Abstract
A new hematopoietic cell transplantation–specific comorbidity index (HCT-CI) was effective in predicting outcomes among patients with hematologic malignancies who underwent HCT at Fred Hutchinson Cancer Research Center (FHCRC). Here, we compared the performance of the HCT-CI to 2 other indices and then tested its capacity to predict outcomes among 2 cohorts of patients diagnosed with a single disease entity, acute myeloid leukemia in first complete remission, who underwent transplantation at either FHCRC or M. D. Anderson Cancer Center (MDACC). FHCRC patients less frequently had unfavorable cytogenetics (15% versus 36%) and HCT-CI scores of 3 or more (21% versus 58%) compared with MDACC patients. We found that the HCT-CI had higher sensitivity and outcome predictability compared with the other indices among both cohorts. HCT-CI scores of 0, 1 to 2, and 3 or more predicted comparable nonrelapse mortality (NRM) among FHCRC and MDACC patients. In multivariate models, HCT-CI scores were associated with the highest hazard ratios (HRS) for NRM and survival among each cohort. The 2-year survival rates among FHCRC and MDACC patients were 71% versus 56%, respectively. After adjustment for risk factors, including HCT-CI scores, no difference in survival was detected (HR: 0.98, P = .94). The HCT-CI is a sensitive and informative tool for comparing trial results at different institutions. Inclusion of comorbidity data in HCT trials provides valuable, independent information.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for many patients diagnosed with acute myeloid leukemia (AML).1 Cytogenetics and, to a lesser extent, age have been the most important factors predicting survival among patients with AML in first complete remission (CR).1–5 However, the literature has been devoid of a systematic assessment of the impact of comorbidities on the ability of AML patients to tolerate HCT. Therefore, it has been suggested to incorporate comorbidities into the risk-adapted decision-making for AML patients to improve outcome prediction, comparison of trial results from different institutions, and design of new clinical trials.6–8 Comorbidity indices have been extensively studied in the field of solid malignancies,9–11 while less work has been done in hematologic malignancies. Recently, a new HCT-specific comorbidity index (HCT-CI) has been modeled to effectively capture comorbidities and predict HCT outcomes in a cohort of patients with various hematologic malignancies, treated at FHCRC.12 It remained to be determined whether this index could yield reproducible information at other institutions and if it could be added to other established prognostic variables to refine estimates of outcomes of newly reported investigational treatments.13 Here, we took additional steps to validate the effectiveness of the HCT-CI. In a group of 224 patients with a single disease entity, AML in first CR, who underwent transplantation at 2 different institutions, Fred Hutchinson Cancer Research Center (FHCRC) and M. D. Anderson Cancer Center (MDACC), we investigated (1) the sensitivity and discriminative capacity of the HCT-CI compared with 2 other comorbidity indices, the Charlson Comorbidity Index (CCI)14 and the Adult Comorbidity Evaluation-27 (ACE-27),15 (2) the ability of the HCT-CI scores to predict outcomes among FHCRC and MDACC patients, and (3) the correlations between HCT-CI scores and patient age.
Patients and methods
Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. This retrospective analysis has been approved by the institutional review boards of the FHCRC and the MDACC.
Patients
Consecutive patients from both institutions, who had comorbidity data available, were included in this study after exclusion of FHCRC patients who had contributed to the initial development of the HCT-CI.12 Nine patients were excluded because of lack of sufficient data for scoring comorbidities. All 244 patients had a diagnosis of AML in first CR. Among those, 177 patients underwent transplantation at FHCRC, between 1990 and 2004, and 67 patients underwent transplantation at MDACC, between 1990 and 2001. Conditioning regimens were either myeloablative, including busulphan (BU) + cyclophosphamide (CY) ± other agents (77 FHCRC and 15 MDACC patients), cyclophosphamide + 12 Gy or higher total body irradiation (TBI) ± other agents (72 FHCRC and 29 MDACC patients), and BU + 12 Gy TBI (8 FHCRC patients); reduced-intensity, including BU + fludarabine (3 FHCRC and 7 MDACC patients), and fludarabine + melphalan (9 MDACC patients); or nonmyeloablative, including fludarabine + 2 Gy TBI (17 FHCRC patients) or fludarabine + cytarabine + idarubicin (7 MDACC patients). Patients and donors were matched for HLA-A, -B, and -C antigens by either intermediate resolution DNA typing or high-resolution techniques. HLA matching for -DRB1 and -DQB1 was done on the basis of allele-level typing.16
Based on cytogenetics, patients were divided into 3 risk groups: favorable, intermediate, and unfavorable, according to the criteria of the Southwest Oncology Group (SWOG)/Eastern Cooperative Oncology Group (ECOG).3
Assessment of comorbidities
Information concerning comorbidities was obtained from medical notes, pathology reports, and laboratory data. Comorbidity data were extracted by M. Sorror for FHCRC patients and by M. Shahjahan for MDACC patients. M. Sorror was assessed for the time required to score a sample of patients for comorbidities. All patients were assigned scores according to 3 comorbidity indices. The CCI was used as a general comorbidity measure, and its scores were deployed according to their original version.14 The ACE-27 has been developed by modifying an older index, the Kaplan-Feinstein index,17 which was originally constructed for patients with diabetes. ACE-27 specifically targeted patients diagnosed with solid malignancies. It represents a comprehensive measure incorporating 27 comorbidities graded as mild, moderate, or severe. The overall comorbidity scores were assigned based on the highest ranked single comorbidity (none [score 0], mild [score 1], moderate [score 2], or severe [score 3]), where score 3 is considered the highest. Per the ACE-27, score 1 was assigned to 1 or more mild comorbidity, score 2 was assigned to any single moderate comorbidity, and score 3 was given to either more than 1 moderate comorbidity or 1 or more severe comorbidities. The definitions of comorbidities per the ACE-27 were applied according to the comorbidity data collection form displayed on the Washington University School of Medicine website.18 Finally, the HCT-CI was used as the comorbidity measure under study. The HCT-CI assigned weights of 1, 2, or 3 to comorbidities according to their impact on nonrelapse mortality (NRM). The final evaluation of the number and seriousness of all comorbidities was represented by a summation of scores with no upper limit.12 The specific criteria of the 17 comorbidities included in the HCT-CI are described in Table 1.
Table 1.
Definitions and weighted scores of comorbidities as summarized by the hematopoietic cell transplantation–specific comorbidity index (HCT-CI)
| Comorbidities | Definitions | HCT-CI weighted scores |
|---|---|---|
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 1 |
| Cardiac | Coronary artery disease,* congestive heart failure, myocardial infarction, or EF of ≤50% | 1 |
| Inflammatory bowel disease | Crohn's disease or ulcerative colitis | 1 |
| Diabetes | Requiring treatment with insulin or oral hypoglycemic, but not controlled with diet alone | 1 |
| Cerebrovascular disease | Transient ischemic attacks or cerebrovascular accident | 1 |
| Psychiatric disturbance | Depression/anxiety requiring psychiatric consult and/or treatment at the time of HCT | 1 |
| Hepatic, mild | Chronic hepatitis, bilirubin >ULN to 1.5× ULN, or AST/ALT >ULN to 2.5× ULN | 1 |
| Obesity | Patients with a BMI of >35 for adults or with BMI-for-age percentile of ≥ 95th percentile for children | 1 |
| Infection | Documented infection or fever of unknown etiology requiring antimicrobial treatment before, during, and after the start of conditioning regimen | 1 |
| Rheumatologic | SLE, RA, polymyositis, mixed CTD, and polymyalgia rheumatica | 2 |
| Peptic ulcer | Requiring treatment | 2 |
| Moderate/severe renal | Serum creatinine >2 mg/dL†, on dialysis, or prior to renal transplantation | 2 |
| Moderate pulmonary | DLco and/or FEV1 66%-80% or dyspnea on slight activity | 2 |
| Prior solid malignancy | Treated at any time point in the patient's history, excluding nonmelanoma skin cancer | 3 |
| Heart valve disease | Except asymptomatic mitral valve prolapse | 3 |
| Severe pulmonary | DLco and/or FEV1 ≤65% or dyspnea at rest or requiring oxygen | 3 |
| Moderate/severe hepatic | Liver cirrhosis, bilirubin >1.5× ULN, or AST/ALT >2.5× ULN | 3 |
Data are from Sorror et al.12
EF indicates ejection fraction; ULN, upper limit of normal; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; CTD, connective tissue disease; DLco, diffusion capacity of carbon monoxide; and FEV1, forced expiratory volume in one second.
One or more vessel-coronary artery stenoses, requiring medical treatment, stent, or bypass graft.
To convert creatinine from milligrams per deciliter to micromoles per liter, multiply milligrams per deciliter by 88.4.
Statistical methods
Survival curves and probabilities were estimated using the Kaplan-Meier method, while cumulative incidence curves and probabilities for NRM were estimated using methods described elsewhere.19 Multivariate hazard ratios (HRs) for NRM and survival outcomes were estimated from Cox regression models, treating NRM and disease relapse/progression as competing risks where appropriate. The c-statistic or concordance index was used, as previously described,12 to compare the discriminative capacity of each of the 3 indices (predictive validity). For a binary end point (eg, NRM within 2 years), the c-statistic is identical to the area under a receiver operating characteristic curve. The c-statistic ranges from 0.5 to 1.0; a value of 0.5 indicates random predictions and a value of 1.0 indicates perfect separation of patients who die from patients who survive. A model with a c-statistic value of more than approximately 0.65 has some use in predicting the response of individual patients. Concurrent validity was evaluated using Pearson correlation coefficient. Although it is very difficult to determine cutoff points for correlation coefficients, because many factors influenced their values,20 correlation coefficients exceeding 0.40 were considered to be moderate, and those exceeding 0.75 were considered to be high.21
Results
Patient characteristics
Patients and disease characteristics are described in Table 2. Patients who underwent transplantation at FHCRC and MDACC had similar median ages (41 vs 39 years) and median intervals (4.8 vs 5.1 months) from diagnosis to HCT. Compared with MDACC patients, FHCRC patients were more frequently given non–TBI-based myeloablative (44% vs 24%), less frequently given reduced-intensity (2% vs 22%), and with similar frequency given TBI-based myeloablative (45% vs 43%) and nonmyeloablative (10% vs 10%) conditioning regimens. Cyclosporine/methotrexate and tacrolimus/methotrexate were the most frequent postgrafting immunosuppressive regimens for FHCRC and MDACC patients, respectively. FHCRC patients more frequently received marrow (65% vs 58%) and unrelated grafts (28% vs 15%), while they less frequently had recipient cytomegalovirus (CMV) seropositivity (55% vs 68%) and unfavorable cytogenetics (15% vs 36%) compared with MDACC patients.
Table 2.
Patient and disease characteristics
| Characteristic | FHCRC patients, n = 177 | MDACC patients, n = 67 |
|---|---|---|
| Median age at transplantation, y (range) | 41 (19-75) | 39 (19-67) |
| Median interval from diagnosis to HCT, mo | 4.8 | 5.1 |
| Conditioning regimens, % | ||
| Myeloablative, TBI-based | 45 | 43 |
| Myeloablative, non–TBI-based | 44 | 24 |
| Reduced-intensity* | 2 | 22 |
| Nonmyeloablative† | 10 | 10 |
| Postgrafting immunosuppression, % | ||
| CSP + MTX | 78 | 22 |
| Tacrolimus + MTX | 2 | 61 |
| CSP + MMF | 9 | 0 |
| Other | 11 | 17 |
| Hematopoietic cell source, % | ||
| G-PBMCs | 35 | 42 |
| Marrow | 65 | 58 |
| Donor type, % | ||
| HLA-matched sibling | 68 | 78 |
| HLA-mismatched related | 4 | 7 |
| HLA-matched unrelated | 22 | 15 |
| HLA-allele–mismatched unrelated | 3 | 0 |
| HLA-antigen–mismatched unrelated | 3 | 0 |
| Patient CMV serostatus, % | ||
| Negative | 45 | 32 |
| Positive | 55 | 68 |
| Cytogenetic risks,%‡ | ||
| Favorable | 3 | 3 |
| Intermediate | 82 | 61 |
| Unfavorable | 15 | 36 |
HCT indicates hematopoietic cell transplantation; TBI, total body irradiation; CSP, cyclosporine; MTX, methotrexate; MMF, mycophenolate mofetil; G-PBMC, granulocyte colony-stimulating factor–peripheral blood mononuclear cells; HLA, human leukocyte antigen; and CMV, cytomegalovirus.
Reduced-intensity conditioning regimens included fludarabine plus busulfan or melphalan.
Nonmyeloablative regimens included 2 Gy TBI plus or minus fludarabine or fludarabine/Ara-C/idarubicin.
Cytogenetic risks were assessed and broken down into 3 risk groups: favorable, intermediate, and unfavorable, according to the criteria of the Southwest Oncology Group (SWOG)/Eastern Cooperative Oncology Group.3
The sensitivity of the HCT-CI compared with CCI and ACE-27
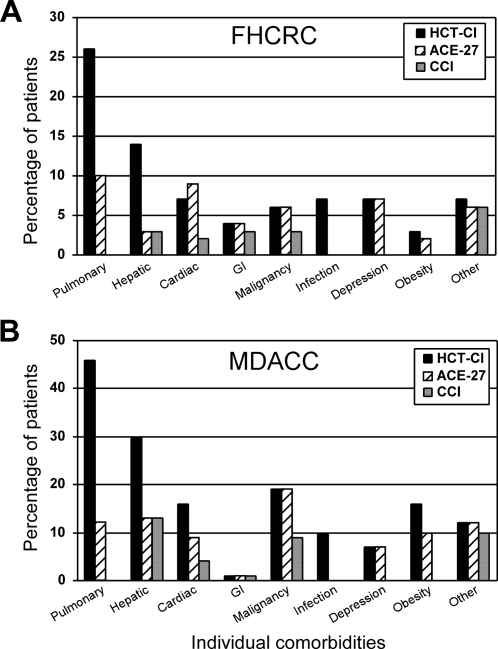
Higher percentages of pulmonary, hepatic, infection, and obesity comorbidities were detected by the HCT-CI compared with ACE-27 and CCI among both FHCRC and MDACC patients (Figure 1). HCT-CI and ACE-27 were comparable in capturing cardiac, prior malignancy, and depression comorbidities but with higher frequencies than the CCI. Almost no differences were seen between the 3 indices in defining other comorbidities.
Figure 1.
The distribution of individual comorbidities. Individual comorbidities among (A) FHCRC and (B) MDACC patients as captured by each of the 3 indices, the HCT-CI, the ACE-27, and the CCI.
Comorbidity scores were collapsed into 3 groups to facilitate comparison between indices and later assessment of the predictive capacity (Table 3). These groups incorporated HCT-CI scores of 0, 1 to 2, and 3 or more, while the cutoffs for the ACE-27 and CCI were scores 0, 1, and 2 or more due to scarcity of patients with scores higher than 2. Nevertheless, the HCT-CI showed higher sensitivity with smaller proportions of patients with scores of 0 and higher proportions of patients with scores 1 to 2 and 3 or more compared with the other 2 indices among both FHCRC and MDACC patients.
Table 3.
Comorbidity groups as stratified by scores of the HCT-CI, the ACE-27, and the CCI among patients who underwent transplantation at FHCRC and MDACC
| Index/scores | Comorbidity group | FHCRC patients, %, n = 177 | MDACC patients, %, n = 67 |
|---|---|---|---|
| HCT-CI | |||
| 0 | Low | 51 | 21 |
| 1 to 2 | Intermediate | 28 | 21 |
| 3 or more | High | 21 | 58 |
| ACE-27 | |||
| 0 | Low | 65 | 48 |
| 1 | Intermediate | 26 | 30 |
| 2 or more | High | 9 | 22 |
| CCI | |||
| 0 | Low | 83 | 70 |
| 1 | Intermediate | 11 | 10 |
| 2 or more | High | 6 | 20 |
HCT-CI indicates hematopoietic cell transplantation–specific comorbidity index; ACE-27, adult comorbidity evaluation-27; and CCI, Charlson comorbidity index.
Using the Pearson correlation coefficient, fairly moderate correlations were found between the scores of all indices. HCT-CI and ACE-27 had the highest correlation (0.65), while the correlations between CCI and ACE-27 or HCT-CI were 0.62 and 0.53, respectively.
A sample of 10 patients was assessed for the median time required to evaluate and score comorbidities by the same investigator using the 3 different indices. The CCI consumed the least median time (5 minutes) compared with HCT-CI (7 minutes) and ACE-27 (15 minutes).
Prediction of HCT outcomes among all patients by the HCT-CI versus other indices
Cox regression models were constructed to assess the predictive capacity of each index for relapse, NRM, and survival among all patients (Table 4). These models were adjusted for age, stem-cell source, cytogenetic risk, donor type, conditioning type, and institution. The HCT-CI comorbidity groups were associated with more consistent linear increases in the adjusted HR for relapse, NRM, and survival compared with those of the ACE-27 and the CCI. The predictive capacity of the HCT-CI scores was more significant than that of ACE-27 or the CCI. The CCI scores were more significant in predicting outcomes compared with ACE-27 scores. Overall, the HCT-CI had higher c-statistics for discriminating NRM (0.69) and survival (0.71) compared with ACE-27 (0.59 and 0.58) and CCI (0.59 and 0.60), respectively.
Table 4.
The predictive capacity of the HCT-CI compared with ACE-27 and CCI for NRM, relapse, and survival among all patients as assessed by Cox regression models taking into account other risk factors
| Index/comorbidity group | Relapse |
NRM |
Survival |
|||
|---|---|---|---|---|---|---|
| HR* | P | HR* | P | HR* | P | |
| HCT-CI | .01 | <.00 | <.00 | |||
| Low | 1.0 | 1.0 | 1.0 | |||
| Intermediate | 2.74 | 3.95 | 3.68 | |||
| High | 2.67 | 7.16 | 5.60 | |||
| ACE-27 | .13 | .27 | .22 | |||
| Low | 1.0 | 1.0 | 1.0 | |||
| Intermediate | 1.54 | 1.53 | 1.59 | |||
| High | 2.10 | 0.69 | 1.23 | |||
| CCI | .03 | .08 | .01 | |||
| Low | 1.0 | 1.0 | 1.0 | |||
| Intermediate | 0.92 | 2.49 | 2.09 | |||
| High | 2.46 | 2.06 | 2.25 | |||
NRM indicates nonrelapse mortality; and HR, hazard ratio.
HR was adjusted for age, stem-cell source, cytogenetic risk, donor type, conditioning type, and institution type.
Impact of HCT-CI comorbidity groups on outcomes of FHCRC compared with MDACC patients
Using the HCT-CI (Figure 1), pulmonary (26% and 46%), hepatic (14% and 30%), cardiac (7% and 16%), prior malignancy (6% and 19%), and obesity (3% and 16%) were the most prevalent comorbidities with lower frequencies among FHCRC compared with MDACC patients, respectively. Hence, more frequent HCT-CI scores of 0 (51% vs 21%) and less frequent HCT-CI scores of more than 3 (21% vs 58%) were found among FHCRC versus MDACC patients, respectively (Table 2).
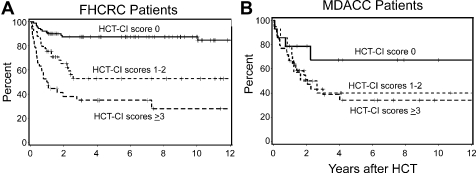
Overall, FHCRC and MDACC patients had 2-year cumulative incidences of NRM of 17% and 22%, respectively, and 2-year survival rates of 71% and 56%, respectively. FHCRC and MDACC patients with low (7% vs 7%, Figure 2A), intermediate (19% vs 21%, Figure 2B), or high (37% vs 27%, Figure 2C) HCT-CI risk groups had comparable cumulative incidences of NRM. Incremental increases of the cumulative incidences of NRM were seen among both cohorts with increasing HCT-CI risk groups. This impact on NRM was reflected in overall survival (OS; Figure 3), where decreasing survival rates were observed with increasing HCT-CI scores among both FHCRC and MDACC patients.
Figure 2.
Cumulative incidences of NRM. Comparison of NRM among FHCRC and MDACC patients as stratified by HCT-CI scores of (A) 0, (B) 1 to 2, and (C) 3 or more. Solid lines represent FHCRC patients; dashed lines represent MDACC patients.
Figure 3.
Kaplan-Meier curves for overall survival. OS as stratified by HCT-CI scores of 0, 1 to 2, and more than or equal to 3 among patients transplanted in (A) FHCRC and (B) MDACC.
Causes of NRM were noninfectious pulmonary complications alone (n = 8), associated with graft-versus-host disease (GVHD; n = 3), or associated with multiorgan failure (n = 1); pneumonia alone (n = 5) or associated with GVHD (n = 5); infection alone (n = 3), associated with renal insufficiency (n = 1), associated with graft failure (n = 2), associated with GVHD (n = 5), or associated with GVHD and renal insufficiency (n = 2); hepatic failure (n = 4); cardiac complications (n = 3); multiorgan failure (n = 2); cerebrovascular accident associated with meningoencephalitis (n = 1); and unknown (n = 1).
In multivariate analyses, HCT-CI scores consistently had the highest HR for risks of NRM and poor survival compared with other risk factors among both FHCRC and MDACC patients (Table 5). When all patients were included in the multivariate models, HCT-CI intermediate- and high-risk groups were independently significant for predicting increased HR of NRM (HR: 3.95 and 7.16, respectively, P < .001) and poor survival (HR: 3.68 and 5.60, respectively, P < .001). Other factors were not statistically significant with the exception of marrow grafts, which compared with peripheral blood grafts had higher risk for NRM (HR: 2.13, P = .05), and unrelated grafts, which compared with related grafts had worse survival (HR: 1.83, P = .01). No statistically significant differences were found between FHCRC and MDACC patients for NRM (HR: 0.69, P = .34) and survival (HR: 0.98, P = .94), after adjustment for other risk factors.
Table 5.
Multivariate analyses of risk factors for NRM and survival among FHCRC and MDACC patients
| Risk factor | NRM |
Survival |
||||||
|---|---|---|---|---|---|---|---|---|
| FHCRC |
MDACC |
FHCRC |
MDACC |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| HCT-CI risk | ||||||||
| Low | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| Intermediate | 3.43 | 1.3-9.2 | 4.93 | 0.4-55 | 3.31 | 1.6-6.9 | 2.60 | 0.7-9.6 |
| High | 8.12 | 3.0-22 | 5.05 | 0.6-43 | 7.45 | 3.6-15 | 2.32 | 0.8-7.1 |
| Cytogenetics | ||||||||
| Favorable/intermediate | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| Unfavorable | 1.61 | 0.6-4.2 | 1.19 | 0.4-3.9 | 1.44 | 0.7-3.1 | 1.07 | 0.5-2.3 |
| Donor | ||||||||
| Related | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| Unrelated | 2.16 | 0.9-5.0 | 0.44 | 0.1-3.7 | 1.91 | 1.0-3.6 | 1.61 | 0.6-4.4 |
| Stem-cell source | ||||||||
| G-PBMC | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| Marrow | 3.39 | 1.2-10 | 1.47 | 0.4-5.5 | 2.62 | 1.2-5.7 | 0.82 | 0.3-2.0 |
| Conditioning | ||||||||
| Ablative | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| Reduced-intensity | 0.00 | 0-Undefined | 2.51 | 0.6-11 | 3.85 | 0.7-20 | 1.62 | 0.6-4.3 |
| Nonablative | 0.81 | 0.1-4.5 | 0.87 | 0.1-11 | 0.87 | 0.3-3.0 | 0.57 | 0.1-3.3 |
| Age | ||||||||
| Younger than 50 y | 1.0 | — | 1.0 | — | 1.0 | — | 1.0 | — |
| 50 y or older | 1.64 | 0.7-3.8 | 0.83 | 0.2-3.6 | 1.74 | 0.9-3.3 | 0.93 | 0.3-2.7 |
— indicates not applicable.
Correlation of HCT-CI scores and age
Patients were separated into 4 age categories: younger than 40, 40 to 49, 50 to 59, and older than 59 years and 3 HCT-CI score groups; 0, 1 to 2, and 3 or more (Figure 4). Increasing age by decade was associated with poorer comorbidity scores. Only 2 patients (11%) older than 59 years had HCT-CI scores of 0 compared with 68 patients (55%) younger than 40 years. HCT-CI scores of 3 or more were found among 42% and 26% of those age groups, respectively.
Figure 4.
Correlation between HCT-CI scores and age. Distribution of HCT-CI scores of 0, 1 to 2, and 3 or more among 4 age categories; less than 40, 40 to 49, 50 to 59, and more than 59 years of all patients (n = 224) with AML in first CR transplanted at FHCRC and MDACC. HCT-CI score 0, white area; HCT-CI scores 1 to 2, gray area; HCT-CI scores more than or equal to 3, black area.
Discussion
Reliable comorbidity information, along with patient demographics, disease features, and treatment characteristics, has been found to be essential for comprehensive risk adjustment in patients with cancer.22–26 Accurate risk adjustment27 is the basis of observational research including comparison of outcomes of different treatment modalities. We are beginning to learn about the influence of multiple comorbidities on treatment outcomes of patients enrolled in HCT trials. Here, AML was chosen as a suitable disease entity for studying the impacts of comorbidities since it has been the disease for which HCT is carried out most frequently.28 Further, AML is a disease of the elderly,27 a population where comorbidities interfere substantially with curative treatment options.29 In addition, uncertainty still exists on how to identify suitable AML patients for different treatment modalities (reviewed in Deschler et al7), in part due to lack of incorporation of accurate assessment of medical problems in decision-making.30 First CR was preferred over advanced stages of AML primarily because comorbidity information may have greater impact in cancer patients with long compared with short life expectancy.15
The HCT-CI was a valid tool in comparing HCT trials for 2 patient cohorts diagnosed with the same disease and treated at 2 different institutions. First, the HCT-CI sensitively mapped the differences in comorbidity burden between FHCRC and MDACC patients (21% and 58% of patients had scores of 3 or more, respectively). Next, the HCT-CI risk groups predicted comparable incidences of NRM among both patient cohorts despite differences in conditioning regimens and other pretransplantation variables. Indeed, the impact of increasing HCT-CI scores on HCT outcomes at the 2 institutions was consistently the most influential risk factor compared with other variables in the multivariate models. Finally, the difference in the unadjusted OS rate in favor of FHCRC compared with MDACC patients (71% vs 56%) turned out to be negligible (HR: 0.98, P = .94) after adjustment for all pretransplantation variables. Lack of adjustment for the higher percentage of HCT-CI scores of 3 or more among MDACC versus FHCRC patients (58% vs 21%) would have resulted in biased conclusions. These results demonstrated how the universal use of comorbidity scoring would enhance the validity of result comparisons of clinical trials conducted at different academic institutions.
In the current analysis, HCT-CI scores correlated directly with age. This finding added to the construct validity of this index since it agreed with findings in the typical geriatric series.29 Improvement in supportive care and the advent of minimally toxic conditioning regimens have allowed to expand the age range for enrollment of AML patients in allogeneic HCT protocols (reviewed in de Lima and Giralt8 and Baron and Storb31). Therefore, comorbidity assessment appears important for treatment decisions in the HCT field. Moreover, SWOG trials of induction chemotherapy have shown that older AML patients presented with poorer performance status, and the combination of advanced age and poor performance status resulted in the worst outcome.5 Comorbidity scores seem to be independent of performance status in predicting treatment outcomes of solid32 as well as blood33,34 malignancies. It will be important in the future to evaluate the correlations and impacts of these 3 prognostic parameters on outcomes of AML patients.
Since we did not have a “gold standard” comorbidity index, we assessed the concurrent validity of the HCT-CI by comparing it with 2 already established indices, the CCI and ACE-27. CCI was considered a general comorbidity index known for its simplicity and wide-scale use.35–42 The ACE-27 had the advantages of being recently developed and incorporated some laboratory data to the definition of comorbidities. Even so, the HCT-CI was more sensitive in assigning scores to current patients (n = 140) compared with the CCI (n = 49) and ACE-27 (n = 97). This was mainly due to the incorporation of laboratory data to refine comorbidity definitions within the HCT-CI resulting in higher detection of pulmonary and hepatic comorbidities. Impaired pretransplantation pulmonary function tests were previously shown to predict both post-HCT noninfectious and infectious pulmonary complications,43–45 which actually constituted the majority of the current nonrelapse deaths.
The higher sensitivity of the HCT-CI resulted in fairly accurate assessment of comorbidity burdens as evidenced by higher predictive capacity for NRM and survival compared with the ACE-27 and the CCI. These findings supported the hypothesis that comorbidity indices that target specific patient populations would be superior to the more general indices. High comorbidity scores were also associated with increased relapse. This might be explained by the reported linkage between comorbidities such as prior malignancy,46 diabetes,47,48 obesity,49,50 autoimmune diseases,51–53 or smoking-associated lung disease54,55 and aggressive leukemia (reviewed in Extermann56). Cytogenetic risks did not independently predict overall mortality in the current analysis, which agreed with results from the SWOG study among patients who underwent allogeneic HCT.3
The HCT-CI was relatively simple to use in comparison with a comprehensive measure such as ACE-27. Comprehensive measures have the limitations of time consumption and possible lack of information needed for accurate assignment. In addition, the applicability of ACE-27 has been limited by the scoring methodology, where only 4 grades were allowed regardless of the numbers of comorbidities. In this report, for example, relatively poor OS (19%) was observed among patients with HCT-CI scores of 5 or more. Information generated from such a sensitive scoring system could be of importance in evaluating which patients would benefit from lower intensity conditioning and which patients would not benefit from HCT at all.
We were not able to address tolerability to different conditioning regimens due to the relatively small numbers of patients receiving nonconventional conditioning regimens. Collection of comorbidity data from large patient cohorts that underwent transplantation at multiple institutions is warranted to investigate such comparisons. Another potential limitation: the 2 cohorts of patients were not exactly concurrent. However, an adjustment for the impact of year of HCT did not alter any of the results (data not shown). The current comorbidity data in patients with AML were derived only from those patients who had already been offered HCT. Two recent reports have used the HCT-CI to define comorbidities and accurately predicted outcomes of elderly untreated AML patients given conventional or novel induction agents.57,58 Future prospective trials should address the roles of comorbidities in treatment decision-making for AML in first CR comparing allogeneic HCT versus autologous HCT versus conventional chemotherapy. The availability of such data could aid in determining the benefit of either one of the 3 treatment modalities for AML patients with low versus high comorbidity burden.
The present report provides an initial step for the use of comorbidity assessment in the understanding of differences between trial results obtained at different institutions. The data suggest that the HCT-CI should be the preferable comorbidity index among patients with hematologic malignancies. The incorporation of the HCT-CI in the future design of HCT clinical trials might improve treatment decision-making and provide the rationale for testing novel treatment modalities.
Acknowledgments
This work was supported by grants CA78902, CA18029, and CA15704 from the National Cancer Institute and grant HL088021 from the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD. Additional support was provided from the Paros Family Fund.
The authors thank the data coordinators, Chris Davis, Gary Schoch, Heather Hildebrant, and Jennifer Freese, and the study nurses, Mary Hinds, John Sedgwick, Michelle Bouvier, and Joanne Greene, for their invaluable help in making the study possible. Bonnie Larson, Helen Crawford, Sue Carbonneau, and Karen Carbonneau provided assistance with paper preparation. We also thank all the Transplant Teams at both institutions.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.L.S. designed the overall study, collected data, analyzed and interpreted the data, and wrote the paper; S.G. and B.M.S. contributed study patients and contributed to study design and to data interpretation; M.D.L. and D.G.M. contributed study patients; M.S. contributed to data collection; H.J.D. and F.R.A. contributed to data interpretation and revised the paper; B.E.S. performed the statistical analysis; R.S. contributed to study design, contributed to data analysis and interpretation, and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mohamed Sorror, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: msorror@fhcrc.org.
References
- 1.Suciu S, Mandelli F, de Witte T, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. 2003;102:1232–1240. doi: 10.1182/blood-2002-12-3714. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial: The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 3.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of pre-remission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 4.Ogawa H, Ikegame K, Kawakami M, et al. Impact of cytogenetics on outcome of stem cell transplantation for acute myeloid leukemia in first remission: a large-scale retrospective analysis of data from the Japan Society for Hematopoietic Cell Transplantation. Int J Hematol. 2004;79:495–500. doi: 10.1532/ijh97.03166. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelbaum FR, Pearce SF. Hematopoietic cell transplantation in first complete remission versus early relapse. Best Pract Res Clin Haematol. 2006;19:333–339. doi: 10.1016/j.beha.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Deschler B, de Witte T, Mertelsmann R, Lubbert M. Treatment decision-making for older patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: problems and approaches. Haematologica. 2006;91:1513–1522. [PubMed] [Google Scholar]
- 8.de Lima M, Giralt S. Allogeneic transplantation for the elderly patient with acute myelogenous leukemia or myelodysplastic syndrome (Review). Semin Hematol. 2006;43:107–117. doi: 10.1053/j.seminhematol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35:181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo JF, Costas I. The impact of comorbidity on outcomes. J Oto-Rhino-Laryngol Related Specialties. 2004;66:180–185. doi: 10.1159/000079875. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602. doi: 10.1097/00005537-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RB. HCT outcomes: a new tool? Blood. 2005;106:2602–2603. [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 16.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 17.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluatin the outcome of diabetes mellitus. J Chronic Dis. 1974;27:387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 18.Clinical Outcomes Research Office. St Louis: Washington University School of Medicine; [Accessed September 13, 2007]. Comorbidity data collection form. http://oto.wustl.edu/clinepi/Forms/com_form.doc. [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 20.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development. Oxford, United Kingdom: Oxford University Press; 2003. [Google Scholar]
- 21.Fleiss JL. The Design and Analysis of Clinical Experiments. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 22.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. The influence of symptoms, age, comorbidity and cancer site on physical functioning and mental health of geriatric women patients. Women Health. 1999;29:1–12. doi: 10.1300/J013v29n03_01. [DOI] [PubMed] [Google Scholar]
- 23.Greimel ER, Padilla GV, Grant MM. Physical and psychosocial outcomes in cancer patients: a comparison of different age groups. Br J Cancer. 1997;76:251–255. doi: 10.1038/bjc.1997.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Given CW, Given B, Azzouz F, Stommel M, Kozachik S. Comparison of changes in physical functioning of elderly patients with new diagnoses of cancer. Med Care. 2000;38:482–493. doi: 10.1097/00005650-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 25.van Spronsen DJ, Janssen-Heijnen ML, Lemmens VE, Peters WG, Coebergh JW. Independent prognostic effect of co-morbidity in lymphoma patients: results of the population-based Eindhoven Cancer Registry. Eur J Cancer. 2005;41:1051–1057. doi: 10.1016/j.ejca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 26.van Spronsen DJ, Janssen-Heijnen ML, Breed WP, Coebergh JW. Prevalence of co-morbidity and its relationship to treatment among un-selected patients with Hodgkin's disease and non-Hodgkin's lymphoma, 1993-1996. Ann Hematol. 1999;78:315–319. doi: 10.1007/s002770050521. [DOI] [PubMed] [Google Scholar]
- 27.Risk Adjustment for Measuring Healthcare Outcomes. Ann Arbor, MI: Health Administration Press; 2003. [Google Scholar]
- 28.Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A, Niederwieser D. Results of the EBMT activity survey 2005 on haematopoietic stem cell transplantation: focus on increasing use of unrelated donors. Bone Marrow Transplant. 2007;39:71–87. doi: 10.1038/sj.bmt.1705555. [DOI] [PubMed] [Google Scholar]
- 29.Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women's Health and Aging Study. J Clin Epidemiol. 1999;52:27–37. doi: 10.1016/s0895-4356(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 30.Sekeres MA, Stone RM, Zahrieh D, et al. Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18:809–816. doi: 10.1038/sj.leu.2403289. [DOI] [PubMed] [Google Scholar]
- 31.Baron F, Storb R. Hematopoietic cell transplantation after reduced-intensity conditioning for older adults with acute myeloid leukemia in complete remission. Curr Opin Hematol. 2007;14:145–151. doi: 10.1097/MOH.0b013e3280168462. [DOI] [PubMed] [Google Scholar]
- 32.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 33.Sorror M, Storer B, Sandmaier BM, et al. The joint role of comorbidity and performance status (PS) in predicting morbidity after allogeneic nonmyeloablative hematopoietic cell transplantation (HCT) [abstract]. Blood. 2006;108(pt 1):180a. Abstract no. 596. [Google Scholar]
- 34.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:954–964. doi: 10.1016/j.bbmt.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Di Iorio B, Cillo N, Cirillo M, De Santo NG. Charlson Comorbidity Index is a predictor of outcomes in incident hemodialysis patients and correlates with phase angle and hospitalization. Int J Artif Organs. 2004;27:330–336. doi: 10.1177/039139880402700409. [DOI] [PubMed] [Google Scholar]
- 36.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies: Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:357–364. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 37.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35:1941–1945. doi: 10.1161/01.STR.0000135225.80898.1c. [DOI] [PubMed] [Google Scholar]
- 38.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–132. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 39.Lubke T, Monig SP, Schneider PM, Holscher AH, Bollschweiler E. Does Charlson-comorbidity index correlate with short-term outcome in patients with gastric cancer? [German]. Zentralblatt fur Chirurgie. 2003;128:970–976. doi: 10.1055/s-2003-44805. [DOI] [PubMed] [Google Scholar]
- 40.Sabin SL, Rosenfeld RM, Sundaram K, Har-el G, Lucente FE. The impact of comorbidity and age on survival with laryngeal cancer. Ear Nose Throat J. 1999;78:578, 581–584. [PubMed] [Google Scholar]
- 41.Sachdev M, Sun JL, Tsiatis AA, Nelson CL, Mark DB, Jollis JG. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol. 2004;43:576–582. doi: 10.1016/j.jacc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 42.Singh B, Bhaya M, Stern J, et al. Validation of the Charlson comorbidity index in patients with head and neck cancer: a multi-institutional study. Laryngoscope. 1997;107:1469–1475. doi: 10.1097/00005537-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Crawford SW, Fisher L. Predictive value of pulmonary function tests before marrow transplantation. Chest. 1992;101:1257–1264. doi: 10.1378/chest.101.5.1257. [DOI] [PubMed] [Google Scholar]
- 44.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172:384–390. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horak DA, Schmidt GM, Zaia JA, Niland JC, Ahn C, Forman SJ. Pretransplant pulmonary function predicts cytomegalovirus-associated interstitial pneumonia following bone marrow transplantation. Chest. 1992;102:1484–1490. doi: 10.1378/chest.102.5.1484. [DOI] [PubMed] [Google Scholar]
- 46.Witherspoon RP, Deeg HJ, Storer B, Anasetti C, Storb R, Appelbaum FR. Hematopoietic stem-cell transplantation for treatment-related leukemia or myelodysplasia. J Clin Oncol. 2001;19:2134–2141. doi: 10.1200/JCO.2001.19.8.2134. [DOI] [PubMed] [Google Scholar]
- 47.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 48.Abe S, Funato T, Takahashi S, et al. Increased expression of insulin-like growth factor i is associated with Ara-C resistance in leukemia. Tohoku J Exp Med. 2006;209:217–228. doi: 10.1620/tjem.209.217. [DOI] [PubMed] [Google Scholar]
- 49.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 50.Chiu BC, Gapstur SM, Greenland P, Wang R, Dyer A. Body mass index, abnormal glucose metabolism, and mortality from hematopoietic cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2348–2354. doi: 10.1158/1055-9965.EPI-06-0007. [DOI] [PubMed] [Google Scholar]
- 51.Patapanian H, Graham S, Sambrook PN, et al. The oncogenicity of chlorambucil in rheumatoid arthritis. Br J Rheumatol. 1988;27:44–47. doi: 10.1093/rheumatology/27.1.44. [DOI] [PubMed] [Google Scholar]
- 52.Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with −7/7q-. Cancer Genetics Cytogenet. 1998;104:94–97. doi: 10.1016/s0165-4608(97)00456-1. [DOI] [PubMed] [Google Scholar]
- 53.Vineis P, Crosignani P, Sacerdote C, et al. Haematopoietic cancer and medical history: a multicentre case control study. J Epidemiol Community Health. 2000;54:431–436. doi: 10.1136/jech.54.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chelghoum Y, Danaila C, Belhabri A, et al. Influence of cigarette smoking on the presentation and course of acute myeloid leukemia. Ann Oncol. 2002;13:1621–1627. doi: 10.1093/annonc/mdf269. [DOI] [PubMed] [Google Scholar]
- 55.Thomas X, Chelghoum Y. Cigarette smoking and acute leukemia (Review). Leuk Lymphoma. 2004;45:1103–1109. doi: 10.1080/10428190310001638904. [DOI] [PubMed] [Google Scholar]
- 56.Extermann M. Interaction between comorbidity and cancer (Review). Cancer Control. 2007;14:13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 57.Giles F, Rizzieri D, Karp J, et al. Cloretazine (VNP40101M), a novel sulfonylhydrazine alkylating agent, in patients age 60 years or older with previously untreated acute myeloid leukemia. J Clin Oncol. 2007;25:25–31. doi: 10.1200/JCO.2006.07.0961. [DOI] [PubMed] [Google Scholar]
- 58.Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. doi: 10.1111/j.1365-2141.2006.06476.x. [DOI] [PubMed] [Google Scholar]