Abstract
The effect of two chronic motor training paradigms on the ability of the lumbar spinal cord to perform an acute instrumental learning task was examined in neonatally (postnatal day 5; P5) spinal cord transected (i.e., spinal) rats. At ∼P30, rats began either unipedal hindlimb stand training (Stand-Tr; 20-25 min/day, 5 days/wk), or bipedal hindlimb step training (Step-Tr; 20 min/day; 5 days/wk) for 7 wks. Non-trained spinal rats (Non-Tr) served as controls. After 7 wks all groups were tested on the flexor-biased instrumental learning paradigm. We hypothesized that 1) Step-Tr rats would exhibit an increased capacity to learn the flexor-biased task relative to Non-Tr subjects, as locomotion involves repetitive training of the tibialis anterior (TA), the ankle flexor whose activation is important for successful instrumental learning, and 2) Stand-Tr rats would exhibit a deficit in acute motor learning, as unipedal training activates the ipsilateral ankle extensors, but not flexors. Results showed no differences in acute learning potential between Non-Tr and Step-Tr rats, while the Stand-Tr group showed a reduced capacity to learn the acute task. Further investigation of the Stand-Tr group showed that, while both the ipsilateral and contralateral hindlimbs were significantly impaired in their acute learning potential, the contralateral, untrained hindlimbs exhibited significantly greater learning deficits. These results suggest that different types of chronic peripheral input may have a significant impact on the ability to learn a novel motor task, and demonstrate the potential for experience-dependent plasticity in the spinal cord in the absence of supraspinal connectivity.
Keywords: spinal cord injury, activity-dependence, posture, locomotion, asymmetry, motor-learning, rehabilitation
1. Introduction
The spinal cord retains an extensive capacity to learn a motor task, such as stepping or postural weight bearing, in the absence of supraspinal input [5,9-11,13,16,19]. Spinally-mediated motor responses to a variety of peripheral stimuli may be exhibited within seconds, minutes, days, or even months, although the responses change over time after a spinal cord injury (SCI) [5,10,11,13,23]. Experiments from our, and other, laboratories show that long-term step and stand training improves functional motor behaviour in adult spinal (i.e., completely spinally transected at the mid-thoracic level) cats and neonatal spinal rats [1,2,9,10,12,14,17,31,34]. For example, adult spinal cats trained to step repetitively exhibit kinematic characteristics and hindlimb muscle activation patterns that approximate those of uninjured controls [1,10]. Similarly, adult spinal cats that were trained daily to stand (i.e., hindlimb weight-bearing) for extended periods of time learned to support their weight for longer durations than non-trained or step-trained cats, although their ability to step was greatly compromised [9]. The spinal cord also can learn a motor task within just moments. Spinal cats stepping on a treadmill exhibit increased limb flexion when a small obstacle is placed in the path of the hind paw during the swing phase of the step, suggesting that the spinal cord acutely “sensed” that there was an obstacle, and responded appropriately to continue stepping [22,26].
Because the supraspinal connections are absent in the complete spinal model, acute and chronic motor learning must result from the nature of the peripheral input provided to the spinal cord, and the dynamic cellular plasticity that remains within the neural circuitry caudal to the lesion site. While it is clear that the spinal cord can learn a range of motor tasks in the absence of supraspinal control, the impact of different types of chronic motor training on the potential of the spinal cord to adapt to a novel peripheral stimulus is not well understood [5,9-14,17,22,35].
The question addressed in the present study, therefore, was whether bipedal step-training (Step-Tr) or unipedal hindlimb stand-training (Stand-Tr) in neonatal spinally transected rats changed the potential to perform a novel, acute instrumental spinal learning task relative to non-trained (Non-Tr) rats [3,4,6,7,25,27]. During the 30 min instrumental learning task, spinally transected rats receive shock to the tibialis anterior (TA) muscle whenever the leg is extended, and learn to maintain the leg in a flexed position, thereby minimizing net shock exposure [25]. Although the TA muscle is the effector of the behaviour and the probable site of the ankle “position sensors,” the flexion response is spinally mediated and dependent on peripheral input, as either severing the sciatic nerve or applying lidocaine on the dorsal surface of the spinal cord abolishes the learning response [6].
As standing and stepping differ considerably from each other in terms of muscle and neural activation patterns (Fig. 1), we anticipated that rats trained in these separate tasks would exhibit different learning potential in the acute motor paradigm. For example, as step training involves the repetitive, alternating recruitment of flexors and extensors in both hindlimbs, we hypothesized that Step-Tr rats would exhibit the most successful levels of learning of the flexor-biased instrumental paradigm. Conversely, since unipedal hindlimb stand training primarily activates hindlimb extensors, but not the ankle flexors in the weightbearing hindlimb , we anticipated that the Stand-Tr group would exhibit a learning deficit on the flexor-biased instrumental learning paradigm relative to naïve Non-Tr and Step-Tr rats. These data have been presented, in part, in abstract form [2].
Fig. 1. Experimental Paradigm.
All rats received a complete spinal cord transection at P5. Motor training began at P30 (see inset). (A) Typical electromyographic signals (EMG) obtained during body weight-supported (BWS) treadmill stepping (75% BWS at 13.5 cm/sec) from P5 spinally transected rats at ∼8-10 wks post-lesion showed alternating extensor (soleus; Sol) and flexor activation (tibialis anterior; TA) of both hindlimbs. (B) During unipedal hindlimb weight bearing at 75% BWS, the weight-bearing ankle extensor (Sol ipsi) and the non-weight-bearing ankle flexor (TA contra) muscles were recruited, while the ipsilateral TA and contralateral Sol were quiescent. a1 and a2 are calibrations for the Sol and TA EMG recordings during bipedal stepping, respectively; b1 is the calibration for the EMG recordings for all muscles shown during unipedal standing. The experimental design and number of non-trained (Non-Tr), step-trained (Step-Tr), and stand-trained (Stand-Tr) subjects used for the acute instrumental learning test are also shown (see text for details). Ipsi refers to the ipsilateral hindlimb, and contra refers to the contralateral hindlimb.
2. Materials and methods
Animal procedures were performed according to standards established by the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals under research protocols approved by the Chancellor's Animal Research Committee at UCLA and the University Laboratory Animal Care Committee at Texas A&M University.
2.1 Surgery
Surgical procedures and chronic motor training were performed at UCLA. Pregnant dams (Rattus rattus, Taconic Sprague Dawley, Germantown, NY) were received at 16-17 days of gestation and were monitored carefully for the day of parturition. At post-natal day 5 (P5), female rat pups (n=28) were deeply anesthetized using inhaled isoflurane (1-1.5%). Under aseptic conditions, the vertebral column was exposed at the mid-thoracic level, and a partial laminectomy was performed. The dura was opened, and the spinal cord was severed completely in the mid-thoracic region using micro-scissors. Small cotton balls were used to separate the cut ends of the spinal cord and a glass probe was passed along the dural and vertebral walls. The completeness of the transection was verified by two surgeons, and then Gelfoam was inserted between the rostral and caudal portions of the spinal cord. The skin was closed with 6.0 silk sutures, and the rat pups were placed in an incubator until they were fully recovered from anesthesia. The pups were cared for by their mothers until they were weaned between P26-30. Thereafter, the rats were housed individually in standard rat cages on a 12 h light:12 h dark cycle (7:00-19:00) and had ad libitum access to standard rat diet and water.
2.2 Motor training
The rats began unipedal stand (n=10) or bipedal step (n=8) training at ∼P30. Non-Tr rats (n=18) served as naïve, non-trained controls. The rats were trained 5 days/wk for 7 wks prior to exposure to the acute instrumental learning paradigm. Hindlimb training was carried out using a manually adjustable weight-supporting counterbalance system to provide quantifiable weight supported assistance and minimize handling during training [12,35]. Each rat was fitted with a cotton vest (made in-house) that was backed with Velcro, and placed into a bodyweight support harness, thereby supporting the torso, while the head, forelimbs, and hindlimbs had full range of movement, as previously described [12,35]. For both stand and step training, the rats were placed in an upright position, bearing ∼25% of their body weight (i.e., 75% body weight support; BWS) on their hindlimbs.
Unilateral stand training: Stand training was performed for 20-25 min/day, during which the ipsilateral, weight-bearing (i.e., Stand-Tripsi) hindlimb was placed on a small raised platform placed directly beneath it, such that it bore weight continuously. The contralateral hindlimb (i.e., Stand-Trcontra) was non-weight bearing, and served as an internal, non-trained control. During the training session, hindlimb extension was initially induced by lifting the hips. Near to full hindlimb extension was maintained for varying consecutive periods of time (mean ± SD = 79±37 seconds, ranging from 15-300 sec/bout of extension). When the hindlimb collapsed or the animal moved such that extension was lost, assistance was provided on an as-needed basis (i.e., every 2-3 min) to reestablish extension by lifting the hips or providing light cutaneous stimulation of the skin over the knee or near the Achilles tendon. Bipedal step training: Step-Tr rats performed bipedal stepping on a motorized treadmill for 20 min/day at speeds ranging from 6 to 21 cm•sec−1. As stepping ability varies from animal to animal and over time following the spinal cord transection, we used this range of treadmill speeds for locomotor training to allow for a “warm-up” period during training, as well as to progressively challenge the neural control of stepping by promoting dynamic peripheral input to the spinal cord. During all motor training sessions, potentially noxious stimuli, such as tail or skin pinching, were avoided.
2.3 Instrumental learning paradigm
After 7 wks of training, subjects were shipped to Texas A&M University. The details of the instrumental learning paradigm have been described in detail elsewhere [6,7,25]. In this model, successful learning is exhibited by prolonged periods of ankle flexion, thereby preventing exposure of the spinal cord circuitry to a noxious stimulus. Briefly, the rats were placed in a restraint tube in a prone position with both hindlimbs hanging freely. Leg shock was administered by placing an intracutaneous electrode above the tibia, with the other end of the lead attached to a shock generator. A steel pin was placed into the belly of the TA muscle, and attached to a second lead. An insulated contact electrode attached at the plantar surface of the foot monitored the degree of ankle flexion. When the ankle extended beyond a certain threshold the electrode touched a saline bath beneath the rat, thus completing the electrical circuit and eliciting an ankle flexion response. A wire attached to the proximal end of that electrode was connected to a Macintosh computer that measures digital input. The shock intensity was adjusted for each subject as the intensity required to elicit an ∼0.4 N flexion force, measured by tying fishing line at one end to the rat foot and the other end to a strain gauge. Two data parameters were measured: 1) response duration, which is derived from the equation (60 s − time in solutioni)/(flexion response numberi + 1) where i is the current time bin, and 2) the number of ankle flexion responses, i.e., the number of times that the electrode made contact with the saline bath, thereby exposing the spinal cord to a nociceptive stimulus. The electrical circuit was monitored at 30 Hz and data for these two behavioural parameters were compiled by the computer system at the end of each 1 min bin and averaged over the course of a 30 min test period.
2.4 Experimental design
The experimental paradigm is summarized in Fig. 1. The acute instrumental learning experiments were performed following 7 wks of motor training. For the Non-Tr (n=18) and Step-Tr (n=8) groups, the hindlimb used for testing, either left or right, was arbitrarily chosen. Because the stand training paradigm was unilateral, the order of the hindlimb used for testing was counter-balanced, such that one-half of the rats (n=5) were tested on the Stand-Tripsi hindlimb and the other half were tested on the Stand-Trcontra hindlimb. After 24 hours, the opposite hindlimb was tested in all Stand-Tr rats, and in a subset of Non-Tr rats (n=10). The experimenters performing the acute instrumental paradigm were blind as to the training status of the rats, and were told only on which hindlimb (left or right) to perform the test.
2.5 Statistical analysis
The data were analyzed using an analysis of variance (ANOVA). Post hoc comparisons were made with Duncan's New Multiple Range Test (SuperAnova, Abacus Concepts, 1991) [15]. Data are presented as the mean values ± S.E.M.
3. Results
3.1 Behavioural and physiological observations from motor training sessions
Stepping and standing each require the activation of specific groups of hindlimb musculature for successful motor performance. Differential activation of specific hindlimb musculature during bipedal stepping and unipedal standing in rats that were spinally transected at P5 was verified using electromyography (EMG) from age-matched spinal rats not included in the instrumental learning experiments. Our EMG surgical implant and recording procedures for rats have been previously published in detail [17]. During hindlimb stepping, the soleus and TA were recruited in an alternating and repetitive fashion in both hindlimbs (Fig. 1A). During unipedal standing, however, the weight-bearing soleus was active, while the ipsilateral TA and contralateral soleus were quiescent (Fig. 1B). An unexpected behavior also was noted, namely that the contralateral hindlimbs were consistently flexed, albeit to varying degrees, during ipsilateral weight bearing. We confirmed the contralateral flexor (TA) activation during a bout of unilateral standing, again, using EMG (Fig. 1B).
3.2 Baseline measures for acute learning
Prior to the acute testing of instrumental learning, flexion force was equated across rats by adjusting shock intensity. Mean shock intensity (±S.E.M.) ranged from 0.16±0.01 to 0.18±0.01 mA across groups. An ANOVA showed that there were no significant group differences (all Fs < 1.0, p > .05). The duration of the first flexion response, obtained as a measure of response vigor, also showed no significant differences (0.13±0.04 to 0.31±0.14 sec; all Fs < 1.0, p> .05). Additionally, there were no obvious behavioral differences in the range of leg movement across groups, suggesting that the overall pattern of the elicited flexion response was comparable independent of training history.
3.3 Effect of prior motor training on acute instrumental learning performance
The impact of motor training on instrumental learning performance is shown in Fig. 2. Non-Tr and Step-Tr rats exhibited a progressive increase in ankle flexion response duration over time, while Stand-Tr rats failed to learn (F(2, 29)=6.10, p < .01; Fig. 2A). Post hoc comparisons of the group means using Duncan's New Multiple Range Test showed that the overall flexion duration of the Stand-Tr group was significantly different from Step-Tr and Non-Tr (p < .05; Fig. 2B). In spite of the differences in flexion duration, all groups exhibited a similar number of flexion responses over the course of the 30 min test period (F < 1.0, p > .05; Fig. 2C-D). Importantly, this implies that although the Stand-Tr rats did not learn, they remained sensitive to the stimulus associated with instrumental learning.
Fig. 2. Instrumental learning in Non-Tr, Step-Tr, and Stand-Tr rats following 7 wks of step training or unilateral hindlimb stand training.
The flexion duration (A) and the number of flexion responses (C) for each 1 min bin over 30 min of testing are shown, as are the overall average test flexion durations (B) and response numbers (D). For flexion duration, Non-Tr and Step-Tr groups were not significantly different, while the Stand-Tr flexion duration was significantly lower relative to both the Step-Tr and Non-Tr groups (B). The average number of flexion responses was not different between groups (D). Data are mean ± S.E.M. *; Significantly different at p < 0.05.
3.4 Bilateral impact of unipedal stand training on instrumental learning
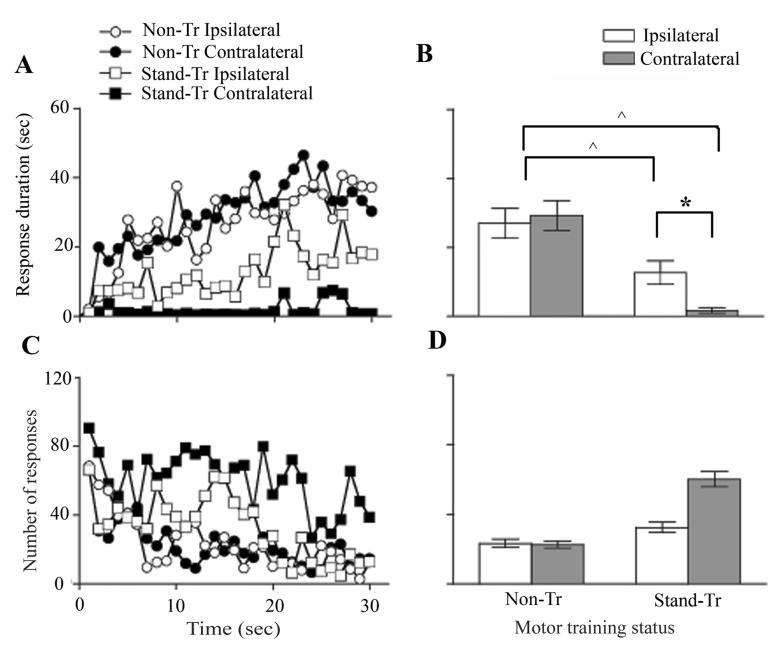
In the first experiments, the Stand-Tr group exhibited a reduced potential to perform the instrumental learning paradigm. However, as those data were counterbalanced such that half were tested on the Stand-Tripsi hindlimb and the other half on the Stand-Trcontra hindlimb, it was not possible to tell which leg exhibited the deficit. Therefore, we examined whether the unilateral stand training paradigm had a bilateral effect with respect to instrumental learning. All Stand-Tr rats received testing on the opposite leg 24 hr following the first test (i.e., those that were originally tested on the Stand-Tripsi hindlimb were then tested on the Stand-Trcontra hindlimb, and vice versa). A subset of Non-Tr rats (n=10) also was tested in a similar manner on the opposite leg to determine whether exposure to the instrumental paradigm on one hindlimb affected the potential of the other hindlimb to learn the same task 24 hrs later. For Non-Tr rats, one hindlimb tested was arbitrarily designated “ipsilateral” (counter-balanced across test days). As we have not previously used a two-day test procedure, we first examined whether the test results varied across days. The mean response duration on Day 1 was correlated with performance on Day 2 (r(24)=0.54, p < .01). An ANOVA verified that the Non-Tr group exhibited superior performance across days relative to Stand-Tr (F(1, 22)=21.5, p < .0001). Neither the main effect of test day, nor its interaction with treatment condition, approached statistical significance (both Fs < 1.0, p > .05). We concluded therefore that instrumental learning performance was not biased by the order in which the hindlimbs were tested on the acute paradigm. Given these results, our subsequent analyses ignored test day as a variable. An ANOVA followed by Duncan's post-hoc tests revealed that Non-Tr rats exhibited successful and equivalent levels of instrumental learning task on both legs, as demonstrated by the increased flexion duration over time (p > .05; Figure 3A, 3B), while Stand-Tr rats showed reduced performance on both legs relative to the Non-Tr rats (all p < .05). In addition, Stand-Tr rats showed better learning on the ipsilateral leg than the contralateral leg (p < .05). As before, the chronic motor training status did not have a significant effect on the number of flexion responses exhibited by any group (both Fs < 3.02, p > .05; Figs. 3 C-D).
Fig. 3. Unilateral stand training induces a bilateral instrumental learning deficit.
Non-Tr and Stand-Tr rats were tested for instrumental learning on both the ipsilateral and contralateral hindlimbs. The flexion duration (A) and the number of flexion responses (C) for each 1 min bin over 30 min of testing are shown, as are the overall average test durations (B) and response numbers (D). For within group analyses, flexion duration did not differ between the ipsilateral and contralateral hindlimbs of Non-Tr rats. In Stand-Tr rats, the contralateral hindlimbs learning showed a significant deficit relative to the ipsilateral hindlimbs (p < 0.05; A-B). Between groups, flexion duration was significantly lower in both the Stand-Tr ipsi and Stand-Tr contra limbs compared either hindlimb of the Non-Tr group (p < 0.05). Chronic motor training did not have a significant effect on the number of flexion responses within or between groups (C-D). Data are the mean ± S.E.M. *, Significantly different within groups at p < 0.05; ^, significantly different between groups at p < 0.05.
4. Discussion
The concept that the spinal cord exhibits use-dependent plasticity after SCI is well known, but questions regarding whether the practice of one type of motor activity influences the performance of another after SCI remain largely unanswered. We therefore examined whether previous motor experience in the form of bipedal step training or unipedal stand training altered the potential to perform a novel instrumental motor task.
4.1 Chronic motor training differentially affects acute instrumental learning
Our results showed three main findings. First, as step training involves the repetitive activation, or “training,” of the hindlimb ankle flexors (Fig. 1A), we anticipated that stepping would confer some advantage towards learning the flexor-biased instrumental paradigm relative to Non-Tr rats. On the contrary, acute instrumental learning performance in Step-Tr rats was similar to Non-Tr rats (Fig. 2). This was surprising, as step training can clearly modulate functional motor recovery and the excitability of the spinal cord relative to Non-Tr spinal animals [10,14,34]. Second, unilaterally Stand-Tr rats exhibited a reduction in overall learning potential (Fig. 2). However, while we expected that the deficit would be isolated to the extensor-trained, Stand-Tripsi hindlimb, the “untrained” (contralateral) hindlimb of the Stand-Tr rats exhibited a significantly greater deficit in learning relative to both Non-Tr rats and to the ipsilateral hindlimb that was trained daily in weight-bearing extension (Fig. 3). We initially anticipated that the Stand-Trcontra limb would serve as an internal, untrained control, and would exhibit similar behavioural properties as the Non-Tr group. An alternate possibility, however, may be that the Stand-Trcontra hindlimb was also trained (or, “detrained”) during unipedal stand training. This is consistent with the observation that during unilateral standing (i.e., Stand-Tripsi) there was significant soleus (ankle extensor) activity but little to no TA (ankle flexor) activity similar to muscle activation patterns that occur in non-injured rats during normal postural weight bearing. However, the non-weight-bearing hindlimb (Stand-Trcontra) during stand training was usually flexed, showing considerable TA flexor activity with little to no soleus extensor activation (Fig. 1B). Because Stand-Trcontra hindlimb flexion often occurred during unipedal stand training, an improved behavioural potential on the instrumental learning paradigm on that hindlimb might be expected. Instead, the nature of the stand-training paradigm modulated the neural circuitry within both the Stand-Tripsi and the Stand-Trcontra sides of the spinal cord such that both exhibited a reduction in instrumental learning capacity.
4.2 Potential role of state-dependent spinal plasticity in acute and chronic motor learning
In an effort to understand these data, one may consider that the physiological “state” of the spinal cord influences the potential for motor behaviour [18,19]. Components of the spinal cord state include 1) peripheral input, i.e., position, force production, etc., 2) the neural circuits recruited by the peripheral input, 3) the biochemical status of the neural circuits, and 4) supraspinal modulation (which is absent in the present experiment). These states may be altered in an activity-dependent manner, and are continually changing over time [14,18,32]. In the present experiment, the ipsilateral weight-bearing extension was commonly temporally paired with contralateral flexion. During instrumental learning, however, both hindlimbs were hanging passively in a non weight-bearing fashion [8]. One possible explanation for the deficit in instrumental performance observed in the Stand-Trcontra limb, therefore, may be that the ankle flexion during unipedal stand training relied on cutaneous and proprioceptive cues associated with weight bearing of the ipsilateral limb. In the absence of Stand-Tripsi weight bearing during the instrumental learning paradigm, the Stand-Trcontra limb may have been unable to engage the appropriate combination of neural circuitry required for the maintenance of the instrumental response, resulting in a reduced learning potential. This concept is supported by data from human subjects, where the ability of one leg to perform a motor task is clearly affected by the physiological state of the contralateral limb [36]. In addition, the physiological states of the limbs and spinal cord continue to influence each other even when supraspinal connectivity is removed. Ferris et al. [21] reported that unilateral stepping in chronic SCI humans induces alternating and rhythmic EMG activity in the non-stepping limb, in a loading-dependent manner. Similar results have been observed in decerebrate cats stepping unilaterally on a treadmill [16].
With respect to the spinal circuitry engaged by the peripheral stimuli used in this study, the stepping and standing paradigms presumably activated cutaneous and propriospinal circuits related to locomotor and postural motor control. Although less is known about the spinal circuits involved in the acquisition of the instrumental response, it presumably involved the activation of nociceptive circuitry, as opposed to propriospinal or cutaneous afferents. It is also known that the L4-S2 spinal cord segments are required for successful instrumental learning because transection or the local application of lidocaine within these segments disrupts learning [30]. Furthermore, instrumental learning depends on temporal contiguity between the response (limb position) and shock onset [25]. The interactions between proprioceptive and nociceptive circuits in response to chronic SCI and/or motor training, however, are not understood. It should be noted that although the flexion response duration was reduced in the Stand-Tr rats relative to the other groups, this inability to learn could not be accounted for by a reduction in the sensitivity to the nociceptive stimuli, as the number of flexion responses was similar between groups. The results do suggest, however, that unilateral stand training bilaterally modulated the interactions between proprioceptive and nociceptive circuitry in a manner that was not observed in Step-Tr or Non-Tr rats.
Finally, biochemical plasticity involving neurotransmitters, receptor levels, and overall synaptic efficacy throughout the spinal cord occurs progressively over time, both in response to SCI [24,32], and to subsequent motor training [14,34]. For example, sprouting of peripheral fibers has been noted after SCI [29], likely impacting nociception [33], autonomic function [28,37], and proprioception [17]. Multiple studies have begun to elucidate a role for plasticity in various neurotransmitter systems in the lumbar spinal cord after SCI and how those changes relate to changes in motor performance [14,24,32,34]. The reduced capacity of the Stand-Trcontra hindlimb for instrumental learning thus may be indicative of state-dependent biochemical and synaptic changes within the spinal circuits that occurred as a direct result of chronic weight-bearing activity of the Stand-Tripsi hindlimb in stand trained rats. On the other hand, while it is known that glycinergic [14], and GABAergic [34] systems change significantly following step training relative to Non-Tr spinal cats, the behavioural potential for instrumental learning in the present experiments was similar in Step-Tr and Non-Tr rats. Whether these neurotransmitter systems are altered in the spinal cord of chronically trained spinal rats is currently under investigation, as is the role of GABA in instrumental learning [20].
5. Conclusions
These results showed that chronic locomotor training had no effect on the ability of the transected spinal cord to learn an acute instrumental paradigm relative to Non-Tr subjects. Ongoing exposure to unilateral postural weight bearing, however, reduced the capacity of both hindlimbs to learn, with the contralateral, non weight-bearing hindlimbs showing the greatest deficit. These results illustrate the strong interdependence between the two sides of the spinal cord and the potential for bilateral plasticity induced by the unipedal stand training paradigm. The possibility of interactions between proprioceptive (i.e., those activated by chronic motor training) and nociceptive (i.e., those presumably activated by instrumental learning) spinal circuits should certainly be given consideration when attempting to define the physiological mechanisms that underlie spinal motor learning. Relative to rehabilitative strategies for SCI patients, these data caution us to not over-generalize as to whether the practice of one motor task might assist, or impede, the performance of other motor tasks.
Acknowledgements
Sincere thanks to Dr. Allan Tobin and Dr. Leif Havton for their insightful comments on our manuscript. Thanks also to Dr. Hui Zhong and Maynor Hererra for surgical support, Jason Guu for assistance with motor training, and Ed Lan for technical support. This work was supported by NIH grants NS40917 (NJKT), NS41548 (JWG), and NIH NS16333 (VRE). AJB was supported by a Roman Reed Predoctoral Fellowship and NASA Graduate Student Research Program Fellowship NGT2-52265.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 2.Bigbee AJ, Crown ED, Ferguson AR, Shah R, Guu JJ, Urdaneta A, Chan B, Zhong H, Roy RR, Tillakaratne NJK, Tobin AJ, Grau JW, Edgerton VR. Instrumental spinal learning is inhibited by unilateral extensor-biased motor training in spinal transected rats. Society for Neuroscience. 2003;#497.12 [Google Scholar]
- 3.Buerger AA, Fennessy A. Long-term alteration of leg position due to shock avoidance by spinal rats. Exp Neurol. 1971;30:195–211. doi: 10.1016/s0014-4886(71)80001-8. [DOI] [PubMed] [Google Scholar]
- 4.Chopin SF, Buerger AA. Graded acquisition of an instrumental avoidance response by the spinal rat. Physiol Behav. 1975;15:155–8. doi: 10.1016/0031-9384(75)90229-2. [DOI] [PubMed] [Google Scholar]
- 5.Cote MP, Menard A, Gossard JP. Spinal cats on the treadmill: changes in load pathways. J Neurosci. 2003;23:2789–96. doi: 10.1523/JNEUROSCI.23-07-02789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiol Behav. 2002;77:259–67. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- 7.Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav Neurosci. 2002;116:1032–51. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- 8.Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. J Neurophysiol. 2001;86:845–55. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- 9.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- 10.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J Neurophysiol. 1998;79:1329–40. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- 11.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J Neurophysiol. 1999;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- 12.de Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002;40:267–73. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- 13.de Leon RD, Roy RR, Edgerton VR. Is the recovery of stepping following spinal cord injury mediated by modifying existing neural pathways or by generating new pathways? A perspective. Phys Ther. 2001;81:1904–11. [PubMed] [Google Scholar]
- 14.de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82:359–69. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- 15.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 16.Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res. 1980;187:321–32. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- 17.Edgerton VR, de Guzman CP, Gregor RJ, Roy RR, Hodgson JA, Lovely RG. Trainability of the spinal cord to generate hindlimb stepping patterns in adult spinalized cats. In: Shimamura SG M, Edgerton VR, editors. Neurobiological basis of human locomotion. Japan Scientific Societies; Tokyo: 1991. pp. 411–423. [Google Scholar]
- 18.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–67. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 19.Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR. Locomotor recovery potential after spinal cord injury. In: Swinnen SP, Duysens J, editors. Neuro-behavioral determinants of interlimb coordination. Kluwer, Dordrecht; Netherlands: 2004. pp. 53–91. [Google Scholar]
- 20.Ferguson AR, Washburn SN, Crown ED, Grau JW. GABA(A) receptor activation is involved in noncontingent shock inhibition of instrumental conditioning in spinal rats. Behav Neurosci. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- 21.Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord. 2004;42:14–23. doi: 10.1038/sj.sc.3101542. [DOI] [PubMed] [Google Scholar]
- 22.Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–7. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- 23.Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–13. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- 24.Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–14. [PubMed] [Google Scholar]
- 25.Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behav Neurosci. 1998;112:1366–86. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc. 1994;26:1491–7. [PubMed] [Google Scholar]
- 27.Joynes RL, Ferguson AR, Crown ED, Patton BC, Grau JW. Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behav Brain Res. 2003;141:159–70. doi: 10.1016/s0166-4328(02)00372-8. [DOI] [PubMed] [Google Scholar]
- 28.Krenz NR, Meakin SO, Krassioukov AV, Weaver LC. Neutralizing intraspinal nerve growth factor blocks autonomic dysreflexia caused by spinal cord injury. J Neurosci. 1999;19:7405–14. doi: 10.1523/JNEUROSCI.19-17-07405.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;85:443–58. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- 30.Liu GT, Ferguson AR, Crown ED, Bopp AC, Miranda RC, Grau JW. Instrumental learning within the rat spinal cord: localization of the essential neural circuit. Behav Neurosci. 2005;119:538–47. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- 31.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of fullweight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–35. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 32.Naftchi NE, Schlosser W, Horst WD. Correlation of changes in the GABA-ergic system with the development of spasticity in paraplegic cats. Adv Exp Med Biol. 1979;123:431–50. doi: 10.1007/978-1-4899-5199-1_27. [DOI] [PubMed] [Google Scholar]
- 33.Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184:373–80. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Tillakaratne NJ, de Leon RD, Hoang TX, Roy RR, Edgerton VR, Tobin AJ. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J Neurosci. 2002;22:3130–43. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol. 2002;88:3108–17. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- 36.Ting LH, Kautz SA, Brown DA, Van der Loos HF, Zajac FE. Bilateral integration of sensorimotor signals during pedaling. Ann N Y Acad Sci. 1998;860:513–6. doi: 10.1111/j.1749-6632.1998.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 37.Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–19. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]