Abstract
Globin gene transfer in autologous hematopoietic stem cells is a promising therapeutic option for subjects with β-thalassemia major. In this approach, high level, erythroid-specific globin transgene expression should correct ineffective erythropoiesis and hemolytic anemia following the delivery of only 1 to 2 vector copies per cell. The generation of vectors that provide high-level globin expression and require low vector copy (VC) integration is therefore essential for both safety and efficacy. We show here the major roles played by 2 lesser-known locus control region elements, termed HS1 and HS4. Partial deletions within HS4 markedly reduce in vivo globin expression requiring multiple VC per cell to correct the anemia. Most strikingly, addition of HS1 to HS2-3-4 increases globin expression by 52%, yielding 9 g Hb/VC in β-thalassemic mice. Thus, while vectors encoding HS2-3-4 provide curative levels of hemoglobin at 1 to 2 copies per cell, adding HS1 is a promising alternative strategy if upcoming clinical trials prove higher levels of expression to be necessary.
Introduction
The β-thalassemias are inherited autosomal recessive anemias caused by mutations that diminish or abolish expression of the β-globin gene.1,2 The only current means to cure the disease is allogeneic bone marrow transplantation (BMT).3,4 In the absence of a histocompatible donor, however, the genetic correction of autologous hematopoietic stem cells (HSCs) represents a highly attractive alternative treatment.4
Achieving therapeutic expression of the human β-globin transgene in hematopoietic chimeras has long posed major challenges in terms of transgene regulation, vector stability and transduction efficiency (reviewed in Persons and Tisdale,5 May and Sadelain,6 and Bank et al7). A careful selection of proximal and distal β-globin transcription control elements and the use of a recombinant HIV-1 genome to generate a stable vector eventually enabled May et al to cure β-thalassemia in mice.8 Their vector, termed TNS9,8 and all subsequent therapeutic globin vectors published to date, express the globin transgene under the control of the β-globin promoter and the HS2, 3, and 4 locus control region (LCR) elements.8–15 The HS2 and HS3 elements are the most powerful single elements within the LCR (reviewed in Li et al16). The relative importance of HS1 and HS4 is less well defined.16
In order to select a vector for clinical evaluation in patients with β-thalassemia, we have therefore undertaken a quantitative analysis of the contribution of the β-globin HS1 and HS4 elements to the specificity, inducibility, long-term in vivo expression, and therapeutic potential of globin lentiviral vectors. It is indeed central to the success of safe stem cell engineering that therapeutic transgene expression be achieved with a low (ideally 1 to 2) vector copy number per cell.17,18 We show here that HS1 and HS4 are major contributors to achieving therapeutic globin expression in β-thalassemic mice.
Materials and methods
All vectors used in this study were derived from the previously described TNS9.8 Detailed description of vector construction and production is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Vector copy number quantification was performed by Southern blot as previously described8 and by TaqMan analysis (primers and probes appear in Document S1). MEL cell transduction and differentiation were done as previously described.8 For β-globin transgene analyses, total RNA was extracted from MEL cells or total mouse peripheral blood (PB) using TRIzol reagent (Invitrogen, Carlsbad, CA). Quantitative primer extension assays were performed as described previously.8 Hbbth3/+ bone marrow (BM) chimeras were generated as described.8,19 BM cells were prestimulated for 12 hours, transduced at 106 cells/mL per well at a multiplicity of infection (MOI) of 20 to 35 for 8 hours and injected into lethally irradiated recipients (5 × 105 to 106 cells per recipient). A detailed description of the transduction procedure appears in Document S1. At several time points after bone marrow transplantation, PB was collected and hemoglobin levels were measured on a Coulter AcT diff. instrument (Beckman Coulter, Brea, CA). Red cell lysates of freshly collected PB were analyzed by cellulose acetate electrophoresis (pH 8.5, Helena Laboratories, Beaumont, TX and quantified as described8 using ImageJ 1.38x software (http://rsb.info.nih.gov/ij/). Statistical analysis was done by the Student t test using SigmaStat 2.03.0 software (Access Softek, San Rafael, CA).
Results and discussion
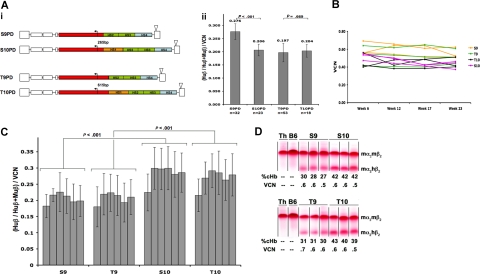
To evaluate the contribution of the 5′HS1 LCR element to lentivirus-encoded human β-globin expression, we created vectors encoding either HS2–3-4 or HS1–2-3–4 (Figure 1Ai). In MEL cells, addition of 5′HS1 had no effect on average transgene expression per vector copy in one vector (T9 vs T10, P = .669, Figure 1Aii), and even significantly decreased the average transgene expression per vector copy in another (S9 vs S10, P < .001). In stark contrast, addition of the 5′HS1 element significantly improved the vectors' performance in vivo (S9 vs S10 and T9 vs T10, Figure 1C). Globin transgene expression, at the mRNA level and per vector copy, increased from 27% (± 6%, mean ± SD, S9 and T9) of endogenous β-globin mRNA to 41% (± 9%, S10 and T10, P < .001). The average copy number ranged from 0.42 for T10 to 0.71 for S9 and remained stable throughout the experiment (Figure 1B). The mRNA data were confirmed on the protein level. All animals (n = 73) showed sustained amelioration of their anemia, without decrease over the period of observation. The T9 and S9 vectors conferred similar levels of chimeric hemoglobin (cHb, mα2:hβ2), on the order of 44% to 54% per vector copy and normalized to endogenous murine Hb, while vectors containing 5′HS1 element (S10 and T10) generated even higher levels of cHb (Figure 1D).
Figure 1.
Effect of HS1 on transgene expression. (Ai) Schematic representation of vector pairs used to evaluate the effect of 5′HS1 element on transgene expression. The human β-globin transgene (gene, promoter, and 3′enhancer) is represented in red. The locus control region (LCR) elements HS2 and HS3 are indicated in green and HS4 in blue. The orange box indicates the HS1 element. The triangle above the 3′LTR indicates deletion in the U3 region making it a self-inactivating (SIN) vector. Numbers between vector pairs indicate the size of promoter used in each vector. The letters “S” and “T” in vector names indicates 265bp and 615bp promoters, respectively. Number “9” in the vector name indicates LCR2-3-4, while “10” stands for LCR1-2-3-4. Through the course of the study, none of the vectors expressed transgene in mouse lymphoma (EL4) cells or undifferentiated MEL cells (data not shown), confirming tissue and differentiation stage specificity of the vectors. (Aii) Quantification of vector expression in independent MEL cell pools. Expression at the RNA level (Huβ/(Huβ+Muβ)) is normalized to vector copy number (VCN). P values were calculated using Student t test. The n values indicate the number of independent MEL cell pools. (B) Long-term stability of vector copy number in vivo assayed by TaqMan analysis. Three sample mice for each vector are shown. (C) Human β-globin transgene mRNA expression in peripheral blood (PB) shown as fraction of total β-globin mRNA and normalized to vector copy [(Huβ/(Huβ + Muβ)/VCN]. For each vector, bars indicate time points during the experiment, in order from left: Week 6, 12, 17, 23, 29, and 37. The number of mice ranged between 8 and 19 per group (total: 73 mice). P values were calculated using Student t test. Error bars in panels Aii and C are SD. (D) Cellulose acetate gel electrophoresis shows chimeric Hb (cHb, mα2:hβ2) levels in vector-transduced bone marrow chimeras. Data shown for 3 representative animals from each group at week 23 after transplantation. Vertical lines have been inserted to indicate repositioned gel lanes. Results were very similar at different time points. Control lanes contain normal C57BL/6 (B6) or HbbTh3/+ (Th) blood samples. The fraction of chimeric Hb (%cHb) relative to total hemoglobin (cHb/cHb+mHb) and vector copy number (VCN) are indicated below each sample.
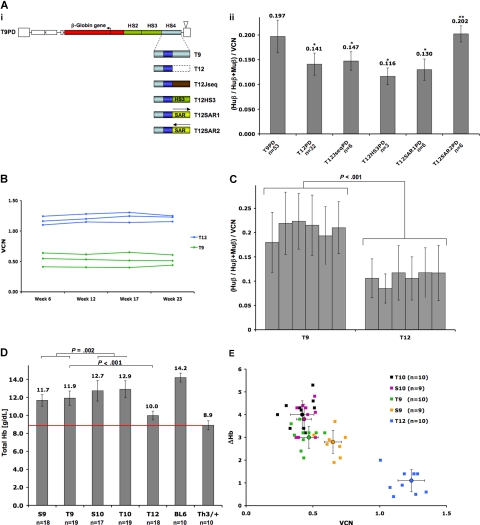
We next examined the contribution of the flanking regions of the HS4 element, which is a staple of TNS98 and other therapeutic globin vectors.9–11,13–15 The T12 vector, which harbors a truncation of the 5′ flanking region of HS4 (Figure 2Ai), showed significantly decreased globin expression both in vivo and in vitro in comparison to T9 (Figure 2Aii and 2C). The average vector copy number of vectors was stable throughout the course of the study (0.41 to 1.24) (Figure 2B). To further characterize the role of 5′ HS4, the 5′ flanking region of HS4 was replaced with different elements shown in Figure 2Ai. Only replacement of 5′ HS4 with the human IFN-β S/MAR20 sequence in forward orientation restored average expression to the level obtained with the T9PD vector in MEL cells (Figure 2Aii), showing that HS flanking regions play an important role in LCR function and do not function solely as spacers between HS cores.
Figure 2.
Evaluation of the effect of HS4 on transgene expression. (Ai) Vector constructs used to study the 5′flanking region of LCR HS4. The dark blue box indicates the core of 5′HS4. The 5′flanking region was truncated (dotted box in T12), replaced with spacer DNA of same size (brown box in T12Jseq), a fragment of HS3 flanking region of same size (green box in T12HS3), or the human IFN-β S/MAR element (light green box) in reverse (T12SAR1) or forward (T12SAR2) orientation. Throughout the course of the experiment, all vectors were tested for stability by Southern blot. The only vector found to undergo rearrangements was the T12HS3 vector (data not shown). Only 3 MEL pools showed no signs of rearrangements and were used in the experiment. In MEL cell experiments, hPGK-DHFR (PD) cassette was inserted between LCR HS4 and 3′LTR. (Aii) Quantification of β-globin mRNA expression (Huβ/(Huβ+Muβ) normalized to vector copy number (VCN) in independent MEL cell pools. The n values indicate the number of independent cell pools; error bars (in panels Aii, C, and D) are SD. P values were calculated using Student t test. *P < .001; **P = .716. (B) Long-term stability of vector copy number in vivo assayed by TaqMan analysis. Three sample mice for each group are shown. (C) Human β-globin transgene mRNA expression in peripheral blood (PB) shown as fraction of total β-globin mRNA and normalized to vector copy [(Huβ/(Huβ+Muβ)/VCN]. For each vector, bars indicate time point during the experiment, in order from left: Week 6, 12, 17, 23, 29, and 37. The number of mice ranged between 8 and 19 per group (total: 37 mice). (D) Total Hb level [g/dL] in peripheral blood (PB) of chimeric mice. Representative data for week 23 is shown. Red line indicates the level of Hb in Th3/+ mice. n indicates number of animals in each group. (E) Correlation between delta(Δ)Hb and provector copy number. ΔHb level was obtained by subtracting Th3/+ Hb ((D) Th3/+ = 8.9g/dL)) from total Hb level for each animal and corroborated by acetate gel electrophoresis (data not shown). Each square represents a single animal. The larger dots represent the average for each group, plus or minus SD. Data collected 23 weeks after bone marrow transplantation. See inset for color-coding of each vector.
Mice treated with vectors containing the 5′HS1 (T10, n = 19 and S10, n = 17) exhibited the highest Hb levels, 129 (± 9) g/L and 127 (± 11g/L, respectively (Figure 2D). When normalized to vector copy, those vectors provide 95g/L and 88g/L of Hb per vector copy (Figure S1B). The total hemoglobin levels in mice treated with T9 (n = 19) and S9 (n = 18) were very similar (119 ± 8 g/L and 117 ± 6 g/L, respectively), and significantly lower than for T10 (n = 19) and S10 (n = 17; P = .002, Figure 2D). When normalized to vector copy, the T9 and S9 vectors provided approximately 64g/L and 42g/L of cHb per vector copy, respectively (Figure S1B). Addition of HS1 thus nearly doubled the hemoglobin output of S9. On the other hand, the T12 vector performed the least well, as mice treated with this vector exhibited significantly lower Hb levels (100 ± 5 g/L; P ≤ .001, compared with T9, Figure 2D), which translated into 9g cHb/L per vector (Figure S1B). Protein expression was directly proportional to β-globin mRNA transcript levels for all vectors as shown in Figure S1A (R2 = 0.98). Figures 2E and S1B summarize the net hemoglobin increase in relation to vector copy number. These results indicate that 1 to 2 vector copies per cell achieve major therapeutic responses with vectors T9, S9, S10 and T10. In contrast, 6 copies per cell of the T12 vector would be required to treat anemia to the same extent as with 1 copy of T9.
Our data clearly demonstrate that HS1 significantly improves LCR function in vivo (P < .001; Figure 1C and Figure S1B). This fits with results of Pasceri et al,21 who found that HS1 enhanced LCR-dependent globin expression in nonviral constructs tested in transgenic mice. These findings were not predicted by MEL cell studies (Figure 1A and other studies22,23), reinforcing the utmost importance of using in vivo disease models to assess complex regulatory elements and the design of therapeutic vectors.
Our studies also support an important role for the intact HS4 element. Truncation of 5′ HS4 indeed significantly decreased β-globin transgene expression at the mRNA (Figure 2C) and protein levels (Figure 2D,E and S1B). Intriguingly, addition of the human IFN-βS/MAR sequence in forward orientation (T12SAR2PD) restored LCR function to the level found in the parental T9 vector. This observation suggests that a functional S/MAR element may be present in the 5′flanking region of HS4, as previously suggested by Cunningham et al24 Alternatively, the IFN-β S/MAR may rescue expression by anchoring proviral sequences to nuclear matrix and positioning them in transcriptional “hotspots.”25
We therefore conclude that HS1 and HS4 are important contributors to achieving therapeutic expression from globin vectors, which directly affects the vector copy number that is required to treat the anemia. Indeed, more than 5 copies per cell are required when using the T12 vector, whereas 1 or 2 copies per cell are sufficient with T9, S9, S10 and T10. This dosage requirement is likely to bear on the safety of HSC transduction, which is thought to be increased by diminishing the vector copy number in single cells.17 The use of tissue-restricted vectors, which should greatly diminish the probability of trans-activating neighboring oncogenes in HSC, progenitor cells, and lymphocytes,18 is expected to further increase the safety of globin gene transfer.
Based on previous findings8 and those reported here, we are preparing a phase I clinical trial to investigate the safety and tolerability of globin gene transfer in subjects with β-thalassemia major who lack a matched donor.4 In this study, which was recently reviewed by the Recombinant DNA Advisory Committee (RAC; June 20, 2007; http://videocast.nih.gov/ram/rac062007.ram), autologous CD34+cells obtained after G-CSF mobilization will be transduced with the TNS9.3 vector and infused following nonmyeloablative conditioning. Should higher levels of globin expression be eventually needed, the S10 and T10 vectors described here will prove to be very useful.
Supplementary Material
Acknowledgments
We thank Dr Stefano Rivella for assistance with cloning the 5′HS1 element, Dr J. Bode for providing the IFNβ S/MAR, and Drs Beata Gajewska and Laurent Beloeil for help with animal work.
This work was supported in part by RO1 HL-57 612 and the Leonardo Giambrone Foundation for the Cure of Thalassemia.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorshiop
Contribution: L.L. designed and executed experiments, and cowrote the manuscript; M.S. designed experiments and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Sadelain, Box 182, MSKCC, 1275 York Ave, New York, NY 10021; e-mail: m-sadelain@ski.mskcc.org.
References
- 1.Weatherall D. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 2.Orkin S, Nathan D. Philadelphia, PA: WB Saunders Co; 1998. Hematology of Infancy and Childhood. pp. 811–886. [Google Scholar]
- 3.Lucarelli G, Clift RA, Galimberti M, et al. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93:1164–1167. [PubMed] [Google Scholar]
- 4.Sadelain M, Boulad F, Galanello R, et al. Therapeutic options for patients with severe beta-thalassemia: the need for globin gene therapy. Human Gene Therapy. 2007;18:1–9. doi: 10.1089/hum.2006.151. [DOI] [PubMed] [Google Scholar]
- 5.Persons DA, Tisdale JF. Gene therapy for the hemoglobin disorders. Seminars in hematology. 2004;41:279–286. doi: 10.1053/j.seminhematol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 6.May C, Sadelain M. A promising genetic approach to the treatment of β-thalassemia. Trends in Cardiovascular Medicine. 2001;11:276–280. doi: 10.1016/s1050-1738(01)00125-6. [DOI] [PubMed] [Google Scholar]
- 7.Bank A, Markowitz D, Lerner N. Gene transfer. A potential approach to gene therapy for sickle cell disease. Ann NY Acad Sci. 1989;565:37–43. doi: 10.1111/j.1749-6632.1989.tb24147.x. [DOI] [PubMed] [Google Scholar]
- 8.May C, Rivella S, Callegari J, et al. Therapeutic haemoglobin synthesis in [beta]-thalassaemic mice expressing lentivirus-encoded human [beta]-globin. Nature. 2000;406:82–86. doi: 10.1038/35017565. [DOI] [PubMed] [Google Scholar]
- 9.Pawliuk R, Westerman KA, Fabry ME, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 10.Imren S, Payen E, Westerman KA, et al. Permanent and panerythroid correction of murine beta thalassemia by multiple lentiviral integration in hematopoietic stem cells. PNAS. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persons DA, Hargrove PW, Allay ER, Hanawa H, Nienhuis AW. The degree of phenotypic correction of murine beta -thalassemia intermedia following lentiviral-mediated transfer of a human gamma -globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- 12.Levasseur DN, Ryan TM, Pawlik KM, Townes TM. Correction of a mouse model of sickle cell disease: lentiviral/antisickling {beta}-globin gene transduction of unmobilized, purified hematopoietic stem cells. Blood. 2003;102:4312–4319. doi: 10.1182/blood-2003-04-1251. [DOI] [PubMed] [Google Scholar]
- 13.Puthenveetil G, Scholes J, Carbonell D, et al. Successful correction of the human {beta}-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- 14.Imren S, Fabry M, Westerman K, et al. High-level β-globin expression and preferred intragenic integration after lentiviral transduction of human cord blood stem cells. J Clin Invest. 2004;114:953–962. doi: 10.1172/JCI21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanawa H, Hargrove PW, Kepes S, Srivastava DK, Nienhuis AW, Persons DA. Extended {beta}-globin locus control region elements promote consistent therapeutic expression of a {gamma}-globin lentiviral vector in murine {beta}-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Peterson KR, Fang X, Stamatoyannopoulos G. Locus control regions. Blood. 2002;100:3077–3086. doi: 10.1182/blood-2002-04-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum C, Dullmann J, Li Z, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2113. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 18.Chang AH, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the LTR, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- 19.Chang AH, Stephan MT, Sadelain M. Stem cell-derived erythroid cells mediate long-term systemic protein delivery. Nat Biotech. 2006;24:1017–1021. doi: 10.1038/nbt1227. [DOI] [PubMed] [Google Scholar]
- 20.Bode J, Kohwi Y, Dickinson L, et al. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992;255:195–197. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- 21.Pasceri P, Pannell D, Wu X, Ellis J. Full activity from human beta -globin locus control region transgenes requires 5′HS1, distal beta -globin promoter, and 3′ beta-globin sequences. Blood. 1998;92:653–663. [PubMed] [Google Scholar]
- 22.Skarpidi E, Vassilopoulos G, Stamatoyannopoulos G, Li Q. Comparison of expression of human globin genes transferred into mouse erythroleukemia cells and in transgenic mice. Blood. 1998;92:3416–3421. [PubMed] [Google Scholar]
- 23.Li Q, Emery DW, Han H, Sun J, Yu M, Stamatoyannopoulos G. Differences of globin transgene expression in stably transfected cell lines and transgenic mice. Blood. 2005;105:3346–3352. doi: 10.1182/blood-2004-03-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham JM, Purucker ME, Jane SM, et al. The regulatory element 3′ to the A gamma-globin gene binds to the nuclear matrix and interacts with special A-T-rich binding protein 1 (SATB1), an SAR/MAR-associating region DNA binding protein. Blood. 1994;84:1298–1308. [PubMed] [Google Scholar]
- 25.Schubeler D, Mielke C, Maass K, Bode J. Scaffold/matrix-attached regions act upon transcription in a context-dependent manner. Biochemistry. 1996;35:11160–11169. doi: 10.1021/bi960930o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.