Abstract
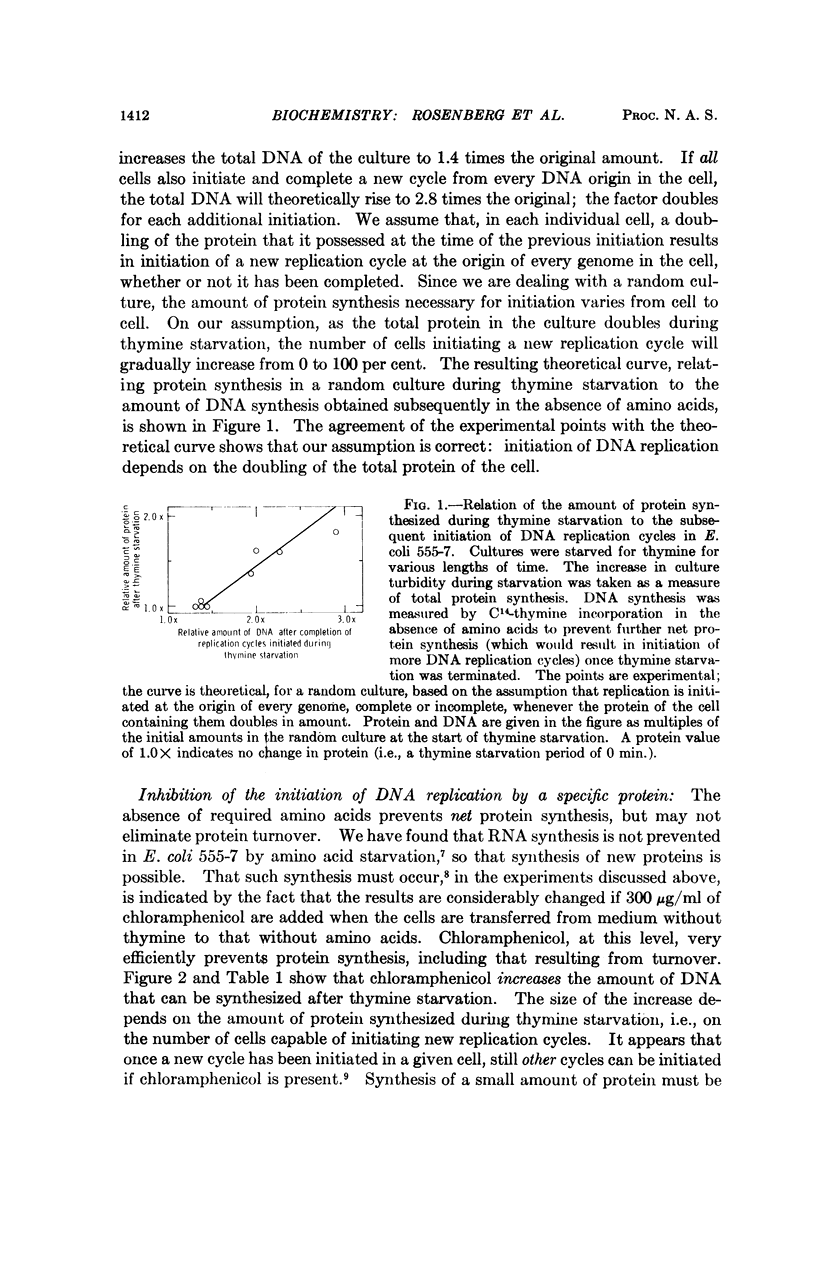
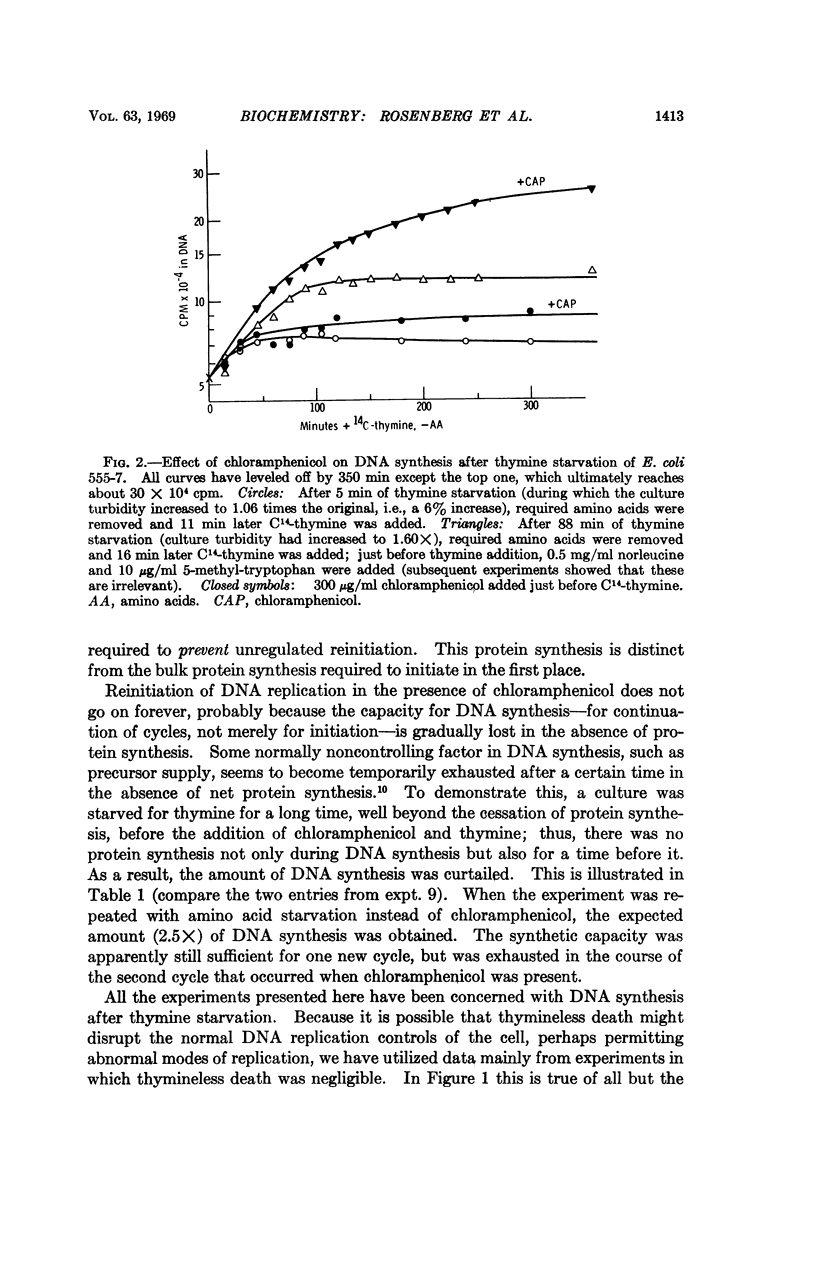
Evidence is presented to show that the initiation of DNA replication in E. coli 555-7 requires synthesis of a protein whose production is correlated with total protein synthesis. Once replication is initiated, however, reinitiation will occur if all further protein synthesis is prevented; a small amount of protein synthesis is sufficient to prevent this unregulated reinitiation. This shows that the initiation of DNA replication is under negative control. A mechanism for the control of DNA replication is proposed; in this mechanism a replication repressor is synthesized periodically, while an antirepressor protein is synthesized continuously. Derepression of initiation results after sufficient accumulation of the antirepressor protein, and repression is re-established by repressor synthesis after the initiation of replication.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R. F., Yanofsky C. The periodicity of RNA polymerase initiations: a new regulatory feature of transcription. Proc Natl Acad Sci U S A. 1968 May;60(1):313–320. doi: 10.1073/pnas.60.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. Transposition of the lac region of Escherichia coli. IV. Escape from repression in bacteriophage-carried lac genes. J Mol Biol. 1967 Dec 28;30(3):529–543. doi: 10.1016/0022-2836(67)90366-x. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



