonsignificant). The difference in tumor rate in the pitKO mice was due to the much higher incidence of nonprolactinomas, which were observed in six of 21 animals (29%), compared with only one such tumor in the control group. All of the nonprolactinoma tumors stained for multiple hormone subtypes (Fig. 5), with tumors exhibiting staining for all hormones from this lineage (Prl/GH/TSH) being the most common.
Table 2.
Incidence of Tumors in PitKO and Control Mice
| Mice | Tumors | Tumor rate | Prolactinoma | Rate | Nonprolactinomaa | Rate | |
|---|---|---|---|---|---|---|---|
| KO | 21 | 10 | 0.48 | 4 | 0.19 | 6 | 0.29 |
| Non-KO | 28 | 5 | 0.18 | 4 | 0.14 | 1 | 0.04 |
| Total | 49 | 15 | 0.31 | 8 | 0.16 | 7 | 0.14 |
| P < 0.05b | P = NS | P < 0.05b |
NS, Not significant.
The nonprolactinoma tumors showed the following: in the WT mouse, the tumor stained for Prl and TSH. In the KO mice, four tumors stained for Prl, TSH, and GH; one tumor stained for Prl and TSH only, and one tumor stained for Prl and GH only.
Comparison of KO vs. non-KO by Fisher’s exact test.
Hormonal Profiles in PitKO Mice
To determine whether the immunohistochemical characteristics of the tumors correlated with serum hormone levels, we measured GH, Prl, and TSH levels in a subset of the mice from the cohort, including 15 pitKOs and 18 control mice (10 WT, eight pitHET), as shown in Table 3. In no case were there any detectable differences in hormone levels between the WT group and the pitHET group (data not shown).
Table 3.
Hormonal Measurements in Study Mice
| N | PRL (ng/ml) ± sd | GH (ng/ml) ± sd | TSH (ng/ml) ± sd | |
|---|---|---|---|---|
| All prolactinomas | 6 | 91.2 ± 46.8a | 12.7 ± 8.7 | 206.3 ± 54.1 |
| Others | 27 | 22.4 ± 20.7 | 18.5 ± 32.4 | 249.6 ± 150.9 |
| All nonprolactinomas | 7 | 29.3 ± 30.8 | 40.0 ± 56.6a | 323.8 ± 248.7 |
| Others | 26 | 36.4 ± 39.7 | 11.4 ± 12.8 | 221.4 ± 93.5 |
| All pitKO | 15 | 34.1 ± 26.4 | 28.3 ± 41.4a | 221.3 ± 107.2 |
| All controls | 18 | 35.6 ± 45.7 | 8.4 ± 6.25 | 257.6 ± 160.2 |
Difference between groups significant at P < 0.01 by Mann-Whitney U test.
Taken as a whole, mice with prolactinomas (n = 6) had significantly higher prolactin levels than mice without these tumors (n = 27) (91.2 ± 46.8 vs. 22.4 ± 20.7 ng/ml, P < 0.01). In these animals, levels of GH or TSH were not significantly different between the groups. Similarly, analysis of all nonprolactinoma tumors showed significant elevation of GH in the tumor group (n = 7) compared with the mice without these tumors (n = 26) (40.0 ± 56.6 vs. 11.4 ± 12.9 ng/ml, P < 0.01). Although there was a slight increase in TSH levels in the nonprolactinoma tumor group (323.8 ± 248.7 vs. 221.4 ± 93.5 ng/ml), this did not reach statistical significance. Prl levels were also not different between the tumor groups. These data indicated that hormonal staining of the tumors correlated well with serum hormone levels.
More importantly, however, when hormone levels from the pitKO mice were compared with the control group, there was a significant elevation in GH production that was observed even in the absence of frank tumors (28.27 ± 41.4 vs. 8.44 ± 20.7 ng/ml, P < 0.01). Despite the increased GH in the pitKO mice, we did not observe any significant differences in weight between the two groups. (35.0 ± 7.3 g vs. 34.2 ± 8.3 g, P not significant). Comparison of the GH levels to mouse weight showed a moderate but statistically significant correlation between these variables (Pearson correlation coefficient 0.36, with a 95% confidence interval of 0.01–0.64 at α = 0.05), which was the same across all groups of animals.
DISCUSSION
Abnormalities of the PKA axis are well established as a cause of human pituitary adenomas. This is best characterized in the case of activating mutations of the stimulatory G protein subunit Gsα (encoded by the GNAS gene, also known as the GSP oncogene) (19). These mutations were also described as causing pituitary tumors in association with the McCune-Albright syndrome (8) and have subsequently been reported in 30–50% of all GH-producing pituitary adenomas (20,21). These mutations lead to constitutive activation of the G protein, with subsequent stimulation of adenylyl cyclase, cAMP generation, and PKA activation. Inactivating mutations in PRKAR1A also cause elevated PKA activity (9), so it was not surprising that CNC patients also develop abnormalities of the GH axis and frank GH-producing pituitary adenomas (4). In contrast to the situation with GNAS mutations, mutations of PRKAR1A in sporadic pituitary tumors are extremely uncommon, as is allelic loss of the gene (22,23,24). The possibility that PRKAR1A expression can be altered by epigenetic means has been raised by recent data showing that immunostaining for PRKAR1A is reduced in GH-producing tumors and that this reduction enhances growth of this particular pituitary cell type (25). Interestingly, a similar study analyzing nonsecreting tumors showed no significant changes in PRKAR1A, either in vivo or in vitro (26).
Knockout of a wide variety of general tumor suppressor genes from the mouse leads to hyperplasia or tumor formation of the intermediate lobe, such as is seen in mice carrying mutations in pRb, p27Kip1, and p18Ink4 (27,28,29,30,31). The relevance of these observations to human tumor pituitary tumorigenesis is unclear (see discussion in Refs. 32 and 33). Pituitary tumors can also be obtained in mice by the overexpression of strong oncogenes (e.g. SV40 T antigen). Again, the relevance to the human condition is uncertain. Alterations of hormone regulation of pituitary cells, such as in GHRH-overexpressing transgenic mice or knockout of dopamine receptors, leads to the formation of pituitary tumors in target adenohypophyseal cells; however, the relevance of these models to human pituitary tumors is also questionable, because human adenomas are known not to harbor similar alterations in the vast majority of cases. Of note, the introduction of a mutation in p27KIP1 into the GHRH-overexpressing line led to a marked potentiation of tumorigenesis (28).
Previous studies of pituitary tumorigenesis relevant to CNC have been carried out in heterozygous KO mice, which are the best genetic model for the human disease. In our studies of Prkar1a heterozygous mice up to 2 yr of age, a pituitary tumor was seen in only one mouse (10), making its connection to genetic modification unclear. Similarly, in another mouse model of Prkar1a mutation, no pituitary tumors were observed up to 19 months (11).
In contrast to these previous studies, the pituitary-specific KO mice in the present study developed pituitary tumors with high frequency, although not until advanced age (Table 2). Similar to the observations in the Prkar1a heterozygous mice, no tumors were observed in the subset of mice characterized as pitHET mice. This observation suggests that haploinsufficiency for Prkar1a is not sufficient to promote tumorigenesis and that complete loss of Prkar1a is necessary for tumor formation. This observation is in keeping with our previous hypothesis regarding the need for complete loss of this protein in tumor formation (10). Given the fact that it took the mice an extended period of time to develop tumors, it is possible that loss of Prkar1a is not sufficient for tumorigenesis and that the long lead time may be explained by the need to accumulate additional genetic hits. At present, there are not enough data to scientifically evaluate this statement. It is interesting to note that in most mouse pituitary tumor models, tumor formation does not occur until the mice reach an advanced age. This may be due to the fact that the pituitary has an extremely low postnatal proliferation rate or the fact that it has a low rate of accumulation of additional hits. More data are needed to address this point.
One of the interesting observations from this study is that although the mice have apparently lost Prkar1a in all Pit1+ cells, the large majority of the tumors expressed GH. The three Pit1 lineages all respond to hormonal input signals via G protein-coupled receptors. For the GH lineage, most of the signal is mediated through the GHRHR, which is a type B1 G protein-coupled receptor that transduces signals through cAMP-mediated signaling (34). In contrast, Prl secretion is primarily mediated in the pituitary by tonic negative feedback by the dopamine D2 receptor, which couples to the inhibitory G protein subunit (Gi). Indeed, KO of the dopamine D2 receptor from mice leads to the frequent production of prolactinomas (35). Prolactinomas are the most common tumor observed in MEN1 patients, and mice heterozygous for Men1 mutations develop prolactinomas by 16 months at a rate fairly similar to human patients (10), suggesting that the connection between Men1 signaling and cell proliferation may be relatively specific for this cell type. Finally, TSH-producing cells respond predominantly to TRH, which transduces its signal through the inositol phosphate-PKC pathway (36). Thus, there appears to be a direct relationship between activation of PKA signaling and hyperactivity of the somatotroph cell type. As is seen in CNC patients, pitKO mice exhibited excess GH secretion even in the absence of frank tumors (4). Cell activation extended beyond hormone secretion and included excess cell growth and tumorigenesis. This model may therefore serve as a new means to address questions of somatotroph growth or as a model to test new therapies aimed at acromegaly in human patients. This may be of particular relevance given that there is a substantial portion of human patients with acromegaly that are not surgically cured of their disease.
Recently, a transgenic mouse model in which cre expression was driven by the rat GH promoter was described (37). In that model, cre expression was observed predominantly in GH-producing cells, with much lower expression of cre in other cell types, including lactotrophs. The authors propose that GH- and Prl-producing cells develop in a parallel fashion, in contrast to previous evidence suggesting that Prl cells arise from somatotrophs. Our data do not provide a means to discriminate between these two models, although our data clearly indicate that these two cell types (in addition to a percentage of thyrotrophs) share a common embryonic precursor, which has been targeted by the rGHRHRcre transgene.
In summary, we have generated a mouse line expressing cre recombinase in the subset of pituitary cells that express the Pit1 transcription factor. This gene, which turns on late in embryogenesis, is required for the development of cells that secrete GH, Prl, and TSH, and mice carrying the transgene express cre in all three of these pituitary cell types. In mice carrying a tissue-specific KO of the CNC gene Prkar1a, pituitary adenomas develop with a moderate frequency and appear to be limited to cells of the appropriate lineage. There is also clear evidence that pitKO mice exhibit hormone abnormalities limited to the GH axis, in close analogy to CNC patients. These mice serve as a good model not only for the pituitary tumorigenesis observed in this human condition but also with more general applicability to studying signaling pathway-specific phenomena in the pituitary and as a potential model to test therapies aimed at treating this human condition.
MATERIALS AND METHODS
Mouse Breeding and Maintenance
The generation of the rGhrhr-cre mice will be presented in full in a separate manuscript (40). The Prkar1a conditional allele has previously been described (10). Animals were bred and maintained in microisolator racks with 12-h light, 12-h dark cycles and allowed to age undisturbed, except for monitoring and periodic weighing of a subset of mice. All animal work described in this manuscript was conducted in accordance with the highest standards of ethical care for experimental animals. The research was conducted under The Ohio State University animal protocol 02-A-0097.
Analysis of Cre Expression
For initial studies of cre expression, transgenic mice were bred to the Z/AP reporter line (16), and tissues were collected from double-transgenic offspring and analyzed for β-galactosidase and alkaline phosphatase activities as described (38). For colocalization of β-galactosidase activity and hormone immunoreactivity, whole pituitaries were removed and fixed in cold 4% paraformaldehyde for 1 h and then stained with X-gal as above. After staining was completed, samples were postfixed in 10% formalin and processed as described below.
Analysis of Mouse Pituitaries
Mice were killed by CO2 inhalation, and their pituitary glands were inspected visually, removed, and placed immediately in 10% buffered formalin. Pituitaries were embedded in paraffin and sectioned sagittally. Paraffin-embedded sections 4–5 μm thick were stained with hematoxylin and eosin (H&E) or the Gordon-Sweet silver method for reticulin matrix. Immunohistochemical stains to localize adenohypophyseal hormones were performed using the streptavidin-biotin peroxidase technique. Primary antisera directed against rat pituitary hormones were used at the following dilutions: GH, 1:2500; Prl, 1:2500; TSHβ, 1:3000; FSHβ, 1:600; LHβ, 1:2500 (all provided through the National Hormone and Peptide Program, Torrance, CA), and prediluted ACTH, further diluted 1:20 (Dako Corp., Carpinteria, CA). Transcription factor expression was determined using a polyclonal antiserum to Pit-1 from Babco (Berkeley, CA) and a monoclonal antibody to Tpit (kindly provided by Dr. J. Drouin, Montreal, Canada). Immunolocalization was detected with the streptavidin-biotin-peroxidase complex technique and 3,3′-diaminobenzidine. Negative controls were performed with normal mouse ascites or normal rabbit serum replacing the primary monoclonal or polyclonal antibody, respectively, after preabsorption of the primary antibody or antiserum with purified antigen. Colocalization studies were performed as previously described (39).
Analysis of Hormone Levels
Mice were anesthetized with CO2, and whole blood volume was withdrawn by cardiac puncture with samples being placed immediately on ice. Samples were spun at 1000 × g for 5 min at 4 C to pellet cells, and the resultant serum was stored at −20 C until completion of the study, when all samples were analyzed in batch. All hormone levels were analyzed in duplicate in a single run at the National Hormone and Peptide Program. Statistical analysis of tumor incidence was performed by Fisher’s exact test. Analysis of hormone levels was analyzed according to Mann-Whitney U analysis.
Acknowledgments
We acknowledge Heiner Westphal and Constantine Stratakis for assistance in the early phases of this project. We also thank William Towns for excellent technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Grants CA112268 (to L.S.K.) and CA16058 (to the Ohio State University Comprehensive Cancer Center).
Disclosure Statement: The authors have no conflicts to disclose.
First Published Online November 1, 2007
Abbreviations: CNC, Carney complex; H&E, hematoxylin and eosin; KO, knockout; MEN1, multiple endocrine neoplasia type 1; PKA, protein kinase A; Prl, prolactin; rGHRHR, rat GHRH receptor; WT, wild type.
References
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE 2004 The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619 [DOI] [PubMed] [Google Scholar]
- Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells Jr SA, Marx SJ 2001 Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Kirschner LS, Carney JA 2001 Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 86:4041–4046 [DOI] [PubMed] [Google Scholar]
- Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA 2000 Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex). J Clin Endocrinol Metab 85:3860–3865 [DOI] [PubMed] [Google Scholar]
- Stergiopoulos SG, Abu-Asab MS, Tsokos M, Stratakis CA 2004 Pituitary pathology in Carney complex patients. Pituitary 7:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares BS, Frohman LA 2004 Isolated familial somatotropinoma. Pituitary 7:95–101 [DOI] [PubMed] [Google Scholar]
- Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, Borson-Chazot C, Estour B, Ciccarelli E, Brue T, Ferolla P, Emy P, Colao A, De Menis E, Lecomte P, Penfornis F, Delemer B, Bertherat J, Wemeau JL, De Herder W, Archambeaud F, Stevenaert A, Calender A, Murat A, Cavagnini F, Beckers A 2006 Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab 91:3316–3323 [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM 1991 Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000 Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet 26:89–92 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns 2nd WH, Carney JA, Westphal H, Stratakis CA 2005 A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65:4506–4514 [DOI] [PubMed] [Google Scholar]
- Veugelers M, Wilkes D, Burton K, McDermott DA, Song Y, Goldstein MM, La Perle K, Vaughan CJ, O’Hagan A, Bennett KR, Meyer BJ, Legius E, Karttunen M, Norio R, Kaariainen H, Lavyne M, Neau JP, Richter G, Kirali K, Farnsworth A, Stapleton K, Morelli P, Takanashi Y, Bamforth JS, Eitelberger F, Noszian I, Manfroi W, Powers J, Mochizuki Y, Imai T, Ko GT, Driscoll DA, Goldmuntz E, Edelberg JM, Collins A, Eccles D, Irvine AD, McKnight GS, Basson CT 2004 Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci USA 101:14222–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman LJ, Burrows HL, Seasholtz AF, Lewandoski M, Muzyczka N, Camper SA 2000 Cre-mediated recombination in the pituitary gland. Genesis 28:167–174 [DOI] [PubMed] [Google Scholar]
- Matsubara S, Sato M, Mizobuchi M, Niimi M, Takahara J 1995 Differential gene expression of growth hormone (GH)-releasing hormone (GRH) and GRH receptor in various rat tissues. Endocrinology 136:4147–4150 [DOI] [PubMed] [Google Scholar]
- McElvaine AT, Korytko AI, Kilen SM, Cuttler L, Mayo KE 2007 Pituitary-specific expression and Pit-1 regulation of the rat growth hormone-releasing hormone receptor gene. Mol Endocrinol 21:1969–1983 [DOI] [PubMed] [Google Scholar]
- Le Y, Miller JL, Sauer B 1999 GFPcre fusion vectors with enhanced expression. Anal Biochem 270:334–336 [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A 1999 Z/AP, a double reporter for cre-mediated recombination. Dev Biol 208:281–292 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999 Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71 [DOI] [PubMed] [Google Scholar]
- Heaney AP, Melmed S 2004 Molecular targets in pituitary tumours. Nat Rev Cancer 4:285–295 [DOI] [PubMed] [Google Scholar]
- Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L 1989 GTPase inhibiting mutations activate the α-chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340:692–696 [DOI] [PubMed] [Google Scholar]
- Spada A, Vallar L 1992 G-protein oncogenes in acromegaly. Horm Res 38:90–93 [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Yu S, Warner DR, Liu J 2001 Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705 [DOI] [PubMed] [Google Scholar]
- Kaltsas GA, Kola B, Borboli N, Morris DG, Gueorguiev M, Swords FM, Czirjak S, Kirschner LS, Stratakis CA, Korbonits M, Grossman AB 2002 Sequence analysis of the PRKAR1A gene in sporadic somatotroph and other pituitary tumours. Clin Endocrinol (Oxf) 57:443–448 [DOI] [PubMed] [Google Scholar]
- Sandrini F, Kirschner LS, Bei T, Farmakidis C, Yasufuku-Takano J, Takano K, Prezant TR, Marx SJ, Farrell WE, Clayton RN, Groussin L, Bertherat J, Stratakis CA 2002 PRKAR1A, one of the Carney complex genes, and its locus (17q22–24) are rarely altered in pituitary tumours outside the Carney complex. J Med Genet 39:e78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Mizusawa N, Nagahiro S, Yamada S, Sano T, Itakura M, Yoshimoto K 2003 GH-secreting pituitary adenomas infrequently contain inactivating mutations of PRKAR1A and LOH of 17q23–24. Clin Endocrinol (Oxf) 58:464–470 [DOI] [PubMed] [Google Scholar]
- Lania AG, Mantovani G, Ferrero S, Pellegrini C, Bondioni S, Peverelli E, Braidotti P, Locatelli M, Zavanone ML, Ferrante E, Bosari S, Beck-Peccoz P, Spada A 2004 Proliferation of transformed somatotroph cells related to low or absent expression of protein kinase a regulatory subunit 1A protein. Cancer Res 64:9193–9198 [DOI] [PubMed] [Google Scholar]
- Mantovani G, Bondioni S, Ferrero S, Gamba B, Ferrante E, Peverelli E, Corbetta S, Locatelli M, Rampini P, Beck-Peccoz P, Spada A, Lania AG 2005 Effect of cyclic adenosine 3′,5′-monophosphate/protein kinase A pathway on markers of cell proliferation in nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 90:6721–6724 [DOI] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA 1992 Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- Teixeira LT, Kiyokawa H, Peng XD, Christov KT, Froh-man LA, Kineman RD 2000 p27Kip1-deficient mice ex-hibit accelerated growth hormone-releasing hormone (GHRH)-induced somatotrope proliferation and adenoma formation. Oncogene 19:1875–1884 [DOI] [PubMed] [Google Scholar]
- Philipp-Staheli J, Kim KH, Liggitt D, Gurley KE, Longton G, Kemp CJ 2003 Distinct roles for p53, p27(Kip1), and p21(Cip1) during tumor development. Oncogene 23:905–913 [DOI] [PubMed] [Google Scholar]
- Sotillo R, Renner O, Dubus P, Ruiz-Cabello J, Martin-Caballero J, Barbacid M, Carnero A, Malumbres M 2005 Cooperation between Cdk4 and p27kip1 in tumor development: a preclinical model to evaluate cell cycle inhibitors with therapeutic activity. Cancer Res 65:3846–3852 [DOI] [PubMed] [Google Scholar]
- Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y 1998 CDK inhibitors p18(INK4c) and p27(Kip1) mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev 12:2899–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa SL 2001 Transgenic and knockout mouse models clarify pituitary development, function and disease. Brain Pathol 11:371–384 [PubMed] [Google Scholar]
- Asa SL, Ezzat S 2002 The pathogenesis of pituitary tumours. Nat Rev Cancer 2:836–849 [DOI] [PubMed] [Google Scholar]
- Harmar AJ 2001 Family-B G-protein-coupled receptors. Genome Biol 2:REVIEWS3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asa SL, Kelly MA, Grandy DK, Low MJ 1999 Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 140:5348–5355 [DOI] [PubMed] [Google Scholar]
- Gershengorn MC 1989 Mechanism of signal transduction by TRH. Ann NY Acad Sci 553:191–196 [DOI] [PubMed] [Google Scholar]
- Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD 2007 Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology 148:1946–1953 [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, Hager JH, Hanahan D, Edlund H, Magnuson MA, Garrett-Beal L, Burns AL, Ried T, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS 2003 Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol Cell Biol 23:6075–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennani-Baiti IM, Asa SL, Song D, Iratni R, Liebhaber SA, Cooke NE 1998 DNase I-hypersensitive sites I and II of the human growth hormone locus control region are a major developmental activator of somatotrope gene expression. Proc Natl Acad Sci USA 95:10655–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Williams-Simons L, Rahwahneh L, Asa S, Kirschner LS, Development of a pituitary-specific Cre line targeted to the Pit-1 lineage. Genesis, in press [DOI] [PubMed] [Google Scholar]