Abstract
Estrogen is known to increase progesterone receptor (PR) levels in the wild-type mouse uterus, and this estrogen induction was thought to be important for progesterone action through the PR. The estrogen receptor α knockout (ERKO) mouse uterus was observed to express PR mRNA that cannot be induced by estrogen. Progesterone action was characterized to determine whether it was diminished in ERKO mice. The PR protein is present in the ERKO uterus at 60% of the level measured in a wild-type uterus. The PR-A and PR-B isoforms are both detected on Western blot, and the ratio of isoforms is the same in both genotypes. Although the level of PR is reduced in the ERKO uterus, the receptor level is sufficient to induce genomic responses, since both calcitonin and amphiregulin mRNAs were increased after progesterone treatment. Finally, the ERKO uterus can be induced to undergo a progesterone-dependent decidual response. Surprisingly, the decidual response is estrogen independent in the ERKO, although it remains estrogen dependent in a wild type. These results indicate that estrogen receptor α modulation of PR levels is not necessary for expression of the PR or genomic and physiologic responses to progesterone in the ERKO uterus.
The reproductive cycle of the female mouse involves a complex interplay of several endocrine organs and hormones. The uterus must be exposed to the proper hormonal environment for successful implantation of fertilized oocytes and full-term development. Ovarian progesterone and estradiol are the primary hormones responsible for preparing the uterine endometrium for implantation. Estrogen is important for the proliferation of the uterine epithelium (1) and also sensitizes it to progesterone action by inducing progesterone receptor (PR) gene expression (2). Progesterone plays a role in the proliferation, differentiation, and maintenance of the stroma (1). In addition, progesterone is critical for initiation and maintenance of the decidualization reaction of the uterine stroma that occurs in response to implantation. This reaction is a massive transformation of uterine stromal cells to decidual cells that occurs at implantation sites.
We have developed and characterized a mouse with a disruption of the estrogen receptor α (ERα) gene that is infertile (3); however, ovarian steroidogenesis is uncompromised, resulting in production of both progesterone and estradiol (4). The ERα knockout (ERKO) uterus lacks an epithelial proliferative response to estrogen as measured by [3H]thymidine incorporation or increased uterine weight (3, 4). Furthermore, the mRNAs of estrogen-responsive genes, such as glucose-6-phosphate dehydrogenase, lactoferrin, and PR, are not induced in the ERKO uterus (4). Because estradiol normally induces PR mRNA and protein in the mouse uterus, estradiol was postulated to be essential for progesterone action. It was presumed that by removing ERα function in the ERKO, PR function would be “ablated” as well (5). However, PR mRNA is detected in the ERKO uterus but not induced by estradiol (4). The amount of PR mRNA in the uterus of ovariectomized (ovex) ERKO is comparable to the constitutive level of PR mRNA in an ovex wild type (WT) (4). Because PR mRNA was detected in the ERKO uterus, the PR protein level was measured by using a R5020 binding assay. The size and relative amounts of PR isoforms were also analyzed by Western blot to determine whether the lack of ERα altered these biochemical characteristics of the PR. To determine whether the PR expressed in the ERKO uterus was functional and of sufficient level to mediate a response to progesterone, induction of the progesterone- responsive genes calcitonin and amphiregulin was measured.
The decidualization reaction is a progesterone-dependent physiologic response of the uterine stroma that occurs at implantation. The ERKO female does not ovulate; therefore, a natural decidual transformation of the ERKO uterine stroma cannot be evaluated. A decidual transformation was induced experimentally to determine whether the PR in the ERKO uterus was capable of mediating this physiological response. Our findings show that, like the progesterone-dependent genomic responses, the lack of ERα-mediated induction of PR levels did not prevent decidualization in the ERKO. Surprisingly, decidualization is estrogen independent in the ERKO but estrogen dependent in the WT. Thus, estrogen induction of PR is not required for progesterone action to occur in the ERKO.
MATERIALS AND METHODS
Animals.
All mice were treated according to an approved National Institute of Environmental Health Sciences (NIEHS) protocol in accordance with National Institutes of Health (NIH) guidelines for humane use of animals in research. The generation and characterization of the ERKO mouse has been reported previously (3, 4, 6).
Materials.
[3H]R5020 and R5020, a synthetic progesterone agonist, were from NEN. Estradiol was from Research Plus (Bayonne, NJ). Progesterone and sesame oil were from Sigma.
Binding Assay.
Uteri were removed from sexually mature females (12–16 weeks old) of all three ERα genotypes, snap frozen in liquid nitrogen, and pulverized with a mortar and pestle. Tissue was homogenized with two to three 10-sec bursts at setting number 7 in TEGTH buffer (10 mM Tris, pH 7.6/1.5 mM EDTA/30% glycerol/3 mM MgCl2/0.1% 3-mercapto-1,2-propanediol) supplemented with a protease inhibitor mixture (50 μg/ml each of antipain, chymostatin, soybean trypsin inhibitor, and 3 mM EGTA) by using a Brinkmann polytron homogenizer. The homogenate was filtered through a Nitex-lined funnel and then centrifuged at 2,500 × g for 10 min at 4°C to pellet the nuclei. The supernatant was collected and centrifuged at 200,000 × g for 50 min at 4°C to clear the cytosol. Nuclei were washed twice with ice-cold TGM (10 mM Tris, pH 7.6/10% glycerol/3 mM MgCl2, and resuspended in TGM. Nuclear suspensions and cytosols were incubated with 20 nM [3H]R5020 with or without a 200-fold excess of unlabeled R5020 either overnight at 4°C (cytosol) or for 45 minutes at 37°C (nuclear suspension). Protein-bound [3H]R5020 was absorbed to hydroxylapetite (HAP) as described previously (7) with the following modifications: the HAP suspension was in TGMTH and the HAP pellets were also washed with TGMTH. The radioactivity in the pellets was quantified by using a liquid scintillation counter (Beckmann), and specific binding was calculated by subtracting nonspecific binding (measured in the presence of 200-fold excess unlabeled R5020) from total binding of [3H]R5020. Values were normalized to total cytosol protein determined by using a Bio-Rad Protein Assay.
Western Blot Analysis.
Cytosols were prepared as described in the previous section, and then ammonium sulfate was added to 40% saturation and mixed for 1 hr at 4°C. The resulting precipitate was pelleted at 16,000 × g at 4°C for 30 min, resuspended in 25 μl TGMTH, and then denatured with 25 μl of 10% SDS. Protein levels were measured by using a bicinchoninic acid assay (Pierce). Fifty μg of cytosolic protein per lane was resolved by SDS/PAGE [8% acrylamide with Acryl Aide crosslinker (FMC)]. Protein was transferred to nitrocellulose by semidry blotting by using a Novoblot apparatus according to the manufacturer’s protocol (Pharmacia LKB). PR was detected by using h928, a monoclonal antibody (kindly provided by Dean Edwards) against the hinge region of the chicken PR (8, 9) and a secondary 125I-labeled anti-mouse IgG F(ab)2 fragment (Amersham).
Northern Analysis of Progesterone Responsive Genes.
Ovex WT or ERKO mice were treated as described below to induce calcitonin (10) or amphiregulin (11, 12) gene expression in the uterus. To induce calcitonin mRNA, ovex ERKO or WT mice were injected s.c. on day 1 with 600 ng estradiol in sesame oil (100 μl) or with vehicle (100 μl sesame oil). On days 2 to 4, mice were injected s.c. daily with 900 ng progesterone (in 200 μl sesame oil) or with vehicle (200 μl sesame oil). On day 5, animals were sacrificed, and the uteri were removed, snap frozen in liquid nitrogen, and pulverized with a mortar and pestle. Total RNA was prepared by using Trizol reagent (Life Technologies) and poly(A)+ RNA was isolated by using oligo(dT)-cellulose (Pharmacia) (13, 14). Three μg of poly(A)+ RNA was resolved on a formaldehyde gel (1% agarose) (15) and transferred by capillary blotting to Hybond N membrane (Amersham Pharmacia). RNA was immobilized on blots by using a Stratagene UV crosslinker and prehybridized as described previously (4). A rat calcitonin cDNA [kindly provided by I. Bagchi (10, 16)] was 32P-labeled by using random priming (Quick Prime kit, Amersham Pharmacia) and used to detect calcitonin mRNA.
To induce amphiregulin gene expression, mice were given a single s.c. injection of 1 mg progesterone (P; in 100 μl sesame oil), 1 mg progesterone + 250 ng estradiol (PE; in 100 μl sesame oil) or vehicle (100 μl sesame oil). Animals were sacrificed 4 hr later, and total uterine RNA was isolated from uterine tissue as described above. Ten μg of total RNA was resolved on a formaldehyde gel [1.2% agarose, Ambion Mix (Ambion, Austin, TX)] separated and transferred as described above. A mouse amphiregulin cDNA (bases 257–721) (GenBank accession no. L41352) was generated by reverse-transcription PCR and inserted into the Srf-1 site of PCRscript (Stratagene). 32P-labeled antisense riboprobe was generated with Ambion MAXIscript kit to detect amphiregulin mRNA.
Decidualization Assay.
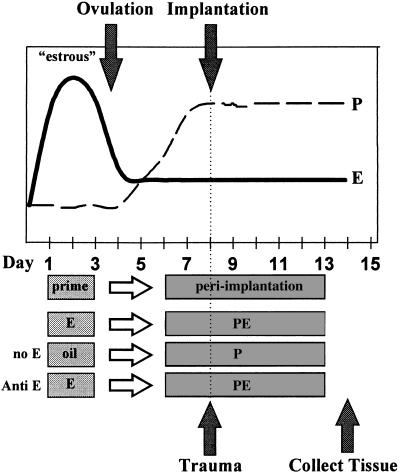
ERKO and WT animals were ovex and 10 days later were treated according to the method of Ledford (17) to mimic the progesterone and estradiol levels of early pregnancy as summarized in Fig. 1. In the priming phase, which mimics estrous, animals were given a daily s.c. injection of 100 ng estradiol (in 100 μl sesame oil) or vehicle (100 μl sesame oil) for 3 days. Two days later, animals were injected s.c. with 6.7 ng estradiol + 1 mg progesterone (PE; in 100 μl sesame oil) (to mimic the periimplantation period) or 1 mg progesterone (P; in 100 μl sesame oil) for 8 more days. Six hr following the third PE injection, the uterus was traumatized. Some mice received an i.p. injection of antiestrogen ICI 182,780 (30 μg in 50 μl DMSO) 30 min before estrogen priming and PE injections. As a control, mice of each genotype were treated with hormones but did not undergo uterine trauma. The morning after the final injection, animals were sacrificed by cervical dislocation and the uterus was removed, weighed, photographed, and fixed for histological analysis. Because of the diminished size of the ERKO uterus (3, 4) established methods to traumatize the uteri were not used. Therefore, a medicine dropper attached to a 3-cc hypodermic syringe was used to force sesame oil through the cervix. A tracheal tube was used to prevent backflow and to allow the oil to be forced through the cervical canals of anaesthetized mice.
Figure 1.
Schematic diagram of hormonal and surgical regimen used to induce decidual response. The top portion of the figure is a schematic representing the trends in progesterone (P) and estradiol (E) levels in the mouse during the estrous cycle and early pregnancy. Estradiol levels peak just before ovulation during the estrous phase and then drop, but remain above the basal level through the periimplantation period. The progesterone level begins to rise after ovulation and is maintained at a plateau if implantation occurs. To mimic this cycle, animals are given a priming dose of estradiol on days 1, 2, and 3 (E). After 2 days with no injection, mice are treated with progesterone and a low dose of estradiol (PE) on days 6–13. Six hr following the third P injection, the uterine lumen is traumatized as described in the Materials and Methods section to mimic implantation. The tissue is collected and analyzed on the day following the final injection. The hormonal regimen was altered to determine the role of estrogen by excluding estrogen from all injections (no E), or cotreating with antiestrogen ICI 182,780 (anti-E) with all injections.
RESULTS
PR levels were measured by [3H]R5020 binding in uterine tissue extracts from WT and ERKO mice. The level of labeled R5020 bound to extracted uterine protein from the WT and heterozygous (not shown) animals was the same (Table 1), although heterozygotes do have approximately one-half the level of ERα (4). The total (nuclear + cytosolic) [3H]R5020 binding in the ERKO uterus is approximately 60% of the WT level, and a greater proportion of binding is in the nuclear compartment compared with WT or heterozygote samples (24% in ERKO vs. 4.5% in WT). The amount of binding was decreased in uterine tissue from ovex WT and ERKO mice. However, ERKO mice retained 80% of the R5020 binding level of the ovexed WT (Table 1). No R5020 binding was detected in the nuclear fraction of uterine tissue samples from ovexed mice of either genotype.
Table 1.
Progesterone binding is decreased in ERKO uteri
| Genotype | Intact | Ovex |
|---|---|---|
| WT | 509 ± 23 [4.5%] | 153 ± 18 |
| ERKO | 338 ± 18 [24%] | 119 ± 26 |
The combined nuclear and cytosol binding of [3H]R5020 was measured in wt and ERKO uteri from intact or ovex animals, as described in Materials and Methods. Binding is expressed as specific (total − nonspecific) mol bound ×10−15 per mg total cystolic protein. Percentages in brackets indicate the percentage of binding detected in the nuclear fraction.
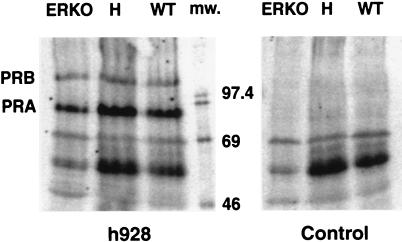
The PR gene encodes two protein isoforms (PR-A and PR-B) that are the products of different promoters (2). PR-B contains additional N-terminal sequences and can be antagonized by PR-A. These PR isoforms do not function identically, and the mechanism regulating their relative expression levels is not known. Because the R5020 binding assay indicated that uterine PR levels were reduced in the absence of ERα, the relative expression levels of the PR isoforms were also analyzed to determine whether their expression ratio was altered. Western blot analysis of uterine cytosolic protein shows that PR-A and PR-B isoforms are present in the same relative amounts in the ERKO and WT uterine extracts (Fig. 2). This result indicates that estrogen action is not required to regulate the relative amounts of PR-A and PR-B isoforms in the uterus.
Figure 2.
PR-A and PR-B isoforms are present in all three ERα genotypes. Cytosol from ERKO, heterozygous (H), and WT animals was analyzed by SDS/PAGE/Western blot as described in Materials and Methods. Two PR isoforms, PR-A and PR-B, are detected with anti-PR antibody h928, but not when primary antibody was not used (control). Size (in kDa) of 14C-labeled molecular weight markers (mw) is indicated.
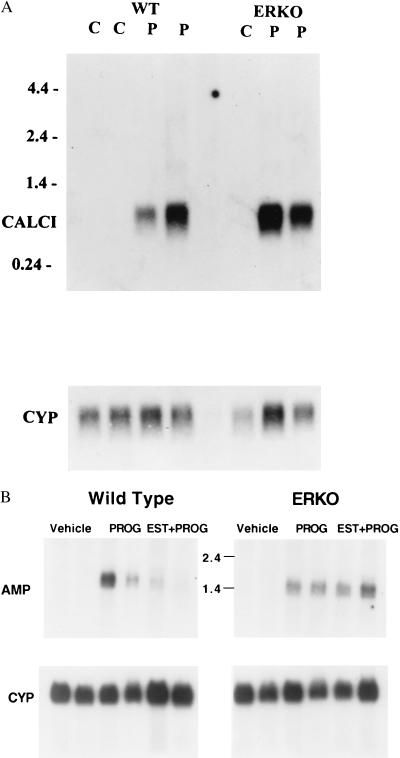
To determine whether the reduced PR levels in the ERKO uterus were still sufficient to mediate genomic responses, induction of progesterone-responsive genes was analyzed. Calcitonin is a hormone that prevents bone resorption and increases calcium secretion in the kidney. In the rat uterus, calcitonin mRNA was shown to be induced at the time of implantation and in response to progesterone treatment (10, 16). Estrogen “priming” or pretreatment, which increases PR levels, is not required for calcitonin induction, but does enhance it about 2-fold (10). Estradiol priming for 1 day followed by 3 days of progesterone treatment caused a robust increase in calcitonin mRNA levels (Fig. 3A) in both WT and ERKO ovex mice. When the level of calcitonin mRNA was normalized to cyclophilin mRNA, which is not regulated by estrogen or progesterone, the average fold induction of calcitonin mRNA in progesterone-treated ERKO mice (24.6-fold) was lower than that of the WT (78.7-fold; Table 2). Although PR levels are lower in the ERKO and are not induced by estrogen “priming” (4), the reduced level of PR is sufficient to mediate calcitonin mRNA induction.
Figure 3.
Calcitonin and amphiregulin mRNA induction by progesterone in the uterus is ERα independent. (A) Uterine poly(A)+ RNA (3 μg/lane) was analyzed by Northern blot as described in Materials and Methods. WT or ERKO mice were ovexed and then treated as described with vehicle (C) or estrogen primed for 1 day and then treated with progesterone for 3 days (P). Duplicate samples were prepared from identically treated animal sets and analyzed in adjacent lanes. The Northern blot was probed with calcitonin cDNA (CALCI) or cyclophilin riboprobe (CYP). The RNA size markers (in kb) are indicated on the upper left. (B) Total uterine RNA (10 μg/lane) was analyzed by Northern blot as described in Materials and Methods. WT or ERKO mice were ovexed and then treated as described with vehicle, progesterone alone (PROG), or estradiol and progesterone (EST + PROG) and sacrificed 4 hr later. Duplicate samples were prepared from identically treated animal sets and analyzed in adjacent lanes. The Northern blot was probed with amphiregulin riboprobe (Top) or cyclophilin riboprobe (Bottom). The position of RNA size markers is indicated in kb.
Table 2.
Calcitonin and amphiregulin are induced by progesterone in the ERKO uterus
| Genotype | WT | ERKO |
|---|---|---|
| Calcitonin | 78.7 ± 48 | 24.6 ± 1.7 |
| Amphiregulin (P) | 1.9 ± 0.6 | 2.55 ± 0.8 |
| Amphiregulin (P + E) | 0.42 ± 0.1 | 2.28 ± 1.5 |
The Northern blots in Fig. 3 were quantified by using a Molecular Dynamics Storm phosphoimager and image quant software. Calcitonin or amphiregulin signals were normalized to cyclophilin and were expressed as fold induction over vehicle control ± range of duplicate samples.
Because induction of calcitonin expression occurred after 3 days of progesterone treatment, a more rapid progesterone-induced gene response was also examined. Amphiregulin is an epidermal growth factor (EGF)-like hormone that activates the EGF receptor and was shown to be induced at the time of implantation in the mouse uterus (12). Amphiregulin mRNA can also be induced after 2 hr of progesterone treatment of ovex mice (12). Therefore, WT and ERKO mice were ovexed and treated with progesterone, and after 4 hr, uterine RNA was isolated and analyzed by Northern blot. Amphiregulin mRNA levels were induced in both genotypes (Fig. 3B; Table 2), indicating the PR levels in the ERKO were sufficient to mediate an acute genomic response. It has been shown previously that amphiregulin mRNA induction by progesterone was inhibited by cotreatment with estrogen (12). However, estradiol does not repress amphiregulin induction by progesterone in the ERKO, indicating ERα was involved in the mechanism of inhibition (Fig. 3B; Table 2).
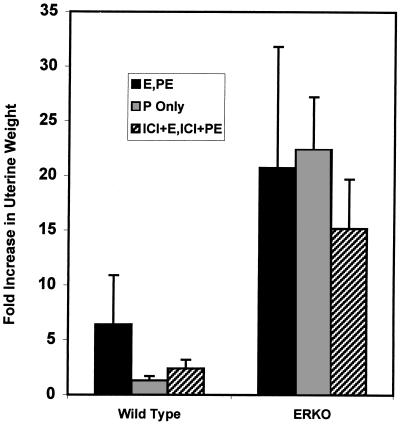
The ERKO mice are infertile because of ovarian dysfunction and a lack of uterine mitogenic response to estrogen (3, 18). Because the above biochemical studies indicate that the PR is present and functional in the ERKO uterus, we wanted to determine whether the ERKO could undergo the progesterone-dependent stromal decidualization that normally occurs in response to implantation. Because of the diminished size of the ERKO uteri (3, 4), physical trauma of the uterine lumen to experimentally induce a decidual reaction was not possible by using established techniques that involve intraluminal scratching or oil injection (5, 17). Instead, sesame oil was forced into the uterine horns through the cervix by using a medicine dropper attached to a 3-cc syringe. In a pilot study using this method on ovex CD-1 mice, five of six mice responded with the development of deciduomas and a 24-fold increase in uterine weight over nontraumatized uteri (data not shown). ERKO mice were estrogen primed and then treated with progesterone to mimic pregnancy and implantation (5, 17) (see Fig. 1). After trauma, 7 of 10 WT and 5 of 7 ERKO uteri responded with deciduoma formation (Table 3, Fig. 4). The ERKO animals averaged a 21-fold increase in uterine weight over nontraumatized controls, while the WT uteri showed only a 6.4-fold increase in uterine weight (Fig. 6).
Table 3.
Decidualization response is estrogen independent in the ERKO
| Treatment | WT | ERKO |
|---|---|---|
| E, PE | 70% (7/10) | 71% (5/7) |
| ICI + E ICI + PE | 10% (1/10) | 100% (5/5) |
| Oil, P only | 0% (0/3) | 100% (2/2) |
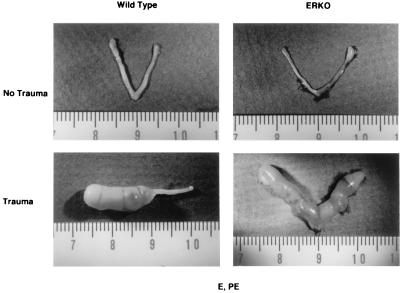
Figure 4.
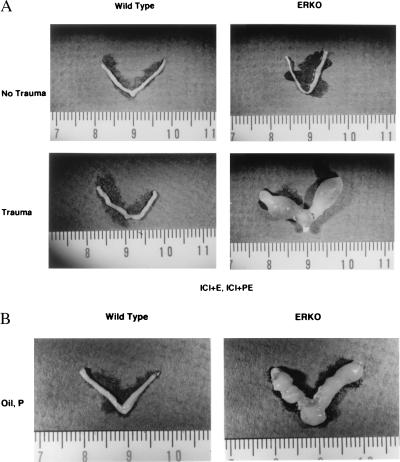
Decidualization occurs in ERKO uterus. WT and ERKO mice were treated as described in Fig. 1 and in Materials and Methods. Uteri (Lower) were traumatized on the third day of PE treatment as described. The WT uterus pictured (Lower) shows a decidual reaction in one horn, indicating that oil may have entered only one horn.
Figure 6.
Fold increase in uterine weight in responding uteri. Uteri were weighed and the average fold increase in weight of responding uteri was calculated in comparison with nontraumatized control uteri of the same genotype treated with the same hormonal regimen. Numbers of determinations: WT E, PE, n = 5; WT P only, n = 3; WT ICI, n = 10; ERKO E, PE, n = 5; ERKO P only, n = 2; ERKO ICI, n = 5.
The decidual response in the ERKO uterus was unexpected, since estrogen priming was thought to be essential for a decidual transformation. To define the role of estrogen in the mechanism of decidualization, mice were given the antiestrogen, ICI 182,780, with the daily estradiol and PE treatments as described in Materials and Methods. Only one of 10 WT uteri displayed a decidual reaction, while five of five ERKO uteri responded (Fig. 5A; Table 3). The increase in wet weight following the decidualization response was more robust in the ERKO group (15-fold increase) than in the WT (2.4-fold increase; Fig. 6). When no estrogen was used either at priming or with the progesterone, two of two ERKO uteri responded with a 22-fold increase in weight (Figs. 5B and 6; Table 3), while zero of three WT uteri responded. Thus, estrogen action through ERα is not necessary for decidualization in the ERKO. The ERKO uterus is fully progesterone responsive in terms of gene regulation and morphological changes of the tissue despite the absence of ERα.
Figure 5.
Decidualization is estrogen dependent in the WT but not in the ERKO. (A) WT and ERKO mice were given the hormonal regimen described above with the addition of the antiestrogen ICI 182–780 each day. Uteri (Lower) were traumatized as described. (B) WT and ERKO mice were treated as described in Fig. 4 with the omission of estrogen at each injection. Both uteri shown were traumatized as described.
DISCUSSION
The actions of estradiol and progesterone in the rodent reproductive tract are interrelated, yet at times oppose one another. For example, estrogen and progesterone treatment of the PR knockout mice resulted in abnormally enlarged fluid-filled uteri with abnormal microscopic appearance, presumably because of unopposed estrogen action (5). Because PR gene transcription is estrogen responsive (5), the ERKO mouse was analyzed to determine whether the activities of progesterone are compromised when there is no ERα-mediated response. PR mRNA is present in the ERKO uterus but does not increase in response to estrogen (4). Furthermore, PR protein is also present in the ERKO uterus, at 60% of the WT level in intact animals, and about 80% of the WT level in ovex mice. Interestingly, there was more progesterone binding in the nuclear fraction of the intact ERKO uterus. The ERKO uterus has a much thinner layer of stroma and fewer epithelial glands (3), therefore the relative size and distribution of cell types in the ERKO uterus differs from that of the WT. Thus, on homogenization, the relative partitioning of the PR into the nuclear compartment might be increased. The PR mRNA is not induced by estradiol in the ERKO; however, the level of PR protein as measured by R5020 binding decreased after ovariectomy (see Table 1). The decrease in PR following ovex indicates that ovarian factors other than estradiol may be involved in regulation of PR levels in the ERKO. Finally, both PR-A and PR-B were detected by Western blot, indicating that the ERα is not necessary for expression of PR or regulation of its isoforms in the mouse uterus. The constitutive level of PR is not induced by ERβ, as no ERβ mRNA can be detected in the uterus of either WT or ERKO mice (19), nor was there any attenuation of PR mRNA in the ERKO uteri following antiestrogen treatment (4).
Progesterone induces calcitonin and amphiregulin gene expression in the ERKO uterus, illustrating that in the absence of estrogen induction, the basal level of PR is capable of modulating expression of progesterone-responsive genes. Although the ERKO lacks estrogen action through ERα, the PR is present, and its biochemical characteristics (R5020 binding, molecular weight, relative amount of isoforms) are the same as those of PR in WT uteri. In addition, the genomic responses of the uterus to progesterone still occur in the ERKO, indicating that constitutive levels of PR are sufficient to mediate genomic and physiologic responses.
The function of progesterone during implantation and reproduction is strongly associated with estrogen and ERα signaling (2, 20). Most interesting is our observation that the decidualization reaction is estrogen dependent in the WT, but not in the ERKO uterus. Others have demonstrated the necessity for estrogen priming (at a time mimicking estrous) as well as a low dose of estrogen (at a time mimicking the periimplantation period) to induce decidualization with oil (see Fig. 1) (21–23). However, more traumatic stimuli, such as crushing the uterine horns, can produce decidualization without estrogen priming in the mouse (24), indicating that the mechanism of decidualization does not absolutely require estrogen. The threshold level of stimulus required for initiation of decidualization might be lowered by estrogen treatment in a WT. Similarly, the estrogen-independent decidualization in the ERKO may indicate that the ovexed ERKO uterine tissue is more sensitive, so that the threshold level of trauma required to initiate decidualization is as low as that of an estrogen-primed WT.
Although the role of estrogen in the mechanism of decidualization has not been defined, it is likely that estrogen induces genes required for progesterone to induce decidual transformation. However, the gene targets of estrogen in initiating and maintaining the deciduoma have not been identified. There is evidence that estrogen can induce several physiologic responses that might be involved in implantation and decidual transformation. These responses include histamine and prostaglandin release (reviewed in ref. 20), increased vascular permeability (23), and induction of various growth factor receptor ligands (12, 26, 27). Induction of PR, especially in the priming phase of the experiment, has been proposed to be a key component of the mechanism. Although PR is increased in both the stroma and epithelium, ERα-mediated induction of PR seems to occur mainly in the stroma, since antiestrogen treatment blocks stromal but not epithelial induction of PR mRNA (25). However, the lack of PR induction by estrogen in the ERKO indicates that the amount of PR expressed constitutively in the ERKO uterus is sufficient to mediate decidualization.
Mice treated with the antiestrogen ICI 182,780 have 20% of the pretreatment level of uterine ERα because of enhanced proteolytic degradation (28). Although the ICI-treated WT uterus is similar to the ERKO uterus with regard to diminished ERα levels, there is no decidual response. Thus, deciduoma induction in the ERKO independent of estrogen is not merely caused by the absence of ERα, but might be caused by altered development of the uterine tissue in the absence of ERα. The ERKO uterine cells may have been imprinted differently because they developed in the absence of estrogen/ERα signaling. This imprinting might alter the regulation of gene products or physiological responses required for decidualization so the uterus is sensitive to progesterone and trauma alone. Alternatively, the uterine cells may respond to decidualization-inducing factors that are estrogen regulated in the WT but expressed constitutively in the ERKO. Histological analysis did not reveal cellular differences between WT and ERKO deciduomas (data not shown), indicating that the ERKO undergoes a genuine decidual transformation.
There are several transgenic mouse models that are deficient in decidualization including the PR knockout mouse (5), the leukemia inhibitory factor (−/−) mouse (29), the COX-2 (−/−) mouse (30), and the Hoxa-10 knockout (31). Study of the expression and estrogen regulation of these gene products may help define the components needed for decidualization. In summary, this study has shown that estrogen action through ERα is not necessary for PR expression or function in terms of genomic response or induction of decidualization. In addition, the lack of ERα in the uterus alters the mechanism of physiological responses in such a way that estrogen dependence of decidualization is lost. Future studies using the ERKO mouse will enable us to explore the mechanism that might be altered.
Acknowledgments
I (S.W.C.) dedicate this paper to my father, Hans F. Weinberger, in honor of his 70th birthday and his retirement, because he gave me a life of science. The authors thank Dr. Joel Mahler for analysis of the deciduoma histology, Dr. Moto Taki for photography of the decidualized uteri, and the Comparative Medicine Branch technicians who bred and cared for the animals used in the studies. We also thank Drs. Gregg Richards and Jeff Webster for critical reading of the manuscript and Dr. Wayne Bocchinfuso for helpful discussions and editorial suggestions.
ABBREVIATIONS
- PR
progesterone receptor
- ERα
estrogen receptor α
- ERKO
ERα knockout
- ovex
ovariectomized
- WT
wild type
- P
progesterone
- PE
progesterone + estradiol
References
- 1.Weitlauf H M. In: The Physiology of Reproduction. Knobil E, Neill J, editors. New York: Raven; 1988. pp. 231–262. [Google Scholar]
- 2.Graham J D, Clarke C L. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 3.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 5.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Shyamala G, Conneely O M, O’Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 6.Couse J F, Davis V L, Tally W C, Korach K S. BioTechniques. 1994;17:1030–1032. [PubMed] [Google Scholar]
- 7.Korach K S. Endocrinology. 1979;104:1324–1332. doi: 10.1210/endo-104-5-1324. [DOI] [PubMed] [Google Scholar]
- 8.Weigel N L, Beck C A, Estes P A, Pendergast P, Altmann M, Christensen K, Edwards D P. Mol Endocrinol. 1992;6:1585–1597. doi: 10.1210/mend.6.10.1448113. [DOI] [PubMed] [Google Scholar]
- 9.Natraj U, Richards J S. Endocrinology. 1993;133:761–769. doi: 10.1210/endo.133.2.8344215. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y Q, Zhu L J, Bagchi M K, Bagchi I C. Endocrinology. 1994;135:2265–2274. doi: 10.1210/endo.135.5.7956949. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty I, Das S K, Wang J, Dey S K. J Mol Endocrinol. 1996;16:107–122. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 12.Das S K, Chakraborty I, Paria B C, Wang X N, Plowman G, Dey S K. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 13.Aviv H, Leder P. Proc Natl Acad Sci USA. 1972;69:1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edmonds M, Vaughan M H, Jr, Nakazato H. Proc Natl Acad Sci USA. 1971;68:1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maniatis T, Fritsch E F, Sarubrook J. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1982. [Google Scholar]
- 16.Ding Y Q, Bagchi M K, Bardin C W, Bagchi I C. Recent Prog Horm Res. 1995;50:373–378. doi: 10.1016/b978-0-12-571150-0.50023-8. [DOI] [PubMed] [Google Scholar]
- 17.Ledford B E, Rankin J C, Markwald R R, Baggett B. Biol Reprod. 1976;15:529–535. doi: 10.1095/biolreprod15.4.529. [DOI] [PubMed] [Google Scholar]
- 18.Korach K S. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 19.Couse J F, Lindzey J, Grandien K, Gustafsson J A, Korach K S. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 20.Weitlauf H M. In: The Physiology of Reproduction. Knobil E, Neill J, editors. New York: Raven; 1988. pp. 231–262. [Google Scholar]
- 21.Finn C A. J Endocrinol. 1966;36:239–248. doi: 10.1677/joe.0.0360239. [DOI] [PubMed] [Google Scholar]
- 22.Finn C A, Pope M. J Endocrinol. 1986;110:93–96. doi: 10.1677/joe.0.1100093. [DOI] [PubMed] [Google Scholar]
- 23.Milligan S R, Mirembe F M. J Reprod Fertil. 1985;74:95–104. doi: 10.1530/jrf.0.0740095. [DOI] [PubMed] [Google Scholar]
- 24.Finn C A. J Endocrinol. 1965;32:223–229. doi: 10.1677/joe.0.0320223. [DOI] [PubMed] [Google Scholar]
- 25.Das S K, Tan J, Johnson D C, Dey S K. Endocrinology. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X N, Das S K, Damm D, Klagsbrun M, Abraham J A, Dey S K. Endocrinology. 1994;135:1264–1271. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- 27.Lim H, Dey S K, Das S K. Endocrinology. 1997;138:1328–1337. doi: 10.1210/endo.138.3.4991. [DOI] [PubMed] [Google Scholar]
- 28.Gibson M K, Nemmers L A, Beckman W C, Jr, Davis V L, Curtis S W, Korach K S. Endocrinology. 1991;129:2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- 29.Stewart C L, Cullinan E B. Dev Genet. 1997;21:91–101. doi: 10.1002/(SICI)1520-6408(1997)21:1<91::AID-DVG11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Benson G V, Lim H J, Dey S K, Maas R L. Dev Biol. 1998;197:141–154. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]