Abstract
The development of automated flow cytometric (FCM) methods for evaluating micronucleus (MN) frequencies in erythrocytes has great potential for improving the sensitivity, reproducibility, and throughput of the traditional in vivo rodent MN assay that uses microscopy-based methods for data collection. Although some validation studies of the FCM evaluation methods have been performed, a comprehensive comparison of these two data collection methods under routine testing conditions with a variety of compounds in multiple species has not been conducted. Therefore, to determine if FCM evaluation of MN frequencies in rodents was an acceptable alternative to traditional manual scoring methods in our laboratory, we conducted a comparative evaluation of MN-reticulocyte (MN-RET) frequencies determined by FCM- and microscopy-based scoring of peripheral blood and bone marrow samples from B6C3F1 mice and Fisher 344 rats. Four known inducers of MN (cyclophosphamide, ethyl methanesulfonate, vincristine sulfate, acrylamide) were assayed in bone marrow and peripheral blood of both mice and rats. In addition, MN-RET frequencies were measured in bone marrow (microscopy) and peripheral blood (FCM) of mice treated with five nongenotoxic chemicals (S-adenosylmethionine chloride, cefuroxime, diphenolic acid, 3-amino-6-methylphenol, pentabromodiphenyl oxide). No significant differences were observed between results obtained by the two methods in either species. These results support the use of FCM for determining MN-RET frequency in rodents after chemical exposure.
Keywords: mice, rats, micronucleus, chromosome damage, genotoxicity, cyclophosphamide, vincristine sulfate
1. INTRODUCTION
Micronuclei (MN) are well-characterized biomarkers of chromosomal damage [1]. Measurements of MN frequencies within a dividing cell population are generally accepted as an alternative to the more laborious slide-based scoring of classical structural chromosomal aberrations (CA). Since MN are formed either from acentric chromosome fragments or from lagging chromosomes that fail to migrate to the poles during anaphase, the determination of MN frequencies is a reliable method for evaluating the potential of a chemical to induce structural and/or numerical chromosomal alterations. Of the variety of in vivo assays used to detect genotoxic chemicals, the most common is the in vivo rodent erythrocyte MN assay [1, 2, 3, 4, 5, 6]. This assay has been used routinely for decades and has typically been conducted by evaluating the frequency of micronucleated erythrocytes in bone marrow or peripheral blood slide preparations.
In mice, two subpopulations of erythrocytes -- immature (reticulocytes, RET) and mature -- may be scored for MN, depending upon treatment regimen. By microscopy, RET and mature erythrocytes are easily differentiated by the presence or absence of residual RNA, respectively, visible by appropriate staining methods (e.g., acridine orange). Because of the kinetics of erythrocyte production and maturation, RET show the effects of recently induced damage (within the past 24–48 hr); using appropriate sampling times, RET can be scored in either bone marrow or peripheral blood. Mature erythrocytes show accumulated damage from repeated chronic dosing within the past 3–30 days; erythrocytes are typically scored for MN in mouse blood smears. Under continuous exposure conditions the frequency of micronucleated erythrocytes reaches steady state in mouse peripheral blood after approximately 30 days (i.e., the average lifespan for mouse erythrocytes) [3]. In rats, due to the rapid and efficient action of the spleen in removing MN-bearing erythrocytes from blood, RET have been scored in bone marrow to completely eliminate the influence of splenic filtration on MN-RET frequency [7].
Although more rapid, less expensive, and more sensitive compared with traditional rodent CA assays, the standard in vivo rodent erythrocyte MN assay has certain limitations. Manually scoring slide preparations for MN can be somewhat subjective and dependent upon staining quality. More importantly, a relatively small number of RET or mature erythrocytes (typically 2000 cells per animal) are evaluated in slide preparations, which can lead to considerable sampling variability due to the rarity of MN.
The development of automated FCM-based methods to measure MN frequencies in erythrocytes represents a technological advance over manual, slide-based scoring methods [8, 9]. FCM scoring eliminates bias and allows for the evaluation of a much greater number of cells per animal, thereby greatly reducing sampling variability. In addition, data from an entire experiment can be obtained in a single day, rather than several days to weeks, and FCM analysis requires only minute quantities of blood (60–120 μL). Furthermore, FCM techniques can target a specific cell surface marker, the transferrin receptor (CD71), which is active for only a short time after a RET enters the peripheral blood from the bone marrow. In organisms such as humans and rats with active splenic selection, targeting the very young CD71+ RET subpopulation permits the interrogation of these cells for MN frequency before the spleen has much opportunity to reduce MN frequencies by sequestering and destroying MN-RET [8]. In standard blood smears, this subpopulation of young RET cannot be identified as precisely, unless the acridine orange supravital staining method is used [10, 11]. Due to the action of the spleen, MN assays in rats traditionally have been based on scoring bone marrow rather than blood [7]. Using FCM techniques to score CD71+ RET in peripheral blood of rats, if accurate results can be obtained with this approach, would allow integration of the rat MN assay into general toxicity tests, since blood samples can be obtained without sacrificing the animal [12, 13, 14, 15].
In addition to the advantages of FCM described above, FCM can simultaneously provide information about the quantity of DNA present in the observed MN, thereby providing an indication of the mechanism by which MN are induced. MN with higher DNA content are more likely to contain a chromosome arising from events producing chromosome loss, whereas MN with lower DNA content are more likely to contain a chromosome fragment arising through breakage events [16].
The National Toxicology Program (NTP) plans to modify its standard microscopy-based in vivo rodent MN test protocols to take advantage of the benefits of FCM for the evaluation of MN frequencies in rodent peripheral blood samples. However, before replacing the routine manual microscope-based bone marrow and peripheral blood scoring methods with automated FCM evaluation of blood samples, we conducted a comparative evaluation in rats and mice to determine the concordance between these two methods of data collection. Initially, we evaluated MN frequencies in peripheral blood and bone marrow of mice treated with five chemicals (S-adenosylmethionine chloride, cefuroxime, diphenolic acid, 3-amino-6-methylphenol, pentabromodiphenyl oxide) nominated to NTP for MN testing, all of which turned out to be negative. Subsequently, we conducted MN assays in both mice and rats with four model genotoxic compounds including three clastogens (cyclophosphamide, ethyl methanesulfonate, acrylamide) and one aneugen (vincristine sulfate). Here, we report our findings and discuss the advantages and disadvantages, based on our experience, with both methods of data collection.
2. MATERIALS AND METHODS
2.1 Chemicals
The nongenotoxic compounds used in these studies were cefuroxime (CAS registry number: 55268-75-2), S-adenosylmethionine chloride (24346-00-7), pentabromodiphenyl oxide (32534-81-9), diphenolic acid (126-00-1), and 3-amino-6-methylphenol (2835-95-2). Known inducers of MN were cyclophosphamide (50-18-0), ethylmethanesulfonate (62-50-0), acrylamide (79-06-1), and vincristine sulfate (57-22-7). The five nongenotoxic compounds were supplied via the NTP Chemistry Support Contract through RTI International (Research Triangle Park, NC) and sent to the testing laboratory (ILS, Inc., Research Triangle Park, NC) as coded aliquots. The four genotoxic compounds were purchased from Sigma-Aldrich (St. Louis, MO) and were assigned code numbers prior to use in the experiments described below.
Cefuroxime, S-adenosylmethionine chloride, pentabromodiphenyl oxide, diphenolic acid, and 3-amino-6-methylphenol were dissolved in corn oil and administered by oral gavage. Acrylamide, ethyl methanesulfonate (both direct-acting clastogens), and cyclophosphamide (a clastogen that requires metabolic activation) were dissolved in phosphate buffered saline (pH 7.4) and administered by oral gavage. Vincristine sulfate, an aneugen, was dissolved in phosphate buffered saline (pH 7.4) and administered by intraperitoneal injection. For nongenotoxic compounds, the high dose was limited by toxicity or, in the absence of toxicity, the internationally accepted limit dose (2000 mg/kg) was used [2]. For the genotoxic compounds, dose-setting information was available from previous studies conducted at ILS, Inc., or from published studies.
2.2 Animal Husbandry
Male B6C3F1 mice and Fisher 344 rats were used for this study. Animals were acclimated for seven days after receipt from the supplier (Charles River Laboratories, Portage, MI). Animals were 8–16 weeks of age at the beginning of treatment; treatment groups consisted of five animals. Animals were maintained in constant temperature rooms (71 ± 3°F) with relative humidity of 30–70% on a 12:12 (5 am–5pm) light:dark cycle. Animals were housed individually in polycarbonate cages with Sanichip Laboratory hardwood bedding (P.J. Murphy Forest Products Corp., Montvale, NJ) and provided food (Purina Certified Rodent Chow 5002, Ralston Purina, St. Louis MO) and tap water ad libitum. The ILS, Inc. Institutional Animal Use and Care Committee (IACUC) approved these studies and the animals were handled strictly in accordance with ILS, Inc. institutional guidelines and NIH regulations for humane treatment of research animals.
The animals were dosed once daily for three consecutive days and sampling of blood and/or bone marrow occurred 24 hr after the third treatment.
2.3 Micronucleus Test
2.3.1 Microscopy-based scoring procedure
To assess MN frequency using traditional protocols [17, 18, 19], peripheral blood smears were prepared from each mouse and rat by either snipping the tail tip and squeezing a drop of blood onto each of two labeled microscope slides or by collection of blood drops from the vena cava after euthanasia. To obtain bone marrow, animals were euthanized using CO2 asphyxiation, the femurs were removed and cleaned of tissue, the bone ends were snipped off, and the contents of the femurs were immediately flushed onto clean, labeled microscope slides using phosphate-buffered saline. Squash preparations were made to create a monolayer of bone marrow cells. Blood and bone marrow slides were air-dried, fixed in absolute methanol for 5–10 minutes, and then air-dried again. Slides were stained with acridine orange (Sigma-Aldrich), a fluorescent DNA- and RNA-specific stain [20], and coded before scoring. For each animal, 2000 uniformly stained RET per tissue were scored at 1000x using epi-illuminated fluorescence microscopy (450–490 nm excitation, 520 emission). In addition, 1000 total erythrocytes (RET and mature) were scored on peripheral blood slides to determine %RET (typically less than 5%) as a measure of chemical-induced bone marrow toxicity. In bone marrow, 200 total erythrocytes were scored per animal to determine %RET (typically 40–60%).
2.3.2. Flow cytometry
To assess MN frequency using FCM, blood samples were processed immediately upon collection as described in the MicroFlow® BASIC Kits (for mouse and rat) from Litron Laboratories (Rochester, NY). The kits contain all the supplies and reagents necessary to process blood samples. Briefly, a 60–120 μL blood sample was collected from the vena cava after euthanasia, diluted in sodium heparin solution, and fixed in ultracold methanol. Fixed blood samples were immediately placed into a −80°C freezer for storage until FCM analysis was conducted. A FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) was used to carry out the analyses. Malaria-infected mouse cells were used as a reference standard to consistently define the MN analysis windows and to establish proper daily photomultiplier tube voltages and compensation [9, 21]. RET were identified by the presence of an active transferrin receptor (CD71+) on the cell surface; mature erythrocytes were identified as CD71-negative. For rat samples, the FCM analysis was restricted to the youngest RET (i.e., the subpopulation of erythrocytes with the highest CD71 expression). MN are detected using the DNA staining dye propidium iodide (PI) in conjunction with RNase treatment. Therefore, MN-RET express high levels of CD71 (CD71+) and PI-associated fluorescence, while MN-erythrocytes are negative for CD71 (CD71−) and show PI-associated fluorescence. Ten to twenty thousand CD71+RET were scored per animal for presence of MN; approximately 1 × 106 total erythrocytes were counted to determine %RET as a measure of chemical-induced bone marrow toxicity.
To potentially evaluate the mechanism of MN formation (aneugenic versus clastogenic activity), the intensity of the PI-associated fluorescence, which is proportional to DNA content of the MN, was determined for 100 MN-RET from each animal within a treatment group for each of the four known inducers of MN. A histogram of the fluorescence intensity was recorded for each animal’s sample, and the median of these values (“median channel”, or the channel number that divides the single parameter histogram into two parts, each containing an equal number of data points) was selected to provide a quantitative description of average DNA content of the MN in a sample. MN with higher DNA content are presumed to contain whole chromosomes and MN with lower DNA content are presumed to contain chromosome fragments [16]. Finally, the mean (± SD) of the median channel values for each dose group was calculated and plotted.
2.4 Statistical analyses
The statistical analyses focused on two aspects of the MN assays. First, do the data from the two scoring procedures (microscopy versus flow cytometry) yield the same conclusion about the in vivo genotoxic potential of the chemical? The ability of each compound to induce MN is based on one-sided tests of the trend of the dose-response relationship and an analysis of pairwise differences between each dosed group and the control group. To maintain the overall significance level at 0.05 with multiple tests, the trend is declared statistically significant if p < 0.025, and pairwise differences from the control group are declared statistically significant if p < 0.025/k where k is the number of dosed groups. Second, are similar %MN-RET values obtained from microscopy-based and flow cytometry-based scoring procedures?
Applying the NTP approach to analyzing MN scores from slides, data for each treatment group were summarized as the mean frequency of MN-RET per 1000 RET, plus or minus the standard error of the mean. The numbers of cells with and without MN were pooled over animals within each exposure group and analyzed for increasing trend over exposure groups using a one-tailed Cochran-Armitage binomial trend test, with an adjustment for variance inflation [22]. In addition, pairwise comparisons between each exposure group and the corresponding control group were made using a one-tailed Pearson chi-square test.
For the FCM -based determinations of MN frequencies, the animal was the experimental unit; data within a treatment group were not pooled prior to analysis. 10,000 RET/animal were scored for frequency of MN for the nongenotoxic chemicals and 20,000 RET/animal were scored for frequency of MN for the genotoxic compounds unless bone marrow toxicity prevented the collection of data from 20,000 RET. The difference in the number of cells scored for MN between the two sets of compounds reflected protocol refinement over time; during the course of these experiments, studies relating sample size and detection sensitivity indicated that 20,000 RET represented an optimal number to score for the presence of MN [23]. MN frequencies were expressed as mean MN-RET per 1000 RET. Approximately 106 erythrocytes were scored per tissue per animal to determine %RET. For each chemical, exposure-related trend was tested using a one-tailed Kendall’s correlation test and each treatment group was compared to the control group using the Mann-Whitney test. Slide-based bone marrow and peripheral blood MN-RET frequencies were compared to flow-based peripheral blood MN-RET frequencies measured in the same animals using Wilcoxon’s signed-ranks test. For the purposes of comparative statistical analysis, we considered RNA-positive reticulocytes observed on acridine orange-stained slides and CD71+ RETs identified by FCM to be equivalent cell populations. Animals having fewer than 10,000 RET counted in FCM-based determinations were excluded from this comparison.
3. RESULTS
Analysis of bone marrow slides stained with acridine orange showed no dose-related increases in MN-RET in mice treated with the five nongenotoxic chemicals: cefuroxime, S-adenosylmethionine chloride, pentabromodiphenyl oxide, diphenolic acid, or 3-amino-6-methylphenol (Table 1). FCM analysis of CD71+RET in blood from these same mice also showed no significant increases in MN-RET. It should be noted that for S-adenosylmethionine chloride and 3-amino-6-methylphenol, a significant increase in MN-RET in bone marrow was observed in the lowest dose group (Table 1); in repeat bone marrow MN tests with each chemical, the positive response at the lowest dose was not replicated (NTP, data not shown).
Table 1.
MN frequency in B6C3F1 mice administered S-adenosylmethionine chloride, cefuroxime, diphenolic acid, 3-amino-6-methylphenol, or pentabromodiphenyl oxide
| S-Adenosylmethionine chloride | |||
|---|---|---|---|
| Microscopy | Flow Cytometry | ||
| BM | PB | ||
| Dose (mg/kg) | N1 | MN-RET/1000 RET2 | |
| 0 | 5 | 0.60 ± 0.19 | 2.29 ± 0.11 |
| 500 | 5 | 3.60 ± 0.51* | 2.06 ± 0.11 |
| 1000 | 4 | 0.88 ± 0.13 | 2.38 ± 0.06 |
| 2000 | 4 | 1.63 ± 0.43 | 2.26 ± 0.17 |
| Trend Test | P = 0.427 | P = 0.607 | |
| CPA3 | 3 | 42.50 ± 1.54* | 23.49 ± 1.77* |
| Cefuroxime | |||
| Microscopy | Flow Cytometry | ||
| BM | PB | ||
|
|
|||
| Dose (mg/kg) | N | MN-RET/1000 RET | |
| 0 | 5 | 1.60 ± 0.24 | 2.28 ± 0.17 |
| 500 | 5 | 1.30 ± 0.37 | 2.22 ± 0.15 |
| 1000 | 4 | 1.38 ± 0.24 | 2.32 ± 0.37 |
| 2000 | 5 | 1.30 ± 0.37 | 2.30 ± 0.17 |
| Trend Test | P = 0.677 | P = 0.413 | |
| CPA | 4 | 35.25 ± 1.53* | 21.34 ± 1.68* |
| Diphenolic Acid | |||
| Microscopy | Flow Cytometry | ||
| BM | PB | ||
|
|
|||
| Dose (mg/kg) | N | MN-RET/1000 RET | |
| 0 | 5 | 1.60 ± 0.40 | 2.45 ± 0.20 |
| 500 | 5 | 2.50 ± 0.76 | 2.31 ± 0.21 |
| 1000 | 5 | 1.50 ± 0.57 | 2.43 ± 0.14 |
| 2000 | 5 | 2.20 ± 0.90 | 2.27 ± 0.30 |
| Trend Test | P = 0.371 | P = 0.736 | |
| CPA | 5 | 27.90 ± 4.37* | 30.56 ± 1.91* |
| 3-Amino-6-methylphenol | |||
| Microscopy | Flow Cytometry | ||
| BM | PB | ||
|
|
|||
| Dose (mg/kg) | N | MN-RET/1000 RET | |
| 0 | 5 | 1.10 ± 0.51 | 2.44 ± 0.18 |
| 100 | 5 | 2.78 ± 0.60* | 2.41 ± 0.19 |
| 200 | 5 | 1.60 ± 0.56 | 2.06 ± 0.24 |
| 400 | 5 | 2.20 ± 0.46 | 2.34 ± 0.16 |
| Trend Test | P = 0.137 | P = 0.590 | |
| CPA | 5 | 39.90 ± 4.46* | 27.99 ± 1.09* |
| Pentabromodiphenyl Oxide | |||
| Microscopy | Flow Cytometry | ||
| BM | PB | ||
|
|
|||
| Dose (mg/kg) | N | MN-RET/1000 RET | |
| 0 | 5 | 2.00 ± 0.42 | 2.59 ± 0.20 |
| 312.5 | 5 | 1.50 ± 0.32 | 2.16 ± 0.10 |
| 625 | 5 | 1.90 ± 0.37 | 2.21 ±-/12 |
| 1250 | 5 | 2.10 ± 0.19 | 2.33 ± 0.19 |
| Trend Test | P = 0.327 | P = 0.790 | |
| CPA | 5 | 33.70 ± 4.14* | 31.81 ± 1.58* |
Significantly different from control (P < 0.025/3 = 0.008)
N = number of animals scored
Data presented as mean ± standard error; see Methods and Materials, Section 2.4 for analysis procedures used for microscopy-based scoring and FCM-based scoring
Cyclophosphamide, positive control administered at 50 mg/kg
The percentage of immature erythrocytes (% RET) in bone marrow (determined in slide preparations) and peripheral blood (measured by FCM) was qualitatively similar, with either no significant alteration seen with either method, or, in the case of diphenolic acid, a small decrease detected at the high dose by both methods (data not shown).
For three of the four genotoxic compounds (cyclophosphamide, ethyl methanesulfonate, and vincristine sulfate) in rats and all four genotoxic compounds in mice, evaluations of acridine orange-stained bone marrow and peripheral blood slides detected a positive dose-response (trend test P < 0.025, Tables 2, Tables 3, Tables 4, 5); FCM measurements of MN-RET in peripheral blood samples from these same animals also showed positive responses (Tables 2 - 5). Furthermore, with one exception, the same dose groups that differed significantly from the control group using slide-based assessments of peripheral blood or bone marrow were also significantly different from the control group using FCM-based assessments of peripheral blood (P < 0.006). For acrylamide in rats, however, FCM and slide evaluations of peripheral blood samples did not agree. Although both slide analysis of bone marrow (the “gold standard” for rat MN evaluation) and FCM analysis of blood gave negative results, positive results were obtained by manually scored peripheral blood slides, based on a significant trend (P = 0.003) and two significantly elevated dose groups (P < 0.006). The magnitude of the increase in MN-RET seen with rat blood slides was quite small, however. The reduced MN response observed with acrylamide in rats compared to mice is consistent with previous studies that have reported greater sensitivity of mice to the genotoxicity of acrylamide compared to rats (consistent with differential blood levels in rats and mice of the genotoxic metabolite glycidamide) [24]. Therefore, for these genotoxic chemicals, FCM analysis of MN-RET frequency gave the same results as traditional acridine orange-stained slide-based evaluations of either bone marrow or peripheral blood smears in 7 of the 8 assays in rats and mice.
Table 2.
MN frequency in peripheral blood and bone marrow of male B6C3F1 mice and F344 rats administered cyclophosphamide by oral gavage
| Mice | ||||
|---|---|---|---|---|
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
| Dose (mg/kg) | N1 | MN-RET/1000 RET2 | ||
| 0 | 5 | 3.16 ± 0.60 | 3.60 ± 0.81 | 2.91 ± 0.18 |
| 25 | 5 | 26.00 ± 1.99* | 18.50 ± 2.77* | 16.65 ± 0.53* |
| 50 | 5 | 37.70 ± 3.14* | 26.44 ± 1.52* | 26.93 ± 0.82* |
| 75 | 5 | 40.88 ± 5.37*3 | 35.03 ± 3.60* | 31.07 ± 1.24* |
| 100 | 5 | 33.88 ± 5.41*3 | 4 | 27.20 ± 1.84* |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
| Rats | ||||
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
|
|
||||
| Dose (mg/kg) | N | MN-RET/1000 RET | ||
|
| ||||
| 0 | 5 | 0.60 ± 0.48 | 0.10 ± 0.10 | 0.35 ± 0.08 |
| 2.5 | 5 | 4.60 ± 1.41* | 1.70 ± 0.62* | 0.95 ± 0.12* |
| 5 | 5 | 10.30 ± 1.15* | 2.00 ± 0.22* | 1.81 ± 0.22* |
| 10 | 5 | 31.10 ± 1.20* | 3.50 ± 0.42* | 2.07 ± 0.48* |
| 20 | 5 | 43.15 ± 2.01* | 3.05 ± 1.20*5 | 6 |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
Significantly different from control (P < 0.025/4 = 0.006)
N = number of animals scored
Data presented as mean ± SEM; see Methods and Materials, Section 2.4 for analysis procedures used for microscopy-based scoring and FCM-based scoring
Only 4 animals available for analysis
Only 2 animals provided sufficient cells for analysis; data not included in analysis
Only 3 animals provided sufficient cells for analysis
Too few RET/animal available for analysis
Table 3.
MN frequency in peripheral blood and bone marrow of male B6C3F1 mice and F344 rats administered ethyl methanesulfonate by oral gavage
| Mice | ||||
|---|---|---|---|---|
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
| Dose (mg/kg) | N1 | MN-RET/1000 RET2 | ||
| 0 | 5 | 2.40 ± 0.43 | 3.20 ± 0.58 | 2.68 ± 0.24 |
| 50 | 5 | 4.60 ± 0.73 | 4.00 ± 0.42 | 3.66 ± 0.16 |
| 100 | 5 | 12.60 ± 1.56* | 9.40 ± 2.18* | 9.05 ± 0.88* |
| 200 | 5 | 23.77 ± 1.36* | 16.70 ± 2.08* | 17.48 ± 2.17* |
| 300 | 5 | 40.20 ± 6.59* | 33.10 ± 1.09* | 40.47 ± 2.08* |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
| Rats | ||||
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
|
|
||||
| Dose (mg/kg) | N | MN-RET/1000 RET | ||
|
| ||||
| 0 | 5 | 0.90 ± 0.10 | 0.60 ± 0.19 | 0.50 ± 0.10 |
| 50 | 5 | 4.40 ± 0.40* | 2.20 ± 0.46* | 2.04 ± 0.14* |
| 100 | 5 | 5.40 ± 0.83* | 4.40 ± 0.37* | 3.62 ± 0.61* |
| 200 | 5 | 9.70 ± 1.56* | 15.91 ± 2.32*3 | 7.17 ± 1.18* |
| 300 | 5 | 11.80 ± 3.71* | -----4 | -----4 |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
Significantly different from control (P < 0.025/4 = 0.006)
N = number of animals scored
Data presented as mean ± SEM; see Methods and Materials, Section 2.4 for analysis procedures used for microscopy-based scoring and FCM-based scoring
Based on 3 animals
Not scorable due to depletion of circulating reticulocytes
Table 4.
MN frequency in peripheral blood and bone marrow of male B6C3F1 mice and F344 rats administered vincristine sulfate in saline by intraperitoneal injection
| Mice | ||||
|---|---|---|---|---|
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
| Dose (mg/kg) | N1 | MN-RET/1000 RET2 | ||
| 0 | 5 | 2.70 ± 0.68 | 2.60 ± 0.24 | 2.96 ± 0.17 |
| 0.0125 | 5 | 3.89 ± 0.833 | 3.70 ± 0.68 | 3.92 ± 0.23 |
| 0.0250 | 5 | 7.10 ± 0.24* | 6.00 ± 0.45* | 6.06 ± 0.32* |
| 0.0500 | 5 | 14.66 ± 2.91* | 12.50 ± 1.47* | 12.88 ± 2.00* |
| 0.0750 | 5 | 43.56 ± 5.54* | 25.50 ± 0.91* | 32.00 ± 3.34* |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
| Rats | ||||
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
|
|
||||
| Dose (mg/kg) | N | MN-RET/1000 RET | ||
|
| ||||
| 0 | 5 | 1.50 ± 0.16 | 0.60 ± 0.10 | 0.35 ± 0.08 |
| 0.0063 | 5 | 2.20 ± 0.56 | 0.40 ± 0.10 | 0.40 ± 0.08 |
| 0.0125 | 5 | 2.20 ± 0.41 | 0.80 ± 0.12 | 0.73 ± 0.12* |
| 0.0250 | 5 | 3.26 ± 0.51* | 1.50 ± 0.42 | 0.88 ± 0.19* |
| 0.0313 | 5 | 7.13 ± 1.35* | 2.10 ± 0.68* | 1.50 ± 0.15* |
| Trend test | P < 0.001 | P < 0.001 | P < 0.001 | |
Significantly different from control (P < 0.025/4 = 0.006)
N = number of animals scored
Data presented as mean ± SEM; see Methods and Materials, Section 2.4 for analysis procedures used for microscopy-based scoring and FCM-based scoring
Based on 4 animals
Table 5.
MN frequency in peripheral blood and bone marrow of male B6C3F1 mice and F344 rats administered acrylamide by oral gavage
| Mice | ||||
|---|---|---|---|---|
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
| Dose (mg/kg) | N1 | MN-RET/1000 RET2 | ||
| 0 | 5 | 2.20 ± 0.49 | 2.00± 0.35 | 2.76± 0.46 |
| 12.5 | 5 | 3.20 ± 1.01 | 3.80± 0.56 | 3.83± 0.54 |
| 25.0 | 5 | 4.20 ± 0.70* | 4.10± 0.29* | 4.13± 0.10* |
| 37.5 | 5 | 4.80 ± 0.48* | 4.50± 0.52* | 4.10± 0.23* |
| 50.0 | 5 | 5.00 ± 0.65* | 4.80± 0.51* | 4.35± 0.20* |
| Trend test | P = 0.002 | P = 0.001 | P = 0.005 | |
| Rats | ||||
| Microscopy | Flow Cytometry | |||
| BM | PB | PB | ||
|
|
||||
| Dose (mg/kg) | N | MN-RET/1000 RET | ||
|
| ||||
| 0 | 5 | 0.60 ± 0.24 | 0.10 ± 0.10 | 0.64 ± 0.15 |
| 12.5 | 5 | 1.30 ± 0.44 | 0.60 ± 0.24 | 0.66 ± 0.09 |
| 25.0 | 5 | 1.10 ± 0.19 | 0.90 ± 0.19* | 0.66 ± 0.04 |
| 37.5 | 5 | 1.20 ± 0.30 | 0.70 ± 0.25 | 0.51 ± 0.10 |
| 50.0 | 5 | 0.80 ± 0.30 | 1.20 ± 0.46* | 0.55 ± 0.03 |
| Trend test | P = 0.382 | P = 0.003 | P = 0.867 | |
Significantly different from control (P < 0.025/4 = 0.006)
N = number of animals scored
Data presented as mean ± SEM; see Methods and Materials, Section 2.4 for analysis procedures used for microscopy-based scoring and FCM-based scoring
Slide-based and FCM-based MN-RET frequencies in peripheral blood were similar for the four genotoxic compounds in both mice and rats, with the exception of acrylamide in rats noted above (Table 6). Slide-based MN-RET frequencies in bone marrow, however, differed significantly from FCM-based frequencies in peripheral blood for all four of the genotoxic compounds in rats and for five of the nine compounds in mice (2 nongenotoxic, 3 genotoxic) (Table 6). For the genotoxic compounds, the FCM-based MN-RET counts in blood, like slide-based counts in blood, were generally lower than the slide-based bone marrow counts. For the two nongenotoxic compounds that showed significant differences between manually scored MN-RET frequencies in bone marrow and FCM-based MN-RET frequencies in blood, the FCM-based blood counts were higher. However, despite these differences, the magnitude of the responses observed between bone marrow and blood were similar and significant increases were detected with the genotoxic compounds at the same dose levels across all three analytical methods (bone marrow slides, blood slides, blood FCM). Thus, FCM scoring did not miss any of the positives, and it correctly identified the negative result in rats with acrylamide.
Table 6.
Wilcoxon signed-ranks p-values comparing slide-based and flow cytometry-based MN-RET frequencies in rats and mice
| Mice | Rats | |||
|---|---|---|---|---|
| Compound | Slide-based BM vs. FCM-based PB | Slide-based PB vs. FCM-based PB | Slide-based BM vs. FCM-based PB | Slide-based PB vs. FCM-based PB |
| Pentabromodiphenyl oxide | 0.011 | |||
| Cefuroxime | <0.001 | |||
| S-adenosylmethionine Cl | 0.185 | |||
| Diphenolic acid | 0.103 | |||
| 3-Amino-6-methylphenol | 0.156 | |||
| Cyclophosphamide | 0.002 | 0.855 | <0.001 | 0.106 |
| Ethyl Methanesulfonate | 0.013 | 0.228 | 0.002 | 0.246 |
| Vincristine sulfate | 0.010 | 0.300 | <0.001 | 0.012 |
| Acrylamide | 0.303 | 0.969 | 0.013 | 0.724 |
As with the nongenotoxic compounds, the % RET values in mice and rats obtained by scoring bone marrow or peripheral blood slides, or by FCM analysis of peripheral blood samples, were qualitatively similar (data not shown). Cyclophosphamide, ethyl methanesulfonate, and vincristine sulfate all produced significant decreases in % RET over the dose ranges tested; % RET values in bone marrow were ~30% of control at the highest dose evaluated, and peripheral blood values, determine by slide analysis or FCM, dropped to 10–20% of the control values at the high dose. Acrylamide, however, was less toxic as evidenced by % RET values, with decreases of 15% in mouse bone marrow and peripheral blood slides, and 30% in mouse peripheral blood scored by FCM at the highest dose compared with the controls. In rats, acrylamide reduced % RET by 15% in bone marrow preparations, and 25 and 35% in blood scored by FCM or slides, respectively. For all four genotoxic compounds tested in rats, % RET determined by FCM was lower than % RET determined by scoring blood slides, although the difference was negligible in rats treated with vincristine sulfate. In mice, % RET values in blood obtained by slide scoring or FCM were similar, except for cyclophosphamide, where the two methods produced similar values in the control group but in the treated groups, values were lower with FCM analysis than with slide scoring. The generally lower FCM-derived % RET values seen in rats compared to slides may reflect the targeting of a smaller subpopulation of erythrocytes by FCM (the most highly expressing CD71+ RET).
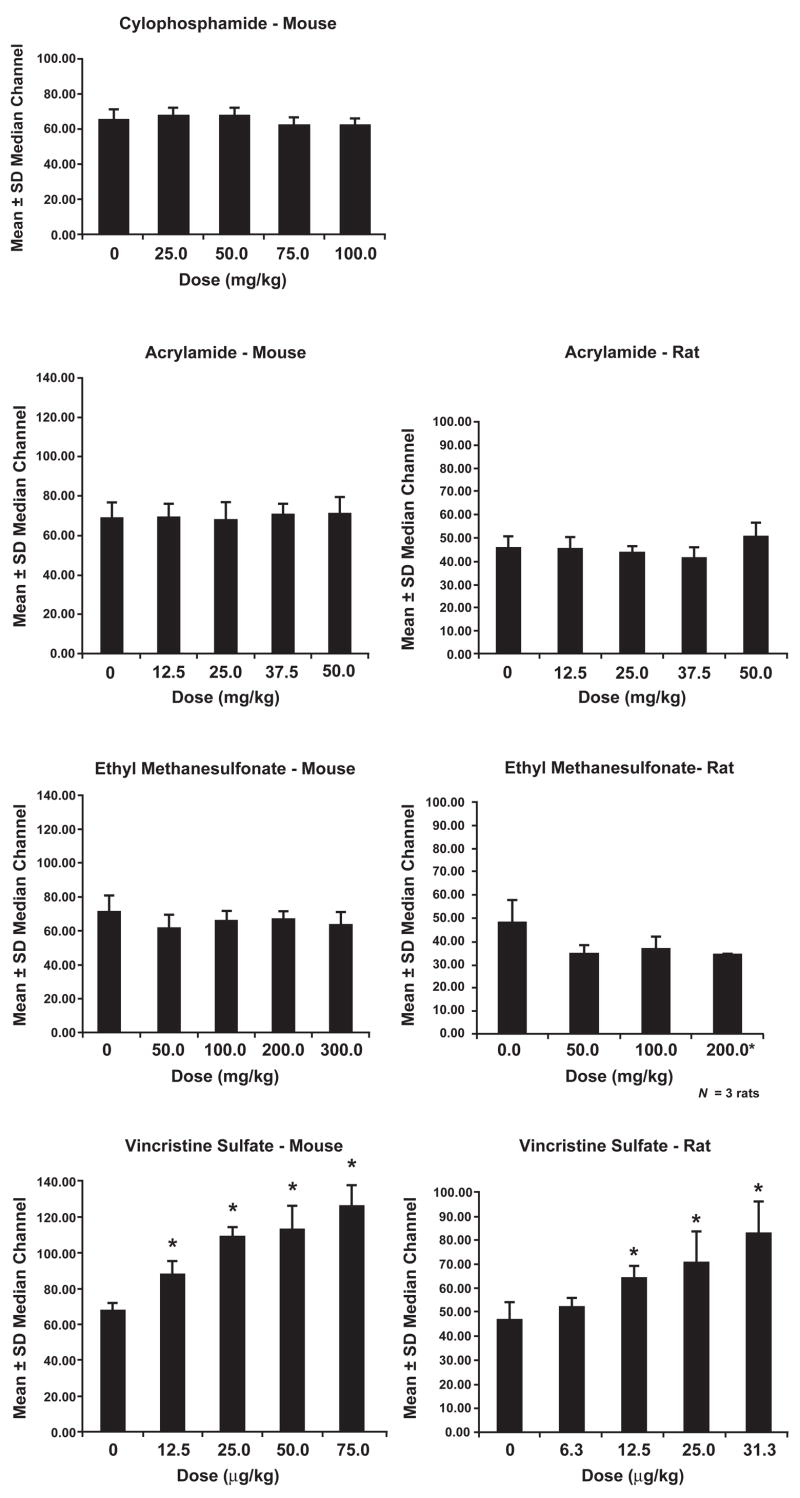
For the genotoxic compounds, analyses of median PI-associated fluorescence signals in mice and rats (Fig. 1) showed that only vincristine sulfate induced a significant shift in MN-associated fluorescence intensity in CD71+ MN-RET. This shift indicates that vincristine sulfate induced a higher proportion of MN containing more DNA (i.e., likely whole chromosomes), which is consistent with its known aneugenic activity. Although cyclophosphamide produced a significant increase in MN-RET in rat blood analyzed by FCM, we did not obtain median channel data for these MN (Fig. 1) due to an extraordinary decline in the %RET in treated rats (reduced at the highest dose level to only 8% of the control value); the number of MN-RET was too low to permit evaluating 100 MN per animal. We also were unable to obtain median channel data for ethyl methanesulfonate-treated rats at the high dose, due to excessive toxicity (%RET = 1.5% of the control value).
Fig. 1.
Propidium iodide staining intensity of DNA in MN of mice and rats treated with genotoxic compounds. Asterisks indicate a significant increase in staining intensity (P<0.05).
4. DISCUSSION
Chromosomal aberrations (CA) contribute to cancer development in humans and experimental animals [25, 26, 27, 28], and elevated lymphocyte CA and MN frequencies have been shown to be biomarkers of cancer risk within a population of healthy subjects [26, 29]. The use of MN as a surrogate for CA is supported by a number of validation studies showing a strong correlation between MN and CA frequencies within the same cell population [30]. In experimental animals, induction of CA or MN in appropriate target cells following defined exposures is considered a biomarker of genotoxic exposure and predictive of an agent’s potential to induce cancer [31, 32, 33]. Because scoring for micronucleated erythrocytes is much easier than scoring for CA in bone marrow cells, the in vivo rodent erythrocyte MN assay is a standard component of safety assessment protocols for environmental agents, industrial and commercial chemicals, and pharmaceuticals (i.e., it is part of the genotoxicity test battery required by regulatory agencies world-wide [34]). The NTP includes the in vivo rodent erythrocyte MN assay as part of its standard genetic toxicology test battery used in the comprehensive toxicological evaluation of substances of public health concern.
The peripheral blood erythrocyte MN assay has advantages over the bone marrow assay because it permits serial sampling of animals over an extended exposure period, permits assessment of the effects of chronic dosing in animals without splenic scavenging, and allows the assay to be combined with other toxicity evaluations. The mouse model has been extensively validated by international collaborative studies [6, 35, 36]. FCM methods have recently emerged as powerful tools for assaying MN in peripheral blood erythrocytes of mice. Studies comparing the traditional slide-based assay to the FCM-based assay have shown the FCM-based assay to be a robust method characterized by high reproducibility and rapid, objective scoring [9]. FCM methods can be used to score over a million cells per animal, if needed, and FCM methods can accurately distinguish subpopulations of erythrocytes so that appropriate target populations can be interrogated for MN based on the specific exposure protocol employed. Distinguishing RET from mature erythrocytes by microscopy relies on the staining of residual RNA, which can be somewhat subjective, while FCM objectively identifies RET by the presence of the CD71 cell surface marker [9].
In rats, MN have traditionally been evaluated in bone marrow RET due to the rapid and efficient removal of MN-RET from the peripheral blood by the rat spleen [35, 37]. However, results of recent studies combined with accumulated published data have suggested that the FCM-based peripheral blood MN assay can be used in place of the slide-based bone marrow assay in rats if analysis is restricted to the most highly expressing CD71 RET (very young RET), and if an adequate number of cells is sampled per animal [12, 13, 14, 15, 38]. Data from the tests conducted with rats in this study revealed generally higher frequencies of MN-RET detected in bone marrow slide preparations than by FCM analysis of peripheral blood (Tables 2–6). This observation is consistent with highly efficient splenic scavenging, even though FCM analysis was restricted to the most highly expressing CD71 subpopulation of erythrocytes. Although frequencies of MN-RET in rats were lower in FCM-analyzed blood samples, the magnitude of the increases induced by the genotoxic chemicals was generally similar in bone marrow and blood (Tables 2–5). The one exception was the CP study in rats, where analysis of BM slides showed a 50-fold increase in MN-RET while FCM analysis of peripheral blood samples showed only a 6-fold increase over baseline at the 10 mg/kg dose level (Table 2).
In general, good agreement was seen in both rats and mice between peripheral blood MN-RET frequencies obtained by microscopy-based and FCM-based methods for both the nongenotoxic and genotoxic compounds (Table 6). In addition, for the genotoxic compounds, the dose response curves in both rats and mice showed a high degree of correspondence between microscopy- and FCM-based MN-RET determinations (Tables 2–6). Thus, the results obtained from peripheral blood samples analyzed either by FCM or microscopy are comparable.
While microscopy-based and FCM-based assessments of MN-RET frequencies in peripheral blood were in close agreement, some significant differences were observed between MN-RET frequencies in bone marrow slide preparations and in blood samples analyzed by FCM (Table 6). In general, the actual counts obtained by scoring bone marrow slides were higher than those obtained through FCM analysis of peripheral blood. Because FCM and microscopic analysis of blood provided similar actual counts, the difference in slide-based counts of bone marrow and FCM-based counts of blood appears to reflect a true biological difference between bone marrow and peripheral blood rather than a difference related to scoring procedure. Furthermore, the difference is not unexpected, particularly in the rats, where the differences between the two scoring methods were most pronounced (Table 6). Despite these differences in absolute counts, the magnitude of the responses (fold-increases) observed with the genotoxic compounds between bone marrow slide evaluation and FCM-based blood evaluation were similar and significant increases were detected at the same dose levels across all three methods (bone marrow slides, blood slides, blood FCM). In fact, for vincristine sulfate in rats, FCM analysis of blood detected a significant rise in MN-RET frequency at a lower dose than did manual scoring of bone marrow or peripheral blood slides (Table 4). Thus, FCM scoring did not miss any of the genotoxic compounds in rats or mice, and in fact, it correctly identified the negative result with acrylamide in rats (comparison with the “gold standard” bone marrow evaluation method of MN frequency in rats). These data indicate that the NTP would not compromise its hazard identification capability by using FCM methods to evaluate MN frequency.
To gain insight into mechanism of MN formation, 100 MN-RET from each animal in each treatment group for each of the four genotoxic agents were analyzed for PI-associated fluorescence intensity, providing a quantitative description of DNA content within the MN-RET [16]. Only MN induced by vincristine sulfate showed an upward shift in distribution of PI-associated fluorescence intensity in rats and in mice, indicating an increased fraction of MN containing larger amounts of DNA, and therefore, whole chromosomes rather than fragments (Fig. 1). These results are consistent with vincristine’s aneugenic mechanism of action. The three other genotoxic chemicals are known clastogens. Because these data are consistent with the primary modes of genotoxicity for each of these four agents, we recommend evaluating this endpoint for all compounds that show a positive response in the MN assay.
Some investigators have expressed concern about the ability to detect MN induced by aneugenic compounds in rat blood samples because the rat spleen preferentially removes RETs with larger sized MN [39]. In the studies reported here, the induction of MN by the aneugenic compound vincristine sulfate was clearly detected in blood of both rats and mice, and median channel fluorescence data showed a clear “signature” of aneugenic potential. Thus, our protocol appears to be compatible with detection of both aneugens and clastogens.
Due to the large number of cells that can be routinely analyzed, the FCM-based assay has a higher power of detection relative to traditional microscopy-based methods. The relationship between sample size (ranging from 2000 to 1 × 106 cells per animal) and statistical power of the FCM-based peripheral blood MN assay was recently examined [23]. Based on their analysis, the authors recommended an ideal sample size of 20,000 RET per animal. Tests reported here with the nongenotoxic compounds were conducted prior to this analysis, and thus, for these five compounds, only 10,000 RET were analyzed per mouse by FCM (still a reasonable number of cells with good statistical sensitivity). For the MN tests with the four genotoxic compounds, conducted at a later date, 20,000 RET were analyzed per blood sample. Overall, the sensitivity of the FCM-based MN assay used with the compounds tested here (clearly genotoxic or nongenotoxic compounds) was not enhanced compared to microscopy-based enumeration of MN-RET. However, because the FCM MN assay has the ability to interrogate very large numbers of cells, even stringent and conservative statistical analyses will at times be expected to detect very small increases in MN-RET (<0.1%) as significant. Thus, it will be important to include experimental reproducibility among the criteria used to evaluate biological relevance.
In conclusion, this study extends previous validation studies of the FCM-based MN assay in mice and rats. FCM-based enumeration of MN-RET has several distinct advantages over microscopy-based scoring and these are supported by the present results as well as by the published data from studies conducted by other laboratories. Based on these results, the NTP has begun to use the in vivo FCM-based rodent peripheral blood MN assay as a standard part of its efforts to evaluate the genetic toxicity of substances of public health concern.
Acknowledgments
The authors appreciate the skilled assistance of Cathy Baldetti, John Winters, and Margaret Kehl at ILS, Inc., in scoring the bone marrow and peripheral blood slides. The authors are grateful to Drs. G. Travlos and D. Shaughnessy, NIEHS, for helpful comments and useful discussions during the preparation of this manuscript.
This work supported by NIEHS Contract N01-ES-35514.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, Tucker JD, Vanparys P, MacGregor JT. Micronuclei as an index of cytogenetic damage: Past, present, and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- 2.OECD. Guideline for the testing of chemicals. Mammalian Erythrocyte Micronucleus Test, Guideline 474, July, 1997. http://www.oecd.org/dataoecd/18/34/1948442.pdf.
- 3.MacGregor JT, Wehr CM, Henika PR, Shelby MD. The in vivo erythrocyte micronucleus test: measurement at steady state increases assay efficiency and permits integration with toxicity studies. Fund Appl Toxicol. 1990;14:513–522. doi: 10.1016/0272-0590(90)90255-i. [DOI] [PubMed] [Google Scholar]
- 4.Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DE, Tice RR, Waters MD, Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat Res. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 5.Witt KL, Knapton A, Wehr CM, Hook GJ, Mirsalis J, Shelby MD, MacGregor JT. Micronucleated erythrocyte frequency in peripheral blood of B6C3F1 mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ Mol Mutagen. 2000;36:163–194. [PubMed] [Google Scholar]
- 6.Hayashi M, Tice RR, MacGregor JT, Anderson D, Blakey DH, Kirsh-Volders M, Oleson FB, Jr, Pacchierotti F, Romagna F, Shimada H, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312:293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel R, MacGregor JT. The persistence of micronucleated erythrocytes in the peripheral circulation of normal and splenectomized Fischer 344 rats: implications for cytogenetic screening. Mutat Res. 1984;127:169–174. doi: 10.1016/0027-5107(84)90018-6. [DOI] [PubMed] [Google Scholar]
- 8.Dertinger SD, Camphausen K, MacGregor JT, Bishop ME, Torous DK, Avlasevich S, Cairns S, Tometsko CR, Menard C, Muanza T, Chen Y, Miller RK, Cederbrant K, Sandelin K, Ponten I, Bolcsfoldi G. Three-color labeling method for flow cytometric measurement of cytogenetic damage in rodent and human blood. Environ Mol Mutagen. 2004;44:427–435. doi: 10.1002/em.20075. [DOI] [PubMed] [Google Scholar]
- 9.Torous DK, Hall NE, Illi-Love AH, Diehl MS, Cederbrant K, Sandelin K, Ponten I, Bolcsfoldi G, Ferguson LR, Pearson A, Majeska JB, Tarca JP, Hynes GM, Lynch AM, McNamee JP, Bellier PV, Parenteau M, Blakey D, Bayley J, van der Leede BJ, Vanparys P, Harbach PR, Zhao S, Filipunas AL, Johnson CW, Tometsko CR, Dertinger SD. Interlaboratory validation of a CD71-based flow cytometric method (Microflow) for the scoring of micronucleated reticulocytes in mouse peripheral blood. Environ Mol Mutagen. 2005;45:44–55. doi: 10.1002/em.20081. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Morita T, Kodama Y, Sofuni T, Ishidate M., Jr The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res. 1990;245:245–249. doi: 10.1016/0165-7992(90)90153-b. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Kodama Y, Awogi T, Suzuki T, Asita AO, Sofuni T. The micronucleus assay using peripheral blood reticulocytes from mitomycin C- and cyclophosphamide-treated rats. Mutat Res. 1992;278:209–213. doi: 10.1016/0165-1218(92)90236-s. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi M, MacGregor JT, Gatehouse DG, Blakey DH, Dertinger SD, Abramsson L, Zetterberg L, Krishna G, Morita T, Russo A, Asano N, Suzuki H, Ohyama W, Gibson D. In Vivo Micronucleus Assay Working Group, IWGT. In vivo erythrocyte micronucleus assay III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussion of non-hematopoietic target cells and a single dose-level limit test. Mutat Res. 2007;627:10–30. doi: 10.1016/j.mrgentox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 13.MacGregor JT, Bishop ME, McNamee JP, Hayashi M, Asano N, Wakata A, Nakajima M, Saito J, Aidoo A, Moore MM, Dertinger SD. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes: II. An efficient method of monitoring chromosomal damage in the rat. Toxicol Sci. 2006;94:92–107. doi: 10.1093/toxsci/kfl076. [DOI] [PubMed] [Google Scholar]
- 14.Dertinger SD, Bishop ME, McNamee JP, Hayashi M, Suzuki T, Asano N, Nakajima M, Saito J, Moore M, Torous DK, MacGregor JT. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes: I. Intra- and interlaboratory comparison with microscopic scoring. Toxicol Sci. 2006;94:83–91. doi: 10.1093/toxsci/kfl075. [DOI] [PubMed] [Google Scholar]
- 15.Cammerer A, Elhajouji A, Suter W. In vivo micronucleus test with flow cytometry after acute and chronic exposures of rats to chemicals. Mutat Res. 2007a;626:26–33. doi: 10.1016/j.mrgentox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Torous DK, Dertinger SD, Hall NE, Tometsko CR. An automated method for discriminating aneugen- vs. clastogen-induced micronuclei. Environ Mol Mutagen. 1998;31:340–344. [PubMed] [Google Scholar]
- 17.Shelby MD, Erexson GL, Hook GJ, Tice RR. Evaluation of a three-exposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environ Molec Mutagen. 1993;21:160–179. doi: 10.1002/em.2850210210. [DOI] [PubMed] [Google Scholar]
- 18.Witt KL, Gulati DK, Kaur P, Shelby MD. Phenolphthalein: induction of micronucleated erythrocytes in mice. Mutat Res. 1995;341:151–60. doi: 10.1016/0165-1218(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 19.Witt KL, Tice RR, Shelby MD, Chhabra RS, Zeiger E. Induction of micronucleated erythrocytes in rodents by diisopropylcarbodiimide and dicyclohexylcarbodiimide: dependence on exposure protocol. Environ Mol Mutagen. 1999;33:65–74. doi: 10.1002/(sici)1098-2280(1999)33:1<65::aid-em8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Sofuni T, Ishidate M., Jr An application of acridine orange fluorescent staining to the micronucleus test. Mutat Res. 1983;120:241–247. doi: 10.1016/0165-7992(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 21.Dertinger SD, Torous DK, Hall NE, Tometsko CR, Gasiewicz TA. Malaria-infected erythrocytes serve as biological standards to ensure reliable and consistent scoring of micronucleated erythrocytes by flow cytometry. Mutat Res. 2000;464:195–200. doi: 10.1016/s1383-5718(99)00183-7. [DOI] [PubMed] [Google Scholar]
- 22.Integrated Laboratory Systems (ILS, Inc) Micronucleus Data Management and Statistical Analysis Software, version 1.4. ILS; PO Box 13501, Research Triangle Park, NC 27707: 1990. [Google Scholar]
- 23.Sumner SC, Williams CC, Snyder RW, Krol WL, Asgharian B, Fennell TR. Acrylamide: a comparison of metabolism and hemoglobin adducts in rodents following dermal, intraperitoneal, oral, or inhalation exposure. Toxicol Sci. 2003;75:260–270. doi: 10.1093/toxsci/kfg191. [DOI] [PubMed] [Google Scholar]
- 24.Aplan PD. Causes of oncogenic chromosomal translocation. Trends Genet. 2006;22:46–55. doi: 10.1016/j.tig.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonassi S, Ugolini D, Kirsch-Volders M, Stromberg U, Vermeulen R, Tucker JD. Human population studies with cytogenetic biomarkers: review of the literature and future prospectives. Environ Mol Mutagen. 2005;45:258–270. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- 26.Rossner P, Boffetta P, Ceppi M, Bonassi S, Smerhovsky Z, Landa K, Juzova D, Sram RJ. Chromosomal aberrations in lymphocytes of healthy subjects and risk of cancer. Environ Health Perspec. 2005;113:517–520. doi: 10.1289/ehp.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yunis JJ. Fragile sites, mutagens and genomic rearrangements in cancer. Basic Life Sci. 1988;43:11–21. doi: 10.1007/978-1-4684-5460-4_3. [DOI] [PubMed] [Google Scholar]
- 28.Bonassi S, Znaor A, Ceppi M, Lando C, Chang WP, Holland N, Kirsch-Volders M, Zeiger E, Ban S, Barale R, Bigatti MP, Bolognesi C, Cebulska-Wasilewska A, Fabianova E, Fucic A, Hagmar L, Joksic G, Martelli A, Migliore L, Mirkova E, Scarfi MR, Zijno A, Norppa H, Fenech M. An increased micronucleus frequency in peripheral blood lymphocytes predicts the risk of cancer in humans. Carcinogenesis. 2007;28:625–631. doi: 10.1093/carcin/bgl177. [DOI] [PubMed] [Google Scholar]
- 29.Kirkland DJ, Hayashi M, Jacobson-Kram D, Kasper P, MacGregor JT, Muller L, Uno Y. Summary of major conclusions from the 4th IWGT, San Francisco, 9–10 September, 2005. Mutat Res. 2007;627:5–9. doi: 10.1016/j.mrgentox.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Shelby MD. The genetic toxicity of human carcinogens and its implications. Mutat Res. 1988;204:3–15. doi: 10.1016/0165-1218(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 31.Shelby MD, Zeiger E. Activity of human carcinogens in the Salmonella and rodent bone-marrow cytogenetics tests. Mutat Res. 1990;234:257–261. doi: 10.1016/0165-1161(90)90022-g. [DOI] [PubMed] [Google Scholar]
- 32.Mateuca R, Lombaert N, Aka PV, Decordier I, Kirsch-Volders M. Chromosomal changes: induction, detection methods and applicability in human biomonitoring. Biochimie. 2006;88:1515–1531. doi: 10.1016/j.biochi.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, MacGregor JT, Gatehouse DG, Adler ID, Blakey DH, Dertinger SD, Krishna G, Morita T, Russo A, Sutou S. In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing, and automated scoring. Environ Mol Mutagen. 2000;35:234–252. [PubMed] [Google Scholar]
- 34.Cimino MC. Comparative overview of current international strategies and guidelines for genetic toxicology testing for regulatory purposes. Environ Mol Mutagen. 2006;47:362–90. doi: 10.1002/em.20216. [DOI] [PubMed] [Google Scholar]
- 35.Morita T, Asano N, Awogi T, Sasaki YF, Sato S, Shimada H, Sutou S, Suzuki T, Wakata A, Sofuni T, Hayashi M. Evaluation of the rodent micronucleus assay in the screening of IARC carcinogens (groups 1, 2A and 2B): The summary report of the 6th collaborative study by CSGMT/JEMS MMS. Mutat Res. 1997;389:3–122. doi: 10.1016/s1383-5718(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 36.Wakata A, Miyamae Y, Sato S, Suzuki T, Morita T, Asano N, Awogi T, Kondo K, Hayashi M. Evaluation of the rat micronucleus test with bone marrow and peripheral blood: summary of the 9th collaborative study by CSGMT/JEMS MMS. Environ Mol Mutagen. 1998;32:84–100. [PubMed] [Google Scholar]
- 37.Torous DK, Hall NE, Murante FG, Gleason SE, Tometsko CR, Dertinger SD. Comparative scoring of micronucleated reticulocytes in rat peripheral blood by flow cytometry and microscopy. Toxicol Sci. 2003;74:309–314. doi: 10.1093/toxsci/kfg143. [DOI] [PubMed] [Google Scholar]
- 38.Cammerer A, Elhajouji A, Kirsch-Volders M, Suter W. Comparison of the peripheral blood micronucleus test using flow cytometry in rat and mouse exposed to aneugens after single-dose applications. Mutagenesis. 2007b;22:129–134. doi: 10.1093/mutage/gel066. [DOI] [PubMed] [Google Scholar]
- 39.Torous DK, Asano N, Tometsko CR, Sugunan S, Dertinger SD, Morita T, Hayashi M. Performance of flow cytometric analysis for the micronucleus assay--a reconstruction model using serial dilutions of malaria-infected cells with normal mouse peripheral blood. Mutagenesis. 2006;21:11–13. doi: 10.1093/mutage/gei053. [DOI] [PubMed] [Google Scholar]