Abstract
In most mammals, prostaglandin F2α (PGF2α) is believed to be a trigger that induces the regression of the corpus luteum (CL), whereby progesterone synthesis is inhibited, the luteal structure involutes, and the reproductive cycle resumes. Studies have shown that the early growth response 1 (EGR1) protein can induce the expression of proapoptotic proteins, suggesting that EGR1 may play a role in luteal regression. Our hypothesis is that EGR1 mediates the actions of PGF2α by inducing the expression of TGF β1 (TGFB1), a key tissue remodeling protein. The levels of EGR1 mRNA and protein were up-regulated in the bovine CL during PGF2α-induced luteolysis in vivo and in PGF2α-treated luteal cells in vitro. Using chemical and genetic approaches, the RAF/MAPK kinase (MEK) 1/ERK pathway was identified as a proximal signaling event required for the induction of EGR1 in PGF2α-treated cells. Treatment with PGF2α increased the expression of TGFB1 mRNA and protein as well as the binding of EGR1 protein to TGFB1 promoter in bovine luteal cells. The effect of PGF2α on TGFB1 expression was mimicked by a protein kinase C (PKC)/RAF/MEK1/ERK activator or adenoviral-mediated expression of EGR1. The stimulatory effect of PGF2α on TGFB1 mRNA and TGFB1 protein secretion was inhibited by blockade of MEK1/ERK signaling and by adenoviral-mediated expression of NAB2, an EGR1 binding protein that inhibits EGR1 transcriptional activity. Treatment of luteal cells with TGFB1 reduced progesterone secretion, implicating TGFB1 in luteal regression. These studies demonstrate that PGF2α stimulates the expression of EGR1 and TGFB1 in the CL. We suggest that EGR1 plays a role in the expression of genes whose cognate proteins coordinate luteal regression.
THE CORPUS LUTEUM (CL) is a transient endocrine gland that develops from the remnants of ovarian follicular cells after ovulation. The CL secretes progesterone for a period of time characteristic of the species and then undergoes luteolysis (disruption of progesterone synthesis and regression of the CL) unless pregnancy ensues (1,2). It is generally accepted that prostaglandin F2α (PGF2α) is the natural luteolysin in most mammals including domestic farm animals like cows, but the cellular and molecular mechanisms that mediate luteolysis remain poorly understood (1).
The initial response of steroidogenic luteal cells to PGF2α involves binding to specific G protein-coupled receptors (3) and subsequent activation of the phospholipase C-diacylglycerol-inositol-1,4,5-trisphosphate-Ca2+-PKC signaling pathway (4). PKC can activate the RAF1/MAP2K1/MAPK3/MAPK1 (also known as the Raf/MEK1/ERK1/2) pathway in luteal cells (5,6,7). Activated ERK1/2 then translocates to the nucleus where it phosphorylates and activates transcription factors, including the transcription factor Elk1 (5) and Jun D (7). Activated Elk1, along with another transcription factor, serum response factor, binds to the serum response element on gene promoters and regulates the expression of the target genes, like FOS, JUN (8), and the early growth response 1 gene (EGR1) (7,9,10,11).
The EGR1 gene belongs to a group of immediate-early response genes and encodes a zinc finger-containing DNA-binding transcription factor (12,13,14,15). Studies have shown that the gonadotropins FSH and LH can induce EGR1 gene expression in ovarian granulosa cells, suggesting that EGR1 may mediate the molecular programs of differentiation and luteinization in the ovary (16). EGR1 knockout mice have a reduced body size, and both males and females are sterile. Additionally, EGR1 null mice fail to synthesize the β-subunit of LH in pituitary and the expression of LH receptors in the ovary is markedly down-regulated (17). This evidence indicates that the EGR1 gene has a central role in the control of reproductive function. Despite the discovery of EGR1 as a cell proliferation-promoting protein, studies in various cell lines have shown that EGR1 can induce the expression of various tissue remodeling and proapoptotic proteins, suggesting that stimulation of the EGR1 gene is also an active part of the apoptotic-signaling cascade (18,19). For example, EGR1 mRNA and protein levels are increased in response to a variety of stresses (9), including ionizing radiation, UV irradiation, shear stress, hypoxia, ischemia/reperfusion injury, and cytotoxic cytokines like TNFα and interferon γ. It has been shown that EGR1, as a transcription factor, can bind to the promoter regions and activate transcription of various genes, including the TGF β1 gene (TGFB1) (20,21). TGFB1 is a multifunctional peptide that influences proliferation, differentiation, and apoptosis in many cell types (22). It also acts as a negative autocrine growth factor and serves as negative regulator of epithelial cell proliferation (23).
Our previous studies show that TGFB1 is produced by the bovine CL (24), and recent studies indicate that TGFB1 mRNA is increased in rat CL during luteal regression (25). Based on the current information we postulate that PGF2α will stimulate the expression of TGFB1 by an EGR1-dependent mechanism in the CL. In this study, we demonstrated that PGF2α stimulates the expression of EGR1 mRNA in bovine CL in vivo and in isolated steroidogenic cells in vitro, and determined the signaling pathway regulating its expression in isolated luteal cells. Furthermore, we demonstrated that EGR1 plays an important role in the regulation of TGFB1 secretion in luteal cells.
RESULTS
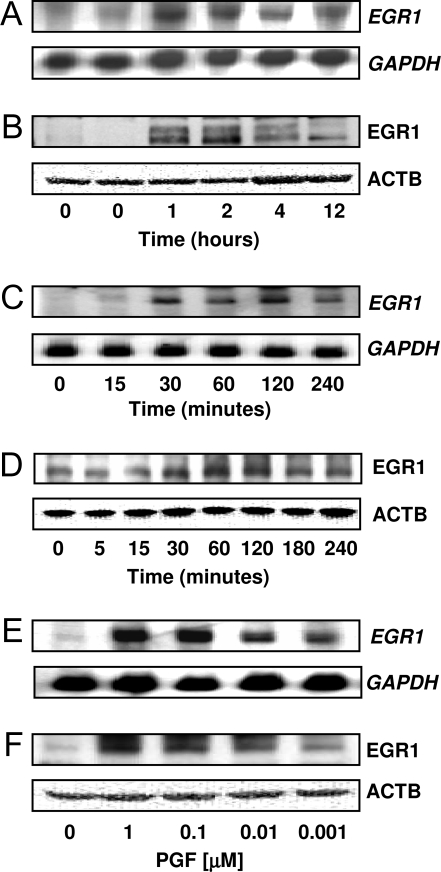
PGF2α Induces the Expression of EGR1
Total cellular RNA was isolated from CL collected from saline-injected controls (0 h) or after 1, 2, 4, and 12 h after injection with a luteolytic dose of a PGF2αanalog. Analysis of luteal tissue showed that EGR1 mRNA (Fig. 1A) and EGR1 protein (Fig. 1B) were elevated within 1–2 h after treatment and gradually declined. In primary cultures of bovine luteal cells the levels of EGR1 mRNA (Fig. 1C) and EGR1 protein (Fig. 1D) increased within 30 min after treatment with 1 μm PGF2α, reached maximal levels after 2 h of treatment, and then gradually declined. Concentration-response studies indicated that EGR1 mRNA (Fig. 1E) and EGR1 protein (Fig. 1F) were elevated in response to treatment with as little as 1 nm PGF2α. Maximal increases in EGR1 expression were observed with 100 nm–1 μm PGF2α.
Figure 1.
PGF2α Induces EGR1 Expression in Vivo and in Vitro
A and B, Angus crossbred cows (two to four animals per time point) were injected im with 25 mg Lutalyse or saline during the mid-luteal phase and CL were removed at 0, 1, 2, 4, and 12 h after injection. The total RNA and protein were extracted as described in Materials and Methods. A, Northern blot analysis of EGR1 mRNA. The same blot was also probed for GAPDH mRNA as a loading control. B, Western blot analysis using an EGR1-specific antibody (1:1000). The same blot was also probed with β-actin (ATCB) (1:5000) as a loading control. C and D, Primary cultures of bovine luteal cells were treated with PGF2α (1 μm) for 5, 15, 30, 60, 120, 180, and 240 min. Cells were extracted and subjected to Northern and Western analysis described above. E and F, Luteal cells were treated for 1 h with increasing concentrations (1 nm–1 μm) of PGF2α). The EGR1 mRNA (C and E) and EGR1 protein (D and F) are shown.
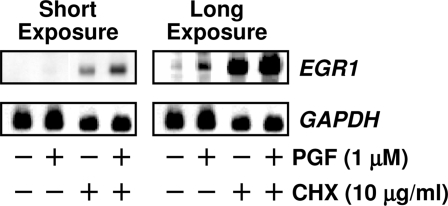
The rapid response to PGF2α indicates that EGR1 is an early response gene in bovine luteal tissue. To investigate whether the induction of EGR1 mRNA by PGF2α requires de novo protein synthesis, we treated luteal cells with or without the protein synthesis inhibitor cycloheximide (CHX, 10 μg/ml) for 4 h followed by treatment with control media or PGF2α (1 μm) for 1 h. Northern blot analysis showed that CHX pretreatment did not block the PGF2α-induced EGR1 mRNA expression (Fig. 2), indicating that EGR1 mRNA induction does not require de novo protein synthesis. Furthermore, CHX caused a super-induction of EGR1 mRNA levels in cells treated with PGF2α (Fig. 2, short exposure), a characteristic of an early response gene.
Figure 2.
PGF2α-Induced EGR1 mRNA Does Not Require de Novo Protein Synthesis
Luteal cells were pretreated with control media or CHX (10 μg/ml) for 4 h before adding PGF2α (1 μm) for another 60 min. Total RNA was extracted and subjected to Northern blot analysis described in Fig. 1. The Northern blot was subjected to a short and long exposure to demonstrate various levels of EGR1 mRNA.
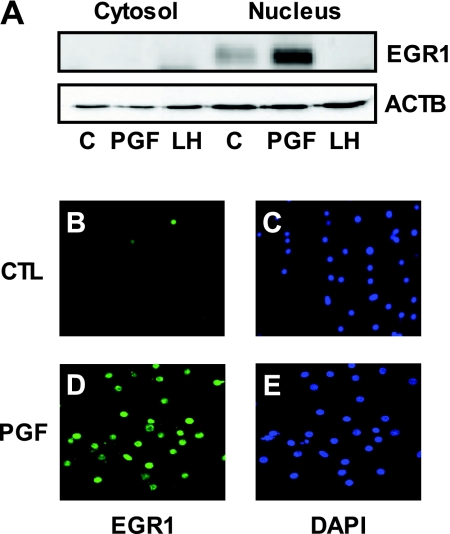
EGR1 Protein Is Localized in the Nucleus in Response to PGF2α Stimulation in Luteal Cells
Subcellular fractionation and immunofluorescence localization studies were performed to examine the subcellular localization of EGR1 protein in response to PGF2α treatment. Analysis of cytosolic and nuclear extracts showed that EGR1 protein was localized primarily in the nucleus of luteal cells. Whereas the levels of EGR1 protein were low in control cells, the levels of nuclear EGR1 protein were greatly enhanced in response to treatment with PGF2α (1 μm, 60 min) (Fig. 3A). Immunofluorescence analysis also confirmed the nuclear localization of EGR1 protein in response to PGF2α treatment (Fig. 3D). Little EGR1 immunoreactivity was observed in the nuclei of control cells (Fig. 3, B–C), but abundant nuclear EGR1 staining was detected after PGF2α treatment (Fig. 3, D–E). No immunofluorescence was detected in the absence of primary antibody (data not shown).
Figure 3.
Nuclear Localization of EGR1 Protein
A, Bovine luteal cells were treated with or without PGF2α (1 μm) or LH (100 ng/ml) for 1 h and then cytoplasmic (Cytosol) and nuclear (Nucleus) fractions were prepared using the Nuclear Extract Kit according to the manufacturer’s instructions. Proteins (40 μg) were separated on 10% SDS-PAGE and transferred to PVDF membranes. EGR1 protein levels were examined by Western blot using the EGR1 antibody (1:1000) and β-actin (ACTB) (1:5000) served as a loading control. B–E, Bovine luteal cells were cultured in four-well Lab-Tek glass chamber slides as described in Materials and Methods. After 24 h in serum-free media, cells were treated with or without PGF2α (1 μm) for 1 h. Photomicrographs showed EGR1 immunofluorescence (green) present in the nucleus of luteal cells after PGF2αtreatment. 4′, 6-Diamidino-2-phenylindole (DAPI) (blue) was used to stain nucleus. Immunofluorescence localization was repeated on cell preparations from three separate ovaries and representative photographs are presented. CTL, Control.
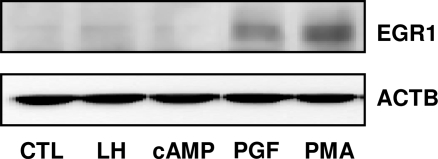
PGF2α and Phorbol 12-Myristate 13-Acetate (PMA), But Neither LH Nor cAMP, Stimulate the Expression of EGR1 Protein in Bovine Luteal Cells
Experiments were conducted to determine whether EGR1 protein expression in luteal cells was responsive to activation of the PKC or protein kinase A signaling pathways. Bovine luteal cells were treated with LH (100 ng/ml), a cell-permeable cAMP analog, 8-Bromo-cAMP (8-Br-cAMP) (2 mm), PGF2α (1 μm), and a PKC activator PMA (20 nm) for 1 h. Both PGF2α and PMA increased EGR1 protein levels. However, neither LH nor 8-Br-cAMP stimulated an increase in EGR1 protein (Fig. 4). In keeping with this observation, LH did not increase concentrations of EGR1 protein in cytosolic and nuclear cell fractions (Fig. 3A). LH and 8-Br-cAMP did, however, stimulate progesterone synthesis in luteal cells (5.7 ± 0.4 and 5.9 ± 0.8, fold increase ± sem, respectively).
Figure 4.
PGF2α and PMA, But Not LH or cAMP, Elevate EGR1 Protein in Luteal Cells
Bovine luteal cells were treated with LH (100 ng/ml), 8-Br-cAMP (2 mm), PGF2α (1 μm) or PMA (20 nm), for 90 min. EGR1 protein levels were examined by Western blot using an antibody specific for EGR1 (1:1000) as described in Fig. 1. The same blot was also probed with β-actin (ACTB) (1:5000) as a loading control. CTL, Control.
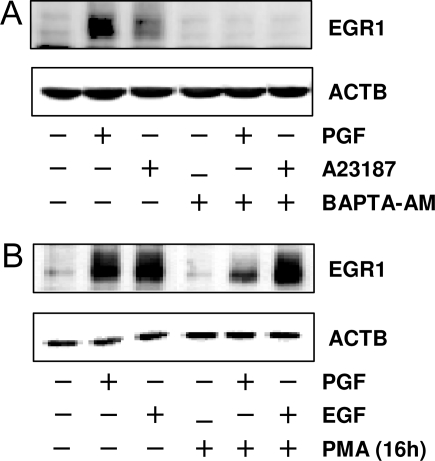
Studies were performed to examine whether calcium and/or PKC were involved in PGF2α induced EGR1 protein up-regulation. Luteal cells were treated with either Ca2+ ionophore, A23187 (10 μm), or PGF2α (1 μm) for 1 h. Both PGF2α and A23187 increased EGR1 protein levels, but the response to A23187 treatment was modest compared with the response to PGF2α (Fig. 5A). The induction of EGR1 by either PGF2αor A23187 was completely abolished by pretreating cells with a membrane-permeable calcium chelator BAPTA-AM (25 μm).
Figure 5.
PGF2α Induces EGR1 Expression through Calcium and PKC-Dependent Pathway
A, Luteal cells were pretreated with BAPTA-AM (25 μm) for 1 h before treatment for 1 h with PGF2α (1 μm) or calcium ionophore A23187 (1 μm). B, Luteal cells were pretreated with PMA (20 nm) for 16 h to deplete phorbol ester-responsive isoforms of PKC. Cells were then treated with either PGF2α (1 μm) or EGF (10 ng/ml) for another 60 min. EGR1 protein levels were examined by Western blot as described in Fig. 1. β-Actin (ACTB) (1:5000) served as a loading control.
To further evaluate whether PKC was involved in PGF2α-induced EGR1 expression, luteal cells were treated with 2 μm PMA for 16 h to deplete phorbol ester-responsive isoforms of PKC as previously reported (26). The chronic treatment with PMA attenuated PGF2α-induced but not epidermal growth factor (EGF)-induced increases in EGR1 protein, indicating a role of phorbol ester-responsive isoforms of PKC in mediating PGF2α-induced EGR1 expression in bovine luteal cells (Fig. 5B).
Inhibition of the MEK1/ERK Signaling Pathway Blocked the PGF2α-Induced Increases in EGR1 Protein
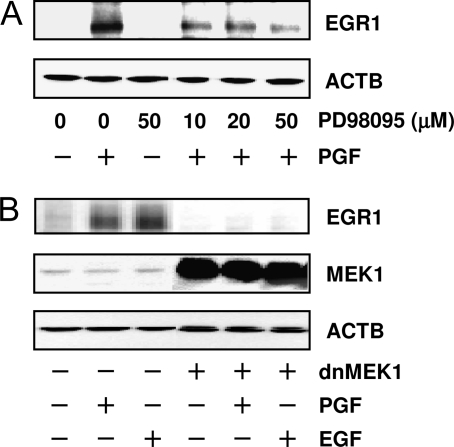
Previous reports demonstrate that PKC can activate MAPK3/1 1/2 (ERK1/2) in cultured bovine luteal cells (26,27). Furthermore, PGF2α-induced ERK1/2 activation can be effectively inhibited by pretreatment with the MEK1 inhibitor PD098095 (26). Herein, studies were performed to examine whether the MEK1/ERK1/2 signaling pathway was also involved in the increase in EGR1 in response to PGF2α. Cultured luteal cells were pretreated with PD098095 (10–50 μm) for 1 h followed by treatment with 1 μm PGF2α for 1 h. The MEK1 inhibitor PD098095 attenuated the stimulatory effects of PGF2α on EGR1 protein levels (Fig. 6A). To further confirm ERK signaling is required for EGR1 expression, we overexpressed dominant-negative (dn) MEK1 (gift from Dr. J. Han) in luteal cells utilizing a well-characterized adenovirus (Ad) (28). Western blots demonstrated that treatment with Ad.dnMEK1 completely blocked the both PGF2α- and EGF-induced increases in EGR1 protein (Fig. 6B). When luteal cells were pretreated with Ad expressing a dn Ras (Ad.dnRas, a gift from Dr. B. K. Kahn) or AG1470, a EGF receptor tyrosine kinase inhibitor, the stimulatory actions of EGF but not PGF2α on EGR1 were abolished (data not shown). Taken together, these observations demonstrate that MEK1/ERK1/2 signaling is required in PGF2α-induced EGR1 expression in luteal cells.
Figure 6.
PGF2α-Induced EGR1 Protein Elevation Was MEK1/ERK Dependent
A, Luteal cells were pretreated with the MEK1 inhibitor PD98095 (50, 25, and 10 μm) for 1 h before treatment for 1 h with PGF2α (1 μm). EGR1 protein was detected by Western blot. Results were representative of three experiments. β-Actin (ACTB) (1:5000) served as a loading control. B, Luteal cells were infected with Ad expressing dominant-negative mutant MEK1 (Ad.dnMEK1) for 24 h as described in Materials and Methods. Cells were then stimulated with either control media, PGF2α (1 μm) or EGF (10 ng/ml) for 1 h. EGR1 protein was detected by Western blot as described in Fig. 1. The membranes were also blotted with antibodies against MEK1 (1:000) to demonstrate the expression of the ectopic protein and for β-actin (ACTB) (1:5000) to verify equal protein loading.
PGF2α Increased TGFB1 Expression in Bovine CL in Vivo and in Luteal Cells in Vitro
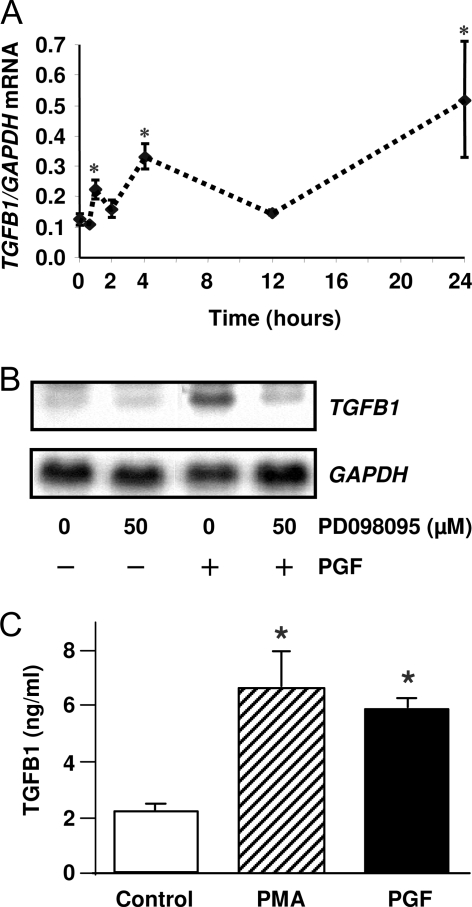
Because it has been shown that the transcription factor EGR1 can bind to the promoter regions and thereby activate transcription of various proapoptotic genes, including TGFB1 (18,19,20), we postulated that in vivo treatment with PGF2α would induce TGFB1 mRNA in bovine CL. To accomplish this, bovine CL were removed at 0, 0.5, 1, 2, 4, 12, and 24 h after injection of a luteolytic dose of PGF2α. Total cellular RNA was isolated and TGFB1 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) mRNA were quantified by real-time RT-PCR. Our data revealed that TGFB1 mRNA was elevated (P < 0.05) as early as 1 h after PGF2α administration (Fig. 7A). In response to the PGF2α injection peak increases in TGFB1 mRNA were observed at 4 and 24 h after the PGF2α administration. To test whether PGF2α induced TGFB1 in vitro, we treated luteal cells with 1 μm PGF2α for 24 h. Northern blot analysis revealed that TGFB1 mRNA was induced in luteal cells after treatment with PGF2α (1 μm). In keeping with a role for MEK1/ERK signaling in response to PGF2α, the induction TGFB1 mRNA was inhibited when luteal cells were pretreated with the MEK1 inhibitor PD098095 (Fig. 7B). Furthermore, treatment with either PGF2α or the PKC activator PMA increased TGFB1 secretion by luteal cells (Fig. 7C). In contrast, treatment with LH or 8-Br-cAMP did not increase TGFB1 secretion (data not shown).
Figure 7.
PGF2α Induces TGFB1 Expression in Vivo and in Vitro
A, Angus crossbred cows were injected im with 25 mg Lutalyse or saline during the mid-luteal phase and the CL were removed at 0.5, 1, 2, 4, 12, and 24 h after injection. Levels of TGFB1 mRNA in bovine CL were quantified by real-time RT-PCR using total CL RNA as described in Materials and Methods. The results are presented in terms of TGFB1 mRNA normalized to levels of GAPDH mRNA. Each time point represents the mean ± sem (n = 3–6 CL each collected from different cows). *, P < 0.05, vs. untreated control. B, Bovine luteal cells were pretreated with PD98095 (50 μm) for 1 h before treatment of PGF2α (1 μm) for 24 h. TGFB1 mRNA was detected by Northern blot. The same blot was probed for GAPDH mRNA as a loading control. Similar results were observed in replicate experiments. C, Luteal cells were treated with control media, PGF2α (1 μm) or PMA (20 nm) for 24 h. The conditioned media were collected for TGFB1 assay utilizing a TGFB1 ELISA as described in Materials and Methods. Each experiment was performed in duplicate, and three separated experiments were performed. *, P < 0.05, vs. control.
EGR1 Mediates PGF2α-Induced TGFB1 Expression
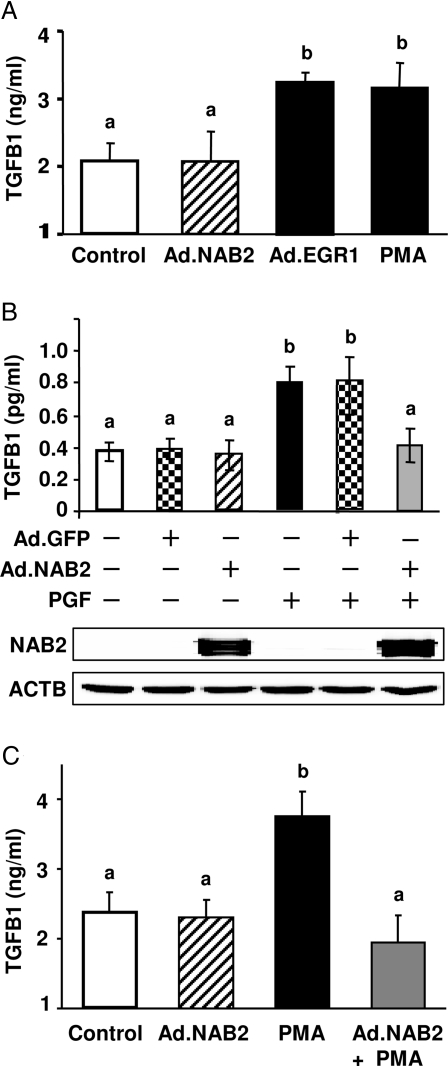
To explore whether the PGF2α-induced expression of TGFB1 was mediated by EGR1, we overexpressed EGR1 protein in primary luteal cells by treatment with an Ad construct that expresses a mutant EGR1 protein (Ad.EGR1) (29). The mutant EGR1 has a point mutation (I293F) in its NGFI-A binding corepressor (NAB)-binding domain, which allows EGR1 to function fully as a transcription factor, while preventing the binding of endogenous inhibitory EGR1 binding proteins 1 and 2 (NAB1 and NAB2, respectively) (30). The effects of Ad.EGR1 were compared with an Ad vector that expresses the EGR1 binding protein NAB2 (Ad.NAB2). We observed that treatment for 24 h with either PMA or Ad.EGR1 significantly (P < 0.05, n = 5) increased TGFB1 secretion (Fig. 8A). In contrast, when compared with controls, treatment with Ad.NAB2 did not alter the amount of TGFB1 secreted by luteal cells.
Figure 8.
EGR1 Mediates PGF2α-Induced TGFB1 Expression
A, Bovine luteal cells were infected with or without Ad (Ad.NAB2 or Ad.EGR1) for 24 h in the presence of serum and then serum-starved for 24 h. On the day of the experiment, the cells were preequilibrated in fresh media for 3 h, and luteal cells were treated without or with PMA (20 nm) for 24 h. TGFB1 in the conditioned media was determined by ELISA. Each experiment was performed in duplicate, and five separate experiments were performed. B, Luteal cells were infected with or without Ad (Ad.GFP or Ad.NAB2) and treated with or without PGF2α (1 μm) for 24 h as described above. TGFB1 in the conditioned media was determined by ELISA. Each experiment was performed in duplicate, and results are the average of four experiments. ACTB, β-Actin. C, Luteal cells were infected with or without Ad.NAB2 and treated with or without PMA (20 nm) for 24 h as described above. TGFB1 in the conditioned media were determined by ELISA. Each experiment was performed in duplicate, and results are the average of five experiments. A–C, Bars with similar letters are not statistically different; bars with different letters (a vs. b) are significantly different, P < 0.05.
It is known that the transcriptional activity of endogenous EGR1 can be repressed by interactions with the EGR1 binding protein NAB2 (31,32). To test whether PGF2α-induced TGFB1 secretion could be attenuated by repressing endogenous EGR1 activity we employed Ad.NAB2 to overexpress the NAB2 protein in luteal cells. An Ad vector that expresses the green fluorescent protein GFP (Ad.GFP) was employed as a control. Our results show that treatment of luteal cells with Ad.NAB2 had no effect on TGFB1 secretion compared with control cells or cells treated with Ad.GFP; but treatment with Ad.NAB2 abrogated PGF2α-induced TGFB1 protein secretion (Fig. 8B). Treatment with Ad.NAB2 also abrogated the stimulatory effect of the PKC activator PMA on TGFB1 secretion (Fig. 8C). In other studies, we observed that treatment with a combination of Ad.EGR-1 and PGF2α increased TGFB1 secretion to a greater extent (1.7 ± 0.1-fold increase, P < 0.05, n = 6) than treatment with either Ad.EGR1 or PGF2α alone.
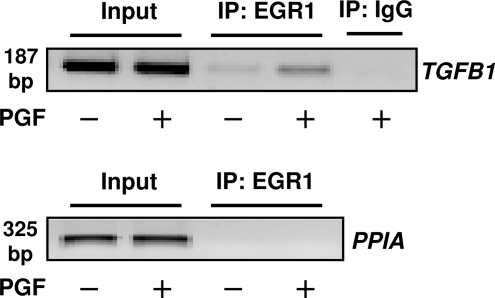
To further explore the role of EGR1 in the response to PGF2α, we tested whether treatment with PGF2α could induce EGR1 binding to the proximal promoter region of the TGFB1 gene in bovine luteal cells. To accomplish this, luteal cell cultures were treated with control media or PGF2α for 60 min and then were subjected to chromatin immunoprecipitation (ChIP) and DNA recovered from immunoprecipitates was PCR amplified to detect the 5′ region of TGFB1 (Fig. 9). We observed that treatment of luteal cells with PGF2α resulted in the association of EGR1 with a 187-bp region of the proximal TGFB1 promoter containing a known EGR1 binding motif (21). As a negative control, we did not observe evidence of TGFB1 promoter PCR products in DNA recovered from IgG immunoprecipitates. The lack of amplification of a cyclophilin A (also known as peptidylprolyl isomerase A) sequence also served as a negative control.
Figure 9.
EGR1 Binds to TGFB1 Promoter in Response to PGF2α in Bovine Luteal Cells
Luteal cells were treated with or without PGF2α (1 μm) for 60 min and DNA and protein cross-linked with formaldehyde as described in Materials and Methods. Extracts were treated with EGR1 antibodies or IgG and ChIP assays performed using the primer pairs specific for the proximal promoter region of TGFB1 or cyclophilin A (peptidylprolyl isomerase A, PIAA) as described in Materials and Methods. Similar results were observed a replicate experiment using a separate cell preparation. IP, Immunoprecipitation.
TGFB1 Antagonizes LH-Induced Progesterone Synthesis
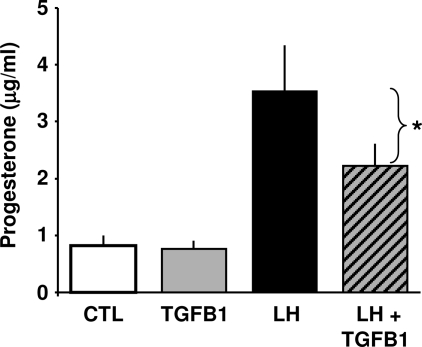
To evaluate the functional significance of the increase in TGFB1, we assessed the role of TGFB1 in progesterone biosynthesis. Cultures of luteal cells were treated with LH (100 ng/ml) with or without TGFB1 (1 ng/ml) for 6 h and progesterone levels were measured by ELISA. Treatment with LH but not TGFB1 increased progesterone secretion. However, treatment with TGFB1 significantly decreased progesterone in response to LH stimulation by 52% (P < 0.05, n = 4) (Fig. 10).
Figure 10.
TGFB1 Inhibits LH-Induced Progesterone Synthesis in Bovine Luteal Cells
Bovine luteal cells were treated with or without LH (100 ng/ml) in the presence or absence of TGFB1 (1 ng/ml) for 6 h. The conditioned medium was collected and progesterone levels were determined by RIA. Results are presented as mean ± sem of four experiments each conducted in triplicate. *, P < 0.05 vs. LH. CTL, Control.
DISCUSSION
Our laboratory has previously demonstrated that the initial response to PGF2α treatment involves binding to specific PGF2α receptors and activating a Ca2+/PKC/RAF/MEK1/ERK signaling pathway in bovine luteal cells (5). However, the molecular mechanisms that link this signaling pathway and various physiological sequelae are largely unknown. In this report, we provide evidence that in response to PGF2α the activation of this signaling pathway induces the expression of EGR1, a transcription factor with a central role in ovarian function. We also demonstrate that the increase in EGR1 expression mediates the stimulatory effect of PGF2α on the expression of TGFB1, a multifunctional protein that regulates tissue growth and development, as well as progesterone synthesis in luteal cells.
EGR1 is known to be involved in diverse pathophysiological responses (13). As an immediate-early response gene, EGR1 mRNA is rapidly induced and translated into new protein after a wide range of extracellular stimuli that activate the ERK1/2 signaling pathway (7,9,10,11). The present studies provide several novel observations that relate to the response of the CL to PGF2α. First, EGR1 mRNA and EGR1 protein were rapidly elevated in luteal tissue in vivo in response to treatment with a luteolytic dose of PGF2α. Similar rapid responses were observed in primary cultures of luteal cells in response to PGF2α treatment. Furthermore, the newly synthesized EGR1 protein was localized in the nucleus in response to PGF2α treatment. Second, pretreatment of luteal cells with the membrane-permeable calcium chelator BAPTA-AM or depletion of phorbol ester-responsive isoforms of PKC blocked PGF2α-induced EGR1 expression, indicating that Ca2+/PKC signaling are involved in the response to PGF2α, presumptively upstream of ERK signaling. Third, pretreatment of luteal cells with chemical inhibitors of MEK1 or an Ad expressing a mutant form of MEK1 (Ad.dnMEK1) prevented PGF2α-induced EGR1 expression, which points to a requirement for MEK1/ERK activation after PGF2α treatment. Furthermore, overexpression of dnRas abolished EGF- but not PGF2α-induced EGR1 up-regulation, suggesting that the induction of EGR1 in response to PGF2α does not require the activation of growth factor receptors. This idea was supported by data indicating that the EGF receptor tyrosine kinase inhibitor AG1470 did not prevent the stimulatory response to PGF2α on ERK activation or EGR1 expression. Fourth, alternative signaling pathways were ruled out by evidence demonstrating that neither LH nor an exogenous cAMP analog increased EGR1 in luteal cells. Collectively, these data support a sequence of events after activation of the PGF2α receptor that includes activation of the MEK1/ERK pathway before induction of EGR1 gene expression.
Important physiological roles of EGR1 have been observed, most notably in the reproductive system. Recent studies demonstrated that EGR1 is essential for the expression of the LH β-subunit in pituitary gonadotropes (17), and that EGR1 knockout mice have a reduced body size and are infertile (17). In developing rat follicles EGR1 can be induced in response to treatment with FSH and LH (16). A more recent study demonstrated that injection of an ovulatory dose of human chorionic gonadotropin transiently induced EGR1 mRNA expression in theca and granulosa cells of preovulatory bovine follicles (33). The latter study also demonstrated that treatment with the adenylyl cyclase activator forskolin or overexpression of EGR1 in granulosa cells resulted in the induction of levels of mRNA for prostaglandin (PG)-endoperoxide synthase 2 (PTGS2), PG E synthase, PG E receptor 2 (PTGER2), and LH/choriogonadotropin receptor (LHCGR) in cultures of bovine granulosa cells. It appears that in preovulatory follicles EGR1 may mediate the expression of genes involved in granulosa cell differentiation and ovulation. In contrast, treatment of bovine luteal cells with LH or 8-Br-cAMP (present studies) did not result in an increase in EGR1 mRNA or protein, despite their ability to increase progesterone synthesis in luteal cells. Although the differential regulation of EGR1 by LH in granulosa cells and luteal cells may indicate differential coupling to various signaling pathways, the fact that LH and cAMP no longer stimulate EGR1 in luteal cells suggests a different mode of gene regulation in the CL. The results also suggest that under the control of PKC/ERK signaling, EGR1 functions differently in the CL than in the follicle. In this regard, studies in various cell lines have shown that EGR1 can induce the expression of proteins (18,19) that influence cellular proliferation, differentiation, and apoptosis (22). Additional studies are required to determine the processes that are regulated by EGR1 in the CL.
Studies in various cell lines have shown that EGR1 protein can induce the up-regulation and activation of various proapoptotic proteins including TGFB1 and other tissue remodeling genes (34). The human TGFB1 promoter (GenBank accession no. J04431) contains a functional EGR1 protein binding site, which is conserved in the bovine TGFB1 promoter (GenBank accession no. EF523342). The present studies allow the conclusion that the PGF2α-induced changes in TGFB1 expression in luteal cells are mediated, at least in part, by EGR1. This conclusion is supported by several key findings in this study. First, similar intracellular signaling mechanisms involving the PKC/MEK1/ERK pathway were required for the induction of EGR1 and TGFB1. Second, overexpression of EGR1 mimicked the stimulatory response to PGF2α on TGFB1 secretion. Third, Ad-mediated overexpression of EGR1 repressor binding protein NAB2 abrogated PGF2α- and PMA-induced TGFB1 secretion; proving strong support for the idea that EGR1 is required for PGF2α-stimulated TGFB1 secretion. Fourth, using ChIP analysis, we demonstrated that EGR1 is capable of binding to the proximal TGFB1 promoter in luteal cells upon treatment with PGF2α.
TGFB1 was identified in cDNA expression array as a PGF2α-inducible gene in the rat CL (25). Wang et al. (35) recently demonstrated that in vivo treatment with PGF2α elevates TGFB1 mRNA in the mouse CL as determined by RT-PCR and in situ hybridization. In the present study, we demonstrate that a luteolytic dose of PGF2α induces TGFB1 mRNA in the bovine CL in vivo. The phasic pattern of TGFB1 mRNA expression in the bovine CL in vivo is similar to the in vivo patterns of expression of other genes in bovine CL after treatment with PGF2α [e.g. like FOS, JUN, TIMP metallopeptidase inhibitor 2 and 3 (TIMP2 and TIMP3)(Ref. 36)]. The pattern of gene expression presumably reflects the induction of immediate-early response genes like EGR1, FOS, JUN, etc., and the subsequent induction/modification of these and other regulatory factors. This seems likely based on the large number of regulatory regions on the TGFB1 gene promoter (34,37). Because PGF2α also induces the expression of FOS and JUN mRNA in the bovine CL (26), it is possible that FOS and JUN proteins bind to activator protein 1 sites on the TGFB1 promoter and also support the induction of TGFB1 mRNA. The posttranscriptional regulation of EGR1 or the presence of other factors may also contribute to the overall response because cells treated for 24 h with both the EGR1 Ad and PGF2α produced more TGFB1 than cells treated with either agent alone. Further studies are required to identify additional regulatory mechanisms that contribute to the induction of TGFB1 and other genes in response to PGF2α.
The activity of the transcription factor EGR1 can be modulated by interactions with members of the NAB family of corepressors (NAB1 and NAB2). Whereas NAB1 is constitutively expressed in most tissues and appears to be a general transcriptional regulator, NAB2 may function as an important inducible regulator of gene expression (38). It has been suggested that down-regulation of NAB2 allows full EGR1 activity and that expression of NAB2 after up-regulation of EGR1 ensures that EGR1 activity will be transient (38). In primary cultures of bovine luteal cells, levels of NAB2 were very low. Results in the present study clearly indicate that PGF2α and PMA induced EGR1 activity was inhibited by ectopic expression of NAB2 in vitro. Although these results suggest a role for NAB2, further studies are required to determine whether endogenous NAB proteins contribute to the physiologic regulation of EGR1 activity in ovarian cells.
Although it is well established that TGFB1 exerts growth inhibitory actions and contributes to the elimination of damaged or abnormal cells from normal tissues in vivo (22) (39), the direct actions of TGFB1 in luteal cells have not received much attention. This study confirms that luteal cells are a direct target for the action of TGFB1. In keeping with previous results (40), we observed that treatment with TGFB1 significantly attenuated LH-induced progesterone secretion. Although it is not currently known whether TGFB1 contributes directly to structural regression of the CL, it has been established that progesterone contributes to the survival of ovarian cells (41) and inhibition of progesterone actions results in the apoptosis of luteal cells in vitro (42,43). Additionally, TGFB1 has been shown to induce apoptosis of theca cells (44). Furthermore, evidence in epithelial cells indicates that TGFB1 can interfere with AKT signaling, which effectively reduces intracellular signals required for cell survival (45). Taken together, these results indicate that EGR1 may contribute to the process of CL regression by increasing the transcription and translation of genes such as TGFB1, which can reduce progesterone secretion and antagonize the actions of survival factors, thereby increasing the sensitivity of luteal cells to apoptotic stimuli.
MATERIALS AND METHODS
Reagents
PGF2α, protease and phosphatase inhibitor cocktail, and a MEK1 inhibitor (PD98059) were purchased from Sigma (St. Louis, MO). M199 and fetal bovine serum were obtained from Cambrex (Walkersville, MD). Type II collagenase was obtained from Atlantic Biologicals (Lawrenceville, GA). RNeasy mini and midi kits and HotStart Taq DNA polymerase were purchased from QIAGEN (Valencia, CA). Hybond-n + nylon membrane, digoxigenin-11-uridine-5′-triphosphate (DIG-UTP), DIG easy hybridization buffer, anti-DIG alkaline-phosphatase-conjugated antibody, and CDP-Star were from Roche Molecular Biochemical (Mannheim, Germany). Antirabbit Alexa 488-conjugated IgG, Ribogreen kit and the AmpliScribe T7-Flash transcription kit were supplied by Molecular Probes, Inc. (Eugene, OR). Mounting medium VECTASHIELD was from Vector Laboratories, Inc. (Burlingame, CA). Nuclear Extract Kit (no. 400-10) was obtained from Active Motif (Carlsbad, CA). A DC protein assay kit was purchased from Bio-Rad Laboratories Inc. (Richmond, CA). Immobilon-P polyvinylidene difluoride (PVDF) (0.45 μm pore size) membrane was from Millipore Corp (Bedford, MA). Specific antibodies against EGR1, Ras, MEK1, and β-actin were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). ECL Plus was from Amersham Pharmacia Biotech (Piscataway, NJ). Primers for real-time PCR and FAM- and BLACKHOLE-modified oligonucleotide probes were synthesized in the Eppley Molecular Biology Core Facility at the University of Nebraska Medical Center (Omaha, NE). Kodak x-ray film was purchase from Fisher Scientific Inc. (Hampton, NH).
Bovine CL Tissue
Angus crossbred cows at mid-luteal phase (d 10) were injected with a luteolytic dose (25 mg) of the PGF2α analog Lutalyse (Upjohn Co., Kalamazoo, MI). CL tissues were collected from saline-injected controls (0 h) or at 0.5, 1, 2, 4, 12, and 24 h after PGF2α injection. CL tissues were either snap frozen in liquid nitrogen and stored at −80 C, or immediately process to isolate total RNA or protein. All procedures were approved by local Institutional Animal Care and Use committees.
Isolation and Culture of Bovine Luteal Cells
Bovine ovaries were collected during early pregnancy (fetal crown rump length <15 cm) from a local slaughterhouse (Conagra Beef, Omaha, NE) and transported to the laboratory in cold M-199. The luteal tissue was dissociated with collagenase as described previously (5,26,46). The cell viability was determined by the trypan blue exclusion test. The luteal cell preparations with more than 90% viability were used. Enriched bovine steroidogenic luteal cells were plated (∼1 × 105 cells/cm2) in basal M-199 medium and 5% fetal bovine serum for 18 h at 37 C in a humidified atmosphere of 5% CO2. The cells were then serum starved for 24 h. On the day of the experiment, the medium was replaced with fresh preequilibrated medium, and cells were equilibrated for 2–3 h before applying various treatments as described in the legends to the figures.
Treatment with Ad
The dnMEK1 was obtained from Dr. J. Han (The Scripps Research Institute, La Jolla, CA). In the Ad.dnMEK1 vector, the MEK1 cDNA has been altered and two crucial serine residues located in the catalytic domain (Ser217 and Ser221) were replaced by alanines (28). The dnRas (obtained from Dr. B. Khan, Harvard Medical School, MA) was created by cloning the human H-ras cDNA with a serine to asparagine (N) substitution at amino acid 17 as reported by Gnudi et al. (47). The generation of recombinant Ad expressing constitutively active EGR1 (AdEGR1), NAB2 (AdNAB2), and AdGFP was described previously (29). The mutant EGR1 carries a point mutation (I293F) in its NAB-binding domain and, thus, does not respond to NAB repression (30). The efficiency of Ad treatment was monitored by the number of cells expressing GFP or by immunostaining for EGR1 protein. In both cases, greater than 90% of the luteal cells stained positive for GFP or EGR1 expression. Preliminary experiments were performed to determine the amount of Ad to be used in this study. In all cases, luteal cells were treated with increasing titers of adenoviral particles and protein expression was monitored by Western blot analysis. This study employed the following treatments based on viral particles/ml: Ad.GFP (1.1 × 109/ml), Ad.EGR1 (5 × 108/ml), Ad.NAB2 (1.2 × 109/ml), Ad.dnMEK1 (5 × 106/ml), Ad.dnRas (5 × 108/ml). Luteal cells were infected with the indicated titer of viruses in the presence of 5% serum for 24 h. Cells were rinsed and then serum-starved 24 h. The cell cultures were preequilibrated for 2–3 h with fresh serum-free media before applying various treatments.
Northern Blot Analysis
After treatment, luteal cells were collected and RNA was isolated using the RNAeasy kit according to the manufacturer’s specifications. RNA was quantified based upon the absorbance value at 260 nm by UV spectrophotometer. TGFB1 Northern blot was conducted as described before using porcine TGFB1 derived 287-bp PCR product as a probe (48). For EGR1 and GAPDH mRNA analysis 3 μg of total RNA from each sample was electrofractionated and transferred to Hybond-n + nylon membrane. The primers used to synthesize EGR1 and GADPH probes are as following: EGR1, forward 5′ TTTCCTCACTCACCCACC 3′, reverse 5′CTCCACCATCGCCTTCTC 3; GADPH, forward 5′-TGGAGCCAAACGGGTCATCATCT-3′, reverse 5′-GAAGAGTGGGAGTTGCTGTTGAA-3′. The membranes were then hybridized with the DIG-UTP-labeled PCR-synthesized probe of EGR1 using the DIG easy Hyb hybridization buffer. The same membranes were also hybridized with a PCR-GAPDH probe to ensure equal RNA loading. Blots were washed twice with 2× SSC, 1% SDS at room temperature (20 min each) followed by two more washes with prewarmed 0.1× SSC, 0.1% SDS at 65 C (20 min each). The probe-target hybrids were detected with an alkaline-phosphatase-conjugated antibody using alkaline phosphatase substrates CDP-Star for chemiluminescence reaction. The membranes were then exposed to the Kodak film for 1–5 min. The analysis was performed in triplicate and involved three cell preparations from different cows.
Western Blot Analysis
Bovine CL tissues or luteal cells were harvested with ice-cold cell lysis buffer [20 mm Tris-HCl (pH 7), 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100 and protease and phosphatase inhibitor cocktails]. The lysed cells were sonicated for 3 sec and cleared by centrifugation at 14,000 × g for 5 min. Protein content was determined using a DC protein assay kit. The cell lysates (40–60 μg protein/lane) were subjected to 10% SDS-PAGE and then electrically (75 V, 2 h) transferred to PVDF membranes. Nonspecific binding was blocked with 5% fat-free milk in TBST [50 mm Tris-HCl (pH 7.5), 0.15 m NaCl, 0.05% Tween 20] at room temperature for 1 h. Membranes were then incubated with appropriate amounts of primary antibodies in TBST-5% BSA (for phospho-specific antibodies) or in 5% fat-free milk in TBST for overnight at 4 C. After four 5-min washes with TBST, the membranes were incubated with antimouse (1:2000 dilution) or antirabbit peroxidase conjugated IgG (1:2000 dilution) for 1 h. The membranes were washed four times with TBST, and bound antibodies were detected ECL with the Western Lightning ECL detection system (PerkinElmer Life Sciences Inc.). Signals were visualized using x-ray film or a Kodak Digital Sciences Image Station 440 (Kodak, Rochester, NY).
Fluorescence Immunocytochemistry
Bovine luteal cells were cultured in four-well Lab-Tek (Nalge Nunc International, Naperville, IL) glass chamber slides. After treatment with 1 μm PGF2α for 1 h, the cells were washed twice with PBS, and fixed with freshly made 4% paraformaldehyde in PBS (pH 7.4) for 30 min. Nonspecific protein-binding sites were blocked with 10% donkey serum, 0.1% Triton X-100, and 0.1% sodium azide in PBS (pH 7.4) for 30 min at room temperature in a humidified chamber. Sections were then incubated with EGR1 antibody (1:200) at 4 C overnight in a humidified chamber. Sections were then rinsed three times for 5 min each in PBS and were incubated with antirabbit antibody conjugated with Alexa-488 (green fluorescence) for 1 h in a humidified chamber at room temperature. Nuclei were stained simultaneously with 4′,6-diamidino-2-phenylindole. After thorough rinsing, sections were mounted with VECTASHIELD and examined under epifluorescence in a Leica Instruments (Nussloch, Germany) DMR research microscope equipped with a Retiga Fast1394 digital camera (Q-Imaging, Burnaby, British Columbia, Canada), and the images were captured using Openlab (Improvision, Lexington, MA) image analysis software.
Real-Time PCR
Total RNA from bovine CL was isolated as described above. RNA was quantified using the Ribogreen kit according to the manufacturer’s protocol. For real-time quantification, mRNA standards for TGFB1 and GAPDH were used in parallel with the sample RNA to obtain true quantitative values. PCR primers and probes were designed based on bovine TGFB1 and GAPDH cDNA sequences using Primer Express software (PerkinElmer, Boston, MA). Sense strand of mRNA for TGFB1 and GAPDH were synthesized using linearized, bovine-specific cDNA and the AmpliScribe T7-Flash transcription kit and quantified using the Ribogreen kit. Its authenticity was verified by routine RT-PCR using the forward and reverse primers designed specifically for real-time RT-PCR. A series of eight standards ranging from 0.1 fg to 1 ng (in 10-fold increments) of specific mRNA per 1.25 μl water was prepared. Duplicates of each standard were reverse-transcribed along with bovine luteal tissue samples. Aliquots of reverse transcribed product of each CL sample were used for real-time quantification of TGFB1 and GAPDH mRNA using the Opticon Thermocycler (MJ Research, Reno, NV). The PCR was continued for 35 cycles after an initial denaturation at 94 C for 15 min to activate the HotStart Taq polymerase. Each cycle of PCR consisted of 30 sec of denaturation at 94 C and 15 sec of annealing at 55 C, followed by a plate reading with a final extension at the end of 35 cycles for 10 min at 72 C. The amount of specific mRNA was calculated from the linear portion of the amplification signal. The authenticity of the PCR signal was verified using tubes containing no RNA or RNA without reverse transcriptase. The sequences for forward and reverse primers and the probes were as follows: TGFB1: forward, 5′-AACAATTCCTGGCGCTACCTC-3′; reverse, 5′-TGCCGCACAACTCCAGTG-3′; probe, 6-FAM-5′-CTCACCGGAGTGGCTGTCCTTTGAC-3′-BLACKHOLE; GAPDH: forward, 5′-TCTGCACCTTCTGCCGATG-3′; reverse, 5′-AGCAGTTGGTGGTGCAGGA-3′; probe, 6-FAM-5′-AACCA CG AGAAGTATAACAACACCCTCAAGATTGTC-3′-BLACKHOLE.
TGFB1 ELISA
Luteal cells were cultured in 24-well plates as described above. After various treatments, conditioned media were collected. The TGFB1 levels in the media were determined using TGFB1Emax ImmunoAssay System (Promega, Madison, WI) according to the manufacturer’s instructions.
ChIP Assay
After treatment, bovine luteal cells (10-cm dishes) were fixed with 1% formaldehyde for 15 min at room temperature to cross-link chromatin-complexes. The complexes were then sonicated to fragment the DNA to an average size of 500-1000 bp. The soluble chromatin was then immunoprecipitated with 25 μl EGR1 antibody (no. 4152; Cell Signaling Technology, Danvers, MA). Rabbit IgG (25 μl; No. I-1000, Vector Laboratories, Burlingame, CA) was used as a negative control. DNA was isolated from the immunoprecipitated samples with QIAGEN DNA extraction kit. Equal volumes of immunoprecipitated DNA were used as templates for PCR using primers specific for the bovine proximal TGFB1 promoter (GenBank accession no. EF523342) (forward 5′-CAGACTTGCCTCAGCTTTCC-3′, reverse 5′-GGTCTCCCGAGGAAAAGGTA-3′). The cyclophilin (peptidylprolyl isomerase A) primers used were 5′-CTCTTTTGAGCTGTTTGCAG-3′ and 5′-GTACCACGTGCTTGCCATCC-3′.
Progesterone RIA
Luteal cells were cultured in 24-well plates and treated as described in figure legends. Conditioned media were collected for progesterone determination using progesterone RIA kit (Diagnostic Systems Laboratories, Inc., Webster, TX) according to the manufacturer’s instructions.
Statistical Analysis
All experiments were performed at least twice using different cell preparations with identical results. The data are presented as the means and se of the averages from multiple experiments or individual representative experiments. The differences in means were analyzed by ANOVA followed by Tukey’s honestly significantly difference test to evaluate multiple responses, or ANOVA followed by the t test to compare two treatment groups. P values < 0.05 were considered statistically significant.
Footnotes
This work was supported by the Department of Veterans Affairs (to J.S.D.), National Institutes of Health Grants R01HD35934-8 (to B.R.R.) and HD048754 (to J.S.D.), and the Olson Center for Women’s Health (to J.S.D.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 4, 2007
Abbreviations: Ad, Adenovirus(es); BAPTA-AM, 1,2-bis-(o-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid, tetraacetoxymethyl ester; 8-Br-cAMP, 8-bromo-cAMP; ChIP, chromatin immunoprecipitation; CHX, cycloheximide; CL, corpus luteum/corpora lutea; dn, dominant negative; DIG-UTP, digoxigenin-11-uridine-5′-triphosphate; EGF, epidermal growth factor; EGR1, early growth response 1; GFP, green fluorescent protein; GAPDH, glyceraldehydes-3-phosphate dehydrogenase; MEK, MAPK kinase; NAB, NGFI-A binding corepressor; PG, prostaglandin; PGF2α, PG F2α; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; PVDF, polyvinylidene difluoride.
References
- Davis JS, Rueda BR 2002 The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci 7:d1949–d1978 [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G 2007 The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Ezashi T, Miwa K, Okuda-Ashitaka E, Houtani T, Sugimoto T, Ito S, Hayaishi O 1994 Molecular cloning and expression of a cDNA of the bovine prostaglandin F2 α receptor. J Biol Chem 269:3881–3886 [PubMed] [Google Scholar]
- Davis JS 1987 Stimulation of intracellular free Ca2+ by luteinizing hormone in isolated bovine luteal cells. Adv Exp Med Biol 219:671–675 [DOI] [PubMed] [Google Scholar]
- Chen DB, Westfall SD, Fong HW, Roberson MS, Davis JS 1998 Prostaglandin F2α stimulates the Raf/MEK1/mitogen-activated protein kinase signaling cascade in bovine luteal cells. Endocrinology 139:3876–3885 [DOI] [PubMed] [Google Scholar]
- Tai CJ, Kang SK, Choi KC, Tzeng CR, Leung PC 2001 Role of mitogen-activated protein kinase in prostaglandin F(2α) action in human granulosa-luteal cells. J Clin Endocrinol Metab 86:375–380 [DOI] [PubMed] [Google Scholar]
- Stocco CO, Lau LF, Gibori G 2002 A calcium/calmodulin-dependent activation of ERK1/2 mediates JunD phosphorylation and induction of nur77 and 20α-hsd genes by prostaglandin F2α in ovarian cells. J Biol Chem 277:3293–3302 [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ 1995 Integration of MAP kinase signal transduction pathways at the serum response element. Science 269:403–407 [DOI] [PubMed] [Google Scholar]
- Jones N, Agani FH 2003 Hyperoxia induces Egr-1 expression through activation of extracellular signal-regulated kinase 1/2 pathway. J Cell Physiol 196:326–333 [DOI] [PubMed] [Google Scholar]
- Gineitis D, Treisman R 2001 Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem 276:24531–24539 [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Bach K, Thiel G 2001 The extracellular signal-regulated protein kinases Erk1/Erk2 stimulate expression and biological activity of the transcriptional regulator Egr-1. Biol Chem 382:1077–1081 [DOI] [PubMed] [Google Scholar]
- Milbrandt J 1987 A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238:797–799 [DOI] [PubMed] [Google Scholar]
- Thiel G, Cibelli G 2002 Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol 193:287–292 [DOI] [PubMed] [Google Scholar]
- Christy BA, Lau LF, Nathans D 1988 A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with “zinc finger” sequences. Proc Natl Acad Sci USA 85:7857–7861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashler A, Sukhatme VP 1995 Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50:191–224 [DOI] [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Gonzales-Robayna I, Pipaon C, Richards JS 2003 Egr-1 induction in rat granulosa cells by follicle-stimulating hormone and luteinizing hormone: combinatorial regulation by transcription factors cyclic adenosine 3′,5′-monophosphate regulatory element binding protein, serum response factor, sp1, and early growth response factor-1. Mol Endocrinol 17:520–533 [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji A, Driancourt MA, Rao CV, Charnay P 1998 Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol 12:107–122 [DOI] [PubMed] [Google Scholar]
- Ragione FD, Cucciolla V, Criniti V, Indaco S, Borriello A, Zappia V 2003 p21Cip1 gene expression is modulated by Egr1: a novel regulatory mechanism involved in the resveratrol antiproliferative effect. J Biol Chem 278:23360–23368 [DOI] [PubMed] [Google Scholar]
- Liu C, Rangnekar VM, Adamson E, Mercola D 1998 Suppression of growth and transformation and induction of apoptosis by EGR-1. Cancer Gene Ther 5:3–28 [PubMed] [Google Scholar]
- Kim SJ, Denhez F, Kim KY, Holt JT, Sporn MB, Roberts AB 1989 Activation of the second promoter of the transforming growth factor-β 1 gene by transforming growth factor-β 1 and phorbol ester occurs through the same target sequences. J Biol Chem 264:19373–19378 [PubMed] [Google Scholar]
- Kim SJ, Glick A, Sporn MB, Roberts AB 1989 Characterization of the promoter region of the human transforming growth factor-β 1 gene. J Biol Chem 264:402–408 [PubMed] [Google Scholar]
- Cohen Jr MM 2003 TGF β/Smad signaling system and its pathologic correlates. Am J Med Genet 116A:1–10 [DOI] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Kitazawa R, Tsukamoto T, Yano S, Tsukada T, Canaff L, Hendy GN, Sugimoto T, Chihara K 2004 Menin inactivation leads to loss of transforming growth factor β inhibition of parathyroid cell proliferation and parathyroid hormone secretion. Cancer Res 64:2222–2228 [DOI] [PubMed] [Google Scholar]
- Gangrade BK, Gotcher ED, Davis JS, May JV 1993 The secretion of transforming growth factor-β by bovine luteal cells in vitro. Mol Cell Endocrinol 93:117–123 [DOI] [PubMed] [Google Scholar]
- Stocco C, Callegari E, Gibori G 2001 Opposite effect of prolactin and prostaglandin F(2α) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology 142:4158–4161 [DOI] [PubMed] [Google Scholar]
- Chen D, Fong HW, Davis JS 2001 Induction of c-fos and c-jun messenger ribonucleic acid expression by prostaglandin F2α is mediated by a protein kinase C-dependent extracellular signal-regulated kinase mitogen-activated protein kinase pathway in bovine luteal cells. Endocrinology 142:887–895 [DOI] [PubMed] [Google Scholar]
- Arvisais EW, Romanelli A, Hou X, Davis JS 2006 AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2α. J Biol Chem 281:26904–26913 [DOI] [PubMed] [Google Scholar]
- Yan Y, Spieker RS, Kim M, Stoeger SM, Cowan KH 2005 BRCA1-mediated G2/M cell cycle arrest requires ERK1/2 kinase activation. Oncogene 24:3285–3296 [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Muhlebach SG, Sohrman S, Leutenegger CM, Lester HA, Davidson N 2000 Modulation of early growth response (EGR) transcription factor-dependent gene expression by using recombinant adenovirus. Gene 258:63–69 [DOI] [PubMed] [Google Scholar]
- Russo MW, Matheny C, Milbrandt J 1993 Transcriptional activity of the zinc finger protein NGFI-A is influenced by its interaction with a cellular factor. Mol Cell Biol 13:6858–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, Milbrandt J 1998 Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Mol Cell Biol 18:512–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J 1996 NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol Cell Biol 16:3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayasith K, Brown KA, Lussier JG, Dore M, Sirois J 2006 Characterization of bovine early growth response factor-1 and its gonadotropin-dependent regulation in ovarian follicles prior to ovulation. J Mol Endocrinol 37:239–250 [DOI] [PubMed] [Google Scholar]
- Liu C, Yao J, de Belle I, Huang RP, Adamson E, Mercola D 1999 The transcription factor EGR-1 suppresses transformation of human fibrosarcoma HT1080 cells by coordinated induction of transforming growth factor-β1, fibronectin, and plasminogen activator inhibitor-1. J Biol Chem 274:4400–4411 [DOI] [PubMed] [Google Scholar]
- Wang Z, Tamura K, Yoshie M, Tamura H, Imakawa K, Kogo H 2003 Prostaglandin F2α-induced functional regression of the corpus luteum and apoptosis in rodents. J Pharmacol Sci 92:19–27 [DOI] [PubMed] [Google Scholar]
- Ricke WA, Smith GW, McIntush EW, Smith MF 2002 Analysis of luteal tissue inhibitor of metalloproteinase-1, -2, and -3 during prostaglandin F(2α)-induced luteolysis. Biol Reprod 66:1387–1394 [DOI] [PubMed] [Google Scholar]
- Liu C, Calogero A, Ragona G, Adamson E, Mercola D 1996 EGR-1, the reluctant suppression factor: EGR-1 is known to function in the regulation of growth, differentiation, and also has significant tumor suppressor activity and a mechanism involving the induction of TGF-β1 is postulated to account for this suppressor activity. Crit Rev Oncog 7:101–125 [PubMed] [Google Scholar]
- Lucerna M, Mechtcheriakova D, Kadl A, Schabbauer G, Schafer R, Gruber K, Koshelnick Y, Muller HD, Issbrucker K, Clauss M, Binder BR, Hofer E 2003 NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J Biol Chem 278:11433–11440 [DOI] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B 2004 Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn 230:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Okuda K, Schweigert FJ, Schams D 1992 Effects of basic fibroblast growth factor, transforming growth factor-β and nerve growth factor on the secretory function of the bovine corpus luteum in vitro. J Endocrinol 135:103–114 [DOI] [PubMed] [Google Scholar]
- Peluso JJ 2006 Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod 75:2–8 [DOI] [PubMed] [Google Scholar]
- Okuda K, Korzekwa A, Shibaya M, Murakami S, Nishimura R, Tsubouchi M, Woclawek-Potocka I, Skarzynski DJ 2004 Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod 71:2065–2071 [DOI] [PubMed] [Google Scholar]
- Rueda BR, Hendry IR, Hendry IW, Stormshak F, Slayden OD, Davis JS 2000 Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biol Reprod 62:269–276 [DOI] [PubMed] [Google Scholar]
- Foghi A, Teerds KJ, van der Donk H, Moore NC, Dorrington J 1998 Induction of apoptosis in thecal/interstitial cells: action of transforming growth factor (TGF) α plus TGFβ on bcl-2 and interleukin-1β-converting enzyme. J Endocrinol 157:489–494 [DOI] [PubMed] [Google Scholar]
- Ju EM, Choi KC, Hong SH, Lee CH, Kim BC, Kim SJ, Kim IH, Park SH 2005 Apoptosis of mink lung epithelial cells by co-treatment of low-dose staurosporine and transforming growth factor-β1 depends on the enhanced TGF-β signaling and requires the decreased phosphorylation of PKB/Akt. Biochem Biophys Res Commun 328:1170–1181 [DOI] [PubMed] [Google Scholar]
- Chakravorty A, Joslyn MI, Davis JS 1993 Characterization of insulin and insulin-like growth factor-I actions in the bovine luteal cell: regulation of receptor tyrosine kinase activity, phosphatidylinositol-3-kinase, and deoxyribonucleic acid synthesis. Endocrinology 133:1331–1340 [DOI] [PubMed] [Google Scholar]
- Gnudi L, Frevert EU, Houseknecht KL, Erhardt P, Kahn BB 1997 Adenovirus-mediated gene transfer of dominant negative ras(asn17) in 3T3L1 adipocytes does not alter insulin-stimulated P13-kinase activity or glucose transport. Mol Endocrinol 11:67–76 [DOI] [PubMed] [Google Scholar]
- May JV, Stephenson LA, Turzcynski CJ, Fong HW, Mau YH, Davis JS 1996 Transforming growth factor β expression in the porcine ovary: evidence that theca cells are the major secretory source during antral follicle development. Biol Reprod 54:485–496 [DOI] [PubMed] [Google Scholar]