Abstract
Advances in immune assessment, including the development of T-cell receptor excision circle (TREC) assays of thymopoiesis, cytokine-flow cytometry assays of T-cell function, and higher-order phenotyping of T-cell maturation subsets have improved our understanding of T-cell homeostasis. Limited data exist using these methods to characterize immune recovery in adult cord blood (CB) transplant recipients, in whom infection is a leading cause of mortality. We now report the results of a single-center prospective study of T-cell immune recovery after cord blood transplantation (CBT) in a predominantly adult population. Our primary findings include the following: (1) Prolonged T lymphopenia and compensatory expansion of B and natural killer (NK) cells was evident; (2) CB transplant recipients had impaired functional recovery, although we did observe posttransplantation de novo T-cell responses to cytomegalovirus (CMV) in a subset of patients; (3) Thymopoietic failure characterized post-CBT immune reconstitution, in marked contrast to results in other transplant recipients; and (4) Thymopoietic failure was associated with late memory T-cell skewing. Our data suggest that efforts to improve outcomes in adult CB transplant recipients should be aimed at optimizing T-cell immune recovery. Strategies that improve the engraftment of lymphoid precursors, protect the thymus during pretransplant conditioning, and/or augment the recovery of thymopoiesis may improve outcomes after CBT.
Introduction
Umbilical cord blood (CB), first demonstrated to have clinical utility by Gluckman et al as a source of hematopoietic stem cells in the setting of Fanconi anemia,1 was later demonstrated to have utility as a source of unrelated donor stem cells for patients lacking matched-sibling donors.2–5 Over the past decade, a large number of studies have demonstrated the clinical utility of CB transplantation (CBT) as a treatment for both malignant and nonmalignant diseases of children and adults.4,6 The establishment of international cord blood banks, advances in supportive care and donor graft selection, and novel clinical approaches aimed at improving engraftment (eg, ex vivo expansion of CB-derived progenitors7,8 and the infusion of pooled unrelated units9) have improved outcomes and led to a dramatic increase in the number of CBTs performed worldwide.
CB grafts obtained from matched unrelated donors offer advantages over bone marrow or peripheral blood stem cells (PBSC) such as noninvasive procurement, more rapid availability without the need for the more prolonged process of screening and obtaining stem cells from a matched unrelated donor (MUD), and the apparently greater tolerance for incompletely human leukocyte antigen (HLA)–matched products.10 These advantages are paramount for recipients in historically underrepresented minority groups, for whom the prospect of locating a MUD registry donor remains relatively diminished. At our institution, more than twice the proportion of CB transplant recipients are minorities relative to MUD marrow or PBSC recipients historically undergoing transplantations. This fact underscores the importance of improving our current approaches to alternative donor transplantation for patients lacking matched-sibling or MUD donors. For these reasons, it remains important for us to clearly define the biologic variables that govern posttransplantation outcomes in these patients.
Of all the clinical challenges that face CBT clinicians, delayed immune reconstitution remains one of the most important causes of morbidity and mortality11–15 (also reviewed in Szabolcs and Niedzwiecki16). Although it is increasingly appreciated that a variety of circulating peripheral blood cell subpopulations may contribute to immune integrity, including B cells, natural killer (NK) cells, peripheral blood monocytes, and dendritic cells, it is also generally accepted that adaptive immune responses mediated by T cells are essential for protective immunity. As is perhaps best illustrated by the HIV/AIDS pandemic, the selective loss of CD4+ T cells is sufficient to trigger profound immunodeficiency that often leads to fatal infection.17 The primary consequence of the loss of CD4+ T-cell help is an attendant loss in the number and/or function of antigen-specific CD8+ T cells, which constitute our primary adaptive response to pathogens, including viruses and fungi.18 A nearly universal characteristic of conditioning regimens used to prepare recipients of unrelated donor grafts is the use of chemotherapeutic agents and/or antibodies that effectively deplete the host of mature T cells. In the setting of CBT, multiple chemotherapy drugs are typically combined with polyclonal antithymocyte globulin to decrease the likelihood of donor graft rejection mediated by surviving host T cells. In this setting, T-cell reconstitution after CBT inherently depends on the survival of adoptively transferred T cells from the CB graft or, alternately, the de novo production of T cells in the recipient thymus.19–22 Although extrathymic production of T cells has been postulated, no conclusive evidence exists that suggests extrathymic maturation contributes significantly to de novo T-cell production in human stem cell transplant (SCT) recipients. Furthermore, CB grafts differ from T-cell–replete PBSC grafts in that they contain fewer T cells that are also uniformly naive (eg, antigen-inexperienced).
Here we report the results of a prospective study of T-cell immune reconstitution in recipients of unrelated CB grafts. We conducted a quantitative analysis of T-cell subsets using immunophenotyping and also performed detailed analyses of superantigen-stimulated and virus-specific T cells using cytokine flow cytometry. We also analyzed the recovery of thymopoiesis using a polymerase chain reaction (PCR)–based assessment of T-cell receptor excision circles (TRECs) in CB transplant recipients. Our results suggest that in our studied population, inadequate thymic regeneration after CBT was associated with lymphopenia, delayed functional immune recovery, and skewing of the T-cell compartment away from naive and early memory T cells.
Patients and methods
Patient selection and GVHD management
A total of 32 patients undergoing CBT at the M. D. Anderson Cancer Center were studied. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki for a correlative laboratory study of immune reconstitution that was approved by the M. D. Anderson Cancer Center Institutional Review Board. All patients received graft-versus-host disease (GVHD) prophylaxis consisting of tacrolimus and low-dose methotrexate (5 mg/m2 on post-CBT days 1, 3, and 6), in addition to thymoglobulin administered during conditioning). In the event of confirmed or suspected GVHD, initial therapy consisted of methylprednisolone (2 mg/kg per day, with a taper based on clinical response). In the absence of GVHD, tacrolimus was tapered after post-CBT day 100.
Immunophenotyping
One million PBMC per sample were stained with monoclonal antibodies against CD4, CD8, CD45RA, and CCR7. Cells were stained in a total of 50 μL staining buffer (PBS + 0.1% BSA + 0.02% sodium azide) and antibodies. Staining was carried out at room temperature for 15 minutes. Cells were then washed with 2 to 3 mL PBS, centrifuged, and resuspended in 200 μL of 1% paraformaldehyde. Cells were acquired on a Cyan flow cytometer (Dako, Fort Collins, CO), and the resulting data were analyzed with FlowJo software (Tree Star, Ashland, OR).
Assessment of functional T-cell recovery using CFC
One million PBMC per sample were coincubated with staphylococcal enterotoxin B (SEB; Sigma, St Louis, MO) and, in cytomegalovirus (CMV)–seropositive patients, a pool of pentadecapeptides spanning CMV pp65 (BD Pharmingen, San Jose, CA) in 96-well V-bottom tissue culture plates in 200 μL of media (RPMI1640; GIBCO Life Technologies, Grand Island, NY) supplemented with 10% fetal calf serum, penicillin, and streptomycin (Sigma). After 1 hour of incubation at 37°C, 5% CO2, brefeldin A (Sigma) was added at a final concentration of 10μg/mL to enable accumulation of effector cytokines in the cytoplasm. The cells were then incubated an additional 5 hours at 37°C, 5% CO2, after which time the cells were moved to 4°C overnight. The cells then underwent lysis and permeabilization with FACSLyse and FACSPerm Solution II, respectively (BD Biosciences), the following day. Cytokine flow cytometry (CFC) analyses were performed using fluorescein isothiocyanate-, phycoerythrin-, PerCP-, and allophycocyanin-conjugated monoclonal antibodies (MAb) specific for human CD4, CD8, CD69, and interferon (IFN)γ (BD Biosciences). After staining at 4°C for 20 to 25 minutes, cells were washed, resuspended in PBS with 1% paraformaldehyde, and acquired by 4-color flow cytometry on a FACSCalibur cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star). For most analyses, at least 30 000 total events were analyzed. Nonspecific activation of cells was tested by incubation of paired samples that were unstimulated.
Quantitative real-time PCR for TREC
Cell preparation.
One million snap-frozen PBMC were lysed overnight (up to 18 hours) at 56°C in lysis buffer (LB) (0.5% Nonidet P-40, 0.1% Tween-20, and 200 μg/mL of molecular grade [nuclease-free] Proteinase K in a standard 1× PCR Buffer containing no magnesium [Invitrogen, Carlsbad, CA]). The samples were then heat inactivated at 95°C for 10 minutes. DNA was purified from cell lysates by adding a 1× volume of phenol-chloroform-isoamyl alcohol (25:24:1; Sigma) for extraction using Phase-Lock light gel separator tubes (Eppendorf, Westbury, NY). The aqueous phase was then transferred to a fresh microfuge tube, and ethanol was precipitated using a 0.2× volume of 7.5 M ammonium acetate and a 2× volume of cold 100% ethanol, placement at −20°C for 1 hour, and then spinning at 16 000g for 15 minutes. The supernatant was aspirated and the DNA pellets were dried down and reconstituted in 35 μL of Tris-EDTA (TE) buffer and stored at −20°C until use in the real-time PCR TREC assay.
Real-time PCR.
Thymic function was assessed using the method of Harris et al.23 Delta-deletion TRECs were amplified and quantified in a Biorad iCycler iQ Real-Time Detection System (BioRad Laboratories, Hercules, CA) using fluorescently labeled oligonucleotides as reporter probes in a 50 μL PCR reaction using 2X iQ Supermix (with additional MgCl2 to a final 3.5 mM concentration) (Biorad). Primers for the TREC sequence were 5′-CCC TTT CAA CCA TGC TGA CAC-3′ (forward) and 5′-GGG TGC AGG TGC CTA TGC-3′ (reverse), which produced an approximately 80-base pair product detected with the probe 5′-FAM-TCT GGT TTT TGT AAA GGT GCC CAC TCC TG-BHQ-1-3′. TREC abundance was normalized to input cell number by a parallel amplification for the β-globin gene. Human β-globin primers were 5′-GAA GAG CCA AGG ACA GGT ACG-3′ (forward) and 5′-CCT GGG AGT AGA TTG GCC AA-3′ (reverse), which produced an 85-base pair product detected with the probe 5′-FAM-CTG TCA TCA CTT AGA CCT CAC CCT GTG-BHQ1-3′. Primers (all from Sigma-Genosys, St Louis, MO) were used at 10 pmol per reaction well, and probes (both from Biosearch Technologies, Novato, CA) were used at 5 pmol per reaction well. Typically, 45 μL of the master mix (appropriate containing either TREC or globin primers and probe) was first added to the 96-well optical grade plate, and then 5 μL per well of standards, controls, and samples were pipetted into the plate and optical-grade tape was applied. All standards were run in duplicate, and all samples were run in triplicate.
Statistical analyses
All statistical analyses were performed using Prism software (GraphPad Software, San Diego, CA). Intergroup comparisons were assessed using the Mann-Whitney U test, with significance defined by P less than .05 using a 2-tailed test. Differences in actuarial survival were determined by the method of Kaplan and Meier.24
Results
Clinical characteristics
A total of 32 CB transplant recipients who underwent transplantation during the study period were assessed, with a median follow-up of 221 days (Table 1).
Table 1.
Clinical characteristics of study subjects
| Characteristic | Data |
|---|---|
| Age, y* | 33.5 (7-57) |
| Follow-up, d* | 221 (31-905) |
| Survival, d* | 224 (31-1309) |
| Sex, n (%) | |
| Female | 9 (28.1) |
| Male | 23 (71.9) |
| Diagnosis, n (%) | |
| ALL | 10 (31.2) |
| AML/MDS | 6 (18.8) |
| CLL | 2 (6.2) |
| CML/MPD | 2 (6.2) |
| Hodgkin's Disease | 8 (25) |
| NHL | 4 (12.5) |
| Prior treatment characteristics, n | |
| No. of prior regimens for AML subjects, n* | 3 (1-8) |
| Hodgkin Disease/NHL patients with prior autologous SC transplantation, n (%) | 12 (100) |
| Preparative regimen, n (%) | |
| Cyclophosphamide/fludarabine/TBI (200 Gy)/ thymoglobulin/rituximab | 1 (3.1) |
| Fludarabine/IV busulfan/thymoglobulin | 7 (21.9) |
| Fludarabine/melphalan/thymoglobulin | 1 (3.1) |
| Fludarabine/melphalan/thymoglobulin/rituximab | 2 (6.2) |
| Fludarabine/melphalan/thiotepa/thymoglobulin/rituximab | 3 (9.4) |
| Fludarabine/melphalan/thiotepa/thymoglobulin | 18 (56.2) |
| Pretransplant CMV seropositivity, n (%) | |
| Positive | 22 (69) |
| Negative | 10 (31) |
| Degree of HLA match, n (%) | |
| 3/6 | 1 (3.1) |
| 4/6 | 18 (56.3) |
| 5/6 | 13 (40.6) |
| Total nucleated cells per kg infused (×106)* | 33.5 (11.1-60.7) |
| CD34 infused/kg (×105)* | 1.23 (0.15-49.9) |
| Complete blood count subsets at day 30, ×109/L* | |
| White blood count | 2.25 (0.4-17.8) |
| Absolute neutrophil count | 1.48 (0.19-16.2) |
| Absolute monocyte count | 0.36 (0.13-1.2) |
| Absolute lymphocyte count | 0.21 (0.1-1.15) |
| Complete blood count subsets at day 60, ×109/L* | |
| White blood count | 4.40 (1.3-21.0) |
| Absolute neutrophil count | 4.44 (0.08-14.91) |
| Absolute monocyte count | 0.44 (0.01-2.45) |
| Absolute lymphocyte count | 0.58 (0.1-2.35) |
ALL represents acute lymphocytic leukemia; AML/MDS, acute myeloid leukemia/myelodysplastic syndrome; CLL, chronic lymphocytic leukemia; CML/MPD, chronic myelogenous leukemia/myeloproliferative disease; NHL, non-Hodgkin lymphoma; and TBI, total body irradiation.
Expressed as median (range).
Clinical outcomes
The majority of patients experienced initial engraftment (28/32), whereas 6 subjects (19%) experienced either primary or secondary graft failure. Neutrophil engraftment occurred at a median of 22.5 days. GVHD occurred after CBT in 20/32 subjects (62.5%). Of the 20 subjects experiencing GVHD, 13 subjects (65%) experienced acute GVHD; in all but 1 of these subjects this was greater than grade 2. Twelve of 20 patients with GVHD experienced chronic GVHD (60%); 7 of these subjects experienced de novo chronic GVHD without antecedent acute GVHD. Five of 12 subjects developed chronic GVHD that was classified as extensive, whereas the remainder had limited disease. Relapse occurred in 15/32 subjects (46%) and was a major cause of death in 12 subjects (37%). Infection was a major cause of death in 10 subjects (31%); in 4 of these subjects, fatal infections occurred in the absence of relapse or GVHD whereas another of these factors contributed to death in the remaining subjects (3 deaths each due to relapse/infection and GVHD/infection). In the 10 patients for whom infection was a major cause of death, the primary infections included the following: suspected or documented fungal pneumonia (4 subjects), presumed infectious pneumonitis without a documented pathogen (4 subjects), CMV colitis with gastrointestinal hemorrhage (1 subject), and polymicrobial infections including herpes simplex virus esophagitis, CMV reactivation, and fungal pneumonia (1 subject).
Persistent lymphopenia and compensatory expansion of NK cells and B cells after CBT
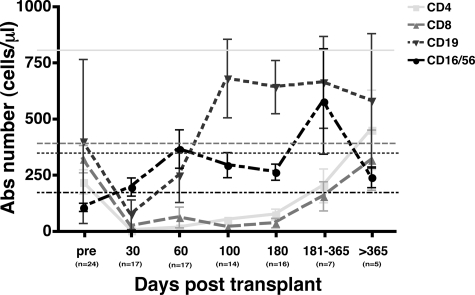
Quantitation of total numbers of CD4+ T cells, CD8+ T cells, CD16/CD56+ NK cells, and CD19+ B cells was prospectively performed on fresh PBMC samples collected after CBT (Figure 1). Compared with previously published normal values for healthy stem cell transplant donors, baseline CD4+ and CD8+ T-cell counts in this group of CB transplant recipients were reduced; furthermore, the typical ratio of CD4/CD8 T cells was inverted. Of note, the absolute number of B cells at baseline was not reduced relative to that expected for healthy subjects, whereas NK-cell numbers were only slightly below expected values. By post-CBT day 30, CD4+ and CD8+ T-cell numbers plummeted and remained extremely low for at least 6 months after CBT. After this interval, increases in absolute CD4+ and CD8+ T-cell counts were observed in surviving subjects; by 1year after CBT, the CD4/CD8 T-cell ratio was clearly positive, and absolute CD8+ T-cell counts approached normal values (as shown for healthy donors previously studied by Storek et al25). In contrast, absolute numbers of NK cells immediately increased over baseline values and remained increased over expected values in control subjects throughout the study interval, though their numbers declined at 1 year in concert with substantial increases in CD4+ and CD8+ T-cell numbers. B cells, like CD4+ and CD8+ T cells, declined after conditioning and were significantly reduced, relative to that seen at baseline and in healthy controls, by post-CBT day 30. Thereafter, absolute B-cell numbers expanded dramatically, though they also declined slightly by the 1-year time point, at which T-cell counts rebounded. Although these results confirm that quantitative T-cell recovery is delayed after CBT, they also suggest that compensatory expansion of non-T lymphoid cells (eg, NK cells and B cells) may accompany T lymphopenia.
Figure 1.
Prolonged T lymphopenia and relative expansion of B cells and NK cells after CBT. T cells (CD3+CD4+ and CD3+CD8+), B cells (CD19+), and NK cells (CD16+ and/or CD56+) were prospectively measured by multiparameter flow cytometry on fresh samples. At baseline and after CBT, CD4+ and CD8+ T cells were relatively reduced, whereas early B-cell and NK-cell recovery was evident. Surviving CB transplant recipients demonstrated rebound of CD4+ and CD8+ T cells at later intervals after transplantation. Horizontal lines depict normal values for healthy stem cell transplant donor previously studied by Storek et al.25 Error bars represent SEM.
Impaired functional T-cell responses after CBT
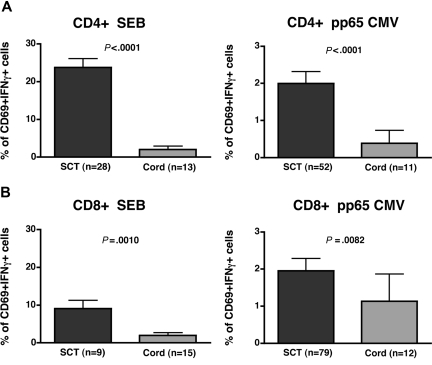
We previously demonstrated that cytokine flow cytometry is a valid measure of T-cell immune reconstitution and that preserved (or reconstituted) responses by CFC are associated with decreased risk of viral reactivation.18,26–28 To examine the extent of functional immune recovery after CBT, we stimulated PBMC from CB transplant recipients with the superantigen SEB and, in the case of recipients at risk for CMV reactivation, with a mixture of pentadecapeptides spanning the dominant CMV capsid antigen pp65. We then assessed the fraction of CD4+ and CD8+ T cells capable of responding functionally, as assessed by intracellular measurement of IFNγ production induced by superantigen or CMV stimulation. Responses in CB transplant recipients were compared with those of other allogeneic SCT recipients assessed at the same time interval (100 days after SC transplantation) using the same assay (Figure 2). Our results demonstrate that the fraction of both CD4+ and CD8+ T cells capable of producing IFNγ after superantigen stimulation was significantly reduced in CB transplant recipients, relative to other SCT patients. Similarly, the proportion of CMV-specific CD4+ T cells that were functionally responsive in the CFC assay was also dramatically reduced relative to other SCT recipients. Although CD8+ CMV-specific T cells were also significantly lower than those of other SCT recipients, the average frequency of CMV-specific CD8+ T cells was greater than 1%, approaching that expected in healthy CMV-seropositive subjects. Overall, a significant proportion of CMV-seropositive recipients assessed by day 100 were found to have circulating CMV-specific CD4+ or CD8+ T cells (4 of 11 with positive CMV-specific CD4+ T-cell responses, 44%; and 6 of 12 with positive CD8+ CMV-specific T cells, 50%) proving definitively that naive cord blood cells, transferred into CBT recipients, may be effectively initiate a primary immune response leading to CMV-specific memory T-cell generation in the early posttransplantation interval.
Figure 2.
Functional CD4+ and CD8+ T-cell recovery is delayed in CB transplant recipients. We used a cytokine flow cytometric assay wherein peripheral blood mononuclear cells are stimulated with a superantigen (SEB) or an overlapping pentadecapeptide mixture derived from the CMV pp65 protein. We compared SEB-stimulated and CMV-specific CD4+ (A) and CD8+ (B) T cells at approximately post-CBT day 100 to those seen in other allogeneic PBSC or marrow SCT recipients previously assessed at the same interval using identical methods. In all cases, CB transplant recipients exhibited reduced functional responses measured by the proportion of CD69+ IFNγ+ T cells after stimulation, relative to allogeneic SC transplantation controls, though differences in CMV-specific T-cell responses were greater in the CD4+ subset (vs the CD8+ subset). These data demonstrate that a significant subset of CB transplant recipients developed positive CMV-specific T-cell responses from adoptively transferred naive cells by day 100 (CD4+: 44% and CD8+: 50%). Results are shown only for those patients surviving to day +100, who were assessable (eg, CMV-seropositive), and who had sufficient events in the relevant gate to draw firm conclusions regarding functional frequencies. Error bars represent SEM.
Thymic regenerative failure after CBT
It has been clearly demonstrated that thymopoiesis persists to a variable extent throughout adulthood and that the recovery of thymopoiesis after autologous and allogeneic SC transplantation is probably an important determinant of naive T-cell recovery and T-cell receptor diversity. In prior cross-sectional studies of a wide range of allogeneic SCT recipients receiving both matched-related PBSC grafts as well as matched-unrelated donor marrow grafts, we found that nearly all recipients had detectable thymic function, albeit to a varying extent, even at early time points (eg, within the first 60 days) after allogeneic SC transplantation (L.S.J., K.V.K., unpublished data, June 2002). Although it is conceivable that graft- or conditioning-related factors might influence the degree of thymopoiesis observed after CBT, we initially expected that the relatively younger age of CB transplant recipients in this prospective study (33 years, Table 1) would be associated with relatively robust recovery of thymopoiesis. We expected that this would offset, at least in part, the relatively reduced number of graft T cells, and the inherent lack of memory T cells, transferred from donors in CB transplant recipients versus allogeneic SCT recipients receiving PBSC grafts.
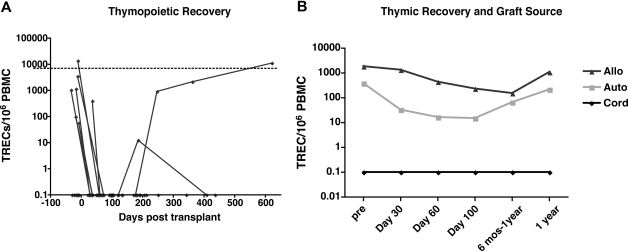
To assess the contribution of thymopoiesis to T-cell immune recovery after CBT, we measured peripheral recent thymic emigrants (RTE) by determining the number of TRECs generated during intrathymic TCR-α rearrangement using a real-time PCR assay, as previously developed in collaboration with Harris et al.29 Longitudinal samples were collected prospectively and were sufficient for analysis in most subjects (n = 26, median age 33) who had samples collected at multiple points after CBT. We also assessed the number of TRECs in 2 groups of longitudinal samples collected prospectively in autologous (n = 54, median age 50) and allogeneic (n = 45, median age 43) SCT recipients who underwent transplantation at the University of Colorado. Individual longitudinal results for all of the CB transplant recipients and median values of TREC levels for all 3 groups are shown (Figure 2A,B, respectively). In addition, we examined a group of healthy donors, matched in age to the CB transplant recipients (n = 18, n = 34) as a reference for the normal values expected in a healthy control population. Our results demonstrate that our heavily pretreated CBT population had markedly impaired thymic function even before transplantation, with only 6 of 21 subjects with samples collected before transplantation having detectable thymopoiesis at baseline. Median baseline TREC values in the 6 subjects with detectable pretransplantation thymopoiesis (1095 TRECs/106 PBMC) were significantly lower than those in healthy donors (7357 TRECs/106 PBMC), suggesting that even those patients had impaired T-cell regenerative potential (Figure 3)
Figure 3.
Thymic regeneration failure after CBT. The frequency of recent thymic emigrants in peripheral blood was quantitated using a real-time PCR assay measuring T-cell receptor (TCR) δ excision circles produced during TCR-α rearrangement. (A) Longitudinal assessment of thymic function in CB transplant recipients (n = 26, median age = 33) demonstrates reduced thymic function in baseline and post-CBT thymopoietic recovery that was measurable in only 2 subjects and significant and sustained in only 1 recipient. The horizontal line depicts the number of TRECs present in a group of healthy control subjects who were age-matched (n = 18, median age = 34). (B) Thymic regenerative failure in CB transplant recipients relative to autologous (auto) and allogeneic (allo) SCT recipients. Median values of TRECs in CBT were dramatically reduced relative to other prospectively analyzed SCT recipients, despite a significantly reduced median age of the CBT population (median age 33 vs 43 for Colorado allogeneic SCT recipients and 50 for Colorado autologous SCT recipients). Values for CB transplant recipients were significantly lower than other groups at all time points (P < .01).
After transplantation, thymopoiesis was undetectable in most CB transplant recipients. Detectable post-CBT thymopoiesis beyond approximately post-SC transplantation day 30 was noted in only 2 subjects; in 1, thymopoiesis recovered and was sustained beyond approximately 8 months after transplantation, and a second subject had only 1 positive post-CBT sample at approximately 6 months posttransplantation. Importantly, average and median TREC values in CB transplant recipients were significantly lower than those seen in autologous and allogeneic SCT recipients at all time points assessed. Notably, the 1 patient with sustained post-CBT thymopoiesis had undetectable TRECs at baseline, demonstrating no correlation between baseline thymic function and eventual thymic recovery. Because of the nearly universal lack of thymic recovery observed, we were unable to examine donor graft (eg, infused stem-cell dose) or host-related factors governing the recovery of thymopoiesis.
Late memory skewing characterizes T-cell recovery after CBT
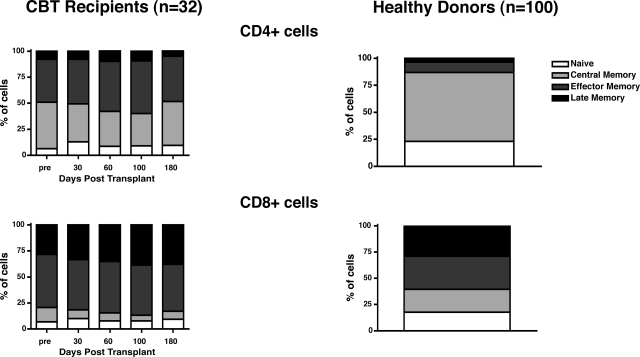
T-cell differentiation may be characterized by the expression of surface markers that demarcate naive, early, and late memory T cells. Over the past several years, multiple models, based on the expression of various surface markers (including the reciprocal isoforms CD45RA and CD45RO, the chemokine receptor CCR7, CD27, CD28, and CD57) have been proposed.30–34 Using the combination of CCR7 and CD45RA, it is possible to define subsets of naive cells (CD45RA+CCR7+), early or central memory cells (CD45RA−CCR7+), effector memory cells (CD45RA−CCR7−), and late effector memory cells (CD45RA+CCR7−).32 At baseline and after transplantation, the CD4+ T-cell compartment in CBtransplant recipients was characterized by a relative paucity of naive cells, with large proportions of central and effector memory T cells. The CD8+ T-cell compartment was similarly characterized by relatively few naive cells, but with fewer central memory T cells than in the CD4+ compartment. The bulk of CD8+ T cells expressed markers consistent with effector memory and late effector memory differentiation. To determine how CB transplant recipients compared with subjects not receiving transplants, we assessed the relative frequencies of T-cell differentiation subsets in samples collected from healthy allogeneic SCT donors. Compared with CB transplant recipients (n = 32), healthy donor subjects had significantly greater proportions of CCR7+ T cells in the CD4+ compartment (wherein CCR7+ T cells constituted the majority of CD4+ T cells in healthy donors) and in the CD8+ compartment (where fewer cells were CCR7+ in healthy subjects, though similarly increased, relative to CB transplant recipients; Figure 4).
Figure 4.
Late memory skewing of CD4+ and CD8+ T cells after CBT. The relative proportions of CD4+ and CD8+ T cells in 4 distinct maturation stages (from least mature to more mature: CD45RA+CCR7+, CD45RA−CCR7+, CD45RA−CCR7−, CD45RA+CCR7−) were assessed using 6-color flow cytometry. Relative proportions of naive and memory T cells in CB transplant recipients (n = 32) were compared with healthy SCT donors (n = 100). CB transplant recipients had, at baseline and after transplantation, relatively reduced proportions of naive and central memory CD4+ and CD8+ T cells and greater proportions of effector and late-effector memory (ie, CCR7−) T cells. Differences relative to healthy donors were statistically significant at all time points (P < .05).
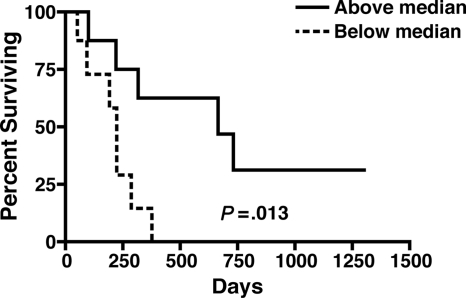
Preservation of CCR7+ CD4+ T cells at day 30 predicts post-CBT survival
In an exploratory analysis, we determined whether the skewing that we observed, toward later memory T-cell subsets, was associated adversely with survival after CBT. Actuarial analysis of survival, stratified by the relative preservation of CCR7+ T cells within the CD4+ compartment, is illustrated in Figure 5. We found that a higher ratio of CCR7+ to CCR7− CD4+ T cells (ie, above the median vs below) at day +30 was associated with significantly longer survival (median survival 240 days vs 700 days, P = .013). When we analyzed the association between CCR7+ T cells within the CD8+ T-cell compartment at day 30 and survival, no similar relationship was seen. We also examined whether the degree of pre-CBT thymic function was associated with survival, given the heterogeneity of baseline thymic function that we observed in this group. In contrast to what has been previously reported in other allogeneic SCT recipients, we found that there was no association between pretransplantation thymic function and survival in this group of CB transplant recipients (data not shown).
Figure 5.
Preservation of CCR7+ CD4+ T cells at day 30 is associated with improved survival after CBT. We examined actuarial survival in patients stratified by the median ratio of CCR7+ to CCR7−CD4+ T cells at post-CBT day 30. Patients above the median ratio had significantly improved survival after CBT (median survival 240 days vs 700 days, P = .013).
Discussion
Although CB is increasingly used as a stem cell source for adults lacking other suitably matched grafts, delayed T-cell reconstitution remains an important source of morbidity and mortality. As our therapeutic approaches to CBT are continually evolving, it remains essential for us to identify areas in which targeted efforts may improve outcomes. For this reason, we conducted a comprehensive analysis of T-cell immune recovery in 32 adult CB transplant recipients consecutively receiving transplants. Our primary findings include the following: (1) In most recipients, we found impaired baseline thymopoiesis and a relatively diminished naive T-cell repertoire before CBT; (2) Prolonged T lymphopenia was evident after CBT; in contrast, relative and absolute expansion of B cells and NK cells was observed; (3) Although impaired functional responses to superantigens and CMV characterized the group overall, a subset of patients had demonstrable recovery of functional CMV-specific T cells by day 100, indicating the ability of naive cord blood T cells to initiate early primary immune responses to pathogens; (4) Thymopoietic failure characterized post-CBT immune reconstitution, in absolute terms and when CB transplant recipients were compared with other SCT recipients examined prospectively and/or in cross-section; and (5) The failure of thymopoiesis was associated with a relative paucity of CCR7+ naive and central memory cells after CBT, with an apparent association between improved posttransplantation outcome and the preservation of CD4+ CCR7+ T cells in the immediate post-CBT interval.
The fact that T-cell lymphopenia characterized the post-CBT state was not surprising. In contrast, we also found that relative, and even absolute, increases in circulating B-cell and NK-cell numbers characterized post-CBT recovery. Previously, increased numbers of circulating NK cells were found in renal allograft recipients receiving T-depleting therapies,35 suggesting that T lymphopenia may actually promote NK-cell recovery. Although CD4+ T-cell help is necessary for promoting B-cell maturation and immunoglobulin class switching, our data also suggest that T lymphopenia may actually facilitate B-cell expansion. Antigen-independent homeostatic B-cell proliferation has been postulated and has shown to occur independent of T cells.36 Recently, studies of 2 groups of patients with T lymphopenia (HIV-infected subjects and others with idiopathic CD4+ T lymphopenia) have demonstrated the expansion of immature/transitional B cells.37,38 Taken together, these data suggest that there may be crosstalk between the T-cell, B-cell, and NK-cell compartments in the setting of lymphopenia. The factors that govern this intercompartmental regulation (eg, cytokines including IL-737 or competition for “niches”) deserve further study.
We observed profound delays in functional immune reconstitution in CBT when considered as a group. In contrast to other allogeneic SCT recipients, subsets of CD4+ and CD8+ T cells capable of responding suitably to stimulation with superantigens were markedly reduced. Because the CFC assay measures the proportion of capable responders within a gated T-cell subpopulation, this approach is less vulnerable (eg, relative to conventional lymphoproliferative assays [LPA]) to the effects of lymphopenia,28 which usually results in diminished LPA responses in patients with CD4+ T-cell counts of less than 50 cells/μL.39 Although it is probable that the transfer of fewer T cells in CBT (vs PBSC grafts) plays a major role in this phenomenon, we cannot discount the effects of nonlymphoid cells (eg, myeloid subsets including monocytes and DC) on these impaired functional responses. When we studied the recovery of CMV-specific CD4+ and CD8+ T-cell responses by CFC, we found that although responses were reduced in CBT overall, a subset of patients had significant frequencies of functional CMV-specific CD4+ and CD8+ T cells by approximately 3 months after SC transplantation. These results confirm and extend the results of Cohen et al, who recently demonstrated, using LPA assays measuring herpesvirus-specific T cells, that many CB transplant recipients had at least 1 detectable assay after CBT that demonstrated successful donor-derived, antigen-specific T-cell recovery.40 Because CFC assays are more quantitative than LPA, our results also provide a more complete window into the quantitative recovery of CMV-specific T cells after CBT.
The most striking finding of our study was the observation that the recovery of thymopoiesis was markedly impaired both at baseline and, in all but 1 subject, at all time points after CBT. Previously, we have conducted cross-sectional studies of more than 140 allogeneic SCT recipients receiving transplants of either marrow or PBSC at our own institution; although thymopoeisis was transiently lower after SC transplantation, we found measurable thymopoiesis in nearly every subject, even at early intervals after SC transplantation. Thymopioesis peaked approximately 6 to 8 months after SC transplantation and predated the recovery of naive CD4+ and CD8+ T cells. Here, we report the results of 2 prospective parallel studies of adult autologous and allogeneic SC transplantation cohorts undergoing transplantation at the University of Colorado. In both groups, consistent with our prior cross-sectional studies, we similarly detected thymic function in nearly all subjects. Thus, the nearly universal lack of thymopoiesis in this group of CB transplant recipients stands out as uniquely different, relative to other SC transplantaion subpopulations. Some studies have previously suggested that thymopoiesis can be detected, even at early intervals, in limited numbers of long-term survivors of CBT.11,41 Given that other (nonsurviving) patients were not assessed in these studies, it is impossible to know what would have been observed if all subjects in those studies had been examined prospectively. Indeed, our preliminary data suggest that patients who fail to preserve naive cells early after CBT are more likely to experience mortality; furthermore, our longest survivor to date is the only patient to recover significant and sustained thymopoiesis. Clearly, these data suggest that larger prospective studies of thymopoietic recovery are needed, especially in adult recipients who are known to have a diminished (though heterogeneous) capacity to recovery thymopoiesis after SC transplantation.
Phenotypic studies using markers of T-cell maturation and differentiation (eg, CD45RA/RO, CD27, CCR7, and CD28) have demonstrated that multiple subcompartments of the memory T-cell repertoire exist, with marked differences in their distribution, with naive and early memory subsets retaining their ability (via CCR7 expression) to home to lymph nodes where primary immune responses occur.30,32 Pantaleo and colleagues have examined the relative subsets of T cells specific for various viral pathogens capable of secreting IFNγ, IL-2, or both cytokines.42 Their results suggest that the subset of T cells that coproduce both IL-2 and IFNγ are a hallmark of protective immune responses to chronic viral pathogens, although cells producing IFNγ alone are often present in the setting of chronic progressive infection (eg, as seen in HIV-1 infection). In recent preclinical studies, we have demonstrated that the cytokine phenotype of late memory cells is distinct from that seen in earlier memory cells after a variety of stimuli, including phorbol esters (eg, phorbol 12-myristate 13-acetate), antibodies stimulating the CD3/TCR complex (eg, OKT3), and superantigens (eg, SEB). Relative to early- or central memory T cells, late effector memory cells produced are skewed toward MIP-1β and IFNγ production, with few cells producing IL-2. In a murine model of adoptive cellular therapy followed by immunization against melanoma-associated antigens, Restifo and colleagues demonstrated that the transfer of late-effector CD8+ T cells (characterized by the production of abundant IFNγ but little IL-2) was associated with progressive tumor growth; in contrast, adoptive transfer of cells with the same specificity but of a central memory differentiation stage resulted in tumor clearance.43
One important caveat is that the patients studied here consisted of a relatively heavily pretreated group of patients. This fact is reflected by the fact that half of the acute myeloid leukemia (AML) recipients received transplants with active disease and overall had received an average of 3 prior treatment regimens and is also demonstrated by the fact that thymopoiesis at baseline, which has previously been demonstrated to correlate with post-CBT immune recovery in 1 pediatric study, was absent in most subjects and markedly reduced (relative to age-matched healthy controls) even in the minority of patients with detectable thymopoiesis before transplantation. Thus, we cannot necessarily generalize these results to other groups of CB transplant recipients who are less heavily pretreated. It should also be noted that all of our recipients received regimens containing antithymocyte globulin (ATG). Although the biologic half-life of ATG is prolonged and is likely to have influenced both central (ie, thymic) and peripheral T cells infused within CB grafts, this intervention is a standard component of most CBT conditioning approaches and appears to be necessary to decrease the otherwise significant risk of graft rejection. It is also probable that stem cell dose, which is reduced in adults relative to children, may also be an important factor in the recovery of lymphoid precursors and thymopoietic function. Given the modest number of patients studied to date, it will be important to conduct larger, multi-institutional studies examining a greater number of immune recovery endpoints before we can draw firm conclusions regarding the broader relevance of the findings of this single-institution study. Ultimately, we need to understand how prior therapies, age, conditioning regimen, and graft characteristics influence thymic regeneration and functional T-cell recovery.
Overall, our data support the generally accepted notion that attempts to improve outcome in the setting of CBT for adults will depend on the success of these approaches in improving T-cell immune reconstitution. Already, encouraging progress has been made in the development of clinical strategies aimed at increasing stem-cell dose, including ex vivo expansion of progenitor cells44,45 and the use of pooled CB units.9,46 It will be important to prospectively assess the influence of these various approaches on thymopoietic recovery and to consider immune recovery endpoints as a primary measure of the success of these trials, in addition to the typical primary endpoints (eg, date of neutrophil and platelet engraftment). Among approaches being considered, the use of Notch ligand-based approaches that may preferentially expand lymphoid progenitors deserves special consideration given our findings.47,48 Additional strategies, already demonstrated to have efficacy in murine models and in human clinical trials, include the use of thymoprotective agents (eg, keratinocyte growth factor or related family members)49–51 and the use of thymopoietic factors (eg, androgen ablation using leuprolide, IL-7 administration, and growth hormone administration).52,53 Finally, the ex vivo expansion of naive cord blood cells in the presence of viral antigens may offer a solution to the elusive goal of adoptively transferring antigen-specific memory to CB recipients.54,55 Although such strategies are attractive, our data also suggest that attention should be paid to the activation status, differentiation stage, and cytokine production signatures of expanded cell populations, because it is possible that in vitro stimulation might favor the production of late-stage memory cells that may be functionally ineffective or proliferatively impaired or even increase the otherwise manageable incidence of GVHD, which remains one of the principal advantages of CBT versus the use of other unrelated donor grafts.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health grants RO1 CA109326 (K.V.K.) and RO1 CA061508 (E.J.S.).
We thank Jeffrey Harris and Mike McCune for helping us to develop and optimize assays of thymopoiesis and for insightful comments on the manuscript. We also acknowledge the technical expertise of Michael Thomas and Michelle Davis and thank the patients, nurses, pharmacists, and staff of the M. D. Anderson Cancer Center stem cell transplant program, without whom this research would not have been possible.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.V.K. designed the study, analyzed the data, and wrote the manuscript; L.S.J. performed the primary research and analyzed the data; M.d.L. and R.E.C. designed the parent clinical trials; J.M. helped to design the parent clinical trials and contributed clinical data; S.R. and I.M. contributed to the study of control subjects; S.G.B., I.K., and S.M. performed the research; E.D.W. contributed valuable flow cytometry expertise; L.W., D.P., and L.J.N.C contributed to the parent clinical trials; J.J.M. assisted with data analysis and interpretation; and E.J.S. designed the parent clinical trials and contributed to writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Krishna V. Komanduri, Transplant Immunology Section, Department of Blood and Marrow Transplantation, M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: kkomanduri@mdanderson.org.
References
- 1.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 4.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplantation. Rev Clin Exp Hematol. 2001;5:87–99. doi: 10.1046/j.1468-0734.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 7.McNiece I, Kubegov D, Kerzic P, Shpall EJ, Gross S. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. doi: 10.1016/s0301-472x(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 8.Hofmeister CC, Zhang J, Knight KL, Le P, Stiff PJ. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39:11–23. doi: 10.1038/sj.bmt.1705538. [DOI] [PubMed] [Google Scholar]
- 9.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 10.Barker JN, Wagner JE. Umbilical cord blood transplantation: current state of the art. Curr Opin Oncol. 2002;14:160–164. doi: 10.1097/00001622-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Talvensaari K, Clave E, Douay C, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood. 2002;99:1458–1464. doi: 10.1182/blood.v99.4.1458. [DOI] [PubMed] [Google Scholar]
- 12.Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001;29:371–379. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 13.Niehues T, Rocha V, Filipovich AH, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children – a Eurocord analysis. Br J Haematol. 2001;114:42–48. doi: 10.1046/j.1365-2141.2001.02900.x. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli F, Maccario R, Comoli P, et al. Hematopoietic and immune recovery after transplantation of cord blood progenitor cells in children. Bone Marrow Transplant. 1996;18:1095–1101. [PubMed] [Google Scholar]
- 15.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 16.Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007;9:111–122. doi: 10.1016/j.bbmt.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdemir E, St John LS, Gillespie G, et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood. 2002;100:3690–3697. doi: 10.1182/blood-2002-05-1387. [DOI] [PubMed] [Google Scholar]
- 19.Poulin JF, Viswanathan MN, Harris JM, et al. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 21.Lewin SR, Heller G, Zhang L, et al. Direct evidence for new T-cell generation by patients after either T-cell-depleted or unmodified allogeneic hematopoietic stem cell transplantations. Blood. 2002;100:2235–2242. [PubMed] [Google Scholar]
- 22.Douek DC, Vescio RA, Betts MR, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- 23.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:547–581. [Google Scholar]
- 25.Storek J, Dawson MA, Maloney DG. Normal T, B, and NK cell counts in healthy donors at 1 year after blood stem cell harvesting. Blood. 2000;95:2993–2994. [PubMed] [Google Scholar]
- 26.Komanduri KV, Donahoe SM, Moretto WJ, et al. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology. 2001;279:459–470. doi: 10.1006/viro.2000.0697. [DOI] [PubMed] [Google Scholar]
- 27.Komanduri KV, Feinberg J, Hutchins RK, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis. 2001;183:1285–1289. doi: 10.1086/319683. [DOI] [PubMed] [Google Scholar]
- 28.Komanduri KV, Viswanathan MN, Wieder ED, et al. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4:953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 29.Harris JM, Hazenberg MD, Poulin JF, et al. Multiparameter evaluation of human thymic function: interpretation and caveats. Clin Immunol. 2005;115:138–146. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 31.Ruprecht CR, Gattorno M, Ferlito F, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Brenchley JM, Karandikar NJ, Betts MR, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 34.d'Angeac AD, Monier S, Pilling D, Travaglio-Encinoza A, Reme T, Salmon M. CD57+ T lymphocytes are derived from CD57- precursors by differentiation occurring in late immune responses. Eur J Immunol. 1994;24:1503–1511. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]
- 35.Klaus G, Mostert K, Reckzeh B, Mueller TF. Phenotypic changes in lymphocyte subpopulations in pediatric renal-transplant patients after T-cell depletion. Transplantation. 2003;76:1719–1724. doi: 10.1097/01.TP.0000100396.81490.0C. [DOI] [PubMed] [Google Scholar]
- 36.Cabatingan MS, Schmidt MR, Sen R, Woodland RT. Naive B lymphocytes undergo homeostatic proliferation in response to B cell deficit. J Immunol. 2002;169:6795–6805. doi: 10.4049/jimmunol.169.12.6795. [DOI] [PubMed] [Google Scholar]
- 37.Malaspina A, Moir S, Chaitt DG, et al. Idiopathic CD4+ T lymphocytopenia is associated with increases in immature/transitional B cells and serum levels of IL-7. Blood. 2007;109:2086–2088. doi: 10.1182/blood-2006-06-031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malaspina A, Moir S, Ho J, et al. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrier RD, Freeman WR, Wiley CA, McCutchan JA. Immune predispositions for cytomegalovirus retinitis in AIDS. J Clin Invest. 1995;95:1741–1746. doi: 10.1172/JCI117851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen G, Carter SL, Weinberg KI, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant. 2006;12:1335–1342. doi: 10.1016/j.bbmt.2006.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 42.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 43.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 45.Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 46.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Curr Opin Immunol. 2006;18:571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 48.Delaney C, Varnum-Finney B, Aoyama K, Brashem-Stein C, Bernstein ID. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossi S, Blazar BR, Farrell CL, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 51.Alpdogan O, Hubbard VM, Smith OM, et al. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood. 2006;107:2453–2460. doi: 10.1182/blood-2005-07-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 53.Alpdogan O, Schmaltz C, Muriglan SJ, et al. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. [DOI] [PubMed] [Google Scholar]
- 54.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 55.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006;108:1770–1773. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]