1. Introduction
Serotonegic neurons in the brain play important roles for the brain functions. These neurons are indispensable for vital functions such as sleep, appetite, nociception, and aggression. Dysfunction of the serotonegic system causes a variety of brain disorders, such as anxiety and depression (Brunton et al., 2006).
Distribution of the serotonergic neurons in the brain was first investigated histochemically with the histofluorescesce method (Dahlströem and Fuxe, 1964). This was followed by a more precise technique of serotonin immunocytochemistry (Steinbusch et al., 1978; Steinbusch, 1981). Serotonergic neurons are distributed in various raphe nuclei in the mesencephalon, pons, and medulla oblongata. Among these raphe nuclei, the dorsal raphe (DR) nucleus is the largest, containing about a half of the total brain serotonergic neurons (Dahlströem and Fuxe, 1965; Descarries et al., 1982; Piňeyro and Blier, 1999). The DR nucleus is the main source of ascending serotonergic pathways which innervate a variety of brain areas such as the cortex, basal ganglia, and amygdala (Azmitia and Segal, 1978; Steinbusch et al., 1981).
We have developed a dissociated cell culture of DR serotonergic neurons from postnatal rats. For this purpose, we used our method of culturing specific brain nuclei. The feature of our method is first to make brain slices of the region containing the nucleus in question, then to isolate and remove the nucleus under the dissecting microscope. Using this method, we have succeeded in making dissociated cultures of cholinergic neurons from the nucleus basalis (Nakajima et al., 1985), noradrenergic neurons from the locus coeruleus (Masuko et al., 1986), dopaminergic neurons from the substantia nigra, dopaminergic neurons from the ventral tegmental area (VTA) (Masuko et al., 1992; Kim et al., 1995), and more recently histaminergic neurons from the tuberomammillary nucleus (Bajic et al., 2004). We have now applied this method to make cultures of serotonergic neurons from the DR nucleus.
There are several reports on culturing embryonic brain serotonergic neurons (Yamamoto et al., 1981; Rudge et al., 1996; Héry et al., 2000; Nishi et al., 2000). Because serotonergic neurons and their nuclei develop during the fetal period (Altman and Bayer, 1981; Lidov and Molliver, 1982; Wallace et al., 1983; Aitken and Törk, 1988; Lautenschlager et al., 2000), and the contour of the DR nucleus is more well-formed in postnatal rats, we decided to make dissociated neuron cultures of the DR nucleus using 9−12 day-old postnatal rats rather than fetal rats.
In this paper, first we describe the method of making dissociated serotonergic neuron cultures from the DR nucleus of postnatal rats. Second, we show data of electrophysiological experiments on those serotonergic neurons. These serotonergic neurons were functional and responded to neurotransmitters such as bombesin and gastrin-releasing peptide. A preliminary account was reported in an abstract (Yasufuku-Takano et al., 2004).
2. Materials and methods
2.1 Dissociated cell cultures
Cell cultures of the DR nucleus were prepared from postnatal Long-Evans rats (Charles River Laboratories, Inc.). We used essentially the same procedures as reported previously (Masuko et al., 1986; Nakajima and Masuko, 1996, 1999), except that, instead of newborn rats, we used mainly 10 to 12-day-old rats. Under ether anesthesia, the brainstem, from the midbrain to the upper part of the cervical spinal cord, was removed aseptically. Immediately afterwards, the animal was decapitated to ensure euthanasia. The removed brainstems were immersed in an ice-cold oxygenated balanced salt solution consisting of: 130 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 33 mM glucose and 5 mM piperazine-N', N'-bis[2-ethanesulfonic acid] (PIPES) buffer (pH 7.4), and were embedded in agar (3.3−3.5%) in the balanced salt solution. The solidified agar block containing the removed brainstems was attached to the bottom of a cutting dish with alpha cyanoacrylate (instant glue), and immersed in the oxygenated ice-cold balanced salt solution. The agar block containing brainstems was then aseptically sectioned with a Vibratome (Lancer 1000) into 400 μm thick slices.
DR is a long nucleus, rostrally located at the level of the caudal end of the oculomotor nucleus complex, and caudally up to the level of the rostral end of the fourth ventricle (Steinbusch et al., 1981; Törk, 1985). When the brains of 9 to 12-day-old rats were serially sectioned at the thickness of 400 μm, the DR nucleus was contained within three slices. The DR nucleus was identified under the dissecting microscope, and excised from the slices using a pair of micro-knives that consisted of a 30G needle attached to a tuberculin syringe. We excised dorsomedial, ventromedial, and lateral regions of the rostral mesencephalic part of the DR nucleus. The excised pieces of DR nuclei were treated with oxygenated Leibovitz's L-15 culture medium (GIBCO BRL) containing 12 units/ml papain (Worthington Biochemical Corp.), 1.27 mM DL-cysteine, and 0.2 mg/ml bovine serum albumin (pH 7.3) for 30 minutes at 37°C. In order to remove the papain, the specimens were then rinsed three times with the culture medium, and dissociated by repeated gentle pipetting (Leifer et al., 1984). Dissociated neurons were then plated on a well (12 mm in diameter) made inside a culture dish (3.5 cm in diameter). The floor of the well was pre-coated with rat tail collagen and a glial feeder layer (Nakajima and Masuko, 1996).
The cultures were kept at 37° C at an atmosphere of 10 % CO2. The culture medium consisted of minimum essential medium with Earle's salt (88%; GIBCO BRL), modified by adding L-glutamine (0.292 mg/ml, final concentration), NaHCO3 (3.7 mg/ml), D-glucose (5 mg/ml), and was supplemented with L-ascorbic acid (10 μg/ml), penicillin (50 units/ml), streptomycin (50 μg/ml), and heat-inactivated rat serum (2%, prepared in our laboratory). The cultures were maintained for 10 to 14 days.
2.2 Immunocytochemistry
Serotonergic neurons in DR cultures were identified by serotonin immunocytochemistry (Steinbusch et al., 1978; 1981) by using the peroxide-antiperoxidase method (Sternberger, 1979) or by using the indirect fluorescence immunocytochemical method.
Fixation
DR neuron cultures were rinsed with cold (4 °C) 0.1 M PBS (pH 7.4) for three times and were fixed first with cold 4 % paraformaldehyde in 0.1 M PBS for 13 minutes, followed by cold 4 % paraformaldehyde in 0.1 M borate buffer (pH 11.0) for 13 minutes.
Immunocytochemical treatment with the peroxide-antiperoxidase method of Sternberger
Following the fixation, cultures were rinsed with 0.1 M PBS containing 0.3% Triton X-100, and were incubated in a blocking solution (5% normal goat serum and 0.3% Triton X-100 in 0.1 M PBS) for 15 minutes at 4° C. The specimens were incubated for an hour at 4° C in a primary antibody solution containing rabbit antiserum to serotonin, which was conjugated to bovine serum albumin (Incstar Corp.; 1: 400 in 0.1 M PBS, containing 1% normal goat serum and 0.3% Triton X-100). This process was followed by washing six times with 0.1 M PBS containing 0.3% Triton X-100, and then by one hour incubation in a secondary antibody solution containing fluorescein-labeled goat antibody against rabbit IgG (heavy and light chains: Organon Teknika Corp.; 1:50 in 0.1 M PBS containing 1% normal goat serum and 0.3% Triton X-100) under gentle agitation at room temperature. After three times washing with 0.1 M PBS, the specimens were treated with a rabbit peroxidase-anti-peroxidase complex solution (Sternberger Monoclonals Inc.; 1:80 in 0.1 M PBS containing 1% normal goat serum) at room temperature for an hour. After three times wash with 0.1 M PBS and additional three times wash with 0.05 M Tris buffer (pH 7.4), the specimens were treated with 0.05 M Tris buffer containing 0.02% 3−3′-diaminobenzidine tetrahydrochloride (DAB) and 0.005% H2O2 for up to 10 minutes. After wash with 0.1 M PBS, the specimens were dehydrated, cleared, and cover-slipped using Histomount (National Diagnostics). This processing produced dark brown staining in serotonin-immunoreactive neurons. Serotonin-BSA conjugate (Incstar Corp., Stillwater, MN) was used for absorption experiment to evaluate the specificity of the anti-serotonin antibody. Controls were treated with the same way except for the absence of the primary antibody.
Immunocytochemistry after electrophysiology
To determine whether a neuron, which was examined electrophysiologically, was serotonergic, we used the indirect fluorescence immunocytochemical method. The location where this neuron was situated was marked with a circle at the bottom of the culture dish. The culture was fixed and rinsed three times before immunocytochemical treatments. We used rabbit antiserum to serotonin, conjugated with bovine serum albumin, as the primary antibody (Incstar Corp.; 1:400 dilution), and fluorescein conjugated IgG fraction of goat anti-rabbit IgG antibody as the secondary antibody (Oregon Teknika Corp.; 1:50 dilution). After incubation with the secondary antibody, the cultures were rinsed with PBS 3 times and mounted on glass slides using fluoromount (Fisher Biotech).
Immunocytochemistry of neurofilaments using the indirect fluorescence method
We used mouse monoclonal antibody to neurofilaments (68 KD) (Boehringer Mannheim Biochemicals; 1:3 dilution) as the primary antibody, and rhodamine conjugated goat anti-mouse IgG (whole molecule) antibody (Organon Teknika Corp.; 1:80 dilution) as the secondary antibody. The same procedure as that used for indirect fluorescence immunocytochemistry of serotonin, was used for the neurofilament immunocytochemistry.
2.3 Electrophysiology
The whole-cell patch-clamp method was used. During electrophysiological experiments, the culture was continuously superfused with an oxygenated external solution. The 5 mM K+ external solution, which was used most often, contained: 145.5 mM NaCl, 5 mM KCl, 2.4 mM CaCl2, 1.3 mM MgCl2, 5 mM HEPES-NaOH, 11 mM D-glucose, and with or without 0.5 μM tetrodotoxin (TTX) (pH 7.4). The 10 mM K+ external solution was prepared as the above described solution except for the NaCl and KCl concentrations which became 140.5 mM NaCl and 10mM KCl. The intra-pipette solution contained 144 mM K-aspartate, 10 mM NaCl, 5 mM HEPES-KOH, 0.5 mM EGTA-KOH, 0.25 mM CaCl2, 3 mM MgCl2, 2 mM ATP, 0.1 mM GTP and ∼5 mM KOH (pH 7.2). Membrane potential values were corrected for the liquid junction potential between the bath and the patch pipette solutions. 8-OH-DPAT was obtained from Research Biochemicals, Inc; phenylephrine from Sigma Chemical Co, bombesin from Peninsula Laboratories, Inc., and gastrin-releasing peptide was from Peninsula Laboratories, Inc. Unless otherwise indicated, experiments were done at the room temperature.
3. Results
3.1. Morphological characteristics of cultured serotonergic DR neurons
Dissociated cultures from a brain nucleus are a mixture of different types of neurons and glia cells. We investigated the proportion of serotonergic neurons among other types of neurons and glia cells in our cultures. This was necessary because our purpose was to investigate the brain serotonergic neurons, and a very low proportion of serotonergic neurons would be ineffectual for the experiments.
To determine the proportion of serotonergic neurons in our culture, we must first identify neurons from glia. For this purpose, we performed immunocytochemistry for neurofilaments: neurofilaments serve as a marker of neurons. As an example, in a culture dish, inside a designated area (i.e., a quarter of the total culture area, which was a circle of ∼11 mm diameter), the number of cells having processes was 226, and all these cells reacted positively to the antibody against neurofilaments. A small number (totally 11) of neurons that were devoid of processes were also positive to the neurofilament antibody. Flat background cells did not show positive reactivity to antibody to neurofilament: they were probably glial cells of the feeder layer. Because all cells with processes were neurons, and only a small percentage of neurons had no processes, we performed a population statistics on cells with processes only: they would represent all neurons with an error of a few percent.
Table 1 summarizes the survey on cells with processes (namely neurons). The cultures were treated with antibody against serotonin using the peroxidase-antiperoxidase method of Sternberger (1979). Serotonergic neurons, as determined by positive serotonin-immunoreactivity, accounted for 62.4% of the total neurons (cells with processes) examined. Four culture batches were used. We found on average 485 serotonergic neurons in one culture dish. Since one rat brain provided five culture dishes, approximately, 2,500 serotonergic neurons were viable after 11 days of culturing originated from a 10-day-old rat brain. Soma diameters were measured on two culture batches. The diameter of the serotonergic and that of the non-serotonergic neurons were essentially the same (Table 1).
Table 1.
Size and occurrence of serotonin-immunoreactive neurons in DR cultures
| Immunoreactivity | Diameter (μm) | Frequency (%) |
|---|---|---|
| Serotonin (+) | 15.8 ± 0.3 (n = 250) | 62.4 (n = 485) |
| Serotonin (−) | 16.7 ± 0.5 (n = 108) | 37.6 (n = 292) |
Cell diameters were determined from two cultures. The frequency measurements were conducted on four cultures. For each batch of cultures, the measurements were conducted on 1/4 of culture area of a dish. All cultured cells were obtained from 10-day-old rats, and were cultured for 11 days. Diameter was defined as a geometrical mean of the major and minor soma diameters. Cultures were treated with the serotonin immunocytochemical method using the peroxide-antiperoxidase method (Sternberger, 1979). Diameters are expressed as means ± S.E.M.
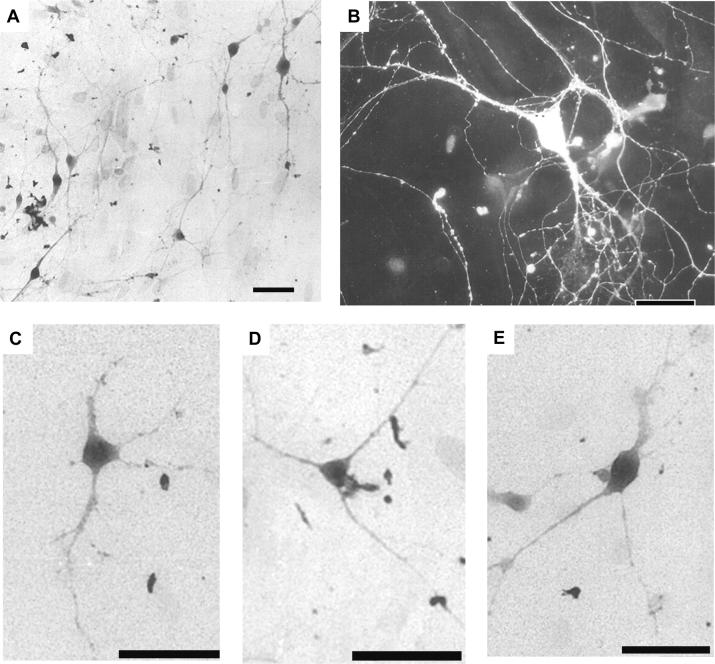
Fig 1A shows serotonergic neurons cultured from DR: they were treated with immunocytochemistry against serotonin. The serotonergic neurons could be classified into two major types, polygonal (Fig. 1B and C), triangular (Fig. 1D), and, less frequently, fusiform (Fig. 1E) with thick processes arising from the opposite poles of the soma. However, non-serotonergic neurons turned out to be also of polygonal or triangular shape. It was impossible to identify serotonergic neurons based on their size and shape.
Fig. 1. Serotonergic neurons in dissociated cultures from DR.
Cells with positive immunoreactivity to serotonin are shown. The peroxide-antiperoxidase method (A and C-E) and the indirect immunofluorescence method (B) were used. A: Low magnification micrograph, showing many serotonin-immunoreaction positive neurons. B-E: Serotonergic neurons. Polygonal neurons (B and C), a triangular neuron (D), and a fusiform neuron (E) are shown. B: A serotonin-immunoreactive neuron treated with the indirect immunofluorescence method clearly shows its thick neuronal processes, rich arborizations, and many varicosities. DR neurons from 10-day-old rats were cultured for 11 days (A), 12 days (C-E), or 16 days (B). Bar: 50 μm.
The main conclusion of this section is that our cultures have abundant cells with processes, and almost all of them are neurons. Out of these neurons (with processes) about 60 % would be serotonergic neurons. Because our cultures contain abundant serotonergic neurons, they will be a useful material for investigating the serotonergic neurons.
3.2. Correlations between the response to 8-OH-DPAT and serotonin-immunoreactivity
According to Aghajanian and Wang (1978), serotonergic neurons in vivo and in slice preparations are inhibited by serotonin which is secreted from their own collaterals that terminate onto the somato-dendritic regions. This auto-inhibition is through 5-HT1A receptors located over the somato-dendritic regions (Feldman and Quenzer, 1983; Barnes and Sharp, 1999). We performed experiments to determine whether the hyperpolarizing response of DR neurons caused by 8-OH-DPAT (1 μM), a selective agonist to 5-HT1A receptors, can be used to identify serotonergic neurons. We recorded the neuronal response to 8-OH-DPAT with the whole-cell patch-clamp method. The same neuron was examined with fluorescence immunocytochemistry using antibody against serotonin.
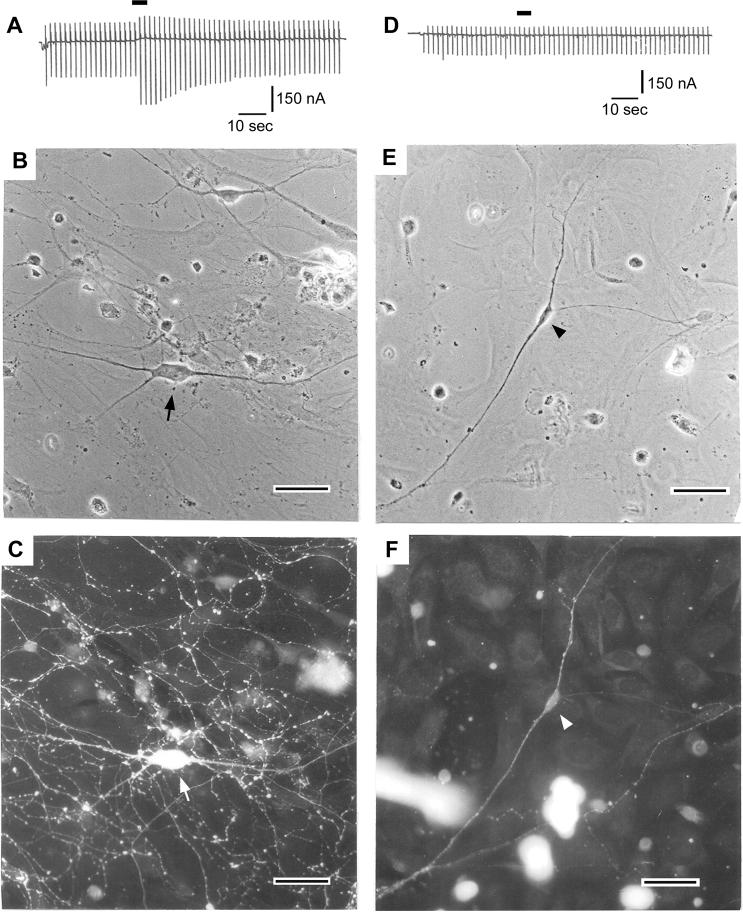
Fig. 2A shows that a neuron in Fig. 2B responded to 8-OH-DPAT with a conductance increase together with a slow outward current at the holding potential (−77 mV). These responses under voltage-clamp reflect a hyperpolrization with a conductance increase under current-clamp conditions. Immunocytochemistry revealed that this neuron (Fig. 2 A, B, C) was reactive to antibody against serotonin (Fig. 2C), indicating that the neuron is serotonergic. In contrast, the neuron in Fig. 2E neither responded to 8-OH-DPAT (Fig. 2D) nor was reactive to serotonin-antibody (Fig. 2F), indicating that this neuron was not serotonergic. We performed these experiments on 39 neurons (Table 2). Among 26 neurons that responded to 8-OH-DPAT with a conductance increase, 24 neurons (92%) were serotonergic. Among 13 neurons with no response to 8-OH-DPAT, 3 neurons (23%) were serotonergic. These results indicate that the positive response to 8-OH-DPAT is an approximate criterion for identifying serotonergic neurons.
Fig. 2.
Correlation between the 8-OH-DPAT response and the serotonin immunoreactivity of cultured DR neurons. (A-C): A DR neuron, which responded to 8-OH-DPAT with a conductance increase, was a serotonergic neuron. (B): A phase-contrast micrograph of a neuron (marked by an arrow) after electrophysiological recording but before fixation, from a 7-day culture made from an 11-day-old rat. (C): The same neuron (pointed by arrow) after fixation showing positive reaction to serotonin-immunofluorescence stain. (D-F): An DR neuron, which did not respond to 8-OH-DPAT, was a non-serotonergic neuron. (E): A phase-contrast micrograph of a neuron (marked by an arrow head) after electrophysiological recording but before fixation from 11-day culture made from 12-day-old rat. (F): The same neuron after fixation showing negative reaction to serotonin-immunofluorescence stain. In A and D, conductance changes were monitored by applying a recurrent command pulse sequence consisting of a square-wave depolarization (20 mV, 50 msec) and hyperpolarization (30 mV, 50 msec). The application of 8-OH-DPAT (1 μM, 5 sec) was conducted by puff application and marked by horizontal bars. The holding potential was −77 mV and 5 mM K+ external solution was used.
Table 2.
Serotonin-immunoreactivity (Serotonin-Immun-R) vs. 8-OH-DPAT response in cultured DR neurons
| Seroronin-Immun-R (+) | Seroronin-Immun-R (−) | |
|---|---|---|
| 8-OH-DPAT response (+) | 24 neurons (*) | 2 neurons |
| 8-OH-DPAT response (−) | 3 neurons | 10 neurons |
The experiments were performed on DR nucleus cultures made from 9 to 12-day-old rats and maintained for 4−16 days. 8-OH-DPAT was applied at 1 μM for 5 sec. The indirect fluorescence immunocytochemical method was used.
3.3. Properties of the 8-OH-DPAT response
We analyzed effects of 8-OH-DPAT, a specific agonist for 5HT1A receptor, on serotonergic neurons. We examined current-voltage (I-V) relationships in 5 mM K+ and 10 mM K+ external solutions (Fig. 3A). In Fig. 3A, five neurons were examined for each external solution. The 8-OH-DPAT (1 μM)-induced currents were obtained by subtracting the resting currents from currents after the application of the agonist. In both external solutions (5 mM K+ and the 10 mM K+ solutions), the 8-OH-DPAT-induced conductance showed inward rectification. The reversal potential for 5 mM [K+]o and that for 10 mM [K+]o were −89.1 and −65.9 mV, respectively. Both of them were near the potassium equilibrium potential, EK (−87.4 mV for 5 mM [K+]o and −69.9 mV for 10 mM [K+]o at 20°C). These results strongly suggest that 8-OH-DPAT, through the 5-HT1A receptor, activated the G-protein-coupled inward rectifier K+ (GIRK; Kir3) channel in DR serotonergic neurons.
Fig. 3.
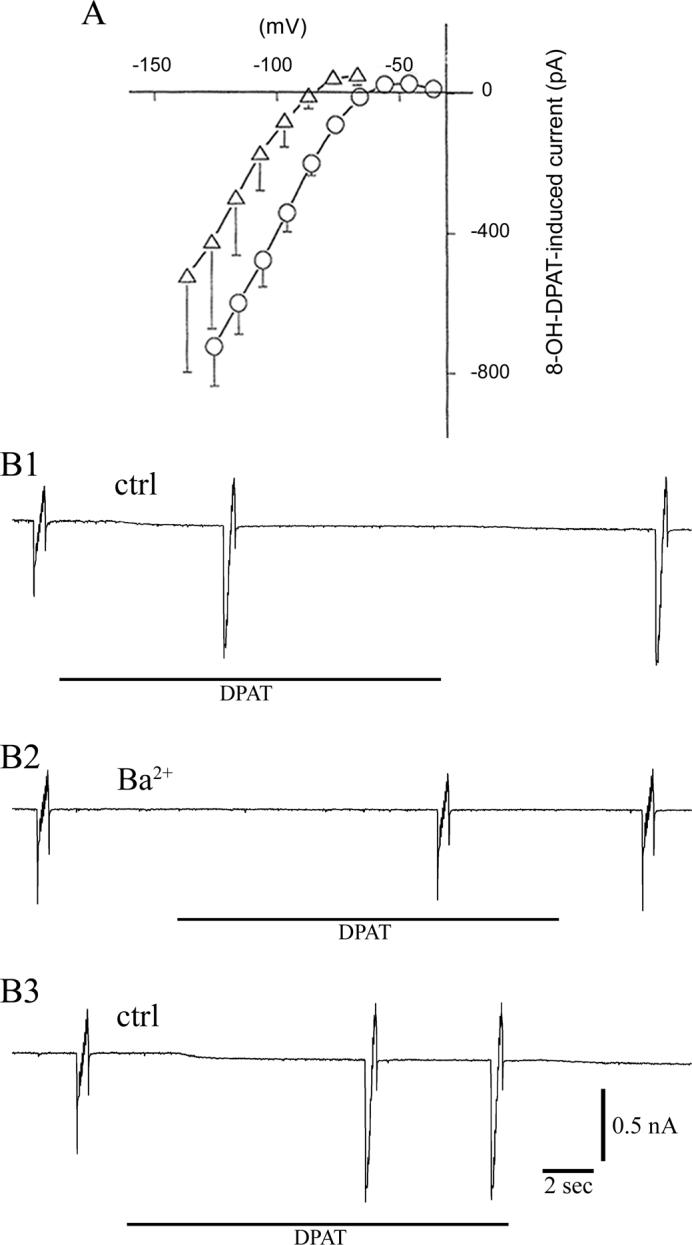
A. Current-voltage (I-V) relation of 8-OH-DPAT-induced response. The current before the application of 8-OH-DPAT was subtracted from the current after the application of 8-OH-DPAT. In both 5 mM K+ (triangle) and 10 mM K+ (circle) external solutions, the conductance showed inward rectification and their reversal potentials were near EK (see the text). To measure the I-V relation, voltage pulses (50 msec in duration) were applied at a 10 mV increment. Vertical lines indicate S.D. Five neurons were examined for each of different external solutions. The holding potential was −77 mV (5 mM K+ external solution) and −76 mV (10 mM K+ external solutions). Neurons cultured for 13 days from 10-day-old rats were used. B. Effects of BaCl2 (300 μM) were investigated on 8-OH-DPAT-induced response. B1. The I-V relation before and after 8-OH-DPAT (1 μM) was measured by imposing staircase-shaped voltage sequence. Application of 8-OH-DPAT produced an inward rectifying current in the same way as in experiments in Fig. 3 A. B2. After applying 300 μM BaCl2, application of 8-OH-DPAT produced hardly any effect. B3. After washing out the BaCl2, the effect of 8-OH-DPAT was recovered considerably. The interval between the beginning of B1 and that of B2 was 176 sec, while the corresponding interval between B2 and B3 was 190 sec. We have also investigated the effects of BaCl2 at a lower concentration (100 μM): at this BaCl2 concentration: the increment of current by the application of 8-OH-DPAT was 22 ± 16% (mean ± sem, n = 5) of the control (i.e, no BaCl2). In both of these experiments neurons were cultured for 10−19 days from 11-day-old rats.
Barium ions are known to inhibit inward rectifier K+ channels (Hagiwara et al., 1978). We examined the effect of Ba2+ on the ability of 8-OH-DPAT to induce the Kir3 (GIRK) channels. As shown in Fig. 3B, we applied a staircase-shaped voltage step (Farkas et al., 1996) to monitor the I-V relation. The record in B1 indicates that application of 8-OH-DPAT (1 μM) produced a large increase in the channel conductance. As expected, the currents created by 8-OH-DPAT (namely, the difference between the currents before and after the 8-OH-DPAT application) revealed I-V relation of an inward rectifying shape, similar to the one in Fig. 3A. We quantified the 8-OH-DPAT effect by using the current amplitude produced by the first step of the staircase voltage imposed (corresponding to a 65 mV hyperpolarization from the holding potential of −76 mV). Subtraction of the current before the 8-OH-DPAT application from that after the 8-OH-DPAT application gave the current increment that was generated by the 8-OH-DPAT application (the average current increment was 297 ± 34 %; mean ± sem, n = 5). Next, we examined the effects of barium ions. In the presence of BaCl2 (300 μM), 8-OH-DPAT hardly activated the K current (Fig. 3B2): the current increment induced by 8-OH-DPAT became only 11 ± 6 % (mean ± sem, n = 5). This means that the Ba2+ treatment reduced the 8-OH-DPAT effect to the level of 4 % (11/297). As shown in B3, the effects of barium ions were partially reversed by washing the barium ions.
The results of this section strongly suggest that the 8-OH-DPAT-induced inhibition of DR serotonergic neurons is largely due to the activation of a hyperpolarizing current; this current shows inward rectification and is eliminated by barium ions. This mode of 8-OH-DPAT effect probably applies to the natural condition, in which serotonin produces inhibitory effects through the 5-HT1 receptor. These results suggest that 8-OH-DPAT, through the 5-HT1A receptor, activated the G-protein-coupled inward rectifier K+ (GIRK; Kir3) channel in DR serotonergic neurons.
3.4. Action potentials
The properties of action potentials recorded from cultured DR neurons were investigated using current clamp recordings at 33° C. Experiments were performed on 15 neurons that were likely to be serotonergic since all of them responded to 8-OH-DPAT. Two neurons out of 15 neurons produced spontaneous repetitive firing, whereas the remaining 13 neurons were silent, but produced action potentials when a depolarizing current was applied. Table 3 shows action potential property of these 15 neurons.
Table 3.
Action potential of cultured serotonergic DR neurons
| Soma diameter (μm) | 19.0 ± 0.7 |
| Resting potential (mV) | −73.0 ± 2.5 |
| Action potential height (mV) | 69.2 ± 2.4 |
| Action potential duration (ms) | 1.70 ± 0.07 |
| Amplitude of after-hyperpolarization (mV) | 20.1 ± 1.8 |
Values are expressed as means ± S.E.M. (n = 15). Experiments were performed on cultures from 10-day-old rats and maintained for 11−14 days. These 15 neurons were likely to be serotonergic neurons, since they were hyperpolarized by the application of 8-OH-DPAT (1 μM). The duration of action potential was measured at threshold. Experiments were conducted at 33° C using a 5 mM K+ external solution.
3.5. Transmitter and agonist effects on action potential firing
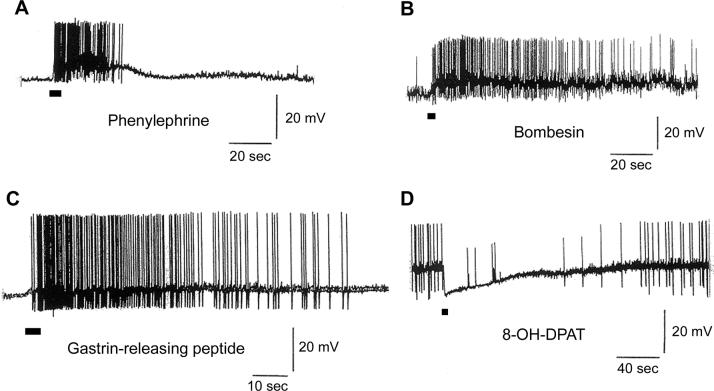
We have investigated the effects of various transmitters and agonists on the initiation of action potentials. We chose neurons that responded with a hyperpolarization to 8-OH-DPAT application since these neurons were likely to be serotonergic neurons. Application of phenylephrine (a selective α1-adrenoreceptor agonist, 50 μM), bombesin (1 μM), or gastrin-releasing peptide (1 μM) depolarized these neurons and produced action potentials (Fig. 5, A, B, C). The effects of these transmitters and agonists are summarized in Table 4.
Fig.5.
Neurotransmitter and agonist effects on cultured serotonergic DR neurons. All neurons used in this experiment were identified as serotonergic based on their hyperpolarizing response to 8-OH-DPAT. Two different transmitters, bombesin and gastrin-releasing peptide as well as two different agonists, phenylephrine and 8-OH-DPAT, were used. These transmitters or agonists were applied for 5 sec. Phenylephrine, bombesin, and gastrin-releasing peptide depolarized neurons and elicited action potentials (A, B, and C). In contrast, 8-OH-DPAT caused hyperpolarization and inhibited action potential firing (D). Experiments were conducted at 33° C. Cultures were made from 10-day-old rats and maintained for 11 days (D), 13 days (A and C), and 15 days (B).
Table 4.
Responses of cultured serotonergic DR neurons to agonists under current clamp
| phenylephrine (50 μM) | bombesin (1 μM) | gastrin-releasing peptide (1 μM) | |
|---|---|---|---|
| Action potentials elicited (n) | 6 | 4 | 6 |
| No response (n) | 0 | 2 | 1 |
| Hyperpolarized (n) | 0 | 0 | 0 |
Agonists were applied for 5 sec. All neurons in this table were likely to be serotonergic, since they responded to 8-OH-DPAT with hyperpolarization. Experiments were conducted at ∼33 °C, except two neurons in the phenylephrine experiments that were recorded at room temperature. Cultures were prepared from 10 to 12- day-old rats and maintained for 10 to 19 days. 5 mM K+ external solution was used. n: number of neurons.
Fig. 5D shows that application of 8-OH-DPAT induced a hyperpolarization and the concomitant inhibition of action potentials.
4. Discussion
4.1. Features of the dissociated DR cultures
One of the features of our dissociated DR cultures is that they originated from ∼10-day-old postnatal rats. This is in contrast to the DR cultures that are obtained from embryonic brains (Yamamoto et al., 1981; Rudge et al., 1996; Héry et al., 2000; Nishi et al., 2000) or from newborn rats (Johnson, 1994). This difference could be important for some types of research such as experiments involving neurotransmitter receptors since certain neuroreceptors are known to increase in number postnatally or to develop postnatally (Dudai et al., 1980; Gonzalez et al., 1989). Another advantage of using older postnatal animals is that the DR nucleus is more developed and better defined, allowing us to dissect out the DR nucleus more precisely.
Highly enriched serotonergic neuron cultures are desirable for various types of experiments. Our cultures are rich in serotonergic neurons: 62% of our cultured DR neurons were serotonergic (Table 1). Cultures containing a high percentage of serotonergic neurons were made by our method, which uses selective excision of the DR nucleus from brain slices under the dissecting microscope.
4.2. Electrophysiological properties of cultured DR serotonergic neurons
Most of serotonergic neurons in our DR cultures did not show spontaneous firing of action potentials. Similarly, DR serotonergic neurons in culture (Johnson, 1994) as well as those in slice preparations are quiescent (Vandermaelen and Aghajanian, 1983; Kirby et al., 2003). In contrast, many DR serotonergic neurons in vivo are firing spontaneously (Aghajanian and Wang, 1978). Absence of spontaneous firing of serotonergic DR neurons in culture and in slice preparations could be due to the absence of excitatory inputs to DR (Baraban and Aghajanian, 1980; Vandermaelen and Aghajanian, 1983; Yoshimura et al., 1985). The duration of action potential (1.7 msec) and the amplitude of after-hyperpolarization (20 mV) obtained from our cultured serotonergic neurons (Table 3) are very similar to those reported for slice preparations (Agajanian and Lakoski; 1984).
4.3. Activation of 5HT1A receptor by serotonin
Serotonergic DR neurons in vivo or in slices are inhibited (hyperpolarized) by serotonin through their autoreceptors (5-HT1A receptors) (Aghajanian and Wang, 1978; Vandermaelen and Aghajanian 1983; Kirby et al. 2003). Aghajanian and Lakoski (1984) noticed that this inhibition is caused by the activation of K+ channels, which were later identified as G protein-coupled inward rectifier channels (Penington et al., 1993; Jin and Akaike, 1998).
Our cultured serotonergic neurons responded to 8-OH-DPAT (a 5-HT1A receptor agonist) with an increase in an inwardly rectifying K+ conductance (Figs. 2 and 3). The neurons responding to 8-OH-DPAT are mostly (92%: 24 out of 27 cells) serotonergic neurons (Table 2, Fig. 2), indicating a very good, though not perfect, correlation between positive 8-OH-DPAT responses and positive serotonin immunocytochemistry (Table 2). A small percentage of non serotonergic neurons responded to the application of 8-OH-DPAT (17%: 2 out of 12 cells). These results agree with the data on brain slice preparations (Kirby et al., 2003), in which a similar correlation existed between the presence of 5HT1A receptors and the presence of serotonin in the neuron.
4.4. Responses to excitatory transmitters and agonists
Phenylephrine (a selective α1-adrenoreceptor agonist)
Noradrenergic afferent fibers innervate DR neurons. Stimulation of these nerve fibers causes tonic excitation in DR serotonergic neurons. This noradrenergic effect is most likely transmitted through the α1-adrenoceptors (Baraban and Aghajanian, 1980; Vandermaelen and Aghajanian, 1983; Yoshimura et al., 1985). Activation of α1-adrenoceptors inhibits K+ conductance. This produces depolarization, resulting in an increase in the firing rate (Aghajanian, 1985).
Most of serotonergic DR neurons in cell cultures as well as in brain slices do not generate spontaneous action potentials (Vandermaelen and Aghajanian 1983; Johnson, 1994; Kirby et al., 2003). These results are probably due to the scarcity of excitatory inputs (including noradrenergic inputs) to the DR neurons in these preparations. In slice preparations of DR serotonergic neurons, application of α1-adrenoceptor agonist restores firing of action potentials (Vandermaelen and Aghajanian 1983; Kirby et al. 2003).
In our cultures, phenylephrine, an α1-adrenoreceptor agonist, produced depolarization and elicited action potentials in all putative serotonergic neurons examined (6 out of 6 cells). In this respect, our primary DR neuron cultures from ∼10 day-old postnatal rats behave like the neurons in brain slice preparation, exhibiting the capability of producing action potentials by phenylephrine application.
Bombesin and gastrin-releasing peptide
In mammalian tissues, there are two kinds of bombesin-like peptides, gastrin-releasing peptide and neuromedin B. These peptides mediate a variety of biological activities, including the control of body temperature, satiety, and the maintenance of circadian rhythms (Battey and Wada, 1991). Three types of receptors for bombesin-like peptides were cloned: the gastrin-releasing peptide receptor, the neuromedin B receptor, and the bombesin receptor subtype 3 (Spindel et al., 1990; Battey et al., 1991; Corjay et al., 1991; Wada et al., 1991; Fathi et al., 1993). These three types of receptors exist in the DR nucleus of rats (Ladenheim et al., 1992; Battey and Wada, 1991; Jennings et al., 2003).
Our cultured serotonergic neurons responded to the application of bombesin and gastrin-releasing peptide with depolarization and action potential firing (Table 4; Fig. 5, B, C). These data approximately agree with those obtained using slice preparations by Pinnock et al. (1994).
4.5. Characteristics and future applications of cultured DR serotonergic neurons
We have developed dissociated cultures of the DR nucleus from ∼10-day-old postnatal rats. The cultures were rich (∼60 %) in serotonergic neurons. Serotonergic and non-serotonergic neurons could not be distinguished by their light microscope morphology. However, we found that neurons responding to the 5-HT1A receptor agonist 8-OH-DPAT with hyperpolarization were most likely (∼90%) serotonergic. The application of transmitters such as phenylephrine, bombesin, and gastrin-releasing peptide to these serotonergic neurons resulted in neuronal excitation. Such serotonergic neurons also produced action potentials similar to those reported in brain slice preparations. These results indicate that our cultured DR serotonergic neurons are useful for physiological studies. By using our dissociated cultures of brain nuclei, such as the locus coeruleus (Masuko et al., 1986), the substantia nigra (Masuko et al., 1992), and the nucleus basalis (Nakajima et al., 1985), we investigated signal transduction mechanisms of neuronal excitation and inhibition using whole-cell and single channel recordings (Grigg et al., 1996; Hoang et al., 2004). We also performed application of toxins such as pertussis toxin for a long time (Hoang et al., 2003), did injection of antisense oligonucleotides (Takano et al., 1997), and transfected DNAs to cultured neurons with a microinjector (Koike-Tani et al., 2005). Recently, using viral vectors, delivery of DNAs and small interference RNAs to primary cultured neurons have become possible. Application of a variety of methods to our DR nucleus cultures would answer many questions in physiology and pathology of the DR serotonergic neurons.
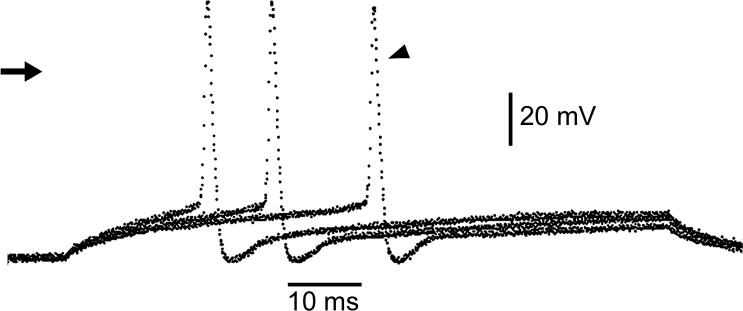
Fig. 4.
Action potentials elicited by depolarizing currents (duration, 93 msec). The neuron was cultured for 13 days from a 10-day-old rat. The neuron was probably serotonergic because of the response to 8-OH-DPAT. Arrow indicates the zero current level. The first action potential (marked by an arrowhead) was elicited by the depolarizing current of 150 pA and the second and third action potentials were activated by 170 pA and 190 pA currents, respectively. The resting potential was −71 mV. The whole-cell version of patch-clamp under constant current was used. The experiment was conducted at 32° C.
Acknowledgments
This work was supported by National Institute of Health Grants MH057837, NS043239. Junko Yasufuku-Takano was in part supported by a Study Abroad Award from College Women's Association of Japan. We thank Christina V. Floreani for suggestions in writing and Lisabeth Duval Pérez and Nicole M. Jones for participating in the experiments of Fig. 3B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985;315:501–3. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Lakoski JM. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984;305:181–5. doi: 10.1016/0006-8993(84)91137-5. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY. Physiology and pharmacology of central serotonergic neurons. In: Lipton MA, Dimascio A, Killam KF, editors. Psychopharmacology: A Generation of Progress. Raven Press; New York: 1978. pp. 171–83. [Google Scholar]
- Aitken AR, Tork I. Early development of serotonin-containing neurons and pathways as seen in wholemount preparations of the fetal rat brain. J Comp Neurol. 1988;274:32–47. doi: 10.1002/cne.902740105. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J Comp Neurol. 1981;198:677–716. doi: 10.1002/cne.901980409. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–67. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bajic D, Hoang QV, Nakajima S, Nakajima Y. Dissociated histaminergic neuron cultures from the tuberomammillary nucleus of rats: culture methods and ghrelin effects. J Neurosci Methods. 2004;132:177–84. doi: 10.1016/j.jneumeth.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–63. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Battey J, Wada E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 1991;14:524–8. doi: 10.1016/0166-2236(91)90005-f. [DOI] [PubMed] [Google Scholar]
- Battey JF, Way JM, Corjay MH, Shapira H, Kusano K, Harkins R, Wu JM, Slattery T, Mann E, Feldman RI. Molecular cloning of the bombesin/gastrin-releasing peptide receptor from Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1991;88:395–9. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton LL, Lazo JS, Parker KL. Goodman & Gilman's The Pharmacological Basis of Therapeutics. eleventh McGraw-Hill; New York: 2006. [Google Scholar]
- Corjay MH, Dobrzanski DJ, Way JM, Viallet J, Shapira H, Worland P, Sausville EA, Battey JF. Two distinct bombesin receptor subtypes are expressed and functional in human lung carcinoma cells. J Biol Chem. 1991;266:18771–9. [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl. 1964;232(SUPPL):1–55. [PubMed] [Google Scholar]
- Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine Neurons in the Central Nervous System. II. Experimentally Induced Changes in the Intraneuronal Amine Levels of Bulbospinal Neuron Systems. Acta Physiol Scand Suppl. 1965;247(SUPPL):1–36. [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult rat: a light and electron microscope radioautographic study. J Comp Neurol. 1982;207:239–54. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Ben-Barak J, Silman I, Gazit H. Ontogenesis and modulation of cholinergic receptors in rat brain. In: Littauer UZ, Dudai Y, Silman I, Teichberg VI, Vogel Z, editors. Neurotransmitters and Their Receptors. Wiley; New York: 1980. pp. 217–239. [Google Scholar]
- Farkas RH, Chien PY, Nakajima S, Nakajima Y. Properties of a slow nonselective cation conductance modulated by neurotensin and other neurotransmitters in midbrain dopaminergic neurons. J Neurophysiol. 1996;76:1968–81. doi: 10.1152/jn.1996.76.3.1968. [DOI] [PubMed] [Google Scholar]
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268:5979–84. [PubMed] [Google Scholar]
- Feldman RS, Quenzer LF. Fundamentals of Neuropsychopharmacology. Sinauer Associates; Sunderland: 1983. Serotonin. [Google Scholar]
- Gonzalez BJ, Leroux P, Bodenant C, Laquerriere A, Coy DH, Vaudry H. Ontogeny of somatostatin receptors in the rat brain: biochemical and autoradiographic study. Neuroscience. 1989;29:629–44. doi: 10.1016/0306-4522(89)90136-x. [DOI] [PubMed] [Google Scholar]
- Grigg JJ, Kozasa T, Nakajima Y, Nakajima S. Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J Neurophysiol. 1996;75:318–28. doi: 10.1152/jn.1996.75.1.318. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hery M, Semont A, Fache MP, Faudon M, Hery F. The effects of serotonin on glucocorticoid receptor binding in rat raphe nuclei and hippocampal cells in culture. J Neurochem. 2000;74:406–13. doi: 10.1046/j.1471-4159.2000.0740406.x. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Zhao P, Nakajima S, Nakajima Y. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol. 2004;92:3183–91. doi: 10.1152/jn.01222.2003. [DOI] [PubMed] [Google Scholar]
- Jennings CA, Harrison DC, Maycox PR, Crook B, Smart D, Hervieu GJ. The distribution of the orphan bombesin receptor subtype-3 in the rat CNS. Neuroscience. 2003;120:309–24. doi: 10.1016/s0306-4522(03)00260-4. [DOI] [PubMed] [Google Scholar]
- Jin YH, Akaike N. Tandospirone-induced K+ current in acutely dissociated rat dorsal raphe neurones. Br J Pharmacol. 1998;124:897–904. doi: 10.1038/sj.bjp.0701922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD. Electrophysiological and histochemical properties of postnatal rat serotonergic neurons in dissociated cell culture. Neuroscience. 1994;63:775–87. doi: 10.1016/0306-4522(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Kim KM, Nakajima Y, Nakajima S. G protein-coupled inward rectifier modulated by dopamine agonists in cultured substantia nigra neurons. Neuroscience. 1995;69:1145–58. doi: 10.1016/0306-4522(95)00326-e. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–83. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike-Tani M, Collins JM, Kawano T, Zhao P, Zhao Q, Kozasa T, Nakajima S, Nakajima Y. Signal transduction pathway for the substance P-induced inhibition of rat Kir3 (GIRK) channel. J Physiol. 2005;564:489–500. doi: 10.1113/jphysiol.2004.079285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim EE, Jensen RT, Mantey SA, Moran TH. Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res. 1992;593:168–78. doi: 10.1016/0006-8993(92)91305-x. [DOI] [PubMed] [Google Scholar]
- Lautenschlager M, Holtje M, von Jagow B, Veh RW, Harms C, Bergk A, Dirnagl U, Ahnert-Hilger G, Hortnagl H. Serotonin uptake and release mechanisms in developing cultures of rat embryonic raphe neurons: age- and region-specific differences. Neuroscience. 2000;99:519–27. doi: 10.1016/s0306-4522(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Leifer D, Lipton SA, Barnstable CJ, Masland RH. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984;224:303–6. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Lidov HG, Molliver ME. Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull. 1982;9:559–604. doi: 10.1016/0361-9230(82)90164-2. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima S, Nakajima Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience. 1992;49:347–64. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima Y, Nakajima S, Yamaguchi K. Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci. 1986;6:3229–41. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Masuko S. A technique for culturing brain nuclei from postnatal rats. Neurosci Res. 1996;26:195–203. doi: 10.1016/s0168-0102(96)01101-7. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Masuko S. Dissociated cell cultures of microdissected brainstem and basal forebrain regions on feeder layers. In: Haynes LW, editor. The Neuron in Tissue Culture. John Wiley & Sons, Lt; West Sussex: 1999. pp. 510–18. [Google Scholar]
- Nakajima Y, Nakajima S, Obata K, Carlson CG, Yamaguchi K. Dissociated cell culture of cholinergic neurons from nucleus basalis of Meynert and other basal forebrain nuclei. Proc Natl Acad Sci U S A. 1985;82:6325–9. doi: 10.1073/pnas.82.18.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington NJ, Kelly JS, Fox AP. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J Physiol. 1993;469:387–405. doi: 10.1113/jphysiol.1993.sp019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–91. [PubMed] [Google Scholar]
- Pinnock RD, Reynolds T, Woodruff GN. Different types of bombesin receptors on neurons in the dorsal raphe nucleus and the rostral hypothalamus in rat brain slices in vitro. Brain Res. 1994;653:119–24. doi: 10.1016/0006-8993(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Eaton MJ, Mather P, Lindsay RM, Whittemore SR. CNTF induces raphe neuronal precursors to switch from a serotonergic to a cholinergic phenotype in vitro. Mol Cell Neurosci. 1996;7:204–21. doi: 10.1006/mcne.1996.0016. [DOI] [PubMed] [Google Scholar]
- Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol Endocrinol. 1990;4:1956–63. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen. A combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol (Paris) 1981;77:157–74. [PubMed] [Google Scholar]
- Steinbusch HW, Verhofstad AA, Joosten HW. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3:811–9. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Sternberger LA. The unlabeled antibody peroxidase-antiperoxidase (PAP) method. In: Sternberger LA, editor. Immunocytochemistry. John Wiley; New York: 1979. pp. 104–69. [Google Scholar]
- Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. Different G proteins mediate somatostatin-induced inward rectifier K+ currents in murine brain and endocrine cells. J Physiol. 1997;502:559–67. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törk I. Raphe nuclei and serotonin containing systems. In: Paxinos G, editor. The Rat Nervous System: Hindbrain and Spinal Cord. Vol. 2. Academic Press; Australia: 1985. pp. 43–78. [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–19. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battery J. cDNA cloning, characterization, and brain region-specific expression of a neuromedin-B-preferring bombesin receptor. Neuron. 1991;6:421–30. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Wallace JA, Lauder JM. Development of the serotonergic system in the rat embryo: an immunocytochemical study. Brain Res Bull. 1983;10:459–79. doi: 10.1016/0361-9230(83)90144-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Steinbusch HW, Jessell TM. Differentiated properties of identified serotonin neurons in dissociated cultures of embryonic rat brain stem. J Cell Biol. 1981;91:142–52. doi: 10.1083/jcb.91.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasufuku-Takano J, Nakajima S, Nakajima Y. Dissociated Serotonergic Neuron Cultures from the Dorsal Raphe Nucleus of Postnatal Rats: Culture Methods and Transmitter Effects.. Society for Neuroscience 34th Annual Meeting; 2004. Program Number 620.14. [Google Scholar]
- Yoshimura M, Higashi H. 5-Hydroxytryptamine mediates inhibitory postsynaptic potentials in rat dorsal raphe neurons. Neurosci Lett. 1985;53:69–74. doi: 10.1016/0304-3940(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Higashi H, Nishi S. Noradrenaline mediates slow excitatory synaptic potentials in rat dorsal raphe neurons in vitro. Neurosci Lett. 1985;61:305–10. doi: 10.1016/0304-3940(85)90481-1. [DOI] [PubMed] [Google Scholar]