Abstract
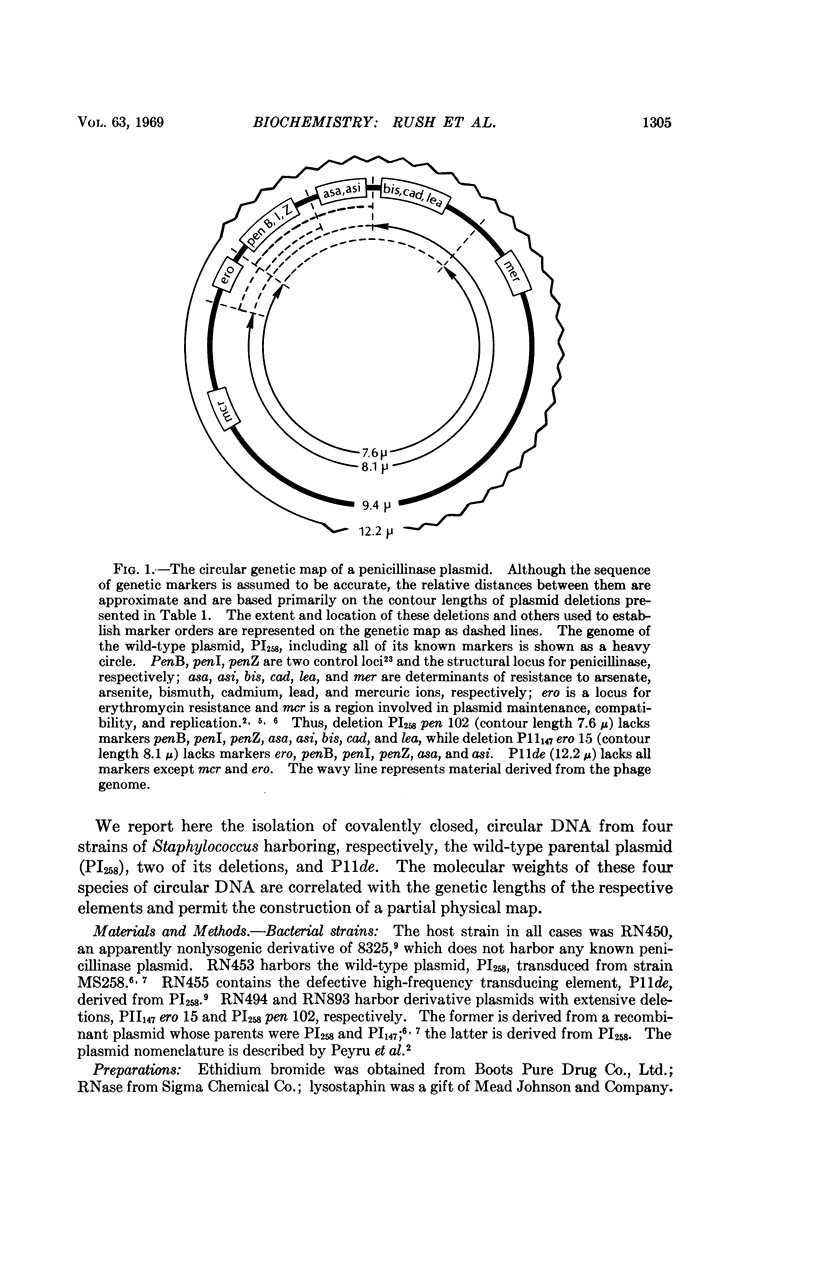
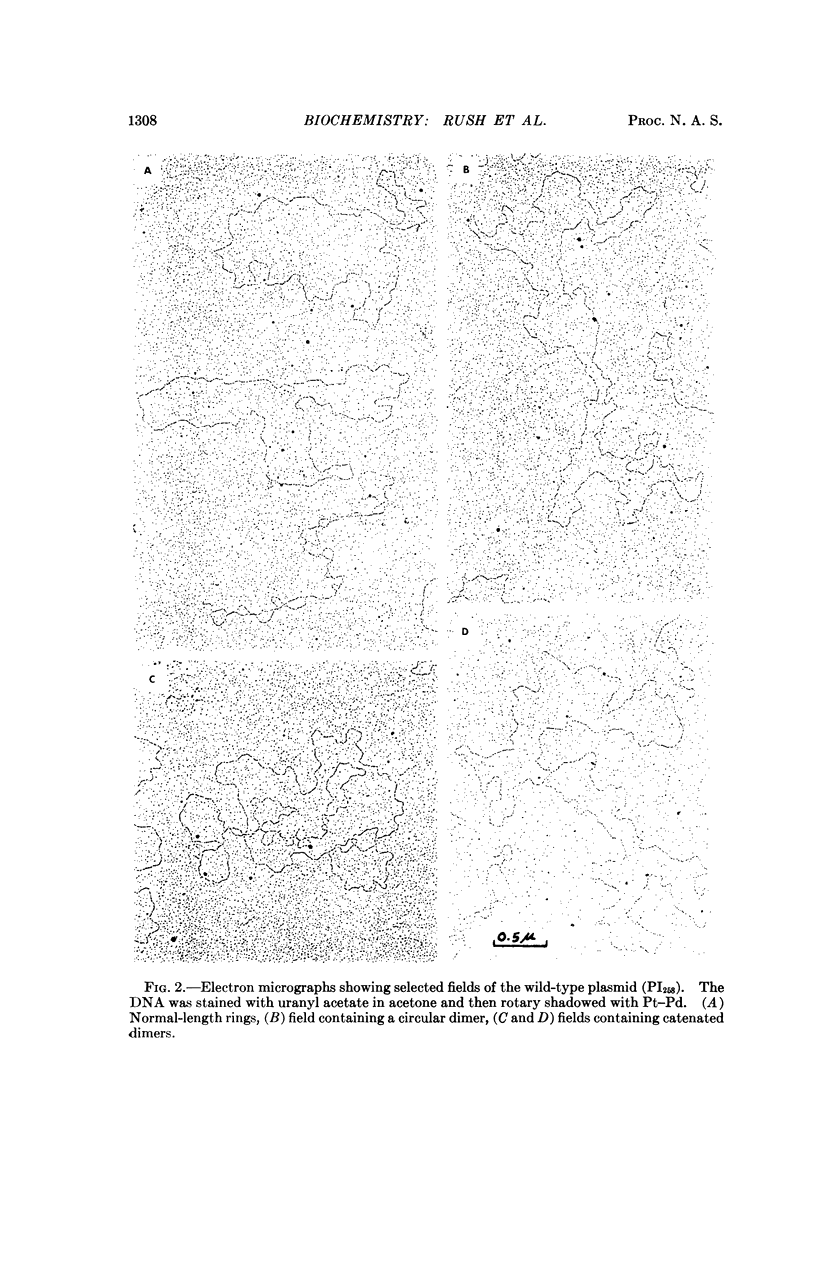
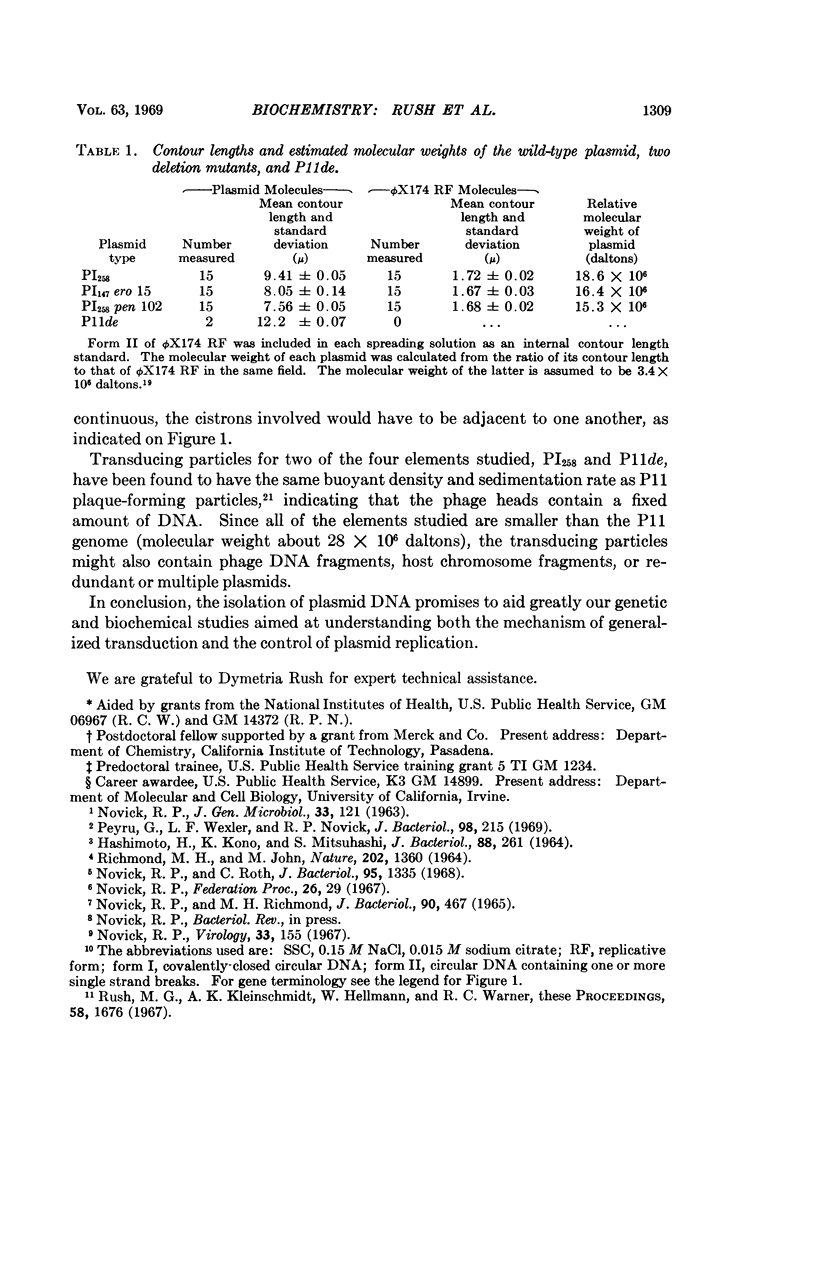
A penicillinase plasmid from Staphylococcus aureus and three of its derivatives, all previously identified as extrachromosomal genetic elements, have been isolated in high yield as circular duplex DNA molecules. The wild-type plasmid was found by contour-length measurements of electron micrographs to have a molecular weight of 18.6 × 106 daltons. Two plasmids with deletions encompassing six and eight of the eleven known plasmid cistrons had molecular weights of 16.4 × 106 and 15.3 × 106 daltons, respectively. This information was used to establish approximate physical distances for the genetic map. A high-frequency transducing element also derived from the plasmid had a molecular weight of approximately 24 × 106 daltons. Although each plasmid preparation appeared homogeneous by ultracentrifugal analysis, electron micrographs always revealed the presence of a low percentage of complex oligomeric forms, particularly circular and catenated dimers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton D. A., Smith C. A., Jordan J. M., Teplitz M., Vinograd J. Occurrence of complex mitochondrial DNA in normal tissues. Nature. 1968 Dec 7;220(5171):976–979. doi: 10.1038/220976a0. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO H., KONO K., MITSUHASHI S. ELIMINATION OF PENICILLIN RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1964 Jul;88:261–262. doi: 10.1128/jb.88.1.261-262.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansz H. S., Pouwels P. H., Schiphorst J. Preparation of double-stranded DNA (replicative form) of bacteriophage phi-X174: a simplified method. Biochim Biophys Acta. 1966 Sep;123(3):626–627. doi: 10.1016/0005-2787(66)90233-4. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Penicillinase plasmids of Staphylococcus aureus. Fed Proc. 1967 Jan-Feb;26(1):29–38. [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H., JOHN M. CO-TRANSDUCTION BY A STAPHYLOCOCCAL PHAGE OF THE GENES RESPONSIBLE FOR PENICILLINASE SYNTHESIS AND RESISTANCE TO MERCURY SALTS. Nature. 1964 Jun 27;202:1360–1361. doi: 10.1038/2021360a0. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. A second regulatory region involved in penicillinase synthesis in Staphylococcus aureus. J Mol Biol. 1967 Jun 14;26(2):357–360. doi: 10.1016/0022-2836(67)90305-1. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Kleinschmidt A. K., Hellmann W., Warner R. C. Multiple-length rings in preparations of phi-X174 replicative form. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1676–1683. doi: 10.1073/pnas.58.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Multiple-length rings of phi-X-174 replicative form, II. Infectivity. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2372–2376. doi: 10.1073/pnas.58.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]







