Abstract
Chimeric proteins joining the histone methyltransferase MLL with various fusion partners trigger distinctive lymphoid and myeloid leukemias. Here, we immunopurified proteins associated with ENL, a protein commonly fused to MLL. Identification of these ENL-associated proteins (EAPs) by mass spectrometry revealed enzymes with a known role in transcriptional elongation (RNA polymerase II C-terminal domain kinase [RNAPolII CTD] positive transcription elongation factor b [pTEFb]), and in chromatin modification (histone-H3 methyltransferase DOT1L) as well as other frequent MLL partners (AF4, AF5q31, and LAF4), and polycomb group members (RING1, CBX8, and BCoR). The composition of EAP was further verified by coimmunoprecipitation, 2-hybrid analysis, pull-down, and colocalization experiments. Purified EAP showed a histone H3 lysine 79–specific methylase activity, displayed a robust RNAPolII CTD kinase function, and counteracted the effect of the pTEFb inhibitor 5,6-dichloro-benzimidazole-riboside. In vivo, an ENL knock-down diminished genome-wide as well as gene-specific H3K79 dimethylation, reduced global run-on elongation, and inhibited transient transcriptional reporter activity. According to structure-function data, DOT1L recruitment was important for transformation by the MLL-ENL fusion derivative. These results suggest a function of ENL in histone modification and transcriptional elongation.
Introduction
Hematopoietic development is controlled by an intricate network of finely tuned transcriptional programs. Consequently, a perturbation of the transcription factors involved can block differentiation. This developmental roadblock cooperates with mutations in pathways that signal growth and/or survival to cause acute leukemias.1 A prime example for such a mechanism is mixed lineage leukemia. In this disease, the gene for the histone methyltransferase MLL participates in chromosomal translocations that eventually create maturation-blocking and therefore leukemogenic MLL fusion proteins.2,3 These protein chimeras consist of N-terminal portions of MLL joined to a variety of mostly unrelated fusion partners that replace the original MLL C-terminus, including the methyltransferase domain (http://atlasgeneticsoncology.org/Genes/MLL.html). MLL fusion proteins are aberrant transcription factors that ectopically activate genes important for hematopoietic development like the abdominal-type Hox genes Hoxa7 and Hoxa9 and their dimerization partner Meis1.4–7
Despite intensive study, little is known about the biological function of MLL fusion partners in normal cells, and it is mostly unclear how these proteins activate the oncogenic potential of MLL. In the rare cases where MLL is joined to a cytoplasmatic protein, domains introduced by the partner force a dimerization of the fusion that is crucial for oncogenic activity.8,9 The overwhelming majority of leukemias with MLL rearrangement, however, involves nuclear proteins as translocation partners. The data available seem to indicate a role of some nuclear MLL partners in transcriptional control and histone modification. Support for this speculation comes from the detection of direct protein-protein interactions of the homologous MLL fusion partners AF4 and AF5q31 that both bind to ENL and the closely related AF9.10,11 ENL in turn interacts with histone H3.11 In addition, another MLL fusion partner, AF10, recruits the histone H3 lysine 79–specific methyltransferase DOT1L that introduces a dimethyl mark during transcriptional elongation.12 The same modification is also a hallmark of genes activated by MLL-ENL.13 Finally, the proteins CBX8 (chromobox 8) and BCoR (BCL6 corepressor) that are involved in chromatin-dependent gene repression have also been found to associate with ENL and AF9.14–16
In order to learn more about the biological function of a classical MLL fusion partner, we identified proteins associated with the “Eleven Nineteen Leukemia” protein (ENL) originally discovered as an MLL fusion partner in the recurrent translocation t(11;19). Here, we describe the purification and analysis of ENL-associated proteins (EAPs) by tandem immunoprecipitation of ENL. This protein assembly contains several other MLL fusion partners, positive transcription elongation factor b (pTEFb), DOT1L, and polycomb group proteins. The composition of EAP suggests that ENL works in a new unit of transcriptional regulation that coordinates transcriptional elongation with chromatin modification.
Materials and methods
Plasmids, cells, antibodies, and software
The cDNA for flagENL (GenBank accession no. NM_00593417), MLL-ENL, and mutants thereof were inserted into the retroviral vectorpMSCVneo. For production of anti-ENL small inhibitory (si) RNA, an oligonucleotide corresponding to ENL positions 489 to 509 (aattccactctctgccttctc) was introduced into the “self-inactivating” retroviral vector pSIRENretroQ (Clontech, Palo Alto, CA). This construct provides a source of small hairpin (sh) RNA for intracellular production of specific siRNA. Derivatives of ENL and mouse Dot1l (a generous gift of B. C. Kone, Houston, TX) for 2-hybrid experiments were cloned either into pGBKT7 as bait construct or pGADT7 for target clones. Fusions with enhanced green fluorescent protein (EGFP) or red fluorescent protein (RFP) were done in pEGFP and pRedExpress (all vectors from Clontech). The luciferase reporter plasmids have been described.4 Primer sequences for qPCR of HOXA9 sequences are available on request.
The 293fENL producer cell line was generated from HEK293 cells by amphotropic transduction with pMSCVneo-fENL viruses.
Anti-flag (M2) agarose and anti-flag antibody were from SIGMA (Taufkirchen, Germany). Monoclonal antibodies against ENL (clones 3.1, 4.1, and 33.1) were produced in-house. Other antibodies were from AbCam (Cambridge, United Kingdom), Santa Cruz Biotech (Santa Cruz, CA), or AbGent (San Diego, CA). Quantification of Western blots was done densitometrically with QuantityOne software (BioRad, München, Germany).
Purification of EAP, chromatography, and mass spectrometry
ENL affinity resin was produced by coupling monoclonal anti-ENL antibodies 3.1, 4.1, and 33.1 to a solid support with SEIZE primary immunoprecipitation reagents (Pierce, Rockford, IL). Extracts from approximately 5 × 108 cells (293fENL or HEK293) were prepared by lysis with bufferP (300 mM NaCl, 20 mM HEPES [pH 7.5], 0.5 mM EDTA, 0.1% Triton X-100, 0.5 mM sodium vanadate, 2 mM NaF, 2 mM DTT, 0.2 mM PMSF, 20 μg/mL leupeptin, 0.4μg/mL aprotinin, and 40 μg/mL pepstatin A). After preclearing with protein A/G agarose (Santa Cruz Biotech) the extracts were incubated overnight with flagM2 agarose. The anti-flag beads were washed in bufferP supplemented with 0.25% bovine serum albumin. Immobilized proteins were eluted with 150 μg/mL flag-peptide (SIGMA) followed by a second precipitation with anti-ENL resin, a wash step in bufferP, and elution by low pH (100 mM glycine [pH 2.7]) and neutralization. Proteins were analyzed by SDS-PAGE and Coomassie and silver staining (ProteoSilver plus; SIGMA). Nucleases were added at 5 U/mL (benzonase) and 0.5 μg/mL (RNAseA).
Gel filtration was done with primary extracts in bufferP (without Triton X-100) on a calibrated Sephacryl S300 column (GE Healthcare, Munich, Germany).
Silver-stained bands were excised from polyacrylamide gels and proteins in each band were digested with trypsin, and the resulting peptide mixtures were analyzed by reverse-phase liquid chromatography–tandem mass spectrometry (LC-MS/MS) using a Thermo Electron (Waltham, MA) linear ion trap (LTQ) mass spectrometer. Peptides were identified by searching acquired MS/MS spectra using Mascot18 against the Human International Protein Index (IPI) protein sequence database version 3.16 with the following search parameters: monoisotopic precursor ion mass with 3 Da mass tolerance, unconstrained search, and allowing for methionine oxidation as a variable modification. Peptide assignments to spectra were statistically validated using PeptideProphet,19 which computes for each peptide assignment in the dataset a probability of being correct given its database search score, the number of tryptic termini and missed cleavages, and other properties. Peptide identifications were assembled into proteins using the protein inference tool ProteinProphet,20 and the probabilities were recomputed at the protein level. All proteins with a probability of being correct greater than 0.9 were retained, which corresponds to the false discovery error rate of less than 1% as estimated by ProteinProphet. In addition, several proteins with lower initial probability were added to the list based on the manual validation of the corresponding peptide assignments to MS/MS spectra. In order to eliminate nonspecific binding proteins, the analysis of ENL-expressing cells was performed in parallel with control 293 cells. Those proteins that were also identified in the control gels were subtracted to create the final list of proteins that interact with ENL.
Yeast procedures, 2-hybrid system, and GST pull-down
For the yeast 2-hybrid experiments, the baits and target plasmids were cotransfected into the yeast strain AH109 (Clontech) according to the manufacturer's instructions. GST pull-down was done with either purified GST or GST fused to full-length ENL and 35S-labeled Dot1l produced by in vitro transcription/translation using TNT reagents from Promega (Madison, WI).
In vitro methylation assay, kinase assay, and in vitro transcription
To test for histone methylation activity in immunoprecipitated material from 293fENL or HEK293 cells, EAP-loaded beads were incubated with 5 μg core histones (Upstate Biotechnology, Charlottesville, VA) and 1 μL 3H-methyl S-adenosylmethionine (3.7 × 1010 Bq/mmol [10 Ci/mmol], stabilized in H2SO4/EtOH; Hartmann Analytik, Braunschweig, Germany) in a total of 40 μL TE plus 1 mM DTT for 1 hour at 30°C. The reaction products were separated on 18% SDS-PAGE gels stained with Coomassie blue and treated with EnHance fluorographic solution (GE Healthcare) before exposure. For immunologic detection of in vitro methylated histones, the reaction was performed in the presence of 32 μM nonradioactive S-adenosylmethionine. After separation and transfer, the modified histones were detected by methylation-specific antibodies in an immunoblot procedure.
To detect pTEFb kinase activity in EAP, the proteins bound to ENL resin were washed once in 20 mM Tris-acetate (pH 7.9), 10 mM magnesium-acetate, 50 mM potassium-acetate, and 1 mM DTT. The beads were incubated with 1 μg recombinant GST-CTD (Calbiochem/Merck, Darmstadt, Germany) and 1 MBq of γ32P ATP in the same buffer at 30°C for 1 hour. Phosphorylated proteins were visualized by SDS gelelectrophoresis, followed by Coomassie staining and autoradiography.
In vitro transcription was done with the HeLa scribe system (Promega) according to the instruction of the manufacturer. Inhibition of CDK9 was achieved by addition of 50 μM 5,6-dichloro-benzimidazole-riboside (DRB; SIGMA) as a 50-mM stock solution in DMSO. The run-off transcription was directed by a linearized DNA template containing the first 244 bases of the mouse Hoxa9 coding sequence under control of the cytomegalovirus (CMV) immediate early promoter.
shRNA-mediated knock-down of ENL
ENL knock-down in HEK293 cells was achieved by serial transfection with pSIRENretroQ vectors capable of producing shRNA directed against ENL or luciferase as control. Cells were subjected at day 1 and again at day 4 to standard Ca transfection (20 μg of pSIRENretroQ vector per 6 × 106 HEK293 cells) before analysis at day 7. For subsequent experiments, equal numbers of living cells as determined by trypan blue exclusion were applied.
ChIP and nuclear run-on assay
Chromatin immunoprecipitation (ChIP) was performed according to the Upstate Biotechnology technical protocol as available at www.upstate.com. Global elongation rates were measured with nuclear run-on experiments according to Carey and Smale.21 In short, nuclei from 5 × 107 cells were prepared by NP40 lysis (10 mM Tris/HCl [pH 7.4], 10 mM NaCl, 3 mM MgCl2, and 0.5% NP40) and centrifugation. The nuclei were resuspended in 50 mM Tris/HCl (pH 8.3), 40% vol/vol glycerol, 5 mM MgCl2, and 0.1 mM EDTA. Aliquots (26.5 μL) were mixed with 7 μL 5× run-on buffer (25 mM Tris/HCl [pH 8.0], 12.5 mM MgCl2, 750 mM KCl, and 1.25 mM each of ATP, GTP, and CTP) and 1.5 μL of α32P-UTP (1.11 × 1014 Bq/mmol [3000 Ci/mmol]). After incubation at 37°C for 15 minutes, RNA was isolated from the reaction mixtures by RNeasy column purification (Qiagen, Hilden, Germany). Purified RNA was spotted in dilutions onto nitrocellulose membranes and dried, and incorporated radioactivity was determined by PhosphoImager quantification (BioRad). For normalization, 18S stable RNA levels in the reaction mixtures were quantified by real-time reverse transcription–polymerase chain reaction (RT-PCR). Primers used were: 18S reverse, cttccttggatgtggtagccg; and 18S forward, tatcaactttcgatggtagtcgc.
MLL fusion protein transformation assay
The transformation capacity of MLL fusion proteins was determined by serial replating assays as previously reported.22 In this experiment, transformation can be visualized as colony formation after repeated replating in semisolid medium. Replating assays were performed at least 3 times per construct.
Results
Purification of EAP
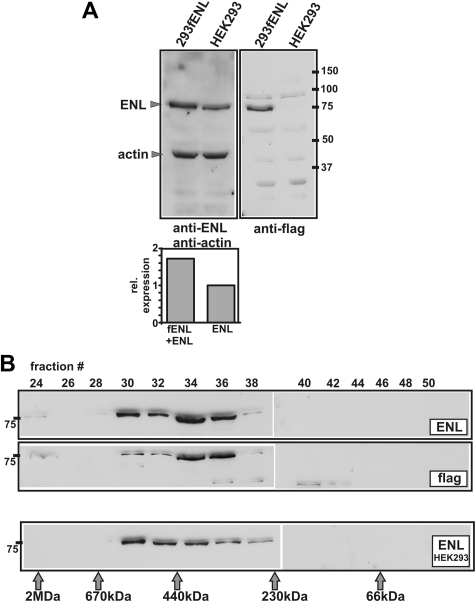
For purification of EAPs, a stable producer cell line was established. In order to achieve low-level stable expression of flag-tagged ENL, the gene was introduced into HEK293 cells by transduction with a pMSCV-based retrovirus (pMSCV-fENL). Immunoblotting with anti-flag and anti-ENL antibodies and densitometric quantification demonstrated successful expression of fENL in 293fENL cells at approximately wild-type levels (Figure 1A). In order to verify that ectopic fENL is incorporated into a potential wild-type ENL complex, extracts of 293fENL cells were analyzed by gel filtration. Elution fractions were probed for the presence of flag-tagged proteins and ENL. In these experiments, the elution profile of ENL and fENL was nearly identical, and the majority of ENL/fENL protein was present in fractions corresponding to a relative molecular weight of approximately 400 kDa (Figure 1B). No uncomplexed fENL could be detected. Compared with the elution profile of wild-type ENL from untransduced HEK293 cells, the peak of flag-ENL elution was shifted slightly toward smaller molecular weights (Figure 1B). However, every elution fraction containing ENL in untransduced cells was also positive for flag-ENL in 293fENL cell extracts. This suggested that at least part of the ectopic fENL was incorporated into the appropriate endogenous complex or complexes.
Figure 1.
Characterization of an fENL producer cell line. (A) Detection of flagENL expression by immunoblot. Extracts from transduced 293fENL and HEK293 parental cells were tested for endogenous and ectopic ENL expression by probing with anti-ENL and anti-flag antibodies. Actin served as loading control. A densitometric quantification of the relative expression levels is shown in the bar graph. (B) Gel filtration on Sephacryl S300. Extracts from 293fENL cells were separated by gel filtration on a precalibrated column as indicated. Individual eluate fractions were tested by immunoblotting with antibodies against ENL and flag. The elution profile of ENL from the parental HEK293 cells is shown in the bottom panel.
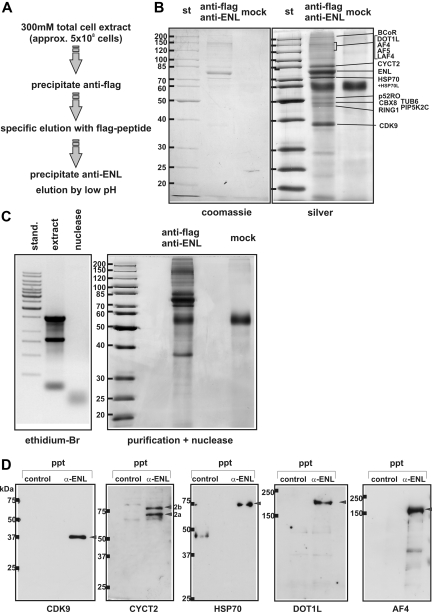
EAPs were purified by a double immunoprecipitation strat-egy (Figure 2A). Triton/salt extracts from approximately 5 × 108 293fENL cells were precipitated with anti-flag agarose. After washing, bound material was selectively eluted with flag peptide and subjected to a second round of immunoprecipitation with a mixture of 3 bead-immobilized monoclonal antibodies targeted against ENL. Beads were washed again and residual material was released by low pH. For controls, the purification procedure was performed with extracts from nontransduced HEK293 cells, or mock precipitations were done with 293fENL cells using unliganded agarose. The final purified product was separated alongside controls by SDS-PAGE, followed by Coomassie and silver staining. This immunopurification reproducibly yielded a preparation of 9 major proteins detectable by Coomassie dye with approximately twice as many bands discernible after silver staining (Figure 2B). An identical pattern of EAPs was also obtained in the presence of nucleases (Figure 2C), excluding the possibility that EAP constituents were inadvertently copurifying due to an affinity for nucleic acids present in the cell extracts.
Figure 2.
Purification of EAP. (A) Schematic flow chart of the purification procedure. (B) Composition of EAP. EAP was purified from approximately 5 × 108 293fENL cells. As a mock control, the same purification strategy was performed using unliganded agarose in place of flag-agarose for the first precipitation step (mock). Eluates were run on a 10% SDS-PAGE, stained with Coomassie (left panel) and subsequently with silver (right panel). The bands were excised, digested, and analyzed by mass spectrometry. All proteins that could be uniquely identified by more than 1 peptide and at least in 2 independent experiments are listed. Where individual band identities could be assigned, they are indicated in the figure. (C) Left panel shows nucleic acid content of cellular extracts used for EAP purification. Nucleic acids from cellular extracts used for EAP purification were isolated by phenol extraction, separated by agarose gel electrophoresis and stained by ethidium bromide. Samples shown are from untreated cellular extracts (extract) and from extracts after nuclease digestion (nuclease). Right panel shows purification of EAP from nuclease-treated cell extracts. EAP was precipitated as described in panel B from cellular extracts after exhaustive nuclease digestion. The figure shows a silver stained gel of EAP proteins in com-parison to a mock control as described in panel B. (D) Coimmunoprecipitation of major EAP components with endogenous ENL. Immunoprecipitations from HEK293 cell extracts were done either with agarose coupled to anti-ENL antibodies (α-ENL) or flag-agarose (control). Coprecipitating proteins were analyzed by immunoblot with antibodies against the pTEFb subunits CDK9, CYCT2, and HSP70, as well as with antibodies specific for DOT1L and AF4. The 2 splice variants of CYCT2 (CYCT2a, 2b) are indicated.
A total of 3 independent EAP preparations and the corresponding controls were subjected to mass spectrometry. The silver-stained gels were cut into slices, and proteins in each fragment were digested by trypsin. The resulting peptide mixtures were analyzed by reverse-phase LC-MS/MS using a linear ion trap mass spectrometer. In total, 15 proteins represented by more than 1 peptide could be unambiguously identified in the EAP samples (Table 1). All proteins (AF5q31, AF4, BCoR, and CBX8) that had been previously found in various 2-hybrid screens as interaction partners of ENL or the homologous AF9 were present also in the EAP preparations. In addition, EAP contained pTEFb, DOT1L, the AF4 homolog LAF4, and RING1, which is known to complex with CBX8, as well as the proteins BCoR, p52RO, TUB6, and PIP5K2C. Surprisingly, pTEFb was exclusively detected in the minor isoform as dimer of CDK9 with CYCT2, whereas approximately 80% of cellular pTEFb consists of CDK9 and CYCT1.23 Also, HSP70, which is known to copurify with CDK9,24 was part of EAP. Identities for individual silver-stained bands could be assigned for most proteins by the expected molecular weight and band-intensity/peptide-frequency correlations. Compared with ENL, the accompanying proteins were present in reproducible but nonequimolar stoichometries, implying that EAP is not a single complex but rather a mixture of different subcomplexes, with ENL as common unit. This assumption was corroborated by the fact that the sum of the calculated molecular weights of all identified EAP components exceeded 1.4 MDa, whereas ENL eluted during gel-filtration at approximately 400 kDa. Alternatively, it cannot be excluded that the conditions applied during size separation might have caused the disruption of a potentially larger complex.
Table 1.
Composition of EAP
| Protein | Accession no. | Molecular weight, kDa | Peptides, no.* | Remarks |
|---|---|---|---|---|
| BCoR | NP_060215 | 188 | 13 | BCL-6–interacting corepressor |
| DOT1L | NP_115871 | 165 | 11 | Histone H3K79 methyltransferase |
| LAF4 | NP_002276 | 133 | 26 | MLL fusion partner, AF4 and AF5q31 homolog |
| AF4 | NP_005926 | 131 | 110 | MLL fusion partner, AF5q31 homolog |
| AF5q31 | NP_055238 | 127 | 741 | MLL fusion partner, AF4 homolog |
| CYCT2 | NP_490595 | 81 | 52 | Cyclin T2, subunit of pTEF-b |
| HSP70 | NP_940833 | 70 | 169 | Heat-shock protein |
| HSP70L | NP_005336 | 70 | 6 | Heat-shock protein–like |
| ENL | NP_005925 | 62 | 167 | MLL fusion partner, tagged for purification |
| p52 RO | NP_003132 | 54 | 47 | Ubiquitin ligase, E3 enzyme |
| TUB6 | NP_997195 | 50 | 3 | Tubulin variant |
| PIP5K2C | NP_079055 | 47 | 2 | Phosphatidylinositol-4-phosphate-5-kinase, type II, γ |
| CBX8 | NP_065700 | 43 | 3 | Chromobox 8 protein |
| CDK9 | NP_001252 | 43 | 55 | Subunit of pTEF-b, RNAPol II CTD kinase |
| RING1 | NP_002922 | 42 | 10 | Member of polycomb repressive complex I |
The table lists proteins that could be uniquely identified by more than 1 peptide and at least in 2 independent EAP samples. In addition, peptides specific for the MLL fusion partners ELL (accession no. NP_006523), AF9 (accession no. NP_004520), and AF10 (accession no. NP_004632) were detected in single experiments. The given molecular weight corresponds to the theoretical calculated value of the prototypical protein as deposited in the database.
All gene-specific peptides that could be unambigously identified by mass-spectrometry across all replicates.
To independently confirm the association of ENL with the major components of EAP, coimmunoprecipitations from unaltered HEK293 cells were performed (Figure 2D). To this end, Triton/salt extracts from native HEK293 cells were incubated either with ENL resin or with flag-agarose as control. The presence of DOT1L and all expected subunits of pTEFb (CDK9, CYCT2, and HSP70) could be successfully proven by immunoblotting. Interestingly, 2 CYCT2 immunoreactive bands were detected that corresponded exactly to the expected molecular weight of the known splice forms of CYCT2 (CYCT2B, 81 kDa; CYCT2A, 73.5 kDa). Mass spectrometry did not pick up the splice variants in purified EAP because no discriminatory peptides were present; however, both CYCT2 isoforms could be detected in EAP by immunoblotting (data not shown). Control blots did not detect any CYCT1 in EAP preparations (data not shown). In addition, the coprecipitation of endogenous ENL with AF4 also could be demonstrated. Suitable antibodies recognizing the AF4 homologs LAF4 and AF5q31 were not available.
ENL interacts directly with Dot1l
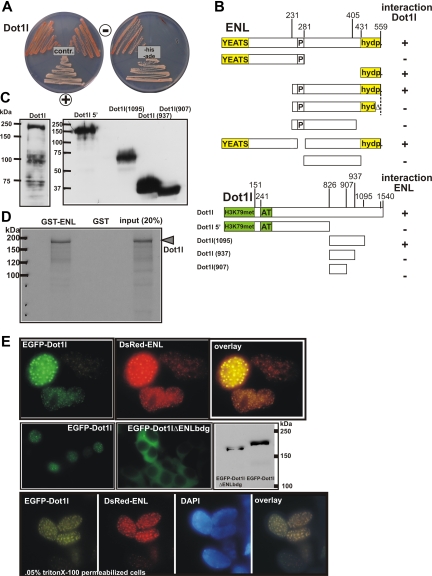
The presence of DOT1L in EAP and the apparent important role for H3K79 methylation in MLL fusion protein mediated transformation12,13 prompted us to analyze the DOT1L-ENL interaction more in detail. Because we were not able to obtain a full-length human DOT1L cDNA, the highly homologous mouse Dot1l was used in these experiments. Yeast 2-hybrid experiments were consistent with a direct association of the 2 full-length proteins (Figure 3A). A deletion analysis localized the ENL-binding domain within the Dot1l C-terminus between amino acids 826 and 1095, a region without any recognizable motif or homology. In contrast, the Dot1l interaction domain in ENL coincided with the highly conserved hydrophobic C-terminus that had been shown to be necessary and sufficient for transformation by MLL-ENL.25 Interestingly, a small deletion of the ultimate 15 amino acids of ENL, a mutation that destroys ENL-dependent transactivation and MLL-ENL–mediated transformation, also precluded binding to Dot1l (Figure 3B,C). A direct interaction between ENL and Dot1l was also supported by GST pull-down experiments. In vitro transcribed and translated Dot1l could be coprecipitated with GST-ENL but not by GST alone (Figure 3D). Finally, EGFP-Dot1l and RFP-ENL fusions colocalized in the nuclei of living cells in a speckled distribution typical for ENL. An EGFP-Dot1l derivative with an internal 191–amino acid deletion within the ENL-binding domain was distributed exclusively in the cytoplasm, indicating an important role of this region for nuclear import und function of Dot1l (Figure 3E). ENL and Dot1l localized exclusively in the nucleus as demonstrated in cells counterstained with DAPI.
Figure 3.
ENL interacts directly with Dot1l. (A) Example of a 2-hybrid experiment using full-length ENL as bait and full-length Dot1l as an interaction target. For comparison, yeast transformed with empty vectors (−) or with plasmids encoding the interacting proteins SNF1 and SNF4 (+) was plated alongside. Growth is shown on control and selective plates. (B) Structure function analysis to determine the ENL-Dot1l association interface. A series of ENL mutants was tested as bait for interaction with full-length Dot1l and vice versa to delineate the respective interaction domains in 2-hybrid experiments. Growth on selective medium is indicated with “+” or “−.” Conserved domains in ENL are labeled “YEATS” (a domain found in several other proteins associated with chromatin modification) and “hydp” (hydrophobic C-terminal domain that has been shown to mediate binding to CBX8). (C) Control for expression of Dot1l derivatives in yeast cells. The correct expression of the Dot1l mutants was checked by immunoblotting with a GAL4-activation domain–specific antibody. The lane designations correspond to the Dot1l mutants shown in panel B. The correct expression of the indicated set of ENL mutants has been published previously.14 (D) GST pull-down experiment. Beads loaded with purified GST or GST fused to full-length ENL were incubated with 35S-labeled Dot1l protein produced by in vitro transcription/translation. After washing, bound proteins were eluted with SDS sample buffer, separated by SDS-PAGE, and visualized by autoradiography. (E) In vivo colocalization of ENL and Dot1l. Top row: vectors encoding fusions of EGFP with Dot1l and RFP with ENL were transfected into HEK293 cells. Fluorescent proteins were detected by microscopy in a nuclear speckled pattern. Photographs in the green and red channels and a software overlay are shown. Middle row: EGFP was fused with a mutant of Dot1l deleting amino acids 937 to 1095 within the ENL-binding domain. The protein was introduced into HEK293 cells (EGFP-Dot1lΔENLbdg), and the expression pattern was compared with wild-type EGFP-Dot1l (EGFP-Dot1l). Correct expression of the respective fusion proteins was controlled by immunoblotting with a GFP-specific antibody. Bottom row: colocalization of EGFP-Dot1l and RFP-ENL in Triton X-100–permeabilized cells to allow counterstaining with the DNA stain DAPI. All microphotographs were taken at room temperature in tissue culture medium with a Canon Coolpix 990 (Krefeld, Germany) electronic camera attached to a Zeiss Axioskop microscope (Oberkochen, Germany) with a Zeiss Neofluar 63×/1.25 NA objective and processed with Corel Draw software (Unterschleißheim, Germany).
EAP has in vitro histone methylase and protein kinase activity
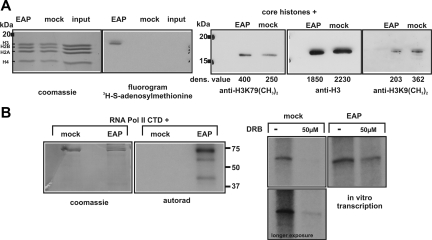
To test if the presence of DOT1L in EAP confers histone methyltransferase activity, purified EAP or a mock preparation obtained by precipitation from 293fENL cells with unliganded agarose beads were incubated with core histones in the presence of 3H-methyl-S-adenosylmethionine. The reaction mixture was separated by SDS-PAGE, stained with Coomassie, and treated with a fluorographic enhancer to allow visualization of 3H-labeled compounds. This assay detected a robust histone H3–specific methyltransferase activity associated with EAP (Figure 4A). In concordance with the known substrate specificity of DOT1L, free recombinant histone H3 was not a substrate for methyl-transfer by EAP (not shown). A specific increase of histone H3 lysine 79 dimethylation could be seen in core histone preparations after incubation with EAP and S-adenosylmethionine, but not in controls. Since DOT1L is the only known methyltransferase able to catalyze H3K79 dimethylation,26 it is highly likely that DOT1L is responsible for the histone-methylating activity in EAP.
Figure 4.
Analysis of EAP histone methyltransferase and CDK kinase activities in vitro. (A) EAP contains a H3K79 dimethylating activity. Left panel: an EAP preparation or a mock control that was done with unliganded agarose as precipitation agent were incubated with core histones and methyl-3H-S-adenosylmethionine. The reaction products and a histone input sample (100% input) were separated on a 18% SDS gel, stained with Coomassie blue, and treated with a fluorographic enhancer solution before exposure to x-ray film (fluorography). Right panel: in an analogous experiment, core histones were incubated with EAP or a mock control as in panel A, and nonradioactive S-adenosylmethionine. After separation of the reaction products by 18% SDS-PAGE, the proteins were blotted and analyzed by immunodetection first with a H3K79 dimethyl–specific antibody and subsequently on the same membrane with a pan-H3–reactive antibody. As a further control, aliquots of the EAP and mock reactions were probed by Western blot for changes of dimethylation at H3K9. The densitometrically obtained concentration values are given underneath the respective lanes. (B) EAP has RNAPolII CTD kinase and pTEFb activity in vitro. Left panel: EAP works as RNAPolII CTD kinase in vitro. Recombinant GST–RNAPolII CTD fusion protein was incubated in the presence of γ32P ATP and either an EAP preparation or a mock control as described. Reactions were separated by SDS-PAGE followed by Coomassie staining and autoradiography. Right panel: EAP supplies pTEFb activity for in vitro transcription. Standard run-off transcription reactions in HeLa nuclear extracts were primed with a template allowing the transcription of the first 244 bp of the Hoxa9 coding sequence under control of the CMV immediate early promoter. The reaction was carried out with α32P-UTP to allow labeling of the transcript. The reaction products were phenolized and separated on a denaturing 6 M urea 6% PAA gel followed by autoradiography. The transcription assays were supplemented either with a mock precipitate as described, or with an EAP preparation. Whereas transcript levels were reduced by addition of the pTEFb inhibitor DRB in unsupplemented samples (left), the presence of EAP rescues a significant amount of transcriptional activity under the same conditions (right).
In an analogous approach, EAP was tested for pTEFb kinase activity in vitro. For this purpose, purified recombinant GST– RNA polymerase II C-terminal domain kinase (RNAPolII CTD) was incubated with EAP or a mock control precipitate in a reaction containing γ32P-ATP. Autoradiography revealed a robust kinase activity of EAP (Figure 4B). The influence of EAP on in vitro transcription was assessed by run-off transcription from a template containing the 5′ portion of the Hoxa9 gene driven by the CMV immediate early enhancer. For detection of transcripts, the reaction was done in the presence of 32P-UTP. As expected, addition of the pTEFb inhibitor DRB caused a severe reduction in transcript levels. However, supplementation of the transcription reactions with EAP partially relieved the inhibitory effect of DRB, suggesting that EAP supplies additional pTEFb activity (Figure 4B).
EAP is required for efficient global and gene-specific H3K79 dimethylation and for efficient transcription
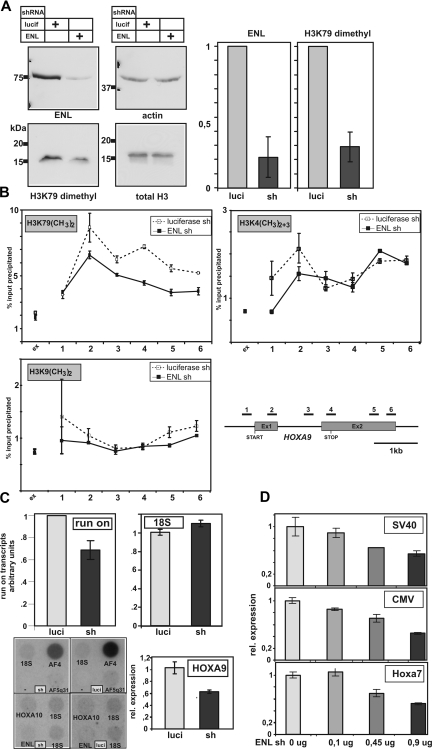
The impact of EAP inhibition on global H3K79 methylation was examined by knock-down of ENL. For this purpose, HEK293 cells were transfected with constructs encoding shRNAs directed against ENL or luciferase as control. shRNAs are processed by cellular enzymes into inhibitory RNAs that trigger the destruction of their complementary mRNAs. The efficiency of ENL knock-down was controlled at the protein level by immunoblotting and densitometric evaluation. Whereas stable knock-down clones with reduced ENL levels could not be obtained (data not shown), transient shRNA transfection reduced endogenous ENL concentrations to approximately 20% of controls after 7 days and did not cause increased cell death compared with a luciferase knock-down control (Figure 5A; data not shown). Correspondingly, global H3K79 dimethylation also decreased to approximately 30% compared with the value obtained in control shRNA–treated cells (Figure 5A). Since it has been shown by independent laboratories that DOT1L is directly responsible for H3K79 dimethylation in HEK293 cells,26,27 it is highly likely that perturbation of EAP and therefore DOT1L function was the cause for the reduced modification levels. The transient knock-down of ENL did not significantly affect expression of pTEFb components in the cells (data not shown).
Figure 5.
EAP function in vivo. (A) ENL is necessary for global H3K79 dimethylation. Endogenous ENL in HEK293 cells was knocked down by transfection with a vector encoding an ENL-specific shRNA. As control, a similar vector with a luciferase-specific shRNA insert was used. Extracts from cells transfected as indicated were subjected to immunoblotting to detect dimethyl-H3K79, ENL, total histone H3, and actin. A densitometric evaluation of 3 different experiments giving average values and standard deviations is shown in the bar graphs. (B) ENL is necessary for gene-specific H3K79 dimethylation. The distribution of dimethylated H3K79 across the HOXA9 coding region was detected by ChIP in control HEK293 cells (□; - - -) transfected with a luciferase-specific shRNA vector or in ENL knock-down cells (■; ▁) after treatment with anti ENL shRNA. As control, H3K4 di/trimethylation and H3K9 dimethylation was determined in analogous experiments. The localization of the primer pairs used for amplification of HOXA9 sequences is shown. An external primer pair amplifying untranscribed genomic sequence was used as control. Input and precipitate was quantified by quantitative PCR in triplicates. The percentage of specifically recovered material compared with input is plotted on the y-axis as average and standard deviation. (C) ENL knock-down affects global run-on elongation and endogenous as well as transient gene expression. Top panels: global elongation rates were measured by nuclear run-on experiments in isolated nuclei. Radioactivity incorporated into nascent RNA during the elongation process was measured in control cells (luciferase specific shRNA) and after ENL knock-down by ENL-specific shRNA as indicated. As control, the amount of stable 18S rRNA was determined in the same samples by quantitative real-time RT-PCR. Average and standard deviations of triplicate experiments are given. Bottom panels: Left panel shows labeled run-on RNA samples from ENL knock-down (sh) and control (luci) cells hybridized to cDNAs of the indicated genes. For normalization, 18S cDNA was included. Right panel shows total HOXA9-specific mRNA in ENL knock-down and control cells determined by qRT-PCR. (D) ENL knock-down affects transient transcription. Luciferase reporter experiments testing the response of 3 different promoters after transfection of increasing amounts of ENL specific shRNA vectors. Averages and standard deviations of triplicate experiments are plotted.
The distribution of dimethyl-H3K79 was determined in ENL knock-down cells across the HOXA9 coding region by ChIP. HOXA9 is expressed in HEK293 cells and it is known that HOXA genes are particularly sensitive to a loss of H2B ubiquitinylation and therefore also H3K79 methylation because the former is a prerequisite for the latter to occur.28 Gene-specific H3K79 dimethylation was moderately but significantly reduced after shRNA-mediated knock-down of ENL (Figure 5B). In contrast, H3K9 dimethylation did not change significantly across the HOXA9 coding region after ENL knock-down. Also, H3K4 di- and trimethylation was largely unaffected except for a localized reduction around the start of the coding sequence. Overexpression of ENL did not increase global or local H3K79 methylation, indicating that ENL is not limiting under normal cellular conditions (data not shown).
Because DOT1L and pTEFb are enzymatic activities associated with elongation, we wanted to determine if a loss of EAP function affects transcription at this level. For that purpose, transcriptional elongation was quantified using a nuclear run-on assay, which measures incorporation of radioactive nucleotides into nascent mRNAs produced in isolated nuclei. Under these conditions, new initiation generally does not occur.21 Therefore, radioactive labeling is proportional to elongation. Nuclei were prepared from HEK293 cells depleted for ENL by shRNA and from control cells transfected with luciferase shRNA. Identical numbers of nuclei were incubated with 32P-αUTP. RNA was purified by spin columns and spotted on membranes, and radioactivity was quantified by phosphoimaging. As a normalization control, the concentration of stable 18S RNA was quantified by real-time RT-PCR. An approximately 30% reduction in global elongation rates was detectable in ENL knock-down cells compared with controls. To determine if the consequences of ENL knock-down are gene specific, labeled run-on RNA was hybridized to select cDNAs. As expected, the effect on elongation varied widely depending on the gene examined. Whereas run-on rates were reduced approximately 40% for AF4, 20% for AF5q31, and 10% for ENL, HOXA10 elongation was not sensitive to reduced ENL levels. Elongation rates for HOXA9 were too low to be detected by a run-on/hybridization assay; however, a knockdown of ENL caused a significant reduction of total HOXA9 mRNA abundance as determined by q-RTPCR (Figure 5C). A dose dependent inhibitory effect of ENL-specific shRNA was also observed in transient luciferase reporter experiments testing 3 different promoters of viral (simian virus 40 [SV40], CMV) and cellular origin (Hoxa7; Figure 5D). In summary, these results underscore an important role for EAP in general transcription in vivo also.
Transformation by MLL-ENL correlates with DOT1L binding
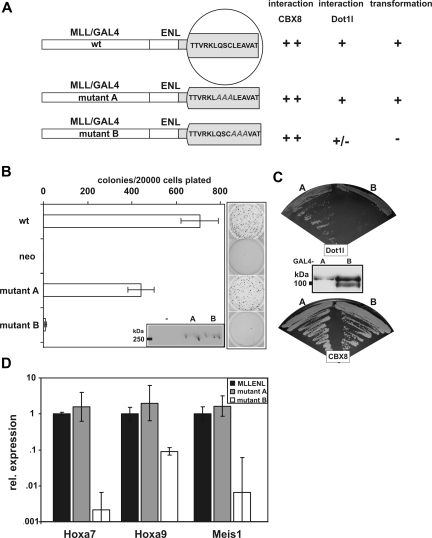
The 15 C-terminal amino acids of ENL are essential for binding of ENL to DOT1L. The same region also is responsible for a direct interaction between ENL and CBX8. In addition, this peptide has been shown to be absolutely required for the transforming ability of the corresponding MLL-ENL fusion protein. The importance of the ENL-DOT1L interaction for the transforming capacity of MLL-ENL was examined by an alanine-scanning mutagenesis. A total of 2 different amino acid triplets within the ENL C-terminus were replaced by alanine. The mutant ENL sequences were introduced into MLL-ENL and into a 2-hybrid bait construct (Figure 6A). Correct expression was demonstrated by immunoblotting of the respective GAL4-DNABD and MLL fusion proteins. Importantly, the transforming capability of the MLL-ENL mutants was highly correlated with the binding affinity for DOT1L. Only ENL mutant A, which bound efficiently to DOT1L, was transforming in the context of MLL-ENL. In contrast, mutant B had significantly reduced affinity for DOT1L and failed to transform when fused to MLL. No such relationship, however, was detected for the interaction of ENL with CBX8, as this parameter was comparable for both mutants (Figure 6B-C). The transforming potential of the individual MLL fusion proteins was perfectly correlated with their ability to induce the expression of the known MLL-ENL target genes Hoxa7, Hoxa9, and Meis1 in primary hematopoietic cells (Figure 6D).
Figure 6.
The transforming capability of an MLL-ENL fusion protein correlates with Dot1l binding. (A) Schematic depiction of alanine insertion mutations introduced into the ENL C-terminus. (B) Results of a serial replating assay with primary hematopoietic precursor cells isolated from bone marrow and transduced either with wild-type MLL-ENL, MLL-ENL mutant A, or MLL-ENL mutant B. Given are the average colony numbers including standard deviations in the third round of replating. Next to the bar graph, a representative example of stained colonies is shown. The insets show Western blot experiments detecting expression of the corresponding GAL4/MLL fusion proteins. (C) 2-hybrid test for interaction of ENL mutants A and B with full-length Dot1l and CBX8. Yeast was cotransformed with a bait plasmid containing mutants A and B as indicated and a Dot1l interaction target. Growth on adenine/histidine deficient selective medium is shown. (D) qRT-PCR analysis of MLL-ENL target gene expression. RNA was isolated from primary hematopoietic cells 1 week after transduction with retroviral constructs coding for wild-type MLL-ENL, mutant A, or mutant B, respectively. Relative RNA levels of the known MLL-ENL target genes Hoxa7, Hoxa9, and Meis1 were determined by qRT-PCR, normalized with respect to actin expression, and plotted with values for wild-type MLL-ENL transduced cells set arbitrarily to 1. Note that the results are displayed on a logarithmic scale. Average and standard deviation of triplicates are plotted.
Discussion
A still largely unsolved question of MLL fusion biology pertains to the biological activity contributed by the fusion partner. The results presented here help to answer this problem by suggesting a function for 1 of the most common nuclear MLL fusion partners, the ENL protein. ENL associates with a set of proteins that can be purified from cells as macromolecular assembly. The composition of EAP as determined here is backed up by a number of previous results, mainly from 2-hybrid screens that indicated a direct interaction of ENL and/or the homologous AF9 fusion partner with AF5q31 and AF410,11 as well as with CBX8 and BCoR.14,16 The fact that AF10 was not reproducibly found in EAP confirms our earlier results that a direct interaction of ENL with AF10 occurs only if AF10 is truncated.11 The internal ENL binding site probably is not accessible under regular cellular conditions. In a similar direction, AF9 should not be a major constituent of EAP because ENL and AF9 occupy the identical binding site in AF4 and AF5q31, respectively.10,11 Therefore, our purification strategy that selects for ENL-bound AF4/AF5q31 should not simultaneously also bring down AF9. Most probably the EAP proteins are not present in a single protein complex. Structure function studies10,11 have shown that AF5q31, AF4, and possibly also the highly homologous LAF4 interact with the same C-terminal domain of ENL. It is very unlikely that 3 proteins with individual molecular weights of more than 130 kDa could simultaneously occupy the identical binding surface on the much smaller ENL protein. Likewise, CBX8 and, as shown in this report, DOT1L share the same interaction domain in ENL, and therefore binding is expected to be mutually exclusive. Considering the stoichometry of the EAP constituents, we propose that ENL and pTEFb are core components that associate depending on context with a select set of the other EAP proteins. Although it cannot be excluded that gel filtration itself might disrupt a larger protein complex, the presence of more than 1 complex that shares ENL as a common subunit would more likely explain the discrepancy of the apparent molecular weight of EAP in gel filtration compared with immunoprecipitation.
The presence of both pTEFb and DOT1L, 2 enzymatic activities necessary for transcriptional elongation, suggests a function for the EAP assembly during this process. pTEFb is a dimer of the cyclin-dependent kinase 9 that, assisted by heat-shock proteins, associates with either cyclin T1 or cyclin T2 to form an active kinase that phosphorylates serine 2 in the heptad repeat of RNA polymerase II.24,29 This modification is necessary for productive elongation during the transcription of many genes. The connection of pTEFb with MLL partner proteins is also supported by a report that identified AF5q31 in affinity-purified pTEFb preparations.30 DOT1L is a non-SET methyltransferase with specificity for histone H3 lysine79 and is the only known enzyme with this histone specificity.26 Previously, we demonstrated that ENL interacts with histone H3 via N-terminal sequences.11 Here, we show ENL also interacts directly with DOT1L. ENL is therefore a prime candidate for serving as an adaptor that tethers DOT1L to its substrate. During the preparation of this manuscript, a report became available that lends further support to a role for EAP in elongation control. Although done exclusively with transiently overexpressed proteins, Bitoun et al31 demonstrated that AF4 associates with AF9, ENL, and pTEFb to stimulate transcriptional elongation through a recruitment of DOT1L and stimulation of H3K79 dimethylation. The same group showed previously that the “robotic” mouse harbors a mutant of AF4 that impairs proteasomal degradation of this protein.32 Robotic mice therefore overexpress AF4, and this overexpression is also accompanied by higher levels of H3K79 dimethylation in the cerebellum, the organ responsible for the “robotic” phenotype. In these studies, CDK9 was expressed in combination with CYCT1, which most likely does not match the endogenous EAP composition, because we could not detect any CYCT1 in our preparations. To our knowledge, EAP is the first instance where a combination of CDK9 with CYCT2a/CYCT2b forms the active unit. EAP components are ubiquitously expressed, and a central role for ENL in cellular biochemistry is supported by genetic ablation experiments in which ENL-deficient embryos die extremely early in utero, even before implantation.33 In summary, all available evidence points clearly to a function of EAP as a major control instance for transcriptional elongation, and therefore EAP seems to be essential for efficient gene expression in all cells.
Surprisingly, EAP also contained minor quantities of 3 proteins (CBX8, RING1, and BCoR) involved in polycomb-mediated repression. The chromoboxprotein CBX8 (also known as human polycomb 3 or HPC3) and RING1 are common components of the polycomb repressive complex 1.34 BCoR has been previously detected in a corepressor complex recruited by the BCL-6 protein.35 Both CBX8 and BCoR have been identified in interaction screens as direct binding partners of ENL or AF9, arguing against an artefactual copurification.14–16 It is therefore tempting to speculate that EAP might also exist in a repressive mode. Indeed, there are reports that a complex of the ENL homolog AF9 and DOT1L is involved in aldosterone-controlled repression of a sodium channel gene in renal cells.36
The identification of EAP also has implications for our understanding of the transforming mechanism of the MLL-ENL fusion protein. Within the context of the leukemogenic MLL-ENL, the biological activities of EAP would be constitutively mistargeted via the fusion partner. As a consequence, elongation and therefore gene expression would be inappropriately stimulated. Indeed, a dramatic increase of H3K79 dimethylation is a hallmark of target genes activated by MLL-ENL.13 This correlates well with our results that mutations in ENL that impair the interaction with DOT1L also inactivate the transforming potential of the corresponding MLL-ENL fusion. Interestingly, the unrelated fusion MLL-AF10 also works through recruitment of DOT1L.12 It remains to be seen if chromatin modification and transcriptional elongation are important also for other MLL fusion proteins. If this is the case, drugs that potentially interfere with these mechanisms might be attractive therapeutic agents.
Acknowledgments
We thank Renate Zimmermann and Gaby Sander for technical support and B.C. Kone for sharing reagents.
This work was supported by DFG grants SL27/6–2 and SFB473/D2 (R.K.S.) and National Institutes of Health grants CA78815 and CA92251 (J.L.H.). M.-P.G.-C. acknowledges funding in part by Dr von Haunersches Kinderspital, Munich. Equipment funding from Jose-Carreras-Stiftung and Curt-Bohnewand-Fond, and travel support by BaCaTec is gratefully acknowledged.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.K.S. designed research; D.M., C.B., D.Z., M.-P.G.-C., S.M., A.S., R.Z., A.N., and A.C. performed research and collected data; A.C., J.L.H., and R.K.S. analyzed and interpreted data; and J.L.H. and R.K.S. drafted the manuscript.
D.M. and C.B. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert K. Slany, Genetics, University Erlangen, Staudtstrasse 5, 91058 Erlangen, Germany; e-mail: rslany@biologie.uni-erlangen.de.
References
- 1.Gilliland DG, Jordan CT, Felix CA. The molecular basis of leukemia. Hematology Am Soc Hematol Educ Program. 2004:80–97. doi: 10.1182/asheducation-2004.1.80. [DOI] [PubMed] [Google Scholar]
- 2.Hess JL. Mechanisms of transformation by MLL. Crit Rev Eukaryot Gene Expr. 2004;14:235–254. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.10. [DOI] [PubMed] [Google Scholar]
- 3.Slany RK. When epigenetics kills: MLL fusion proteins in leukemia [review]. Hematol Oncol. 2005;23:1–9. doi: 10.1002/hon.739. [DOI] [PubMed] [Google Scholar]
- 4.Schreiner SA, Garcia-Cuellar MP, Fey GH, Slany RK. The leukemogenic fusion of MLL with ENL creates a novel transcriptional transactivator. Leukemia. 1999;13:1525–1533. doi: 10.1038/sj.leu.2401534. [DOI] [PubMed] [Google Scholar]
- 5.So CW, Cleary ML. Common mechanism for oncogenic activation of MLL by forkhead family proteins. Blood. 2003;101:633–639. doi: 10.1182/blood-2002-06-1785. [DOI] [PubMed] [Google Scholar]
- 6.Zeisig BB, Milne T, Garcia-Cuellar MP, et al. Hoxa9 and Meis1 are key targets for MLL-ENL-mediated cellular immortalization. Mol Cell Biol. 2004;24:617–628. doi: 10.1128/MCB.24.2.617-628.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisig BB, Schreiner S, Garcia-Cuellar MP, Slany RK. Transcriptional activation is a key function encoded by MLL fusion partners. Leukemia. 2003;17:359–365. doi: 10.1038/sj.leu.2402804. [DOI] [PubMed] [Google Scholar]
- 8.Martin ME, Milne TA, Bloyer S, et al. Dimerization of MLL fusion proteins immortalizes hematopoietic cells. Cancer Cell. 2003;4:197–207. doi: 10.1016/s1535-6108(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 9.So CW, Lin M, Ayton PM, Chen EH, Cleary ML. Dimerization contributes to oncogenic activation of MLL chimeras in acute leukemias. Cancer Cell. 2003;4:99–110. doi: 10.1016/s1535-6108(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 10.Erfurth F, Hemenway CS, de Erkenez AC, Domer PH. MLL fusion partners AF4 and AF9 interact at subnuclear foci. Leukemia. 2004;18:92–102. doi: 10.1038/sj.leu.2403200. [DOI] [PubMed] [Google Scholar]
- 11.Zeisig DT, Bittner CB, Zeisig BB, Garcia-Cuellar MP, Hess JL, Slany RK. The eleven-nineteen-leukemia protein ENL connects nuclear MLL fusion partners with chromatin. Oncogene. 2005;24:5525–5532. doi: 10.1038/sj.onc.1208699. [DOI] [PubMed] [Google Scholar]
- 12.Okada Y, Feng Q, Lin Y, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Milne TA, Martin ME, Brock HW, Slany RK, Hess JL. Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Res. 2005;65:11367–11374. doi: 10.1158/0008-5472.CAN-05-1041. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Cuellar MP, Zilles O, Schreiner SA, Birke M, Winkler TH, Slany RK. The ENL moiety of the childhood leukemia-associated MLL-ENL oncoprotein recruits human Polycomb 3. Oncogene. 2001;20:411–419. doi: 10.1038/sj.onc.1204108. [DOI] [PubMed] [Google Scholar]
- 15.Hemenway CS, de Erkenez AC, Gould GC. The polycomb protein MPc3 interacts with AF9, an MLL fusion partner in t(9;11)(p22;q23) acute leukemias. Oncogene. 2001;20:3798–3805. doi: 10.1038/sj.onc.1204478. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan RS, de Erkenez AC, Hemenway CS. The mixed lineage leukemia fusion partner AF9 binds specific isoforms of the BCL-6 corepressor. Oncogene. 2003;22:3395–3406. doi: 10.1038/sj.onc.1206361. [DOI] [PubMed] [Google Scholar]
- 17.GenBank. National Center for Bioinformatic Information. [Accessed July 11, 2007]; http://www.ncbi.nlm.nih.gov/Genbank/index.html.
- 18.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 20.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 21.Carey MS, Smale ST. Transcriptional regulation in eukaryotes. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 22.Lavau C, Szilvassy SJ, Slany R, Cleary ML. Immortalization and leukemic transformation of a myelomonocytic precursor by retrovirally transduced HRX-ENL. EMBO J. 1997;16:4226–4237. doi: 10.1093/emboj/16.14.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Luca A, De Falco M, Baldi A, Paggi MG. Cyclin T: three forms for different roles in physiological and pathological functions. J Cell Physiol. 2003;194:101–107. doi: 10.1002/jcp.10196. [DOI] [PubMed] [Google Scholar]
- 24.O'Keeffe B, Fong Y, Chen D, Zhou S, Zhou Q. Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J Biol Chem. 2000;275:279–287. doi: 10.1074/jbc.275.1.279. [DOI] [PubMed] [Google Scholar]
- 25.Slany RK, Lavau C, Cleary ML. The oncogenic capacity of HRX-ENL requires the transcriptional transactivation activity of ENL and the DNA binding motifs of HRX. Mol Cell Biol. 1998;18:122–129. doi: 10.1128/mcb.18.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng Q, Wang H, Ng HH, et al. Methylation of H3-lysine 79 is mediated by a new family of HMTases without a SET domain. Curr Biol. 2002;12:1052–1058. doi: 10.1016/s0960-9822(02)00901-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Hayashizaki Y, Kone BC. Structure and regulation of the mDot1 gene, a mouse histone H3 methyltransferase. Biochem J. 2004;377:641–651. doi: 10.1042/BJ20030839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu B, Zheng Y, Pham AD, et al. Monoubiquitination of human histone H2B: the factors involved and their roles in HOX gene regulation. Mol Cell. 2005;20:601–611. doi: 10.1016/j.molcel.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Estable MC, Naghavi MH, Kato H, et al. MCEF, the newest member of the AF4 family of transcription factors involved in leukemia, is a positive transcription elongation factor-b-associated protein. J Biomed Sci. 2002;9:234–245. doi: 10.1007/BF02256070. [DOI] [PubMed] [Google Scholar]
- 31.Bitoun E, Oliver PL, Davies KE. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum Mol Genet. 2007;16:92–106. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- 32.Bitoun E, Davies KE. The robotic mouse: unravelling the function of AF4 in the cerebellum. Cerebellum. 2005;4:250–260. doi: 10.1080/14734220500325897. [DOI] [PubMed] [Google Scholar]
- 33.Doty RT, Vanasse GJ, Disteche CM, Willerford DM. The leukemia-associated gene Mllt1/ENL: characterization of a murine homolog and demonstration of an essential role in embryonic development. Blood Cells Mol Dis. 2002;28:407–417. doi: 10.1006/bcmd.2002.0525. [DOI] [PubMed] [Google Scholar]
- 34.Bardos JI, Saurin AJ, Tissot C, Duprez E, Freemont PS. HPC3 is a new human polycomb orthologue that interacts and associates with RING1 and Bmi1 and has transcriptional repression properties. J Biol Chem. 2000;275:28785–28792. doi: 10.1074/jbc.M001835200. [DOI] [PubMed] [Google Scholar]
- 35.Huynh KD, Fischle W, Verdin E, Bardwell VJ. BCoR, a novel corepressor involved in BCL-6 repression. Genes Dev. 2000;14:1810–1823. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem. 2006;281:18059–18068. doi: 10.1074/jbc.M601903200. [DOI] [PMC free article] [PubMed] [Google Scholar]