Abstract
Signal transducer and activator of transcription 6 (STAT6) is critical for IL-4 and IL-13 responses, and necessary for the normal development of Th2 cells. We previously generated mice that express a constitutively active STAT6 (STAT6VT) under control of the CD2 locus control region, which directs expression to the T-cell compartment. We now describe that a small proportion of these mice (∼5%) develop a spontaneous lymphoproliferative disease (LPD) that results in dramatic splenomegaly. The cell populations observed in the LPD spleens can be divided into 2 categories, those that are composed of mixed lineage cells and those that are predominantly T cells with a phenotype similar to that in autoimmune lymphoproliferative syndrome (ALPS) patients. These data suggest that while active STAT6 is not a transforming factor, expression in T cells predisposes toward the development of lymphoproliferative disorders.
Introduction
Signal transducer and activator of transcription 6 (STAT6) is critical for the response to the Th2 cytokines IL-4 and IL-13.1 The use of STAT6-deficient mice has demonstrated the importance of this factor in Th2 immune responses in vivo.2–4 STAT6 is found to be constitutively active in a number of tumors, though how it contributes to a transformed phenotype is unclear.5
We have recently generated mice that express a constitutively active STAT6 in T cells.6 The mutant STAT6, termed STAT6VT, was identified through scanning alanine mutagenesis and does not require cytokine signaling or Janus kinase activation to become phosphorylated and active.7 Mice expressing STAT6VT in T cells have decreased T-cell and increased B-cell numbers in the spleen and enhanced Th2 activity in vivo.6 In this report, we show that mice expressing active STAT6 in vivo are predisposed toward developing a lymphoproliferative disorder (LPD).
Materials and methods
The generation of Stat6VT transgenic mice has been previously described.6 All experiments were approved by the Indiana University institutional animal care and use committee (IACUC) and animals were maintained under specific pathogen-free conditions. Flow cytometry was performed using reagents from BD Pharmingen (San Diego, CA). All other experiments were performed using standard procedures.6 Data are presented as representative of at least 3 experiments.
Results and discussion
We have previously shown that expression of a constitutively active STAT6 in T cells alters lymphocyte homeostasis.6 Approximately 5% of mice (21/419) expressing STAT6VT develop a lymphoproliferative disorder (LPD) characterized by splenomegaly and in some cases, lymphadenopathy (Figure 1A,B), whereas LPD was never observed in nontransgenic littermates. In mice with LPD, spleen size, by mass, can increase 40-fold and leukocyte cell number can increase an average of 5-fold. LPD was observed in 3 separate STAT6VT transgenic founder lines, indicating that this is not an effect of transgene integration.
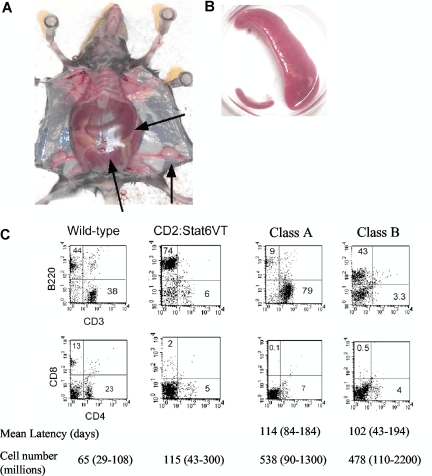
Figure 1.
Lymphoproliferative disease in Stat6VT transgenic mice (A) Photograph of mouse with lymphoproliferative disease. Spleen is extended through entire peritoneum ( ). Lymphadenopathy is also apparent. (B) Comparison of spleen size from wild-type mouse (bottom left) compared with spleen from mouse with LPD. (C) Flow cytometric analysis of splenocytes from wild type, Stat6VT transgenic and 2 Stat6VT transgenic mice with LPD. Numbers in quadrants represent percentage of total spleen cells. Average latency (the age of the mouse, in days, at which the LPD was characterized) and averages of spleen cell numbers (range shown in brackets) are shown below (n = 11 for class A and n = 10 for class B).
). Lymphadenopathy is also apparent. (B) Comparison of spleen size from wild-type mouse (bottom left) compared with spleen from mouse with LPD. (C) Flow cytometric analysis of splenocytes from wild type, Stat6VT transgenic and 2 Stat6VT transgenic mice with LPD. Numbers in quadrants represent percentage of total spleen cells. Average latency (the age of the mouse, in days, at which the LPD was characterized) and averages of spleen cell numbers (range shown in brackets) are shown below (n = 11 for class A and n = 10 for class B).
The phenotype of cells in LPD can be divided into 2 classifications. In class A LPD, cells are predominantly T cells (CD3+, αβ TCR+) but are largely CD4- and CD8-negative (Figure 1C). In class B LPD, the splenocytes still have a mixed phenotype with a predominance of B220+cells and an expansion of non-B, non-T cells. There is no difference between the 2 classes in the numbers of mice affected, the onset of disease or in the overall size of the spleen (Figure 1C). Moreover, development of both classes of LPD was dependent on the presence of endogenous IL-4 since we observed no occurrence of LPD in Il4−/− STAT6VT transgenic mice (0/177, P < .001 using Fisher exact test), despite constitutive activation of STAT6VT in the absence of endogenous IL-4.
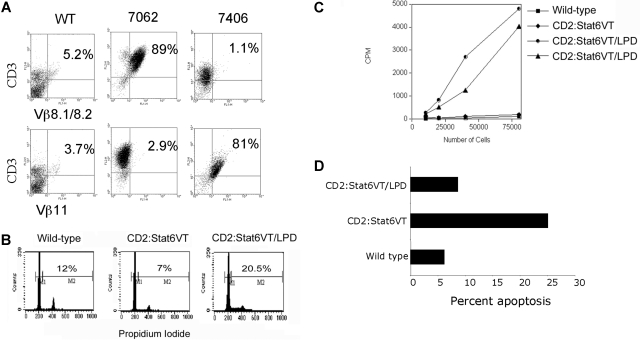
We further examined the phenotypes of T cells in class A LPD. Using flow cytometry we analyzed the clonality of T cells using antibodies specific for TCR Vβ regions. While several Vβ specificities were observed and PCR of TCR rearrangements indicated multiple clones, there was an expansion of cells expressing one Vβ segment in these mice, though the particular Vβ expansion varied among the affected mice (Figure 2A). Isolated spleen cells demonstrated an increased percentage of cells in the S or G2/M phases of cell cycle (Figure 2B) and had an ability to spontaneously proliferate in culture (Figure 2C).
Figure 2.
T-cell expansion in class A LPD mice. (A) FACS analysis of spleen cells from wild type mice and 2 Stat6VT transgenic mice with class A LPD. Data are shown for staining of 2 TCR Vβ regions on the ordinate. Samples were counterstained for CD3 on the abscissa. Numbers on plots represent percentage of total spleen cells. (B) Total splenocytes from mice of the indicated genotypes were fixed and stained with propidium iodide to analyze DNA content. Numbers shown are the percentages of CD3+cells that are in S + G2/M phases of the cell cycle. (C) Isolated splenocytes from wild type or Stat6VT transgenic mice or 2 mice with class A LPD were incubated in 96-well plates and proliferation was assessed using uptake of 3H-thymidine. (D) CD3+ splenocytes from mice of the indicated genotypes were tested for propidium iodide exclusion as a measure of apoptosis.
The reason for the low penetrance of this phenotype is unclear. It is likely that the activated STAT6 provides a “first-hit” in the development of LPD. Additional environmental stimuli might then provide further signals to promote cell expansion, as immunization increases lymphoma frequency in STAT5 transgenic mice.8 The requirement for IL-4 in the development of LPD in the STAT6VT transgenic mice further supports a requirement for immune stimulation in the development of LPD and suggests an involvement of STAT6-independent signaling in the development of class A LPD. The requirement for IL-4 in the development of class B LPD is a trans effect because non-T cells have limited expression of STAT6VT in these transgenic mice (data not shown).
The class A phenotype is reminiscent of the T-cell phenotype in autoimmune lymphoproliferative syndrome (ALPS), which can arise from mutations in FAS, FASL, CASP8, CASP10 and NRAS, is characterized by decreased apoptosis in the affected cell populations and a predisposition toward the development of lymphoma.9,10 While we have not observed consistently altered expression of Fas or FasL on STAT6VT-expressing T cells (data not shown), there are lower levels of spontaneous apoptosis in STAT6VT LPD cells than in normal STAT6VT T cells (Figure 2D). In addition, some ALPS patients have altered Th2 cytokine production that correlates with pathogenesis.11,12 It is intriguing to speculate that, given our result with IL-4–deficient transgenic mice, Th2 cells are a contributing factor to disease in some patients rather than a result of altered immune homeostasis.
It is now clear that STAT proteins contribute to cellular transformation. Particular attention has been paid to STAT3 and STAT5, as they are observed to have increased expression and activation in a wide variety of tumors.13 Active STAT6 is observed in a high percentage of Hodgkin lymphoma samples, as well as other T- and B-cell lymphomas and prostate cancer.5 The critical issue is whether STAT6 can contribute to the transformation process. In this report, we have shown that constitutively active STAT6 can predispose toward a lymphoproliferative disorder when expressed as a transgene. Identification of additional factors that cooperate with STAT6 in the generation of LPD should provide some insight into the development of lymphoproliferative disorders and lymphoma.
Acknowledgments
We thank Dr M. Robertson for review of this manuscript.
This work was supported by National Institutes of Health grants AI070448 (M.H.K.), T32DK007519 (H.C.C.) and T32AI060519 (J.T.O.). A.N.M. and H.A.B. were predoctoral fellows of the American Heart Association.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
All authors performed research, H.A.B. and M.H.K. designed research, and M.H.K. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark H. Kaplan, PhD, 702 Barnhill Drive, RI 2600, Indianapolis, IN 46202; email: mkaplan2@iupui.edu.
References
- 1.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biological functions. Ann Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MH, Whitfield JR, Boros DL, Grusby MJ. Th2 cells are required for the Schistosoma mansoni egg-induced granulomatous response. J Immunol. 1998;160:1850–1856. [PubMed] [Google Scholar]
- 3.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal Transducer and Activator of Transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban JF, Jr., Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 5.Bruns HA, Kaplan MH. The role of constitutively active Stat6 in leukemia and lymphoma. Crit Rev Oncol Hematol. 2006;57:245–253. doi: 10.1016/j.critrevonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003;170:3478–3487. doi: 10.4049/jimmunol.170.7.3478. [DOI] [PubMed] [Google Scholar]
- 7.Daniel C, Salvekar A, Schindler U. A gain-of-function mutation in STAT6. J Biol Chem. 2000;275:14255–14259. doi: 10.1074/jbc.c000129200. [DOI] [PubMed] [Google Scholar]
- 8.Kelly JA, Spolski R, Kovanen PE, et al. Stat5 synergizes with T cell receptor/antigen stimulation in the development of lymphoblastic lymphoma. J Exp Med. 2003;198:79–89. doi: 10.1084/jem.20021548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oliveira JB, Bidere N, Niemela JE, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. Br J Haematol. 2006;133:124–140. doi: 10.1111/j.1365-2141.2006.05993.x. [DOI] [PubMed] [Google Scholar]
- 11.Fuss IJ, Strober W, Dale JK, et al. Characteristic T helper 2 T cell cytokine abnormalities in autoimmune lymphoproliferative syndrome, a syndrome marked by defective apoptosis and humoral autoimmunity. J Immunol. 1997;158:1912–1918. [PubMed] [Google Scholar]
- 12.Kim YJ, Dale JK, Noel P, et al. Eosinophilia is associated with a higher mortality rate among patients with autoimmune lymphoproliferative syndrome. Am J Hematol. 2007;82:615–624. doi: 10.1002/ajh.20851. [DOI] [PubMed] [Google Scholar]
- 13.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]